Abstract
Motivated by auditory and speech deficits in autism spectrum disorders (ASD), the frequency dependence of superior temporal gyrus (STG) 50 msec (M50) and 100 msec (M100) neuromagnetic auditory evoked field responses in children with ASD and typically developing controls were evaluated. Whole-cortex magnetoencephalography (MEG) was obtained from 17 typically developing children and 25 children with ASD. Subjects were presented tones with frequencies of 200, 300, 500, and 1,000 Hz, and left and right STG M50 and M100 STG activity was examined. No M50 latency or amplitude Group differences were observed. In the right hemisphere, a Group × Frequency ANOVA on M100 latency produced a main effect for Group (P 5 0.01), with an average M100 latency delay of 11 msec in children with ASD. In addition, only in the control group was the expected association of earlier M100 latencies in older than younger children observed. Group latency differences remained significant when hierarchical regression analyses partialed out M100 variance associated with age, IQ, and language ability (all P-values < 0.05). Examining the right-hemisphere 500 Hz condition (where the largest latency differences were observed), a sensitivity of 75%, a specificity of 81%, and a positive predictive value (PPV) of 86% was obtained at a threshold of 116 msec. The M100 latency delay indicates disruption of encoding simple sensory information. Given similar findings in language impaired and nonlanguage impaired ASD subjects, a right-hemisphere M100 latency delay appears to be an electrophysiological endophenotype for autism.
Keywords: autism spectrum disorders, M50, M100, magnetoencephalography, language impairment, auditory evoked response
INTRODUCTION
Autism spectrum disorders (ASD) are a set of disabilities diagnosed in childhood that have significant impact throughout development and into adulthood. The ASD spectrum encompasses Autism Disorder, Asperger's Disorder, and Pervasive Developmental Disorder Not Otherwise Specified (PDD-NOS), and thus this disorder is characterized by great phenotypic heterogeneity. As a neurodevelop-mental disorder,itis likelythatabetterunderstanding of the neurobiological abnormalities associated with ASD is needed to allow advances in the diagnosis and treatment of individuals included under this umbrella diagnosis. Specifically, whereas clinical diagnosis and ongoing assessment is presently made on the basis of observed behavioral characteristics, underlying structural and functional brain abnormalities may better characterize this heterogeneous disorder, allowing for more effective treatment and therapy monitoring [Edgar, Keller, Heller, & Miller, 2007].
As early auditory processes are hypothesized to be impaired in ASD, several studies have examined the 50 and 100 msec auditory responses [for reviews, see Bomba & Pang, 2004; Jeste & Nelson, 2009]. Although both the latency and amplitude of early auditory responses have been examined, the present study focuses on latency, as group latency differences appear more often than amplitude differences.
With regard to latency, some electroencephalographic (EEG) studies find earlier 100 msec (N1) auditory responses in subjects with autism than controls [Dawson, Finley, Phillips, & Galpert, 1986; Martineau, Garreau, Barthelemy, & Lelord, 1984; Oades, Walker, Geffen, & Stern, 1988]. For example, Ferri et al. [2003] observed shorter N1 latencies in males with autism and concomitant mental retardation (MR) than controls. In contrast, comparing participants to controls, Bruneau, Roux, Adrien, and Barthelemy [1999] showed a longer N1 latency in children with autism, Seri, Cerquiglini, Pisani, and Curatolo [1999] longer N1 latencies in very young autistic children with tuberous sclerosis complex, Korpilahti et al. [2007] a longer N1 latency in children with Asperger's Syndrome, and Sokhadze et al. [2009] a longer N1 latency in children with autism. Similarly, Dunn, Vaughan, Kreuzer, and Kurtzberg [1999] reported longer N1 latencies in response to words in a target-detection paradigm in nonmentally retarded children with autism compared to age-matched controls. Some studies, however, have reported no 100 msec latency group differences [Kemner, Verbaten, Cuperus, Camfferman, & van Engeland, 1995; Lincoln, Courchesne, Harms, & Allen, 1995; Novick, Vaughan, Kurtzberg, & Simson, 1980].
