Abstract
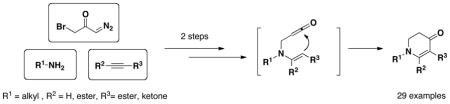
Cyclic 6-membered enaminones were synthesized from three components (bromo diazoacetone, primary amine, and alkyne) in high yields via aza-Michael addition, Wolff rearrangement and nucleophilic ketene cyclization.
Ketenes, first discovered by Staudinger in 1905, are among the best studied intermediates in organic chemistry.1 Their versatile reactivity provides access to a number of valuable structural motifs. Ketenes can be readily prepared from several precursors including diazoketones and acid halides, which further underscores their value in synthetic organic chemistry.2
Reactions involving ketenes can be categorized into two major types: cycloaddition reactions and nucleophilic additions to ketenes. The so-called Staudinger reaction is a well-known example of a cycloaddition reaction, where a ketene reacts with an imine or a ketone to provide a β-lactam or a β-lactone, respectively.3 Ketenes can also undergo cycloadditions with alkenes or alkynes to form a C–C bond at the sp center of the ketene.4
The other type of reaction, the nucleophilic addition to a ketene,5 is exemplified by the Arndt-Eistert homologation, where the acid functionality is homologated via the formation of a diazo intermediate, followed by a Wolff rearrangement, and subsequent nucleophilic addition to the intermediate ketene.6 In nucleophilic additions to electrophilic ketenes, most often water, alcohol and amines are used whereas carbon nucleophiles are used less frequently.7 Although organometallic reagents such as RMgX and RLi have been explored in reactions with ketenes, few methods are of significant synthetic utility.8
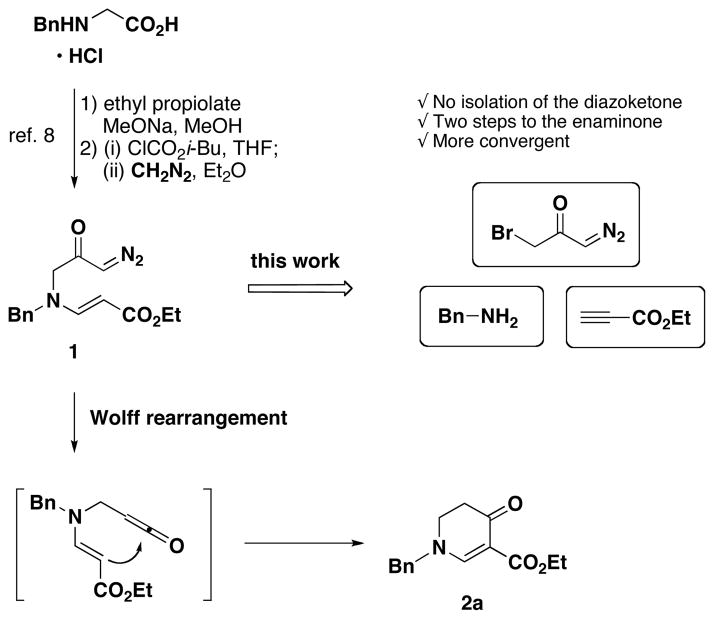
Recently we reported a novel C–C bond forming cyclization with a ketene to synthesize 6-membered enaminones, under very mild conditions (Figure 1).9 In this cyclization, a ketene is generated from diazoketone 1, employing a silver-catalyzed Wolff rearrangement, which subsequently reacts with a pendant vinylogous amide as a neutral nucleophile to form 6-membered enaminones such as 2a.
Figure 1.
Two Approaches for the Synthesis of Diazoketone 1.
In our recently reported method, the diazoketone precursors were obtained from amino acids using diazomethane. Although the incorporation of chirality derived from an amino acid into the enaminone is advantageous, the use of diazomethane as well as the limited solubility of amino acids in organic solvents diminishes the scale and scope of this method. To address these issues, an alternative approach to synthesize the diazoketones was sought. We envisioned that the diazoketones can be derived from three components: a primary amine, an alkyne, and a bromo diazoacetone.10
Herein, we are communicating a new synthetic method to obtain cyclic enaminones from amines and alkynes in two steps. This disconnection enabled us to vary the substituents on the enaminone structure in a convergent fashion and to limit the use of diazomethane to the preparation of the common diazoacetone intermediate 3a (Scheme 1). The enaminone reaction products are well-known to be versatile intermediates for alkaloid synthesis.11
Scheme 1.
