Abstract
Multidrug resistance (MDR) remains one of the key determinants in chemotherapeutic success of cancer patients. Often, acquired resistance is mediated by the overexpression of ATP-binding cassette (ABC) drug transporters. To study the mechanisms involved in the MDR phenotype, investigators have generated a variety of in vitro cell culture models using both multi-step and single-step drug selections. Sublines produced from multi-step selections have led to the discovery of several crucial drug transporters including ABCB1, ABCC1 and ABCG2. Additionally, a number of mechanisms causing gene overexpression have been elucidated. To more closely mimic in vivo conditions, investigators have also established MDR sublines with single-step drug selections. Here we examine some of the multi-step and single-step selected cell lines generated to elucidate the mechanisms involved in the development of MDR in cancer cells.
Keywords: Multidrug resistance, multi-step selection, single-step selection, ABC transporter, gene amplification, epigenetic changes
Introduction
Cancer is one of the top ten leading causes of death in the world. In the United States alone, one of every four deaths will be due to cancer in 2008 (1). Advancements in early detection and cancer treatments have yielded significant progress, yet cancer deaths still outnumber deaths due to heart disease in people less than 85 years of age in the United States. A major factor in therapeutic failure for cancer involves the development of drug resistance to a variety of structurally unrelated anti-cancer drugs, also known as multidrug resistance (MDR) (2).
MDR can develop in several different ways, with the predominant mechanism being the overexpression of ATP-binding Cassette (ABC) drug transporters on the plasma membrane of tumor cells. These transporters act as energy driven pumps (3), and as such, maintain intracellular drug concentrations below toxic levels. Thus, the tumor survives, rendering the treatment ineffective. Tumors can demonstrate intrinsic drug resistance in which the tumor is innately resistant to treatment; this occurs in tumors originating from epithelial cells such as renal or adrenal tumors which naturally express ABC drug transporters (4). Acquired resistance, on the other hand, arises following therapy, and tumors normally present with the MDR phenotype subsequent to various genetic changes.
ABC drug transporters belong to the largest superfamily of transporter proteins (5). Members of this family are recognized by a consensus ATP-binding site from 90 to 110 amino acids in length which also includes a linker region between two Walker motifs. In addition to two ATP-binding sites, ABC transporters normally possess two transmembrane domains. The 50 human ABC transporter genes are further subdivided into seven subfamilies (A-G) based on similar gene structure, order of the domains, and on sequence homology in their consensus domains (6). ABC drug transporters such as ABCB1, ABCC1 and ABCG2 are expressed in normal and tumor cells and are localized to different plasma membrane surfaces; the normal function of a number of these transporters is to efflux endogenous and xenobiotic metabolites from the cell (7). The substrate specificity for ABCB1, ABCG2, and the various ABCC family members overlaps extensively although the primary sequences of these transporters vary significantly (7). This phenomenon makes treatment of multidrug-resistant cancer unsuccessful in spite of the multitude of drugs available. Reports show that patients with ABCB1-positive tumors are three times more likely to fail a course of therapy than those who have tumors which are ABCB1-negative (8). Even more taxing for patients are tumors which express multiple ABC transporters, since overexpression is not mutually exclusive and a tumor can overexpress several MDR-linked ABC transporters in tandem.
Although over 12 of these transporters have been linked to MDR (9), little is known about the regulation of these transporters. Often multi-step drug selections have been employed to study the MDR phenotype. Several drawbacks are associated with this technique. The multi-step selections utilize higher concentrations of drug than those found in patients as well as extended periods of exposure. These factors produce pleiotropic effects. To avoid such issues, we recently employed a single-step selection to evaluate ABC transporter regulation. We reported that ABC transporter mRNA expression patterns vary with single vs. multi-step selection with doxorubicin in MCF-7 breast cancer cells (10). In multi-step selections with doxorubicin, ABCB1 is often the dominant ABC transporter causing MDR; we have shown that following a single-step selection using low concentrations of doxorubicin other transporters including ABCC2, ABCC4 and ABCG2 are overexpressed. In this chapter, we will review a number of the multi-step and single-step selected cancer cell lines which have been established and used extensively to investigate MDR (Table 1). In addition, we will discuss the mechanisms that have been ascertained in the development of these drug-resistant cell lines.
Table 1.
List of Select Multi-step and Single-Step Selected Cell Lines Overexpressing ABC Transporters
| Selection Regimen | Cell Line | Drug | ABC Transporter Overexpressed |
|---|---|---|---|
| Multi-step | KB-3-1 | Colchicine | ABCB1 (13) |
| Doxorubicin | ABCB1 (13) | ||
| Vinblastine | ABCB1 (13) | ||
| Multi-step | OVCAR-8 | Doxorubicin | ABCB1 (14; 15) |
| Multi-step | MCF-7 | Doxorubicin | ABCB1 (17) |
| Multi-step | MES-SA | Doxorubicin | ABCB1 (19) |
| Multi-step | MCF-7 | Docetaxel | ABCB1 (20) |
| Multi-step | MDA-MB-231 | Docetaxel | ABCB1 (20) |
| Multi-step | MCF-7 | Doxorubicin | ABCB1 and ABCG2 (21; 22) |
| Multi-step | MCF-7 | Paclitaxel | ABCB1 and ABCG2 (21; 22) |
| Multi-step | NCI-H69 | Doxorubicin | ABCC1 (23) |
| Multi-step | COR-L23 | Doxorubicin | ABCC1 (24; 25) |
| Multi-step | MOR | Doxorubicin | ABCC1 (24; 25) |
| Multi-step | GLC4 | Doxorubicin | ABCC1 (26) |
| Multi-step | MCF-7 | Etoposide | ABCC1 (27) |
| Multi-step | NCI-H69 | Etoposide | ABCB1 and ABCC1 (28) |
| Multi-step | MCF-7 | Flavopiridol | ABCG2 (29) |
| Multi-step | MCF-7 | Doxorubicin and verapamil |
ABCG2 (30) |
| Multi-step | MCF-7 | Mitoxantrone | ABCG2 (31) |
| Multi-step | S1 | Mitoxantrone | ABCG2 (32) |
| Multi-step | IGROV-1 | Topotecan | ABCG2 (33) |
| Multi-step | IGROV-1 | Mitoxantrone | ABCG2 (33) |
| Multi-step | SF295 | Mitoxantrone | ABCG2 (34) |
| Multi-step | GLC4 | Mitoxantrone | ABCA2 (35) |
| Single-step | MCF-7 | Doxorubicin | ABCC4 and ABCG2* (10) |
| Single-step | MCF-7 | Etoposide | ABCG2 (10) |
| Single-step | IGROV-1 | Doxorubicin | ABCG2 (10) |
| Single-step | S1 | Doxorubicin | ABCG2 (10) |
| Single-step | MES-SA | Doxorubicin | ABCB1 (57) |
| Single-step | MES-SA | Paclitaxel | ABCB1 (59) |
| Single-step | NCI-H82 | Epirubicin | ABCC1 (62) |
| Single-step | GLC4 | Doxorubicin | ABCC1 (63) |
In all cases the overexpression of an ABC transporter was demonstrated at the functional level and the references are given in the parenthesis.
