Abstract
The search for a cell-supporting scaffold with controlled topography and surface chemistry is a constant topic within tissue engineering. Here we have employed M13 phages, which are genetically modifiable biological nanofibers (~880 nm long and ~6.6 nm wide) non-toxic to human beings, to form films for supporting the growth of mesencymal stem cells (MSCs). Films were built from nearly parallel phage bundles separated by grooves. The bundles can guide the elongation and alignment of MSCs along themselves. Phage with peptides displayed on the surface exhibited different control over the fine morphologies and differentiation of the MSCs. When an osteogenic peptide was displayed on the surface of phage, the proliferation and differentiation of MSCs into osteoblasts was significantly accelerated. The use of the grooved phage films allows us to control the proliferation and differentiation of MSCs by simply controlling the concentrations of phages as well as the peptides displayed on the surface of the phages. This work will advance our understanding on the interaction between stem cells and proteins.
Keywords: M13 phage, mesenchymal stem cells, differentiation, topography, surface chemistry
Introduction
Tissue engineering involves a system with specific biochemical functions and cells within an artificially-created scaffold. Finding a proper artificially-created scaffoldis vital for achieving engineered tissues. Cellular behaviors depend on substrate surface topography and surface chemistry[1]. Therefore, an effective scaffold includes one with a well-organized structure that allows cellular adhesion and growth. Another crucial property is a signaling environment that regulates resident cells’ behavior[2–5]. The organized fibrous structure guides tissue morphogenesis and remodeling[6]. To mimic the native fibrous structure, various nanofibrous scaffolds have been fabricated from biological and synthetic polymers, which yield tremendous potential for use in tissue engineering applications[7]. The main advantage of using nanofibersis their larger surface area to volume ratio, allowing for greater attachment of native extracellular matrix (ECM) ligands and growth factors onto fiber surfaces[7, 8]. Some groups have demonstrated that the patterning of nanofibers could easily be fabricated to affect cellular processes[9, 10]. However, a facile way to introduce signaling motifs onto the fiber surface for controlling cellular processes is still not available.
MSCs are multipotent stem cells that can differentiate into osteoblasts, chondrocytes and adipocytes[1]. Because of their broader differentiation ability, a significant number of studies have been focused on MSCs for their potential applications in the repair of fat tissue, cartilage, and more significantly, bone regeneration[11, 12]. By investigating the cellular behavior of MSCs cultured in vitro on a specifically functionalized scaffold, it would be possible to provide an understanding of the effects that the local niche may have on the stem cell response as well as obtain a strategy for directing the formation of bone in vivo.
The response of MSCs to different nanomaterials including titania nanotubes[13], virus films[14], and carbon nanotubes[15], has been studied. There have been many reports on the response of MSCs to different surface chemistry such as small functional groups[16, 17] and proteins[18]. However, there has been no report on the response of MSCs on scaffolds bearing different genetically displayed peptide sequences. Filamentous phage is unique in this application because it is easy to introduce different peptides to the surface of the phage by genetic means. As a result, it will enable us to systematically investigate the proliferation and differentiation of MSCs on a scaffold with same architectures but bearing different peptides..
The M13 bacteriophage used in this work is a flexible nanofiber with a width of ~6.6 nm and a length of ~880 nm[19]. The benefit of phage display technology lies in the fact that the surface chemistry of a phage nanofiber is easily controlled by genetically displaying foreign functional peptides on phage major coat proteins. At high concentrations, both wild type and engineered phages can self-assemble into long-range ordered micro-filamentous structures that effectively mimicnatural extracellular matrix fibers[20–22]. Investigations using animal studies have shown that genetically engineered phage nanofibers can be placed directly into animal or human bodies for disease treatment: (1) phage can carry drugs on its coat, be infused into nose, enter the brain and then deliver the drugs to brain to treat brain diseases without inducing adverse events in animals[23]; (2) phage can be circulated in the body to identify a peptide that recognizes a specific tissue or organ (e.g., brain)[24]; (3) phage with peptides displayed on the coat can be injected into the human body to bind to tumors in a human cancer patient without inducing obvious toxicity and immune response[25]. It has been demonstrated that filamentous phage can serve as targeting vehicles for delivering drugs and imaging probes for human medical treatments[26, 27]. Currently, there have been no reports on toxic effects on mammalian cells caused by phage possibly due to specificity against their host bacterial cells rather than mammalian cells[27]. These facts suggest that phage is a promising candidate for medical application.
In this work, we employed wild type (WT-) and genetically engineered M13 phages to form films for supporting MSCs proliferation and differentiation. For engineered phages, two peptides PDPLEPRREVCE (PD-phage) and YGFGG (YG-phage) were displayed on the sidewalls of the phages, i.e., fused to the major coat protein of phage. The PDPLEPRREVCE peptide is derived from a bone protein called osteocalcin (OCN) [28] and YGFGG is the core domain of the typical cell growth peptide known as osteogenic growth peptide (OGP)[29].
Methods and materials
Phage film fabrication
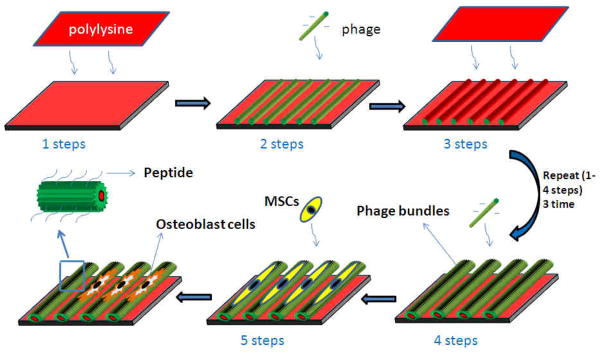
Phage films in this work were prepared by applying electrostatic self-assembly approach developed by our group (Scheme 1) [30]. Briefly, the pre-cleaned glass chips were soaked into a poly-lysine solution for 30 min, allowing for the adsorption of the first cationic polyelectrolyte layer onto the chips. Subsequently, the chips were washed in ultra-pure water (5 min, continuous water flow) and dried in 96 or 24-well plate under a pressurized air stream. Then100μl phage solution with different concentrations (1014pfu/ml, 1013pfu/ml, 1012pfu/ml) was added into the well, which was dried under a pressurized air stream, followed by washing with ultra-pure water (5 min). This process was repeated three times. The films were thus terminated with a phage layer.