Synthesis of Diazoketone 1
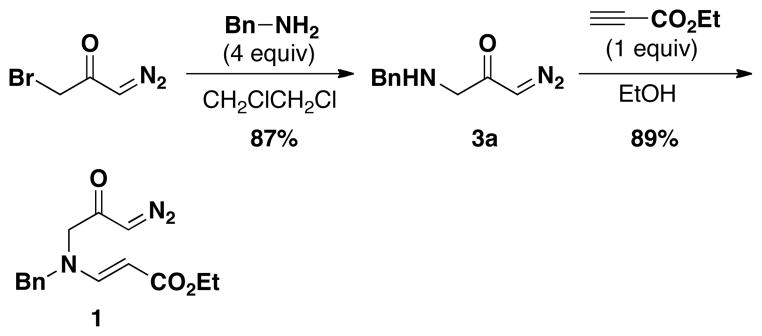
To test our hypothesis, we first investigated the synthesis of known diazoketone 1. We found that the desired product could be readily synthesized by aza-Michael addition of benzylamino diazoacetone to ethyl propiolate in ethanol (Scheme 1). Benzylamino diazoacetone (3a) was prepared by the treatment of readily available bromo diazoacetone12 with an excess of benzylamine.
Encouraged by the successful synthesis of diazoketone 1, amino diazoketones 3a–3h (Table 1) were prepared in the same fashion using bromo diazoacetone and alkyl amines. A variety of primary alkyl amines (entry 1–6), as well as a chiral amine (entry 7), and an amino acid-derived amine (entry 8) afforded the corresponding amino diazoketones in good yields.
Table 1.
Synthesis of Amino Diazoketones
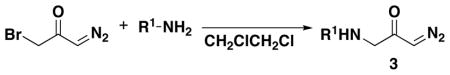 | |||
|---|---|---|---|
| entrya | product (3) | yield | |
| 1 | R1 = Bn | 87% | 3a |
| 2 | R1 = Et | 65% | 3b |
| 3 | R1 = n-Pr | 63% | 3c |
| 4 | R1 = Allyl | 72% | 3d |
| 5 | R1 = n-Bu | 80% | 3e |
| 6 | R1 = -CH2Cy | 81% | 3f |
| 7 |
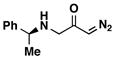
|
93% | 3g |
| 8b |
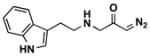
|
73% | 3h |
Reaction conditions: Bromo diazoacetone was treated with the amine (4 equiv) in dichloroethane (0.25 M) at 50 °C.
Bromo diazoacetone was treated with tryptamine hydrochloride (2 equiv) in a 0.5 M MeONa/MeOH solution (0.25 M) at 50 °C.
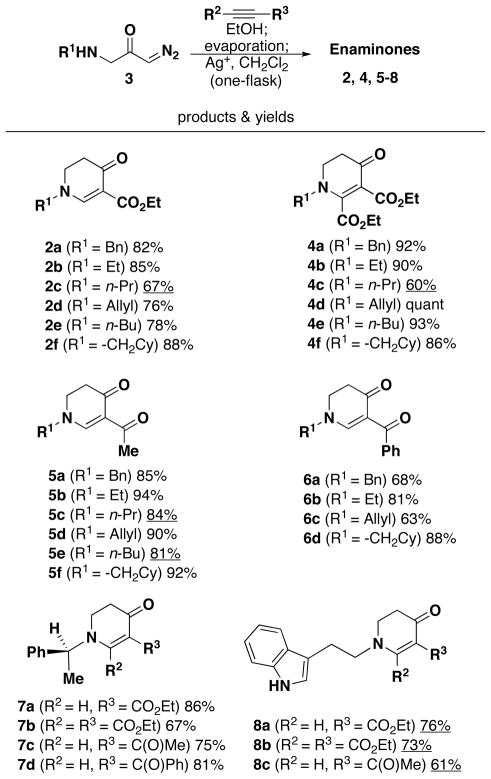
With amino diazoketones 3a–3h in hand, we carried out the aza-Michael addition to the alkynes13 and subsequent Wolff rearrangement in a one-flask procedure. In order to obtain optimal yields, the use of two different solvents was necessary. Ethanol was employed for the aza-Michael addition and dichloromethane for the Wolff rearrangement. Using these reaction conditions, the enaminone reaction products 2, 4, 5–8 were obtained in good to excellent yields (Scheme 2). In several instances, Ag2O was found to be a better catalyst for the Wolff rearrangement than PhCO2Ag.