ABCG2 is the ABC transporter responsible for MDR and ABCC4 does not confer resistance to doxorubicin.
Multi-Step Selected Cell Lines
To study MDR in vitro, investigators have utilized drug selections to generate resistant cell lines for over 30 years. Selections can be performed on individual clones or on mass populations of cells (11). To establish individual clones, the cells must be cloned so that an individual cell is the source for the entire population which will then be selected with the drug of choice. This technique boosts one major advantage in that a single gene will be responsible for the MDR. Alternatively, multifactorial MDR will result when an entire cell population is selected (11). The selection of a cell population, on the other hand, more closely mimics the clinical situation and can stem from a spectrum of mechanisms.
One of the original multi-step selected cell lines was established by the selection of an individual clone of KB epidermoid carcinoma cells with colchicine (12). The subsequent resistant sublines were generated with increasing single-step selections beginning with KB-3-1, an individual clone from a population of the KB cells. The MDR1 (ABCB1) gene was first identified from these cells. Using this same methodology, four additional KB sublines were created with selections in high concentrations of colchicine, vinblastine or doxorubicin (13). These sublines were selected with a step-wise selection, and all express high levels of ABCB1. Since their establishment, the various resistant sublines of KB cells have been widely used to investigate MDR mediated by ABCB1.
In contrast to the KB cells, MCF-7 breast cancer cells were selected with doxorubicin using the mass population method, yet also expressed ABCB1 (14). This original selection was performed with increasing concentrations of doxorubicin beginning with 10 nM. The final resistant subline, MCF-7 AdrR, was capable of surviving in 10 μM doxorubicin. Later these original doxorubicin-resistant MCF-7 cells were determined to actually be OVCAR-8 ovarian cancer cells, which were resistant to doxorubicin (15). Their nomenclature has changed accordingly to NCI/ADR-Res or OVCAR-8/ADR (16). Other laboratories independently generated doxorubicin-resistant MCF-7 cells, and one such subline was established by culturing MCF-7 cells in 0.025 μg/ml doxorubicin and increasing the selection pressure by 2-fold until the cells grew in the presence of 2 μg/ml doxorubicin (17). Interestingly, these resistant cells also overexpressed ABCB1 and were karyotyped to match MCF-7 cells from ATCC (18). Doxorubicin-resistant sarcoma cells (MES-SA/Dx5) were also one of the early MDR models expressing ABCB1; these cells were selected with increasing concentrations of doxorubicin (19). Lastly, MCF-7 and MDA-MB-231 breast cancer cells exposed to increasing concentrations of docetaxel (up to 30 μM), known as MCF-7 TAX30 and MDA-MB-231 TAX30, were also found to overexpress ABCB1 (20). Moreover, ABCB1 was involved in MDR in highly resistant cell lines derived from both the individual clone and population selection techniques.
Yet in other more recent studies with MCF-7 cells using multi-step selections with lower concentrations of doxorubicin, both ABCG2 and ABCB1 were expressed (21; 22). In these studies concentrations ranging from 9 nM to 300 nM doxorubicin were employed. Similarly, MCF-7 cells selected with a multi-step selection with paclitaxel, 0.56 nM to a final concentration of 6.6 nM, also express both transporters, but at a lower level than the doxorubicin-selected MCF-7 cells (21; 22). Remarkably, paclitaxel is not a substrate of ABCG2, yet its selection pressure caused the overexpression of ABCG2. Furthermore, the investigators demonstrated that the clones were isogenic and that MDR was a consequence of adaptation and not a selection of a clone within the population. Involvement of other ABC transporters at lower selection concentrations suggests that ABCB1 may be dominant only at the later stages of MDR in particular cell types.
ABCC1 was first reported in a resistant cell line produced with a stepwise doxorubicin selection in a small cell lung cancer cell line, NCI-H69; this was called H69AR and did not express ABCB1 (23). Large-cell (COR-L23), small-cell (H69) and adenocarcinoma (MOR) lung tumor lines continuously selected with increasing concentrations of doxorubicin were also found to express ABCC1 (24; 25). Another small cell lung carcinoma cell line, GLC4, was utilized in resistance studies, and ABCC1 was overexpressed when selected with doxorubicin concentrations augmented from 18 nM to 1,152 nM (26). Surprisingly, etoposide-selected MCF-7 cells also showed overexpression of ABCC1 instead of ABCB1. These cells were generated with a stepwise selection in increasing concentrations of etoposide starting with 200 nM up to 10 μM, and revertants were prevented by occasional reselection in 4 μM etoposide (27). Investigators reported that etoposide-selected H69 small cell lung cancer cells expressed both ABCB1 and ABCC1 at the mRNA and protein levels (28). During this selection process, ABCC1 expression preceded that of ABCB1. At a moderate level of ABCC1 expression, rather than continue increasing the expression of ABCC1, the cells turned on the ABCB1 gene (28). It also appears that cell type dictates the particular ABC transporter which is induced and that cells can activate more than one ABC transporter.
Investigators have also prepared a variety of resistant cell lines overexpressing ABCG2. For instance, MCF-7 cells selected with increasing concentrations of flavopiridol, MCF-7/FLV1000, expressed wild-type ABCG2 (29). MCF-7 cells selected in the presence of both doxorubicin and verapamil also overexpressed ABCG2; these cells are known as MCF-7 AdVp3000 (30). Unlike the MCF-7/FLV1000, the MCF-7 AdVp3000 expressed the mutant ABCG2 R482T. MCF-7 cells were also exposed to mitoxantrone and were found to overexpress wild-type ABCG2 (31). When S1 human colon carcinoma cells were selected with mitoxantrone, ABCG2 was also overexpressed (29). Additional sublines were generated when these original S1-M1-3.2 (32) were exposed to higher concentrations of drug, up to a final concentration of 80 μM. These S1 sublines also expressed a mutant R482G ABCG2 protein. Another example of MDR mediated by ABCG2 overexpression occurred in IGROV-1 human ovarian carcinoma cells selected with either topotecan or mitoxantrone (33). Similarly, SF295 human glioblastoma cells showed ABCG2 overexpression when selected in increasing concentrations of mitoxantrone ( 50 to 500 nM) (34). Unexpectedly, when a mitoxantrone-resistant small cell lung cancer cell line, GLC4-MITO, was established, ABCA2 upregulation was found (35). In the case of ABCG2-overexpressing cell lines in vitro, two gain of function mutations have been identified (R482T and R482G). To our knowledge, no such mutations have been reported in clinical samples positive for ABCG2 to date.