A handful of more recent studies have used magnetoencephalography (MEG) to examine auditory processes in ASD. An advantage of MEG is that, compared to EEG, separate measures of left- and right-hemisphere auditory activities are more easily obtained [see Edgar et al., 2003]. Despite this advantage, MEG 100 msec (M100) latency findings in ASD are also mixed. Presenting 200 and 1,000 Hz tones, Gage, Siegel, Callen, and Roberts [2003a] observed a later M100 in males with ASD than controls. In contrast, using a paired-tone (1 kHz/2 kHz) paradigm, Oram Cardy, Ferrari, Flagg, Roberts, and Roberts [2004] observed no M50 or M100 group latency differences to the first tone. Similarly, examining M100 right-hemisphere activity in children with autism and controls administered an oddball task, Tecchio et al. [2003] observed no M100 group latency differences.
In addition to simply examining the latency of the auditory responses in ASD, several studies have sought to examine other features of the 100 msec auditory response. As an example, neural correlates of the recognition of basic features of auditory stimuli are observed in the latency of auditory event-related field (ERF) components as early as 100 msec [Roberts & Poeppel, 1996], with an earlier M100 response to high-frequency than low-frequency tones [Roberts, Ferrari, Stufflebeam, & Poeppel, 2000]. A few studies have examined associations between 100 msec latency and tone frequency in ASD. Examining M100 latencies to 200, 500, and 1,000 Hz tones, Gage et al. [2003a] noted that whereas the general form of the M100 latency response as a function of tone frequency was intact in children with ASD and typically developing controls (conforming to a model in which latency is inversely associated with frequency), the dynamic range in children with ASD (the latency difference between M100 responses to 200 and 1,000 Hz stimuli) was reduced in the right hemisphere of children with autism.
A few studies have examined associations between 100 msec latency and age. Paetau, Ahonen, Salonen, and Sams [1995] discuss the changing form of electrophysio-logical responses to auditory stimulation (using both tones and speech elements) as a function of typical childhood and adolescent development. They noted that auditory components differ as a function of age, and observed a tendency for major ERPs (e.g., N1) to become stronger and to occur at an earlier latency with increasing age [see also Tonnquist-Uhlen, Borg, & Spens, 1995]. Examining auditory responses to 1,000 Hz tones, Oram Cardy et al. [2004] noted later M50 and M100 responses in children than adults. In another study, whereas M100 latency was associated with age in both hemispheres in a group of typically developing controls, M100 latency was associated with age only in the left hemisphere in ASD subjects [Gage, Siegel, & Roberts, 2003b].
Finally, there is evidence suggesting that the latency of the 50 and/or 100 msec auditory response may be associated with language ability. For example, Eggermont, Ponton, Don, Waring, and Kwong [1997] measured the latency of the EEG 50 msec (P1) component in deaf children with cochlear implants who had undergone prolonged auditory deprivation prior to implant and reported that time-to-maturation of the P1 in the children with implants was delayed by a duration roughly equal to the duration of their deafness. Jirsa and Clontz [1990] observed increased N1 latencies in children with auditory processing disorders compared to controls. Examining M50 (the magnetic analog of the P50) and M100 data from children and adult controls, children with autism, children with Asperger's syndrome, and children with specific language impairment (SLI), Oram Cardy, Flagg, Roberts, and Roberts [2008] noted that longer M50 latencies predicted worse receptive language ability.