Scheme 2.
Synthesis of an Enaminone Library via the Sequence of aza-Michael Addition, Wolff Rearrangement, and Ketene Cyclizationa
(a) Reaction conditions: The amino diazoacetone was reacted with an alkyne (1.2 equiv) in EtOH (0.2 M). Upon evaporation, the reaction mixture was treated with the Ag catalyst (20 mol %, PhCO2Ag, underline: Ag2O) in dichloromethane (0.2 M) in the dark.
In summary, we have discovered an efficient, convergent synthesis of cyclic enaminones from bromo diazoacetone, primary amines, and alkynes. Aza-Michael addition, Wolff rearrangement, followed by nucleophilic ketene cyclization were carried out sequencially in one flask, providing facile access to an enaminone library. Exploration of other C-nucleophiles in this methodology is currently ongoing.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (GM081267), and the University of Minnesota through the Vince and McKnight Endowed Chairs. We thank Dr. Subhashree Francis (University of Minnesota) for mass spectrometry assistance.
Footnotes
Supporting Information Available: Experimental procedures and full spectroscopic data for all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Staudinger H. Chem Ber. 1905;38:1735. [Google Scholar]; (b) Tidewell TT. Ketenes. 2. John Wiley & Sons, Inc; Hoboken, New Jersey: 2006. [Google Scholar]
- 2.(a) Meier H, Zeller KP. Angew Chem. 1975;87:52. [Google Scholar]; (b) Kirmse W. Eur J Org Chem. 2002:2193. [Google Scholar]; (c) Tidwell TT. Eur J Org Chem. 2006:563. [Google Scholar]; (d) Staudinger H. Chem Ber. 1911;44:1619. [Google Scholar]
- 3.For review: Hyatt JA, Raynolds PW. Org React. 1994;45:159.Georg GI, Ravikumar VT. Stereocontrolled Ketene-Imine Cycloaddition Reactions. In: Georg GI, editor. The Organic Chemistry of β-Lactams. Verlag Chemie; New York: 1993. p. 295.
- 4.(a) Lee SY, Kulkarni S, Burbaum BW, Snider BB. J Org Chem. 1988;53:1848. [Google Scholar]; (b) Liebenskind LS. Tetrahedron. 1989;45:3053. [Google Scholar]; (c) Moore HW, Yerxa BR. Chemtracts. 1992;5:273. [Google Scholar]; (d) Snider BB. Chem Rev. 1988;88:793. [Google Scholar]
- 5.Examples of C–X bond forming ketene cyclizations: Rahman SS, Wakefield BJ, Roberts SM, Dowle MD. J Chem Soc, Chem Commun. 1989:303.Boeckman RK, Pruitt JR. J Am Chem Soc. 1989;111:8286.Vernier JM, Hegedus LS, Miller DB. J Org Chem. 1992;57:6914.Barton DHR, Quinkert G. J Chem Soc. 1960:1.Quinkert G, Kleiner E, Freitag BJ, Glenneberg J, Billhardt UM, Cech F, Schmieder KR, Schudok C, Steinmetzer HC, Bats JW, Zimmermann G, Dürner G, Rehm D. Helv Chim Acta. 1986;69:469.Quinkert G, Billhardt UM, Jakob H, Fischer G, Glenneberg J, Nagler P, Autze V, Heim N, Wacker M, Schwalbe T, Kurth Y, Bats JW, Dürner G, Zimmermann G, Kessler H. Helv Chim Acta. 1987;70:771.Quinkert G, Nestler HP, Schumacher B, Delgrosso M, Durner G, Bats JW. Tetrahedron Lett. 1992;33:1977.Dillon JL, Gao Q, Dillon EA, Adams N. Tetrahedron Lett. 1997;38:2231.Coutts IGC, Saint RE, Saint SL, Chambers-Asman DM. Synthesis. 2001:247.Tojino M, Uenoyama Y, Fukuyama T, Ryu I. Chem Commun. 2004:2482. doi: 10.1039/b408746a.