Mechanisms of Overexpression in Multi-Step Selected Cell Lines
Gene amplification is the most common method for drug-resistant cells to overexpress a particular ABC transporter. In a comprehensive analysis of 23 drug-resistant cancer cell lines derived from 10 different human cancers, it was revealed that changes in gene copy number were implicated in acquired drug resistance (36). Comparative genomic hybridization (CGH) was executed on drug sensitive and their corresponding drug-resistant sublines, and the regions of gain within the drug-resistant cell lines were consistent with regions encoding ABC transporters in 19 of the 23 cell lines. These changes were further confirmed by fluorescence in situ hybridization (FISH) analysis in these cells. Of particular interest were ABCA3, ABCB1 and ABCC9 which had a greater than 2-fold increase in copy numbers. Furthermore, gene amplification was in line with gene expression changes present in these resistant cells.
Amplified genes are either present in homogeneously staining regions or on extrachromosomal elements such as double-minutes. Investigations of resistant KB cells also showed gene amplification of the ABCB1 gene (37; 38). Double-minute chromosomes were identified in these KB resistant cell lines. Investigators also determined that KB cells could easily lose their resistance when no selection pressure was present because the gene amplification was only found in the form of double-minutes. Furthermore, in this KB resistant series, it was uncovered that in the less resistant sublines the ABCB1 was activated while in the more highly resistant sublines gene amplification occurred (39). For instance, later studies showed that with the higher colchicine selection pressure, double-minutes were stably maintained even after several months of continuous passaging in culture. The exact formation of the double-minutes in the sequential series of resistant sublines was closely examined using gel electrophoresis. Submicroscopic extrachromosomal circular DNA was revealed in the less resistant sublines. Consequently, the double-minutes uncovered in the more resistant sublines were formed by multimerization of these submicroscopic circular DNAs (39).
Gene amplification has also been reported for some of the other multi-step selected cell lines previously described. MES-SA/Dx5, doxorubicin-resistant sarcoma cells, displayed 7q21 alterations and gene amplification (40). However, these cells, unlike the KB resistant cells, did not possess double-minutes as seen by FISH analysis. In the two breast cancer cell lines selected with docetaxel, MCF-7 TAX30 and MDA-MB-231 TAX30, gene amplification of chromosome 7q in the region which encodes for ABCB1 was discovered using CGH (20).
ABCB1 overexpression can also be caused at the transcription level. Investigators have found that in drug-sensitive cells only one transcription start site is used for ABCB1; nonetheless, drug-resistant cells which do not exhibit gene amplification often exploited more than one downstream transcription start site for ABCB1 (41). This substitution of other downstream transcription start sites for ABCB1 within the same cell line was a distinct mechanism which led to the identification of the MED-1 (Multiple start site Element Downstream) in many of the genes with a TATA-less promoter which have multiple start sites such as ABCB1 (42; 43). This MED-1, GCTCCC/G, was crucial for ABCB1 expression in drug-resistant cells in the cell lines examined. Likewise MEF1, MDR1 promoter-enhancing factor 1, also activated transcription but through an upstream promoter element, −118 to −111 (44).
Often drug selections can also cause alterations in genes that appear as gene rearrangements through nonhomologous recombinations. For example, investigators first reported a hybrid ABCB1 mRNA resulting from such a chromosomal rearrangement in a doxorubicin-selected S48-3s human colon adenocarcinoma cell line (45). In these cells, there was a 4;7 translocation resulting with the 3′ end of ABCB1 adjacent to a transcriptionally active chromosome 4 gene, thus triggering the activation of ABCB1 by the promoter sequences on the adjacent chromosome 4. For this particular gene rearrangement, the subsequent ABCB1 protein structure remained unaltered due to the rearrangement occurring within the 5′ region of ABCB1. Follow-up studies illustrated that eight other selected cells and two clinical samples had gene rearrangements (45; 46). Unexpectedly, these gene hybrids differed, suggesting that ABCB1 merely required a sufficiently active promoter to activate it and that the specific promoter was irrelevant. Other ABCB1 mRNA hybrids have also been reported (47). These hybrids were regulated by nearby genomic sequences with similarity to a retroviral element. Nevertheless, no chromosomal rearrangements were discovered in these hybrids.
Epigenetic changes have also been reported to activate ABCB1. In the repressed state, the ABCB1 promoter is methylated and enriched in methyl-CpG binding protein 2 (MeCP2). In MCF-7 cells exposed to a stepwise selection with doxorubicin, ABCB1 is overexpressed and in the resistant cells the ABCB1 promoter is completely unmethylated (48). The promoter methylation status of ABCB1 is inversely correlated to the expression of the ABCB1 gene. The loss of methylation at the promoter facilitates the activation of ABCB1 in these resistant cells.
In the resistant cell lines which displayed ABCC1 overexpression, gene amplification was also the most common mechanism. The original ABCC1-overexpressing cell line H69AR demonstrated gene amplification with Southern blot analysis (23). Many double-minutes of chromosome 16 were discovered in the COR-L23/R cells, while the MOR cells exhibited an enlarged copy of chromosome 16 with homogeneously staining regions (24). As with the cells overexpressing ABCB1, highly resistant cell lines such as GLC4/ADR (26) , COR-L23/R and MOR/R predominantly displayed gene amplification of ABCC1 as the mode of gene overexpression. On the contrary, transcriptional activation of ABCC1 was solely responsible for gene overexpression in the weakly resistant SW-1573 30.3M subline, which had been selected with low concentrations of doxorubicin (49) . Of the highly resistant cell lines, only MOR/R presented a combination of gene amplification and gene activation, whereas gene amplification was the main mechanism for gene overexpression in the GLC4/ADR and COR-L23/R selected cell lines.