The goal of the present study was to examine the auditory evoked response latency in a well-characterized sample of typically developing children/adolescents and children with ASD. In all subjects, the latency and amplitude of auditory activity at 50 and 100 msec was examined in response to 200, 300, 500, and 1,000 Hz tones. Rather than examine activity in sensor space (as most previous studies have), activity was examined in source (brain) space to more directly examine brain activity. In particular, MEG was used to model auditory activity in the left and right superior temporal gyri. The relevant MEG literature points to superior temporal gyrus (STG) as the 50 msec generator [e.g., Edgar et al., 2003; Huotilainen et al., 1998; Makela, Hamalainen, Hari, & McEvoy, 1994; Pelizzone et al., 1987; Reite, Teale, Zimmerman, Davis, & Whalen, 1988; Yoshiura, Ueno, Iramina, & Masuda, 1995; Yvert, Crouzeix, Bertrand, Seither-Preisler, & Pantev, 2001]. Investigators using either intraoperative electrocorticography [Liegeois-Chauvel, Musolino, Badier, Marquis, & Chauvel, 1994] or chronic subdural electrodes [Lee et al., 1984] have also reported that the EEG P50 is a near-field potential in the primary auditory cortex. Picton et al. [1999] noted that, although multiple brain regions contribute to N1, the major activity underlying the scalp-recorded EEG N1 wave is located in the supratemporal plane. Because MEG does not detect activity from radial current configurations, M100 is well described as being generated by a pair of equivalent current dipoles (one in each hemisphere) located in the region of the planum temporale [e.g., Hari, 1990].
The following predictions were made. Replicating the several studies that have observed delayed auditory latencies in ASD, longer M100 latencies were expected in children with ASD (Hypothesis 1). Replicating Gage et al. [2003a], a decreased dynamic range between the 200 and 1,000 Hz tones in children with ASD was expected (Hypothesis 2). These two results would indicate that a primary feature of auditory perception in ASD may be an encoding deficit, manifested as a delayed M100 response. As a result of the predicted latency delays in ASD, it was expected that the developmental relationship of earlier latencies in older subjects would not be observed in the children with ASD (Hypothesis 3). Finally, as a relationship between a delayed evoked response component and language impairment (LI) was reported in a previous study [Oram Cardy et al., 2008], a similar association was expected in the present study. Specifically, it was predicted that a delayed M50 (and perhaps M100) response would be most prominent in a group of ASD subjects with concomitant LI (Hypothesis 4).
Methods
Participants
Subjects with ASD were recruited from the Regional Autism Center of The Children's Hospital of Philadelphia (CHOP), the Neuropsychiatry program of the Department of Psychiatry of the University of Pennsylvania School of Medicine, and from local and regional parent support groups such as ASCEND (Asperger Syndrome Information Alliance for Southeastern Pennsylvania), Autism Society of America—Greater Philadelphia Chapter, and local chapters of Autism Speaks. All children screened for inclusion in the ASD sample had a prior ASD diagnosis made by an expert clinician, typically a developmental pediatrician in the Regional Autism Center at the Children's Hospital of Philadelphia. The original diagnosis was made after an extensive clinical interview, documentation of DSM-IV criteria for ASD, and use of various ASD diagnostic tools, such as the Childhood Autism Rating Scale and, in many cases, the ADOS. Subjects with typical development (TD) were recruited through local newspaper advertisements and from pediatric practices of the CHOP primary care network.
Research participants made two visits to CHOP. During the first visit (2–3 weeks prior to the MEG exam), clinical and diagnostic testing was performed to confirm the referral ASD diagnosis, to administer neuropsychological tests, and to ensure that the TD children met study inclusion/exclusion criteria. Assessments were performed by licensed child psychologists with expertize in autism (L.B., S.W.). Given the extensive clinical evaluations upon which original diagnosis was made, an abbreviated diagnostic battery was used to confirm the original diagnosis. Specifically, the ASD diagnosis was confirmed with standard diagnostic tools, including direct observation with the Autism Diagnostic Observation Schedule [ADOS; Lord et al., 2000] and parent report on the Social Communication Questionnaire [SCQ; Rutter, Bailey, & Lloyd, 2003]. Dimensional symptom severity ratings were also obtained by parent report on the Social Responsiveness Scale [SRS; Constantino & Gruber, 2005]. Asperger's disorder symptomatology was measured with the Krug Asperger's Disorder Index [KADI; Krug & Arick, 2003]. For final inclusion in the ASD group, children were required to exceed established cut-offs on both the ADOS and SCQ. Children 1 point below ADOS cut-offs were included if they exceeded cut-offs on at least two parent questionnaires (one ASD subject met ADOS criteria and had a best-estimate diagnosis of ASD by clinician judgment, but exceeded cut-offs on only one parent questionnaire).