Scheme 1.
Schematic illustration of the formation of the phage film by using layer-by-layer method and the MSCs growth on the phage film. The polylysine was employed as a cross-linker to fabricate the phage film. After several layers alternately deposited, the phage nanofibers were assembled into parallel alignment bundles separated by grooves. As a result, the MSCs were oriented and elongated along the phage bundles. The functional peptide displayed on the phage surface and the effect of stretching stimulated the MSCs differentiation into osteoblast cells.
Methylthiazoletetrazolium (MTT) assay
The cells were plated at a density of 4×103 cells/well on the phage films in 96-well plates in a standard growth medium for 72h. Measurement of cell viability was done by a 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. MTT was used as an indicator of cell viability as determined by its mitochondrial-dependent reduction to formazone. MTT (5 mg/ml, 20μl/well, Sigma, USA) was then added to the cell cultures for an additional 4 h period. The supernatant was then discarded, followed by the addition of dimethyl sulfoxide (DMSO, 150μl/well, Sigma, USA) and agitated for 30 seconds to dissolve the crystal completely. The values of optical density (OD) were measured at a 490 nm wavelength on a plate reader (Biotek, USA).
Scanning Electron Microscopy (SEM) for Examining the Morphologies of Scaffolds and Cells
MSCs were added onto the phage film at a density of 1 × 104 cells/mL. After cultured for 24 hours, the cells on the phage film were washed with 1× PBS, fixed with 2.5% glutaraldehyde in 0.1× PBS for 1 h and washed three times with 1× PBS times. The cells were then dehydrated in a graded series of ethanol (50%, 70%, 90%, and 100%) for 30 min each and left in 100% ethanol until they were totally dried by a supercritical point CO2. The dried samples were sputter-coated with very thin gold for SEM characterization. The morphology of the phage film as well as the resident cells was monitored by SEM (XL30, FEI Corporation). Cell surface area were measured by the computer-assisted planimetry. Forty five cells in 5–6 fields were examined in each experiment.
Immunofluorescence of Actin and Osteopontin/Osteocalcin
After 2 weeks of culture in osteogenic media, the cells on the phage film substrates were fixed using70% ethanolin 1× PBS for 15 min at room temperature. Once fixed, the cells were washed twice with 1× wash buffer (1× PBS containing 0.05% Tween-20). To induce the permeability of the cells, 0.1% Triton X-100 in 1× PBS solution was added for 5 min and then washed twice with a wash buffer. Afterwards, the samples were incubated for 1 h at room temperature in 5% BSA/1× PBS followed by the addition of anti-osteopontin (anti-OPN) antibody (1:1000, Abcam Biotechnology) and anti-osteocalcin (anti-OCN) antibody (1:1000, Abcam Biotechnology), and incubated overnight at 4 °C. Following incubation, the cells were washed 3 times for 5 min with a 1× wash buffer. Goat anti-rabbit IgG-TRITC (1:500, Santa Cruz Biotechnology) and FITC-conjugated phalloidin (1:40, Invitrogen) in 1× PBS was added for double staining and the cells were then incubated again for 1 h at room temperature. The cells were washed with a 1× wash buffer for 5 min three successive times. Then, the samples were stained by DAPI (1:1000, Chemicon) for nucleus staining. The samples were then inverted onto coverslips, mounted, visualized, and photographed by a fluorescence microscope (N-storm, Nickon Microsystems).
Real-time PCR
Total RNA and reverse transcription were prepared with cells-to-cDNA kit(Ambion). Real-time PCR using Power SYBR Green PCR master mix (Applied Biosystems) was carried out in triplicate in a miniFast real-time PCR system (BIORAD) according to the manufacturer’s instructions. Analysis was performed using the software supplied with the instrument. The specificity of the PCR amplification was confirmed by agarose gel electrophoresis. The PCR reaction conditions utilized include: 45 cycles of PCR, 95 °C for 30 s, 58 °C for 30 s, and 72°C for 30s.
The sequences of gene primers were as follows:
Osteopontin (OPN),
Forward primer, 5′–GACGGCCGAGGTGATAGCTT–3′,
-
Reverse primer 5′-CATGGCTGGTCTTCCCGTTGC–3′;
Osteocalcin (OCN),
Forward primer, 5′-AAAGCCCAGCGACTCT–3′,
-
Reverse primer 5′-CTAAACGGTGGTGCCATAGAT–3′;
Runx2,
Forward primer, 5′–GCTTCTCCAACCCACGAATG–3′,
-
Reverse primer 5′–GAACTGATAGGACGCTGACGA–3′;
Arbp (Housekeeping gene),
Forward primer, 5′-CGACCTGGAAGTCCAACTAC–3′,
Reverse primer 5′–ATCTGCTGCATCTGCTTG–3′.
Mineralized bone matrix formation assay
The cells were plated at a density of 4×103 cells/well on the phage films generated from different types of phage in 96-well plates. The medium was changed every three or four days and allowed to grow for 2 weeks. Mineralized nodules were stained with Alizarin red S[31]. The dye taken up by extracellular calcium deposits was dissolved in 0.1N sodium hydroxide and quantified using the spectrophotometer at 548 nm.