- 6.(a) Bachmann WE, Struve WS. Org React. 1942:38. [Google Scholar]; (b) Seikaly HR, Tidwell TT. Tetrahedron. 1986;42:2587. [Google Scholar]; (c) Matthews JL, Braun C, Guibourdenche C, Overhand M, Seebach D. Enantiosel Synth β-Amino Acids. 1997:105. [Google Scholar]
- 7.Non-organometallic C-nucleophile addition to ketenes: Hickmott PW, Giasuddin Ahmed M, Ahmed SA, Wood S, Kapon M. J Chem Soc, Perkin Trans. 1985:2559.Hickmott PW. S Afr J Chem. 1989;42:17.Yerxa BR, Moore HW. Tetrahedron Lett. 1992;33:7811.Byeon CH, Hart DJ, Lai CS, Unch J. Synlett. 2000:119.
- 8.For ketene reactions with organo lithium/magnesium reagents, see: Haener R, Laube T, Seebach D. J Am Chem Soc. 1985;107:5396.Baigrie LM, Seiklay HR, Tidwell TT. J Am Chem Soc. 1985;107:5391.For reactions with other type of carbon nucleophiles, see: Rathke MW, Sullivan DF. Tetrahedron Lett. 1973:1297.Kita Y, Matsuda S, Kitagaki S, Tsuzuki Y, Akai S. Synlett. 1991:401.Negri G, Kascheres C. J Heterocycl Chem. 2001;38:109.
- 9.Seki H, Georg GI. J Am Chem Soc. 2010;132:15512. doi: 10.1021/ja107329k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.A recent report of a three-component synthesis using a ketene: Leon F, Rivera DG, Wessjohann LA. J Org Chem. 2008;73:1762. doi: 10.1021/jo7022125.
- 11.(a) Comins DL, Joseph SP. Adv Nitrogen Heterocycl. 1996;2:251. [Google Scholar]; (b) Joseph S, Comins DL. Curr Opin Drug Discovery Dev. 2002;5:870. [PubMed] [Google Scholar]; (c) Comins DL, O’Connor S, Al-awar RS. In: Comprehensive Heterocyclic Chemistry III. Alan RK, Christopher AR, Eric FVS, Richard JKT, editors. Elsevier; Oxford: 2008. p. 41. [Google Scholar]; (d) Turunen BJ, Georg GI. J Am Chem Soc. 2006;128:8702. doi: 10.1021/ja0609046. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Niphakis MJ, Turunen BI, Georg GI. J Org Chem. 2010;75:6793. doi: 10.1021/jo100907u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Niphakis MJ, Georg GI. J Org Chem. 2010;75:6019. doi: 10.1021/jo101051w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Niphakis MJ, Georg GI. Org Lett. 2011;13:196. doi: 10.1021/ol1023954. [DOI] [PubMed] [Google Scholar]
- 12.Bromo diazoacetone is currently commercially available or can be prepared as described in the following references: Pace V, Verniest G, Sinisterra J-V, Alcantara AR, De Kimpe N. J Org Chem. 2010;75:5760. doi: 10.1021/jo101105g.Padwa A, Austin DJ, Precode L, Zhi L. J Org Chem. 1993;58:1144.
- 13.The alkynes are commercially available with the exception of 1-phenylprop-2-yn-1-one, used for the synthesis of enaminones 6, which was prepared using a reported method: Chassaing S, Kueny-Stotz M, Isorez G, Brouillard R. Eur J Org Chem. 2007:2438.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.