To confirm the presence/absence of LI, all subjects were evaluated with the Clinical Evaluation of Language Fundamentals—4th edition [CELF-4; Semel, Wiig, & Secord, 2003]. The ASD group with LI (ASD1LI) was comprised of subjects with a CELF-4 Core Language score below the 16th percentile. The ASD group without LI (ASD LI) performed at or above the 16th percentile on the CELF-4. To rule out global cognitive delay, all subjects were required to score at or above the 5th percentile (SS>75) on the Perceptual Reasoning Index (PRI) of the Wechsler Intelligence Scale for Children-IV [WISC-IV; Wechsler, 2003]. In all subjects, the WISC-IV Verbal Comprehension Index (VCI) was also obtained.
Inclusion criteria for the TD children included scoring below the cut-off for ASD on all domains of the ADOS as well as parent questionnaires, and performance above the 16th percentile on the CELF-4. In addition to the above inclusion/exclusion criteria, all subjects and families were native English speakers and had no known genetic syndromes or neurological (e.g., cerebral palsy, epilepsy), or sensory (hearing, visual) impairments. The study was approved by the CHOP Institutional Review Board and all participants’ families gave written informed consent. As indicated by institutional policy, where competent to do so, children over the age of seven additionally gave verbal assent.
Auditory Stimuli
Auditory stimuli were presented using Eprime v1.1 experimental software (Psychology Software Tools Inc., Pittsburgh, PA). Auditory stimuli were delivered via a sound pressure transducer and sound conduction tubing to the subject's peripheral auditory canal via eartip inserts (ER3A, Etymotic Research, Illinois). Prior to the MEG exam, each participant's hearing threshold was determined, and the auditory stimuli were presented 45 dB SPL above threshold. During the MEG exam, 200, 300, 500, and 1,000 Hz sinusoidal tones of 300 msec duration were binaurally presented (digitized at 1,041.7 Hz with a 10 msec rise time). Tones were randomly presented, with a 1 sec interstimulus interval (jittered ± 100 msec). Over approximately 10 min of recording time, 105 tones at each of the 4 frequencies were presented.
MEG Recordings
Recordings were performed at the Lurie Family Foundations’ MEG Imaging Center of the Department of Radiology in a magnetically shielded room using a whole-cortex 275-channel MEG system (VSM MedTech Inc., Coquitlam, BC). At the start of the session, three head-position indicator coils were attached to the scalp. These coils provided continuous specification of the position and orientation of the MEG sensors relative to the head. Because it was necessary for the participants’ heads to remain in the same place in the MEG dewar across the recording session, foam wedges were inserted between the side of each participant's head and the inside of the dewar to ensure immobility. To minimize subject fatigue and encourage an awake state during acquisition, subjects viewed (but did not listen to) a movie projected on to a screen positioned at a comfortable viewing distance.
To aid in the identification of eye-blink activity, the electro-oculogram (EOG; bipolar oblique, upper and lower left sites) was collected. Electrodes were also attached to the left and right collar bone for electrocardiogram (ECG) recording. After a band-pass filter (0.03–150 Hz), EOG, ECG, and MEG signals were digitized at 1200 Hz with 3rd order gradiometer environmental noise reduction for the MEG data.