Statistical analysis
Data were analyzed by using SPSS 10.0 statistical software (SPSS 10.0 for Windows). One-way-ANOVA and post hoc multiple comparison tests were performed. Each value represents a mean ± standard error (SE) in all the figures. A significant level set at 0.05 was defined for the differences between the groups.
Results
Characterization of the phage films
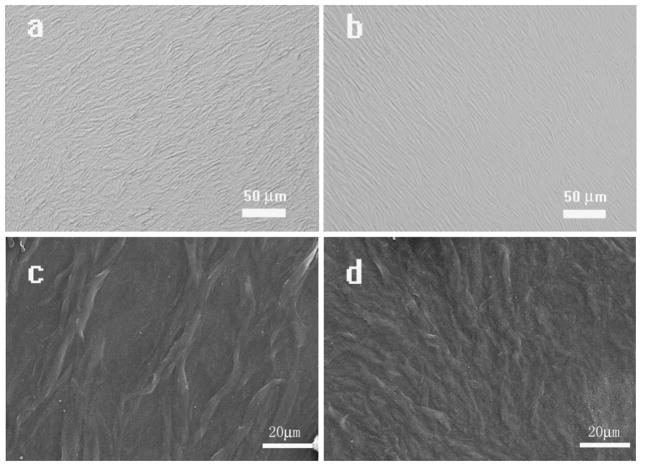
In this study, we used the typical electrostatic self-assembly technique to generate phage films[32]. In our study, positively charged polylysine molecules were employed to modify the chip surface to crosslink with negatively charged phage layers. As illustrated in Fig 1, the fabricated film had a slightly rough surface with alternating phage bundles and grooves. The surface of the film generated from the high concentration (1014pfu/ml) of the phage is more rough than the film surface generated at the lower concentration (1012pfu/ml). The phage bundles arranged in parallel were separated by grooves (Figures S1-S3), giving rise to a micro-textured surface morphology. In addition, the phage bundle sizes could be controlled using different concentrations of phage solution. As seen in Figure 1, the bundles generated by the high concentration of the phage (1014 pfu/ml) were larger in magnitude than those generated by the low concentration (1012pfu/ml). Also the grooves on the phage film assembled from the high concentration (1014 pfu/ml) phages are wider compared to those on the phage films generated by the 1012pfu/ml phage concentration (Figure 1). Furthermore, the depth of the grooves is dependent on the bundle size and phage concentration. The higher concentration of the phage (1014pfu/ml) generated a deeper grove than the lower phage concentration (1012 pfu/ml).
Figure 1. M13 phage film assembled from different concentrations (1012 and 1014 pfu/ml) of phage.
The filamentous phages self-assemble into bundles which tend to be in parallel and constitute a large area with a high level of organization. The films in a and c are derived from phage with a higher concentration (1014 pfu/ml) whereas those in b and d are assembled from the phage with a lower concentration (1012 pfu/ml) (a,b: Optical Microscopy; c,d: SEM)
Morphology of MSCs on phage films
It was reported that grooved and micro-textured surfaces could provide directional cues for the morphogenesis of cells with a preferred direction[33]. In order to test what changes could be brought to resident MSCs by phage films, we systematically studied the morphology varieties of MSCs on the phage films prepared with different phages (WT-, PD-, YG-phage) and phage concentrations (1014 pfu/ml, 1013 pfu/ml, and1012 pfu/ml).
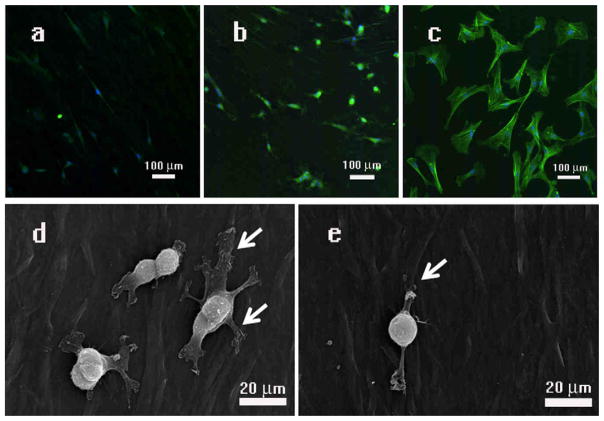
As shown in Fig. 2 and Figure S2, MSCs on the WT-phage films generated from 1013 pfu/ml of phage experienced significant elongation and alignment along the phage bundles, all of which are characteristics that lead to a spindle-like cellular morphology. However, not all resident MSCs observed gave the same morphology. With different phage concentrations and fused peptides (PD-/YG-), slight variations in the morphologies of resident cells were apparent. Specifically, we found that the films generated from a higher concentration of phage induced a higher degree of spatial orientation among the resident MSCs than the films from a lower concentration of phage (Figure 3). Moreover, the degree of stretching of the resident MSCs was also dependent on the phage concentration. As a proof of this concept, we observed the surface area of seeded cells on the film generated by lower concentration of phage (1012pfu/ml) is significantly larger than the cells seeded on the film generated by the higher concentration of phage (1013pfu/ml, 1014pfu/ml)(P<0.01, P<0.01, respectively) (Figure 3h). Fused foreign peptides can also affect cell morphologies. Usually, MSCs seeded on the polylysine film show spindles bipolar to polygonal fibroblastic morphology (Figure 3d and Figure 4c)[1]. As shown in Figures4 and S4, the cells seeded on the WT- or YG-phage film were found to be more stretched and oriented compared to the cells seeded on the PD-phage. And most of the cells show spindles bipolar on WT- or YG-phage film (Fig 4a and S4). But from the Figure 4b and Figure 4d, most of the MSCs seeded on the PD-phage film have several filopodias and are more spread out than cells attached on the WT- &YG-phage film.