For resistant cell lines overexpressing ABCG2, Southern analysis of MCF-7 AdVp3000 and S1-M1-80 sublines uncovered that only MCF-7 AdVp3000 had gene amplification (50). In later studies, these cell lines as well as MCF-7/MX were further characterized by CGH, FISH, spectral karyotyping and Southern blotting (51). No amplification was confirmed in the S1-M1-80 subline, while the other two cell lines showed amplification with multiple translocations of chromosome 4. Other investigators evaluated the MCF-7/MX subline and also showed gene amplification by Southern blot analysis (52). In the SF295 MX selected cells, ABCG2 was found amplified by Southern analysis. The sublines selected with the lower concentrations displayed double-minutes containing the ABCG2 gene when examined with FISH and spectral karyotyping. At 500 nM mitoxantrone selection pressure, a homogeneously staining region was found integrated into the chromosome causing ABCG2 overexpression (34). Furthermore, Boonstra and colleagues also found gene amplification with CGH in the GLC4-MITO cells for ABCA2 on chromosome 9 (35).
Analogous to results from ABCB1 promoter studies in drug-resistant cells overexpressing ABCB1, ABCG2 has also been shown to have multiple transcription start sites in drug-selected cells (53). Investigators have also reported the expression of novel 5′UTR variants of transcripts that possess different translation efficiencies. Thus, the ABCG2 protein expression is directed at the posttranscriptional level as a consequence of these 5′UTR variants. However, no gene rearrangements in the 5′UTR region were seen.
Various epigenetic changes have also been found in multi-step selected cell lines that overexpress ABCG2. Chromatin immunoprecipitation (ChIP) has been used to identify histone modifications in these multi-step selected cells. In the S1-M1-80 cells, which show no gene amplification, the ABCG2 proximal promoter displayed histone H3 acetylation (54). Further epigenetic changes were present in this subline as HDAC1 and HDAC3 bound less to the proximal promoter of ABCG2. More importantly, Pol II binding, an indicator of ABCG2 promoter activity, was enhanced in these resistant cells.
MicroRNAs (miRNA) have also been implicated in the regulation of genes. Recent reports investigated the effects of miRNA on ABCG2 expression. S1 colon cells possessed a longer 3′UTR in the ABCG2 mRNA where a putative hsa-miR-519c binding site exists (55). This miRNA binds to this site, causing translational repression and mRNA degradation in the sensitive parental cells. Conversely, the S1-M1-80 cells utilize a noncanonical AUUAAA poly (A) signal to yield a much shorter 3′UTR lacking this miRNA binding site, thus eluding degradation mediated by hsa-miR-519c. It appears that a combination of epigenetic and miRNA mediated changes are responsible for the overexpression of ABCG2 in the highly resistant S1-M1-80 cells.
Single-Step Selected Cell Lines
These and many other multidrug resistant cancer cell lines have been established in vitro through continual drug selection. Although they offer a sufficient means for investigating the regulation of ABC transporter expression and function, rarely do these continual drug selections emulate what is found in the clinical setting. Thus, insight into the development of MDR by ABC transporters at clinically relevant concentrations and ascertaining which factors induce upregulation of ABC transporters during the initial steps of MDR will afford more advantageous measures to circumvent MDR. Our goal was to evaluate the expression of ABC transporters following a short low dose selection which would simulate the conditions encountered in vivo and to compare the gene expression levels of MDR-linked ABC transporters in these sublines selected by a low-dose single step to an established high-dose doxorubicin selected subline (17) and to determine if overexpression of the same ABC transporters occurs.
We have recently found that ABC transporter mRNA expression patterns vary with single- vs. multi-step treatment with doxorubicin in MCF-7 breast cancer cells. We established single-step doxorubicin-selected MCF-7 sublines using very low concentrations (14 or 21 nM) (10). Individual clones were selected from a population of 10,000 cells in a 100 × 20mm tissue culture dish exposed to drug for 10 days. Clones were then maintained in drug-free medium following the initial drug selection. We compared these single-step sublines to a previously established multi-step doxorubicin-selected MCF-7 subline (17) known to overexpress only ABCB1 at the mRNA and protein levels (Figure 1) due to gene amplification. We evaluated a number of ABC transporters and found that ABCC2, ABCC4 and ABCG2 were overexpressed at the mRNA level in these single-step selected sublines (Figure 2). Yet, only ABCC4 and ABCG2 were overexpressed at the protein level. Both 14 and 21 nM single-step doxorubicin-selected sublines exhibited nearly 5-fold resistance to doxorubicin compared to parental MCF-7 cells. However, ABCC4 did not confer resistance to this drug, suggesting that ABCG2 was the major transporter responsible for the development of doxorubicin resistance. Sequencing of ABCG2 in the single-step selected sublines revealed that our in vitro selection resulted in the overexpression of the wild-type ABCG2 and not the gain of function mutations either G or T at amino acid 482. SiRNA studies further confirmed that mainly ABCG2 confered drug resistance in these clones. We also observed that the upregulation of ABCG2 was facilitated by histone hyperacetylation of H3 at the proximal promoter of ABCG2. Similar to what was found in the S1-M1-80 cells, Pol II binding was increased while HDAC1 binding was decreased in the single-step selected sublines. This was the first report of ABCG2 overexpression in MCF-7 cells following a short term low dose selection with doxorubicin.
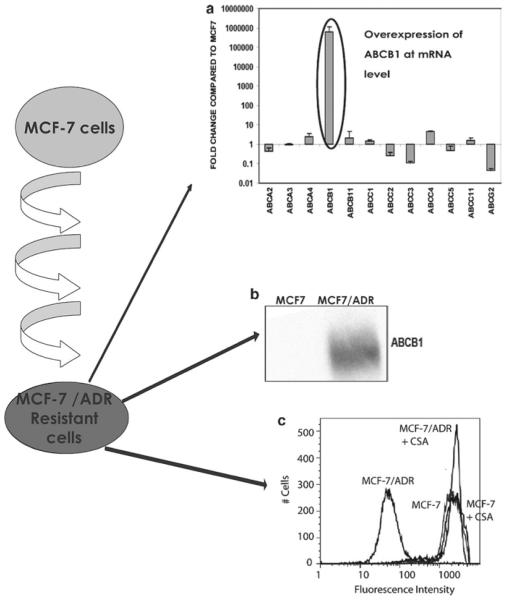
Figure 1. Analysis of ABC transporter expression and function in the multi-step doxorubicin-selected MCF7/ADR cell line.