MEG Data Analysis
M50 and M100 source localization was done blind to participant group. Epochs 500 msec pre-stimulus to 500 msec post-stimulus were defined from the continuous recording. To correct for eye blinks, a typical eye blink was manually identified in the raw data (including EOG) for each participant. The pattern search function in BESA 5.2 (MEGIS Software GmbH, Graäfelfing, Germany) scanned the raw data to identify other blinks and computed an eye-blink average. An eye blink was modeled by its first component topography from principal component analysis (PCA), typically accounting for more than 99% of the variance in the eye-blink average. In addition to eye-blink activity, a heartbeat average was obtained and heartbeat activity was modeled by the first two PCA components topographies of a heartbeat average, typically accounting for more than 85% of the variance in the heartbeat average. Scanning the eye blink and heartbeat-corrected raw data, epochs with artifacts other than blinks and heartbeat were rejected by amplitude and gradient criteria (amplitude > 1200 fT/cm, gradients > 800 fT/cm/sample). Noncontaminated epochs were averaged according to stimulus type and a 1 Hz (6 dB/octave, forward) to 40 Hz (48 dB/octave, zero-phase) band-pass filter was applied.
Using all 275 channels of MEG data, determination of the strength and latency of M50 and M100 sources in the left and right STG was accomplished by applying a standard source model to transform each individual's raw MEG surface activity into brain space (MEG data co-registered to the Montreal Neurologic Institute (MNI) averaged brain) using a model with multiple sources [Scherg, 1990; Scherg & Berg, 1996; Scherg & von Cramon, 1985]. In particular, the standard source model applied to each subject was constructed by including (1) left and right STG dipole sources (placed at Heschl's gryus), and (2) nine fixed regional sources that modeled brain background activity and serve as probe sources for additional oscillatory activity. The eye-blink and heartbeat source vectors derived for each participant were also included in each participant's source model to remove eye-blink and heartbeat activity [Berg and Scherg, 1994; Lins, Picton, Berg, & Scherg, 1993]. The final source model served as a source montage for the raw MEG [Scherg & Ebersole, 1994; Scherg, Ille, Bornfleth, & Berg, 2002]. As such, the MEG sensor data was transformed from channel space into brain source space where the visualized waveforms were the modeled source activities. This spatial filter disentangled the source activities of the different brain regions that overlapped at the sensor level. Of note, although the amplitude and latency of the 50 and 100 msec STG responses were obtained using a dipole source placed at a standard location, in each subject left- and right-hemisphere dipoles were oriented at the maximum of the M50 and M100. As such, orientation of the standard STG sources was optimized in each subject.
To measure M50 (40–90 msec) and M100 (90–180 msec) STG amplitude and latency, prestimulus baseline activity ( —400 to —100 msec) was subtracted, and left and right M50 and M100 STG peak source strength (measured in nano-Ampere-meters, nAm) and latency were calculated from the largest point in the M50 and M100 scoring windows using in-house MatLab software (Mathworks, Natick, MA). These slightly extended M50 and M100 latency ranges allowed capturing responses observed in younger children and with low frequency stimuli.
Group Comparisons
For all MEG analyses, subjects more than three standard deviations from the group mean were excluded (typically one to two subjects per variable). In each hemisphere, Group × Frequency (200, 300, 500, 1000 Hz tones) ANOVAs examined differences in source strength and latency separately for M50 and M100. If significant group differences were observed, receiver operator curve (ROC) analyses assessed the sensitivity and specificity of the measure.
Given expected group differences in CELF-4 and IQ scores, to examine the relationship between cognitive and language ability, and how cognitive and language ability may differ as a function of diagnostic status and M50/M100 amplitude and latency, hierarchical regression was performed in which CELF-4 or IQ was entered first, group second, and their interaction last, with the M50 and M100 measures of interest entered as the dependent variable. Finally, as M100 latency is affected by age [Gage et al., 2003b; Oram Cardy et al., 2008], and as an association between age and M100 latency was hypothesized in the control but not patient group, regression analyses with age were also performed.