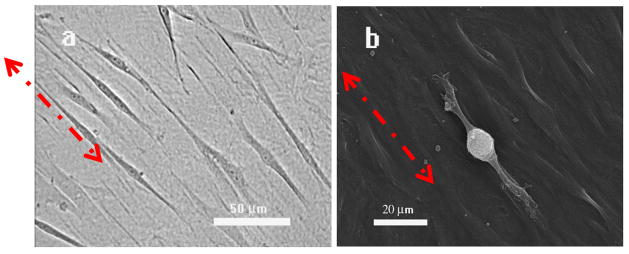
Figure 2. The typical morphology of MSCs on the phage film.
The bright field (a), and SEM (b) images show the MSC cells are elongated along one direction of phage bundles. In a and b, red arrows highlight the orientation of the phage bundles and MSCs.
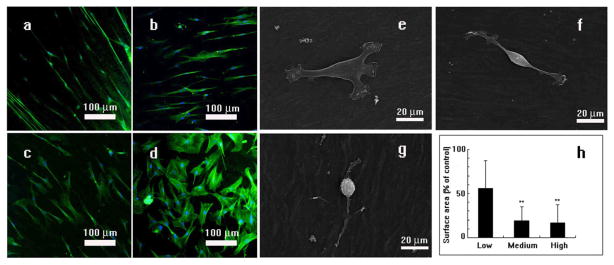
Figure 3. Effect of phage concentrations on the morphology of MSCs.
Typical fluorescent images and SEM images of the phage films derived from phages with different concentrations (a and g, 1014pfu/ml; b and f, 1013pfu/ml; c and e, 1012pfu/ml; d, slides coated with polylysine) show that the phage concentration can affect the degree of cell alignment and stretching. MSCs were arranged at a higher degree of order and extension on phage films derived from phage with the higher concentration (1014 pfu/ml, a) in comparison with those seeded on the film generated from phage with a lower concentration (1012 pfu/ml). In all images, cell nuclei are stained by DAPI (blue) and the F-actin of cells are stained by FITC-labeled phalloidin (green). The cells surface area depends on the phage concentration (* p<0.05, ** p<0.01) as shown in (h). At a higher phage concentration (g), MSCs are highly stretched, resulting in a lower surface area. At lower phage concentration (e), MSCs are spread out, resulting in a higher surface area.
Figure 4. Fluorescent images and SEM images show MSCs cultured on phage films derived from phages with different surface chemistry.
(a and e) wild type phage; (b and d) engineered phage with a foreign peptide PDPLEPRREVCE displayed on side wall; and (c) without phage but with polyLysine (control). MSCs cultured on the film generated by the wild type phage were elongated and aligned (a and e). MSCs cultured on the film generated by the engineered phage were aligned as (a and e), but displayed multiple pseudopods (b and d, highlighted by white arrows) compared to those on the wild type phage film (a) and control (c). In all images, cell nuclei are stained by DAPI (blue) and the F-actin of cells are stained by FITC-labeled phalloidin (green).
Proliferation of MSCs on phage film
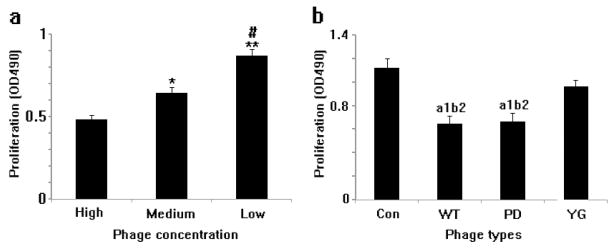
We have studied the proliferation of MSCs seeded on both WT-phage and engineered phage assembled films (Figure 5). However, the growth rate of the MSCs on phage films depended on both the phage concentration and the peptides displayed on the side wall of the phage. After cultured for 3 days and assayed by the MTT method, MSCs seeded on films generated under different concentrations of phage depicted a different growth rate (Figure 5a). As the concentration of phage is increased, the measured OD490 value decreased (1012pfu/ml or 1013pfu/ml groups vs. 1014pfu/ml group, P<0.01, P<0.01), indicating a decreased growth rate of resident MSCs. By monitoring the growth of MSCs on WT-, PD- and YG-phage films, we noticed that MSCs on the YG-phage film grew faster than those on WT-phage or PD-phage (P<0.05, P<0.05) films, however, there is no significant difference between the growth rate of MSCs on WT- and PD-phage films. Finally, the MSCs seeded on polylysine films grew faster than those on the WT- and PD-phage film (P<0.01, P<0.01, respectively) (Figure 5b).
Figure 5. Proliferation of MSCs on the phage films derived from phages with different concentration (a) and surface chemistry (b).
MSCs cultured at the low concentration (1012 pfu/ml) of phage film grew faster than those at the higher concentration (1014pfu/ml) of film (a). MSCs seeded on the phage film grew slower than those on the glass slides (b). The growth rate of cells on the YG-phage film is faster than those on the WT- or PD-phage film. CON: glass slide coated with polylysine as control; YG: phage with osteogenic peptide displayed on the side wall; WT: wild type phage; PD: phage with osteocalcine-derived peptide (PDPLEPRREVCE) displayed on the side wall. (when compared with High group, *P<0.05, **P<0.01; compared with Medium group, #P<0.01; compared with control group, a1P<0.01; compared with YG group, b2P<0.05).
Differentiation of MSCs on phage film
Specific differentiation of resident MSCs towards osteoblasts is a popular area of study in tissue engineering. Therefore, we wanted to test if MSCs could specifically differentiate into osteoblasts on our phage films. Specific differentiation of MSCs towards osteoblasts can be characterized by monitoring the increased expression of two bone formation marker proteins, osteopontin (OPN) and osteocalcin (OCN)[34].