A) The delta-delta CT method was used to determine the fold change of ABC transporter gene expression in the multi-step doxorubicin-selected cells, MCF7/ADR (17), compared to their parental line, MCF-7. The values represent the mean and standard deviation (n=2). The overexpression of ABCB1 is highlighted by the black circle. B) Using C219, the ABCB1-specific monoclonal antibody, the relative quantities of ABCB1 were determined for MCF7/ADR and MCF-7 in whole-cell lysates. Lane 1, MCF-7 control and lane 2, MCF7/ADR, (100,000 cells for all samples). C) Calcein-AM efflux assays were performed using flow cytometry. Assays compared MCF-7 and MCF7/ADR. Charcoal grey histogram, MCF7/ADR; dark grey histogram, MCF7/ADR cells in the presence of 10 μM cyclosporine A (CSA); black histogram, MCF-7, and light grey histogram, MCF-7 in the presence of cyclosporine A. The schematic on the far left side depicts the multi-step selection with doxorubicin in 0.025 μg/ml doxorubicin and increasing the selection pressure by 2-fold until the cells grew in the presence of 2 μg/ml doxorubicin.
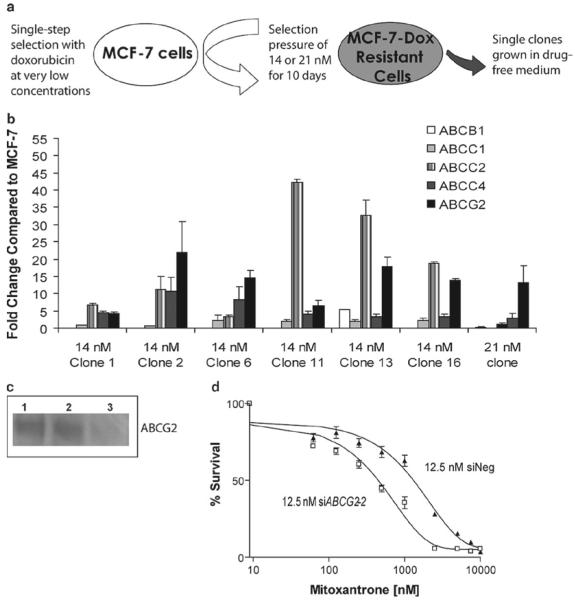
Figure 2. Single-step doxorubicin-selected clones overexpress ABCG2.
A) The schematic of the single-step selection for the MCF-7 cells with doxorubicin. B) Characterization of selected ABC transporter gene expression levels in several single-step clones. Doxorubicin-resistant MCF-7 clones were established employing a single step selection with either 14 or 21 nM treatment for 10 days followed by culturing continuously in drug-free medium. The average fold change compared to parental MCF-7 cells ± SD (n=4) was calculated using delta delta Ct method from real-time RT-PCR data. Reference gene is PMCA4 (64). The key for selected five ABC transporters is given in the figure. C) Western blotting analysis of ABCG2 protein using BXP-21 antibody following no treatment (lane 1), 50 nM negative siRNA treatment (lane 2) and 50 nM G2-2 siRNA treatment (lane 3). D) Cytotoxicity assays with mitoxantrone evaluating the effect of silencing ABCG2 in the 21 nM single-step clone. Dose response curves were derived from three independent experiments using the CCK-8 assay. White box, 21 nM cells with 12.5 nM G2-2 siRNA and black triangle, 21 nM cells with 12.5 nM negative siRNA. Error bars indicate standard deviation (n=3).
To further evaluate if this ABCG2 overexpression was drug and cell line independent, we generated additional sublines of MCF-7 cells with a single-step selection using 300 nM etoposide and two different cancer cell lines, IGROV-1 ovarian cancer cells and S-1 colon tumor cells, with 14 and 21 nM doxorubicin, respectively. To ensure that we were not selecting for a resistant clone, several lower etoposide concentrations, 50, 100 and 200 nM, were first evaluated. These lower selections indicated that all MCF-7 parental cells were able to survive. For IGROV-1 cells, only the 14 nM doxorubicin selection yielded resistant cells. Five sublines derived from IGROV-1 cells obtained using 14nm doxorubicin and S-1-resistant clones, obtained at a 21 nM doxorubicin concentration were further characterized. Furthermore, we were also able to replicate the upregulation of the same ABC transporters in the MCF-7 cells using this single-step selection with 300 nM etoposide. ABCG2 was also the dominant overexpressed ABC transporter for these additional sublines. This suggests that even a low dose selection can bring about MDR and that ABCG2 overexpression mediates the early stages of MDR development in certain cell lines. ABCG2 may be protecting against the cytotoxic effects of drugs in our single-step-selected clones, as it does in stem cells (56). Analysis of other mammary stem cell markers in our single-step sublines demonstrated that we did not enrich for cancer stem cells during the single-step selections of these clones. Taken together with the epigenetic alterations that were discovered in these resistant sublines, adaptation as opposed to selection appears to be the mitigating factor for this selection process.
Single-step selections have also been performed with the MES-SA, human sarcoma cell line. This protocol used the mass population selection technique, where cells were first plated in a 96-well plate, treated for two weeks with 40 nM doxorubicin, grown drug free for two additional weeks, and then individually harvested from each well (57). As with the MES-SA/Dx5, all clones examined expressed ABCB1. The authors used fluctuation analysis to determine that the doxorubicin-resistant clones were derived due to spontaneous mutations. Additionally, no chromosomal alteration or gene amplification was discovered in these single-step mutants (40). When either etoposide or paclitaxel was used in single-step selections with MES-SA cells, authors found that either no ABCB1 overexpression occurred (58) or that only 44% of the clones expressed ABCB1 (59), respectively. Etoposide-selected MES-SA cells showed a reduction in topoisomerase II but no ABC transporter increases. This suggests that ABCB1 substrates have different effects when selecting for ABCB1-expressing clones. Furthermore, a single-step selection with 40 nM doxorubicin in the presence of an ABCB1 inhibitor, PSC833, also produced no detectable levels of ABCB1 but rather decreased levels of topoisomerase II α (60).
In recent follow-up studies, the authors found that an increase in acetylated H3 modified the chromatin structure of ABCB1 far upstream, 968-bp proximal to the upstream promoter, and initiated upstream transcripts for these single-step selections (61). Equally important, the authors confirmed that these upstream ABCB1 transcripts were spontaneous in nature given that a clonal variant expanded to several million cells without any drug selection also produced these ABCB1 upstream variants.
Other single-step selections have generated sublines which overexpress ABCC1. One such example was the H82, a variant of small cell lung cancer, which was selected for 18 hours with 69 nM epirubicin. This initial selection yielded a drug-resistant cell line which was then subsequently selected for 18 hours with 14 nM epirubicin, producing an even more resistant line known as H82/E8 (62). These sublines displayed two- to nine-fold resistance. Remarkably, the H82/E8 subline remained stably resistant for over two years without further drug treatment. Investigators also selected H69 cells with eight treatments of 14 nM epirubicin followed by maintenance in drug-free medium. This subline is referred to as H69/E8. Only the H82/E8 increased ABCC1 expression (62) while neither cell line expressed ABCB1. Other investigators using a 50 nM single-step doxorubicin-selection with GLC4 small cell lung cancer cells attributed an increase in ABCC1 expression to the activation of the JNK pathway (63).