Results
Excluded Subjects
Sixty-two subjects entered the study. Twenty subjects were excluded (19 ASD referrals, 1 TD control). Nine ASD referred subjects who did not meet current criteria for ASD were excluded, as were three ASD subjects with a full scale IQ < 75. Seven ASD subjects were excluded because MEG recordings were too noisy (due to metal artifact), because they were unable to tolerate the MEG procedure, or because they did not arrive for the imaging appointment. One control subject who scored above the ADOS cut-off threshold was excluded. After exclusions, 25 children with ASD and 17 TD children remained.
Demographics
Of the final sample, two children with ASD were left-handed and 1 child with ASD was ambidextrous (self-report). In the patient group, two were receiving medications for ADHD, two were receiving ADHD medications and antidepressants, and two were receiving ADHD medications and antipsychotics: five of the six subjects were receiving methylphenidate type medications (RitalinTM, DaytranaTM, ConcertaTM, FocalinTM), with the remaining subject taking amphetamine (AdderallTM). None of the control subjects were taking prescription medications. Of the participants, 22 identified themselves as White (22 European American, 0 as Hispanic), 2 as African American and 1 as bi-racial (European and African American). Of the controls, 10 identified themselves as white (8 European Americans, 2 Hispanics), 6 as African American and 1 as Hispanic.
Other demographics are reported in Table Ia. ANOVAs examining group differences showed groups were similar in age, t(40) = 0.88, ns. As expected, controls had higher PRI, t(40) = 2.42, P < 0.05, VCI, t(39) = 2.10, P < 0.05, and SRS scores, t(40) = –11.5, P < 0.01. Comparing the ASD groups, as shown in Table Ib, the ASD+LI and the ASD—LI groups did differ in age, t(23) = 2.13, P < 0.05, in this small sample. As expected, given the inclusion and exclusion criteria, the two ASD groups differed on VCI, t(23) = 4.10, P < 0.01, SRS, t(23) = 2.43, P < 0.05, and CELF-4 scores, t(23) = 6.94, P < 0.01.
Table I.
Demographic Information: (a) Controls and Children with ASD and (b) ASD–LI and ASD+LI
| Controls (N = 17) |
ASD (N = 25) |
|||
|---|---|---|---|---|
| Groups | Mean (years) | SD | Mean (years) | SD |
| (a) | ||||
| Age | 10.77 | 1.98 | 10.20 | 2.15 |
| PRI* | 110.65 | 12.20 | 100.32 | 14.35 |
| VCI* | 107.13 | 13.10 | 97.36 | 15.35 |
| SRS SS** | 43.80 | 5.04 | 77.64 | 11.43 |
| CELF** | 109.94 | 9.72 | 87.96 | 20.97 |
| ASD–LI (N = 16) |
ASD+LI (N = 9) |
|||
|---|---|---|---|---|
| Mean (years) | SD | Mean (years) | SD | |
| (b) | ||||
| Age* | 10.83 | 2.19 | 9.06 | 1.62 |
| PRI** | 106.06 | 13.66 | 90.11 | 9.20 |
| VCI** | 104.69 | 11.86 | 84.33 | 12.04 |
| SRS SS* | 81.44 | 11.90 | 70.89 | 6.81 |
| CELF** | 100.63 | 11.32 | 65.44 | 13.70 |
Group differences significant at P < 0.05.
Group differences significant at P < 0.01.
Groups did not differ in maximum head displacement, t(40) = 1.01, P = 0.32. During the recording, head motion may be transient and return to baseline position. Analyses indicated that 36 out of 42 subjects did not exceed 1 cm transient displacement in more than 10% of the trials (with 18 subjects never exceeding 1 cm displacement). Analyses excluding the 6 subjects with transient movement on more than 10% of the trails did not change the significance of any finding. Although children with ASD tended to have weaker hearing (detection thresholds ~5 dB higher than the TD group), the controls and children with ASD did not differ significantly in hearing thresholds in the left, t(40) = 1.60, P = 0.12, or right ear, t(40) = 1.25, P = 0.22. As noted above, stimuli were presented 45 dB above individually determined thresholds, controlling for individual hearing threshold differences.