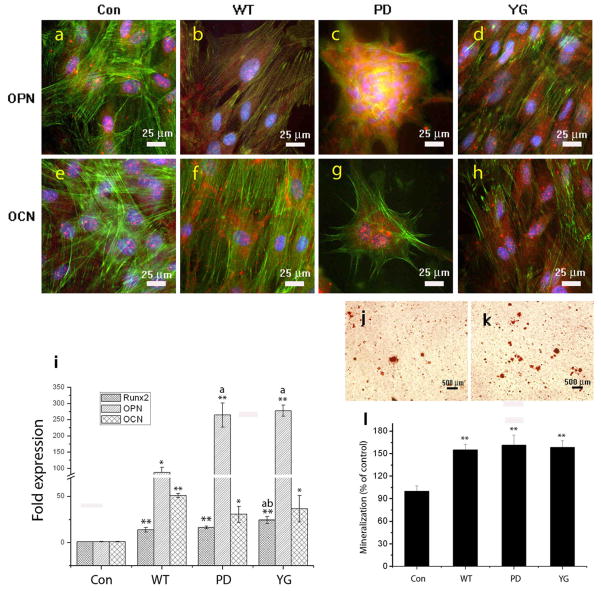
In our study, phage films were able to increase the expression of both OPN and OCN in resident MSCs under the osteogenic media. After cultured for 2 weeks, MSCs seeded on phage films generated by the WT-, YG-, and PD-phage each expressed greater levels of OPN and OCN in the cytoplasm (Figure 6b, c, d, f, g, and h) compared to MSCs grown on polylysine films (Figure 6a and e). During the 2 weeks culturing process in osteogenic media, the mature cells seeded on the PD-phage film aggregated to form calcified nodule-like structures. The high OCN and OPN activity observed inside the formed nodules suggest the existence of matured osteoblast cells. However, the cells cultured on the WT- or YG-phage displayed more fibroblast characteristic morphology, which lacks the critical nodule formation.
Figure 6. Differentiatiom and mineralization of MSCs on the WT- or engineered phage film.
Fluorescent images (a, b, c, d, e, f, g, h) show the effect of phages with different surface chemistries but constituting the films on the differentiation of MSCs into osteoblasts. The phage film (b, c, d, f, g, h) show strong osteocalcin and osteopontin expression compared to those in the control groups (i.e, cells on glass slides with polylysine coated) (a,e). The phage film derived from phage displaying the peptide (PDPLEPRREVCE) can stimulate MSCs aggregation to form a calcified nodule-like substance. Cell nuclei are stained by DAPI (blue) and F-actin is stained by FITC-labeled phalloidin (green). Osteopintin and Osteocalcin are stained by rhodamine-labeled antibody (red). Real-time PCR analysis (i) for Runx2, OPN, and OCN mRNA expression. Phage film can stimulate Runx2, OPN, OCN mRNA expression compared to control group (i.e., glass slides with polylysine coated). YG-phage film can significantly induce Runx2 expression compared to the WT- or PD-phage film (p<0.01, p<0.05, respectively). Phage film (WT-, PD-, and YG-) can induce OCN expression compared to the control groups (p<0.01, p<0.05, p<0.05, respectively). YG- and PD-phage film significantly induced OPN expression in comparison to the WT-phage film (p<0.01, p<0.01, respectively), but there is no significant difference between PD- and YG-phage groups. (When compared with Control group, **P<0.01, *P<0.05; When compared with WT group, aP<0.01; When compared with PD group, bP<0.05). Mineralization (j, k, l) on films derived from different phages assayed by Alizarin red S at 14 days (**p<0.01). Optical images of mineralized nodules on MSCs cultured on films derived from glass slide with polylysine coated (control) (j) and PD-phage (k). The levels of mineralization formed by MSCs seeded on the different types of substrates (l).
To further support the immuno-fluorescent staining results described above, osteoblast gene expression (OCN, OPN, and Runx2) was also studied by real-time PCR analysis after 14 days of incubation as shown in Fig. 6i. The phage film (WT- or engineered) displayed the higher up-regulation of all selected osteoblastic genes compared to the control group (i.e., glass slides with polylysine coated) at 14 days of incubation. According to the results, MSCs on phage film showed significantly higher levels of OCN expression than control group (WT-, PD- or YG-vs. Con, p<0.01, p<0.05, p<0.05, respectively). However, there is no significant difference of OCN expression between the cells grown on WT- and engineered phage film. The OPN gene was significantly up-regulated by the phage film in comparison to the control group (WT-, PD- or YG-vs. Con, p<0.05, p<0.01, p<0.01, respectively). The OPN expression levels of MSCs seeded on engineered phage is higher than those of the cells on the WT-phage film (PD- or YG-vs. WT, P<0.01, P<0.01, respectively). Runx2 is a master transcription factor in MSCs differentiation and we found higher levels of induced runx2 by the phage film compared to the control group (Con vs. WT-, PD-, or YG-, p<0.01, p<0.01, p<0.01, respectively). Among the phage film groups, YG-phage can significantly induce runx2 expression in contrast to those on the WT- or PD-phage film (YG-vs. WT- or PD-, P<0.01, p<0.05, respectively).
Calcium-containing mineral deposits in the ECM are also markers for testing bone formation. To further support the immune staining results, mineralized calcium-containing matrices prepared under the same conditions were stained using Alizarin red S and quantitatively characterized by a spectro photometer at OD548. Figure 6(j, k, l) shows both the quantitative and qualitative evidence that the phage films significantly promoted calcium deposition in mineralized nodules. Almost 1.5 times more calcium-containing matrix could be obtained from MSCs on phage films than without phage (p<0.01, p<0.01, p<0.01, respectively). However, no significant quantitative difference was observed between MSCs on the WT-phage, PD-phage and YG-phage films. From the above results, the phage films can stimulate the MSCs to undergo differentiation and mineralization under osteogenic media.