Conclusion
Drug selections with both clonal and cell populations have aided in the study of MDR mediated by ABC transporters. For instance, these in vitro techniques have led to the identification of at least three of the most influential ABC drug transporters for MDR. The overexpression of a particular ABC transporter during drug selection appears to depend on a multitude of factors which include but are not limited to the cell type, the selection regimen, the drug used for selection pressure as well as the concentrations utilized. These factors suggest that a number of ABC transporters should be evaluated following drug selection in addition to ABCB1. The single-step selection is capable of generating sublines with the MDR phenotype at clinically relevant concentrations while eliminating pleiotropic effects due to long-term drug exposure. With advancements in techniques for analysis at the molecular level and better understanding of gene regulation in the presence of drug, future studies should focus on translational research to improve the success rate of cancer therapies.
Acknowledgements
We thank Mr. George Leiman for editorial assistance. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 3.Dean M, Annilo T. Evolution of the ATP-Binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet. 2005;6:123–142. doi: 10.1146/annurev.genom.6.080604.162122. [DOI] [PubMed] [Google Scholar]
- 4.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 5.Gottesman M, Ambudkar S. Overview: ABC transporters and human disease. J Bioenerg Biomembr. 2001;33:453–458. doi: 10.1023/a:1012866803188. [DOI] [PubMed] [Google Scholar]
- 6.Dean M, Rzhetsky A, Allikmets R. The Human ATP-Binding Cassette (ABC) Transporter Superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 7.Haimeur A, Conseil G, Deeley R, Cole S. The MRP-related and BCRP/ABCG2 multidrug resistance proteins: biology, substrate specificity and regulation. Curr Drug Metab. 2004;5:21–53. doi: 10.2174/1389200043489199. [DOI] [PubMed] [Google Scholar]
- 8.Tan B, Piwnica-Worms D, Ratner L. Multidrug resistance transporters and modulation. Curr Opin Oncol. 2000;12:450–458. doi: 10.1097/00001622-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 10.Calcagno AM, Fostel JM, To KK, Salcido CD, Martin SE, Chewning KJ, Wu CP, Varticovski L, Bates SE, Caplen NJ, et al. Single-step doxorubicin-selected cancer cells overexpress the ABCG2 drug transporter through epigenetic changes. Br J Cancer. 2008;98:1515–1524. doi: 10.1038/sj.bjc.6604334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottesman MM, Cardarelli C, Goldenberg S, Licht T, Pastan I. Selection and maintenance of multidrug-resistant cells. Methods Enzymol. 1998;292:248–258. doi: 10.1016/s0076-6879(98)92019-5. [DOI] [PubMed] [Google Scholar]
- 12.Akiyama S, Fojo A, Hanover JA, Pastan I, Gottesman MM. Isolation and genetic characterization of human KB cell lines resistant to multiple drugs. Somat Cell Mol Genet. 1985;11:117–126. doi: 10.1007/BF01534700. [DOI] [PubMed] [Google Scholar]
- 13.Shen D, Cardarelli C, Hwang J, Cornwell M, Richert N, Ishii S, Pastan I, Gottesman M. Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J. Biol. Chem. 1986;261:7762–7770. [PubMed] [Google Scholar]
- 14.Batist G, Tulpule A, Sinha B, Katki A, Myers C, Cowan K. Overexpression of a novel anionic glutathione transferase in multidrug- resistant human breast cancer cells. J. Biol. Chem. 1986;261:15544–15549. [PubMed] [Google Scholar]
- 15.Scudiero DA, Monks A, Sausville EA. Cell line designation change: multidrug-resistant cell line in the NCI anticancer screen. J Natl Cancer Inst. 1998;90:862. doi: 10.1093/jnci/90.11.862. [DOI] [PubMed] [Google Scholar]
- 16.Liscovitch M, Ravid D. A case study in misidentification of cancer cell lines: MCF-7/AdrR cells (re-designated NCI/ADR-RES) are derived from OVCAR-8 human ovarian carcinoma cells. Cancer Lett. 2007;245:350–352. doi: 10.1016/j.canlet.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Mehta K. High levels of transglutaminase expression in doxorubicin-resistant human breast carcinoma cells. Int. J. Cancer. 1994;58:400–406. doi: 10.1002/ijc.2910580316. [DOI] [PubMed] [Google Scholar]
- 18.Devarajan E, Chen J, Multani AS, Pathak S, Sahin AA, Mehta K. Human breast cancer MCF-7 cell line contains inherently drug-resistant subclones with distinct genotypic and phenotypic features. Int J Oncol. 2002;20:913–920. [PubMed] [Google Scholar]
- 19.Harker WG, Sikic BI. Multidrug (pleiotropic) resistance in doxorubicin-selected variants of the human sarcoma cell line MES-SA. Cancer Res. 1985;45:4091–4096. [PubMed] [Google Scholar]
- 20.McDonald SL, Stevenson DA, Moir SE, Hutcheon AW, Haites NE, Heys SD, Schofield AC. Genomic changes identified by comparative genomic hybridisation in docetaxel-resistant breast cancer cell lines. Eur J Cancer. 2005;41:1086–1094. doi: 10.1016/j.ejca.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Guo B, Villeneuve DJ, Hembruff SL, Kirwan AF, Blais DE, Bonin M, Parissenti AM. Cross-resistance studies of isogenic drug-resistant breast tumor cell lines support recent clinical evidence suggesting that sensitivity to paclitaxel may be strongly compromised by prior doxorubicin exposure. Breast Cancer Res Treat. 2004;85:31–51. doi: 10.1023/B:BREA.0000021046.29834.12. [DOI] [PubMed] [Google Scholar]
- 22.Villeneuve DJ, Hembruff SL, Veitch Z, Cecchetto M, Dew WA, Parissenti AM. cDNA microarray analysis of isogenic paclitaxel- and doxorubicin-resistant breast tumor cell lines reveals distinct drug-specific genetic signatures of resistance. Breast Cancer Res Treat. 2006;96:17–39. doi: 10.1007/s10549-005-9026-6. [DOI] [PubMed] [Google Scholar]
- 23.Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 24.Barrand MA, Heppell-Parton AC, Wright KA, Rabbitts PH, Twentyman PR. A 190-kilodalton protein overexpressed in non-P-glycoprotein-containing multidrug-resistant cells and its relationship to the MRP gene. J. Natl. Cancer Inst. 1994;86:110–117. doi: 10.1093/jnci/86.2.110. [DOI] [PubMed] [Google Scholar]
- 25.Twentyman PR, Fox NE, Wright KA, Bleehen NM. Derivation and preliminary characterisation of adriamycin resistant lines of human lung cancer cells. Br J Cancer. 1986;53:529–537. doi: 10.1038/bjc.1986.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zijlstra JG, de Vries EGE, Mulder NH. Multifactorial Drug Resistance in an Adriamycin-resistant Human Small Cell Lung Carcinoma Cell Line. Cancer Res. 1987;47:1780–1784. [PubMed] [Google Scholar]
- 27.Schneider E, Horton JK, Yang CH, Nakagawa M, Cowan KH. Multidrug resistance-associated protein gene overexpression and reduced drug sensitivity of topoisomerase II in a human breast carcinoma MCF7 cell line selected for etoposide resistance. Cancer Res. 1994;54:152–158. [PubMed] [Google Scholar]
- 28.Brock I, Hipfner DR, Nielsen BS, Jensen PB, Deeley RG, Cole SP, Sehested M. Sequential coexpression of the multidrug resistance genes MRP and mdr1 and their products in VP-16 (etoposide)-selected H69 small cell lung cancer cells. Cancer Res. 1995;55:459–462. [PubMed] [Google Scholar]
- 29.Robey RW, Medina-Perez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, Senderowicz AM, Ross DD, Bates SE. Overexpression of the ATP-binding Cassette Half-Transporter, ABCG2 (MXR/BCRP/ABCP1), in Flavopiridol-resistant Human Breast Cancer Cells. Clin. Cancer Res. 2001;7:145–152. [PubMed] [Google Scholar]
- 30.Lee JS, Scala S, Matsumoto Y, Dickstein B, Robey RW, Zhan Z, Altenberg G, Bates SE. Reduced drug accumulation and multidrug resistance in human breast cancer cells without associated P-glycoprotein or MRP overexpression. J. Cell. Biochem. 1997;65:513–526. [PubMed] [Google Scholar]
- 31.Volk EL, Rohde K, Rhee M, McGuire JJ, Doyle LA, Ross DD, Schneider E. Methotrexate cross-resistance in a mitoxantrone-selected multidrug-resistant MCF7 breast cancer cell line is attributable to enhanced energy-dependent drug efflux. Cancer Res. 2000;60:3514–3521. [PubMed] [Google Scholar]
- 32.Rabindran SK, He H, Singh M, Brown E, Collins KI, Annable T, Greenberger LM. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res. 1998;58:5850–5858. [PubMed] [Google Scholar]
- 33.Maliepaard M, van Gastelen MA, de Jong LA, Pluim D, van Waardenburg RC, Ruevekamp-Helmers MC, Floot BG, Schellens JH. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res. 1999;59:4559–4563. [PubMed] [Google Scholar]
- 34.Rao VK, Wangsa D, Robey RW, Huff L, Honjo Y, Hung J, Knutsen T, Ried T, Bates SE. Characterization of ABCG2 gene amplification manifesting as extrachromosomal DNA in mitoxantrone-selected SF295 human glioblastoma cells. Cancer Genet Cytogenet. 2005;160:126–133. doi: 10.1016/j.cancergencyto.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Boonstra R, Timmer-Bosscha H, van Echten-Arends J, van der Kolk DM, van den Berg A, de Jong B, Tew KD, Poppema S, de Vries EG. Mitoxantrone resistance in a small cell lung cancer cell line is associated with ABCA2 upregulation. Br J Cancer. 2004;90:2411–2417. doi: 10.1038/sj.bjc.6601863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yasui K, Mihara S, Zhao C, Okamoto H, Saito-Ohara F, Tomida A, Funato T, Yokomizo A, Naito S, Imoto I, et al. Alteration in copy numbers of genes as a mechanism for acquired drug resistance. Cancer Res. 2004;64:1403–1410. doi: 10.1158/0008-5472.can-3263-2. [DOI] [PubMed] [Google Scholar]
- 37.Shen D, Fojo A, Chin J, Roninson I, Richert N, Pastan I, Gottesman M. Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification. Science. 1986;232:643–645. doi: 10.1126/science.3457471. [DOI] [PubMed] [Google Scholar]
- 38.Fojo AT, Whang-Peng J, Gottesman MM, Pastan I. Amplification of DNA sequences in human multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci U S A. 1985;82:7661–7665. doi: 10.1073/pnas.82.22.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoenlein PV, Shen DW, Barrett JT, Pastan I, Gottesman MM. Double minute chromosomes carrying the human multidrug resistance 1 and 2 genes are generated from the dimerization of submicroscopic circular DNAs in colchicine-selected KB carcinoma cells. Mol Biol Cell. 1992;3:507–520. doi: 10.1091/mbc.3.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen GK, Lacayo NJ, Duran GE, Wang Y, Bangs CD, Rea S, Kovacs M, Cherry AM, Brown JM, Sikic BI. Preferential expression of a mutant allele of the amplified MDR1 (ABCB1) gene in drug-resistant variants of a human sarcoma. Genes Chromosomes Cancer. 2002;34:372–383. doi: 10.1002/gcc.10067. [DOI] [PubMed] [Google Scholar]
- 41.Ince TA, Scotto KW. Differential utilization of multiple transcription start points accompanies the overexpression of the P-glycoprotein-encoding gene in Chinese hamster lung cells. Gene. 1995;156:287–290. doi: 10.1016/0378-1119(94)00907-a. [DOI] [PubMed] [Google Scholar]
- 42.Ince TA, Scotto KW. A conserved downstream element defines a new class of RNA polymerase II promoters. J Biol Chem. 1995;270:30249–30252. doi: 10.1074/jbc.270.51.30249. [DOI] [PubMed] [Google Scholar]
- 43.Ince TA, Scotto KW. Stable transfection of the P-glycoprotein promoter reproduces the endogenous overexpression phenotype: the role of MED-1. Cancer Res. 1996;56:2021–2024. [PubMed] [Google Scholar]