Discussion
Natural filamentous phages form longrod-likeshapes (~880 nm by 6.6 nm) and their monodispersity drives them to self-assemble into highly controlled periodic nanostructures (FigureS1)[20, 21]. Their monodispersity in water is superior to any available commercial rod-like colloid or polymer. At certain high concentrations, the phage exhibits a cholesteric liquid crystalline phase in which all phages are aligned in parallel[35]. On the other hand, it is well known that divalentmetal ions or positively charged polymers can laterally aggregate protein rods by cross-linking neighboring fibers[36]. For phage film fabrication, positively charged polylysine was employed to immobilize the phage on the slide surface. In this overlapped layer, phages with a relatively high local concentration might form a cholesteric liquid crystalline phase and were cross-linked by free polylysine, meanwhile the thin meniscus formed at the contact line in the middle of the well might enable the formation of parallel phage bundles on the surface separated by grooves[37] (Figure 1 and Scheme 1). At a higher concentration, a greater amount of phages were cross-linked by polylysine on the surface, resulting in the formation of larger bundles. Higher phage concentrations induce deeper grooves between the phage bundles which correspond to increased phage bundle size[38]. Therefore, the depth of the microgrooves of the film generated by the phage could be regulated by the phage concentration.
Previous reports have demonstrated that grooves with a width of 500nm and depth of 150nm could induce a highly qualitative cell alignment[39]. In our work, MSCs seeded on the grooves structure consisting of phage were found to be significantly stretched and aligned along parallel phage bundles (Figure 2, 3, and 4) leading to linear cellular shapes. The cells also attach onto the phage bundles (Figure S2). This phenomenon of the orientation and alignment of cells on micro-groove-like pattern is usually called contact guidance[33]. The selective distribution of ECM proteins on the surface was proposed to be possible mechanisms[33, 40]. ECM proteins serve as a base for cells to adhere and spread out onto substrates[40, 41]. The selective distribution of ECM protein aggregates could induce the changes of cell morphology, such as cell elongation[40]. Earlier studies have demonstrated that the substrate surface modified with functional groups could affect the cell morphology because of the ECM distribution[31]. Within an initial incubation time, some proteins in the media from the serum adsorb to the surface and act as the preliminary ECM, which can help cell adhesion at the earliest stages of culture[40]. And several factors such as charge of the substrate[42] and geometry of the surface[41] could affect the ECM distribution. On our phage films, most of the ECM protein aggregates were only found attached to the phage bundles (Figures S2 and S3). This may have induced the elongation and alignment of resident MSCs along these phage bundles (Figures 2, 3, and 4). We observed a higher degree of elongation of MSCs on films generated with the high phage concentration (1014 pfu/ml) compared to that on the low concentration. It is possible that the grooves generated from the high concentration of phage is wider and deeper than those from the low concentration, inducing the preferential ECM distribution (Figure S3). A part from the concentration of the phage, the presence of fusion peptide could also have an impact on the MSCs morphology (Figure 4). The negative charge of the WT-phage and PD-phage was confirmed by zeta potential measurement (−27 and −35mv for WT- and PD-phage, respectively). Such charge, could be the main cause for the observed morphology changes of the MSCs seeded on phage films since cells on the more negative PD-phage film appeared more filopodial than the cells on the less negative WT-phage film.
In our experiment, the growth rate of the MSCs on phage films depended on the phage concentration and the type of fused functional peptide (Figure 5). The growth rate of resident MSCs decreased upon increasing the phage concentration (Figure 5a). The deeper grooves and larger bundles generated from the high concentration of the phage induce a higher level of cell actins alignment in one direction (Figure 6b and 6f), leading to a restriction of motion direction of resident cells so as to decrease the cell growth rate[43]. As figure S4 shows, the cells grow on the phage film just along the oriented direction. Therefore, changing the concentration of the phage used to form scaffolds allows a convenient way to control the cell proliferation rate by this phage-based scaffold.
Besides phage concentration, fused functional peptides could also affect the cellular growth rate. MSCs on the YG-phage film grew faster than those on WT- or PD-phage films. The YGFGG peptide is the functional domain of the osteogenic growth peptide which, typically regulates cell proliferation, alkaline phosphatase activity, and matrix mineralization[44]. At the molecular level, the stimuli effect of OGP is probably triggered by the activation of membrane receptor so as to promote Gi protein-MAP kinase signaling cascade[44]. Meanwhile, MAP kinases have a key role in proliferation stimulation of MSCs[44]. As a result, the YG-phage film can accelerate the growth rate of resident MSCs compared to WT- and PD-phage films.
It was established earlier that the physical stresses from the topography can accelerate stem cell differentiation into a specific cell lineage. For example, Dalby et al have confirmed that the elongation and stretching of the hMSCs favor the preferential differentiation into osteogenic lineage[41]. The molecular mechanism is related to the RhoA-Rho Kinase (ROCK) singaling pathway which serves as a key regulator of the actin cytoskeleton. The stretching of the actin can activate the RhoA and ROCK pathway so as to induce the MSCs differentiation into osteoblast cells[45]. In our experiment, the phage films causes a significant increase in runx2 activity, OPN and OCN production, and mineralization in MSCs compared to those on the polylysine film. It is likely that the phage film (even the WT-phage) stretched the resident cells, triggering the ROCK pathway which induced the MSCs differentiation. A part from the physical stresses, certain chemical groups immobilized on the substrate surface could also stimulate the stem cell differentiation[16, 17]. For example, the substrate coated with OGP peptide could induced the MSCs differentiation into the osteoblast cells[29]. Our results (Figure 6) show that the engineered phage films (PD- and YG-) stimulated not only the MSCs differentiation into osteoblast cells, but also the formation of calcified nodule-like substances.
Though there is no significant difference in inducing the MSCs differentiation between the engineered and WT-phage films, we still found that the runx2 mRNA expression levels of MSCs on YG-phage film were more up-regulated than those on the WT- and PD-phage films. Runx2 is a key transcript factor promoting osteocalcin gene expression which stimulates MSCs differentiation into osteoblast cells[46]. YG peptide stimulates the MSCs growth and differentiation into osteoblast cells via runx2 parthway[44]. The YG peptide displayed on phage has the potential to encourage MSC differentiation. The mineralization results show little difference between the phage films. But in PD-phage film, many cells aggregate nodules have been found compared to the WT- or YG- phage film, and strong OCN and OPN activity was observed inside the nodules. The previous studies have showed that the cell constituting the nodules are more easily to form mineralized matrix[14]. The strong negative charge (−35mV) on PD-phage surface, which attracts the calcium ions to induce mineralization, may be the reason why many mineralized nodules were formed.
Conclusion
We employed wild type and genetically engineered M13 phages to form films with grooved structures to support MSCs proliferation and differentiation. The films can be used as a scaffold for directing MSCs proliferation and differentiation. The morphology, proliferation and differentiation of MSCs seeded on the phage film could be controlled by the varying concentration of phage and types of chemical groups modified on the phage nanofibers. Our results indicate that incorporating genetically engineered phages into a complicated scaffold is a feasible method for controlling its surface topography and surface chemistry, offering a promising model matrix for the study of tissue generation and cell differentiation niches. Further studies will be focused on controlling the differentiation of MSCs seeded on the phage film displaying different types of functional peptides.
Acknowledgments
We thank the National Science Foundation (CBET-0854414) and National Institutes of Health (R03AR056848-01) for support of this work. This work is also in part supported by National Science Foundation (DMR-0847758, CBET-0854465), National Institutes of Health (R21EB009909-01A1, R01HL092526-01A2), and Oklahoma Center for the Advancement of Science and Technology (HR06-161S).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Ma PX, Zhang RY. Synthetic nano-scale fibrous extracellular matrix. J Biomed Mater Res. 1999;46:60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Hirano Y, Mooney DJ. Peptide and protein presenting materials for tissue engineering. Adv Mater. 2004;16:17–25. [Google Scholar]
- 4.Ma PX, Choi JW. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 2001;7:23–33. doi: 10.1089/107632701300003269. [DOI] [PubMed] [Google Scholar]
- 5.Li CM, Vepari C, Jin HJ, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115–24. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Patel S, Kurpinski K, Quigley R, Gao HF, Hsiao BS, Poo MM, et al. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007;7:2122–8. doi: 10.1021/nl071182z. [DOI] [PubMed] [Google Scholar]
- 7.Davis ME, Motion JPM, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, et al. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–50. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–5. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 9.Lim JY, Donahue HJ. Cell sensing and response to micro- and nanostructured surfaces produced by chemical and topographic patterning. Tissue Eng. 2007;13:1879–91. doi: 10.1089/ten.2006.0154. [DOI] [PubMed] [Google Scholar]
- 10.Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials. 1999;20:573–88. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 11.Horwitz EM, Gordon PL, Koo WKK, Marx JC, Neel MD, McNall RY, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–7. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci USA. 2002;99:4397–402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J, Bauer S, von der Mark K, Schmuki P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007;7:1686–91. doi: 10.1021/nl070678d. [DOI] [PubMed] [Google Scholar]
- 14.Kaur G, Valarmathi MT, Potts JD, Wang Q. The promotion of osteoblastic differentiation of rat bone marrow stromal cells by a polyvalent plant mosaic virus. Biomaterials. 2008;29:4074–81. doi: 10.1016/j.biomaterials.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 15.Park SY, Park SY, Namgung S, Kim B, Im J, Kim Y, et al. Carbon nanotube monolayer patterns for directed growth of mesenchymal stem cells. Adv Mater. 2007;19:2530–4. [Google Scholar]
- 16.Benoit DSW, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nature Mater. 2008;7:816–23. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curran JM, Chen R, Hunt JA. The guidance of human mesenchymal stem cell differentiation in vitro by controlled modifications to the cell substrate. Biomaterials. 2006;27:4783–93. doi: 10.1016/j.biomaterials.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Lei YG, Segura T. DNA delivery from matrix metal lop roteinase degradable poly(ethylene glycol) hydrogels to mouse cloned mesenchymal stem cells. Biomaterials. 2009;30:254–65. doi: 10.1016/j.biomaterials.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Souza GR, Christianson DR, Staquicini FI, Ozawa MG, Snyder EY, Sidman RL, et al. Networks of gold nanoparticles and bacteriophage as biological sensors and cell-targeting agents. Proc Natl Acad Sci USA. 2006;103:1215–20. doi: 10.1073/pnas.0509739103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo PJ, Nam KT, Qi JF, Lee SK, Park J, Belcher AM, et al. Spontaneous assembly of viruses on multilayered polymer surfaces. Nature Mater. 2006;5:234–40. doi: 10.1038/nmat1596. [DOI] [PubMed] [Google Scholar]