- 44.Scotto K. Transcriptional regulation of ABC drug transporters. Oncogene. 2003;22:7496–7511. doi: 10.1038/sj.onc.1206950. [DOI] [PubMed] [Google Scholar]
- 45.Mickley LA, Spengler BA, Knutsen TA, Biedler JL, Fojo T. Gene rearrangement: a novel mechanism for MDR-1 gene activation. J Clin Invest. 1997;99:1947–1957. doi: 10.1172/JCI119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huff LM, Lee J-S, Robey RW, Fojo T. Characterization of Gene Rearrangements Leading to Activation of MDR-1. J. Biol. Chem. 2006;281:36501–36509. doi: 10.1074/jbc.M602998200. [DOI] [PubMed] [Google Scholar]
- 47.Huff L. Mickley, Wang Z, Iglesias A, Fojo T, Lee J-S. Aberrant Transcription from an Unrelated Promoter Can Result in MDR-1 Expression following Drug Selection In vitro and in Relapsed Lymphoma Samples. Cancer Res. 2005;65:11694–11703. doi: 10.1158/0008-5472.CAN-04-1349. [DOI] [PubMed] [Google Scholar]
- 48.Chekhun VF, Kulik GI, Yurchenko OV, Tryndyak VP, Todor IN, Luniv LS, Tregubova NA, Pryzimirska TV, Montgomery B, Rusetskaya NV, et al. Role of DNA hypomethylation in the development of the resistance to doxorubicin in human MCF-7 breast adenocarcinoma cells. Cancer Lett. 2006;231:87–93. doi: 10.1016/j.canlet.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 49.Eijdems EW, De Haas M, Coco-Martin JM, Ottenheim CP, Zaman GJ, Dauwerse HG, Breuning MH, Twentyman PR, Borst P, Baas F. Mechanisms of MRP over-expression in four human lung-cancer cell lines and analysis of the MRP amplicon. Int J Cancer. 1995;60:676–684. doi: 10.1002/ijc.2910600518. [DOI] [PubMed] [Google Scholar]
- 50.Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T, et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 51.Knutsen T, Rao VK, Ried T, Mickley L, Schneider E, Miyake K, Ghadimi BM, Padilla-Nash H, Pack S, Greenberger L, et al. Amplification of 4q21-q22 and the MXR gene in independently derived mitoxantrone-resistant cell lines. Genes Chromosomes Cancer. 2000;27:110–116. [PubMed] [Google Scholar]
- 52.Volk EL, Farley KM, Wu Y, Li F, Robey RW, Schneider E. Overexpression of wild-type breast cancer resistance protein mediates methotrexate resistance. Cancer Res. 2002;62:5035–5040. [PubMed] [Google Scholar]
- 53.Nakanishi T, Bailey-Dell KJ, Hassel BA, Shiozawa K, Sullivan DM, Turner J, Ross DD. Novel 5′ untranslated region variants of BCRP mRNA are differentially expressed in drug-selected cancer cells and in normal human tissues: implications for drug resistance, tissue-specific expression, and alternative promoter usage. Cancer Res. 2006;66:5007–5011. doi: 10.1158/0008-5472.CAN-05-4572. [DOI] [PubMed] [Google Scholar]
- 54.To KKW, Polgar O, Huff LM, Morisaki K, Bates SE. Histone Modifications at the ABCG2 Promoter following Treatment with Histone Deacetylase Inhibitor Mirror Those in Multidrug-Resistant Cells. Mol Cancer Res. 2008;6:151–164. doi: 10.1158/1541-7786.MCR-07-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.To KKW, Zhan Z, Litman T, Bates SE. Regulation of ABCG2 Expression at the 3′ Untranslated Region of Its mRNA through Modulation of Transcript Stability and Protein Translation by a Putative MicroRNA in the S1 Colon Cancer Cell Line. Mol. Cell. Biol. 2008;28:5147–5161. doi: 10.1128/MCB.00331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou S, Schuetz JD, Bunting KD, Colapietro A-M, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. 2001. pp. 1028–1034. [DOI] [PubMed]
- 57.Chen G, Jaffrezou JP, Fleming WH, Duran GE, Sikic BI. Prevalence of multidrug resistance related to activation of the mdr1 gene in human sarcoma mutants derived by single-step doxorubicin selection. Cancer Res. 1994;54:4980–4987. [PubMed] [Google Scholar]
- 58.Jaffrezou JP, Chen G, Duran GE, Kuhl JS, Sikic BI. Mutation rates and mechanisms of resistance to etoposide determined from fluctuation analysis. J Natl Cancer Inst. 1994;86:1152–1158. doi: 10.1093/jnci/86.15.1152. [DOI] [PubMed] [Google Scholar]
- 59.Dumontet C, Duran GE, Steger KA, Beketic-Oreskovic L, Sikic BI. Resistance mechanisms in human sarcoma mutants derived by single-step exposure to paclitaxel (Taxol) Cancer Res. 1996;56:1091–1097. [PubMed] [Google Scholar]
- 60.Beketic-Oreskovic L, Duran GE, Chen G, Dumontet C, Sikic BI. Decreased mutation rate for cellular resistance to doxorubicin and suppression of mdr1 gene activation by the cyclosporin PSC 833. J Natl Cancer Inst. 1995;87:1593–1602. doi: 10.1093/jnci/87.21.1593. [DOI] [PubMed] [Google Scholar]
- 61.Chen KG, Wang YC, Schaner ME, Francisco B, Duran GE, Juric D, Huff LM, Padilla-Nash H, Ried T, Fojo T, et al. Genetic and epigenetic modeling of the origins of multidrug-resistant cells in a human sarcoma cell line. Cancer Res. 2005;65:9388–9397. doi: 10.1158/0008-5472.CAN-04-4133. [DOI] [PubMed] [Google Scholar]
- 62.Su GM, Davey MW, Davey RA. Induction of broad drug resistance in small cell lung cancer cells and its reversal by paclitaxel. Int J Cancer. 1998;76:702–708. doi: 10.1002/(sici)1097-0215(19980529)76:5<702::aid-ijc15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 63.Shinoda C, Maruyama M, Fujishita T, Dohkan J, Oda H, Shinoda K, Yamada T, Miyabayashi K, Hayashi R, Kawagishi Y, et al. Doxorubicin induces expression of multidrug resistance-associated protein 1 in human small cell lung cancer cell lines by the c-jun N-terminal kinase pathway. Int J Cancer. 2005;117:21–31. doi: 10.1002/ijc.21094. [DOI] [PubMed] [Google Scholar]
- 64.Calcagno AM, Chewning KJ, Wu CP, Ambudkar SV. Plasma membrane calcium ATPase (PMCA4): a housekeeper for RT-PCR relative quantification of polytopic membrane proteins. BMC Mol. Biol. 2006;7:29. doi: 10.1186/1471-2199-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]