- 21.Rong JH, Lee LA, Li K, Harp B, Mello CM, Niu ZW, et al. Oriented cell growth on self-assembled bacteriophage M13 thin films. Chem Comm. 2008:5185–7. doi: 10.1039/b811039e. [DOI] [PubMed] [Google Scholar]
- 22.Merzlyak A, Indrakanti S, Lee SW. Genetically engineered nanofiber-like viruses for tissue regenerating materials. Nano Lett. 2009;9:846–52. doi: 10.1021/nl8036728. [DOI] [PubMed] [Google Scholar]
- 23.Frenkel D, Solomon B. Filamentous phage as vector-mediated antibody delivery to the brain. Proc Natl Acad Sci USA. 2002;99:5675–9. doi: 10.1073/pnas.072027199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380:364–6. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 25.Krag DN, Shukla GS, Shen GP, Pero S, Ashikaga T, Fuller S, et al. Selection of tumor-binding ligands in cancer patients with phage display libraries. Cancer Res. 2006;66:7724–33. doi: 10.1158/0008-5472.CAN-05-4441. [DOI] [PubMed] [Google Scholar]
- 26.Ngweniform P, Abbineni G, Cao BR, Mao CB. Self-Assembly of Drug-Loaded Liposomes on Genetically Engineered Target-Recognizing M13 Phage: A Novel Nanocarrier for Targeted Drug Delivery. Small. 2009;5:1963–9. doi: 10.1002/smll.200801902. [DOI] [PubMed] [Google Scholar]
- 27.Frenkel D, Solomon B. Filamentous phage as vector-mediated antibody delivery to the brain. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5675–9. doi: 10.1073/pnas.072027199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen-Marsh CM, Richards MP, Hauschka PV, Thomas-Oates JE, Trinkaus E, Pettitt PB, et al. Proc Natl Acad Sci USA. Vol. 102. 2005. Osteocalcin protein sequences of Neanderthals and modern primates; pp. 4409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore NM, Lin NJ, Gallant ND, Becker ML. The use of immobilized osteogenic growth peptide on gradient substrates synthesized via click chemistry to enhance MC3T3-E1 osteoblast proliferation. Biomaterials. 2010;31:1604–11. doi: 10.1016/j.biomaterials.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Liu AH, Abbineni G, Moo CB. Nanocomposite Films Assembled from Genetically Engineered Filamentous Viruses and Gold Nanoparticles: Nanoarchitecture- and Humidity-Tunable Surface Plasmon Resonance Spectra. Adv Mater. 2009;21:1001–05. [Google Scholar]
- 31.Vanella L, Kim DH, Asprinio D, Peterson SJ, Barbagallo I, Vanella A, et al. HO-1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage. Bone. 2010;46:236–43. doi: 10.1016/j.bone.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Beucken JJJP, Vos MRJ, Thune PC, Hayakawa T, Fukushima T, Okahata Y, et al. Fabrication, characterization, and biological assessment of multilayered DNA-coatings for biomaterial purposes. Biomaterials. 2006;27:691–701. doi: 10.1016/j.biomaterials.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Gerecht S, Bettinger CJ, Zhang Z, Borenstein JT, Vuniak-Novakovic G, Langer R. The effect of actin disrupting agents on contact guidance of human embryonic stem cells. Biomaterials. 2007;28:4068–77. doi: 10.1016/j.biomaterials.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahnert J, Fan X, Case N, Murphy TC, Grassi F, Sen B, et al. The role of nitric oxide in the mechanical repression of RANKL in bone stromal cells. Bone. 2008;43:48–54. doi: 10.1016/j.bone.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao CB, Flynn CE, Hayhurst A, Sweeney R, Qi JF, Georgiou G, et al. Viral assembly of oriented quantum dot nanowires. Proc Natl Acad Sci USA. 2003;100:6946–51. doi: 10.1073/pnas.0832310100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fowler WE, Aebi U. Polymorphism of Actin Paracrystals Induced by Polylysine. J Cell Biol. 1982;93:452–8. doi: 10.1083/jcb.93.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin Y, Balizan E, Lee LA, Niu ZW, Wang Q. Self-Assembly of Rodlike Bio-nanoparticles in Capillary Tubes. Angew Chem Intl Ed. 2010;49:868–72. doi: 10.1002/anie.200904993. [DOI] [PubMed] [Google Scholar]
- 38.Merzlyak A, Lee SW. Phage as templates for hybrid materials and mediators for nanomaterial synthesis. Current Opin Chem Biol. 2006;10:246–52. doi: 10.1016/j.cbpa.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Oakley C, Jaeger NAF, Brunette DM. Sensitivity of fibroblasts and their cytoskeletons to substratum topographies: Topographic guidance and topographic compensation by micromachined grooves of different dimensions. Exp Cell Res. 1997;234:413–24. doi: 10.1006/excr.1997.3625. [DOI] [PubMed] [Google Scholar]
- 40.Oh S, Brammer KS, Li YSJ, Teng D, Engler AJ, Chien S, et al. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci USA. 2009;106:2130–5. doi: 10.1073/pnas.0813200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nature Mater. 2007;6:997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 42.Nakaji-Hirabayashi T, Kato K, Arima Y, Iwata H. Oriented immobilization of epidermal growth factor onto culture substrates for the selective expansion of neural stem cells. Biomaterials. 2007;28:3517–29. doi: 10.1016/j.biomaterials.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 43.Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–9. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 44.Gabarin N, Gavish H, Muhlrad A, Chen YC, Namdar-Attar M, Nissenson RA, et al. Mitogenic G(i) protein-MAP kinase signaling cascade in MC3T3-E1 osteogenic cells: Activation by C-terminal pentapeptide of osteogenic growth peptide [OGP(10–14)] and attenuation of activation by cAMP. J Cell Biochem. 2001;81:594–603. doi: 10.1002/jcb.1083. [DOI] [PubMed] [Google Scholar]
- 45.Woods A, Beier F. RhoA/ROCK signaling regulates chondrogenesis in a context-dependent manner. J Biol Chem. 2006;281:13134–40. doi: 10.1074/jbc.M509433200. [DOI] [PubMed] [Google Scholar]
- 46.Huang J, Zhao L, Xing LP, Chen D. MicroRNA-204 Regulates Runx2 Protein Expression and Mesenchymal Progenitor Cell Differentiation. Stem Cells. 2010;28:357–64. doi: 10.1002/stem.288. [DOI] [PMC free article] [PubMed] [Google Scholar]