Left- and right-hemisphere STG source strength and latency
M50
Peak STG M50 latency values for each hemisphere and frequency are reported in Table II. A Group (ASD, controls) × Frequency (200, 300, 500, 1000 Hz) ANOVA on STG M50 latency indicated longer left, F(1,26) = 26.33, P < 0.001, and right STG M50 latencies, F(1,16) = 16.58, P < 0.001, for lower than higher frequency tones. Groups did not differ in M50 source strength values in the left or right hemisphere (P's > 0.05).
Table II.
M50/M100 STG Latency Mean and Standard Deviation (SD) Values
| M50 | 200 Hz latency (ms) and SD | N | 300 Hz latency (ms) and SD | N | 500 Hz latency (ms) and SD | N | 1000 Hz latency (ms) and SD | N |
|---|---|---|---|---|---|---|---|---|
| Controls | ||||||||
| Left STG | 76.54 (13.04) | 13 | 78.33 (14.7) | 15 | 71.38 (14.33) | 16 | 62.41 (24.32) | 17 |
| Right STG | 85.8 (23.35) | 10 | 75.46 (15.86) | 13 | 66.14 (28.11) | 14 | 60.81 (29.22) | 11 |
| ASD | ||||||||
| Left STG | 86.67 (23.14) | 18 | 85 (15.80) | 22 | 74.16 (14.15) | 24 | 65.45 (7.43) | 22 |
| Right STG | 82.67 (11.87) | 12 | 85.38 (13.5) | 16 | 78.05 (8.98) | 21 | 72.3 (9.85) | 20 |
| M100 | 200 Hz latency (msec) and SD | N | 300 Hz latency (msec) and SD | N | 500 Hz latency (msec) and SD | N | 1000 Hz latency (msec) and SD | N |
|---|---|---|---|---|---|---|---|---|
| Controls | ||||||||
| Left STG | 131.15 (18.12) | 13 | 130.38 (21.25) | 13 | 121.62 (21.50) | 13 | 120.17 (17.90) | 12 |
| Right STG | 129.5 (14.10) | 16 | 124.25 (16.08)* | 16 | 112.63 (14.85)** | 16 | 110.43 (7.90) | 14 |
| ASD | ||||||||
| Left STG | 143.29 (15.17) | 14 | 135.8 (15.83) | 15 | 118.63 (20.72) | 16 | 110.79 (20.30) | 19 |
| Right STG | 139.67 (19.50) | 21 | 136.55 (19.03) | 22 | 125.58 (14.32) | 24 | 115.75 (10.73) | 24 |
Although analyses relied on ANOVAs, for the reader's convenience, Table II shows the Ns in each group for each measure. Although the right-hemisphere Group × Frequency interaction term was not significant, t-tests results at each frequency are provided to allow comparison with other studies.
Controls vs. ASD P ≤ 0.05.
Control vs. ASD P ≤ 0.01.
M100
Peak M100 latency values for each hemisphere and frequency are reported in Table II. A Group (ASD, controls) × Frequency (200, 300, 500, 1000 Hz) ANOVA on STG M100 latency indicated longer left, F(1,20) = 32.08, P < 0.001, and right STG M100 latencies, F(1,33) = 61.47, P < 0.001, for lower than higher frequency tones, a finding consistent with previous literature [Roberts & Poeppel, 1996; Roberts et al., 2000]. None of the left-hemisphere M100 Group × Frequency terms were significant. Supporting Hypothesis 1 (Figs. 1 and 2), children with ASD had delayed right-hemisphere M100 responses across all frequencies, Group F(1,33) = 7.09, P < 0.05. The right-hemisphere Group × Frequency interaction was not significant, indicating that the direction of the Group main effect did not differ over the 4 frequencies. Groups did not differ in M100 source strength values in the left or right hemisphere (all P's > 0.30).
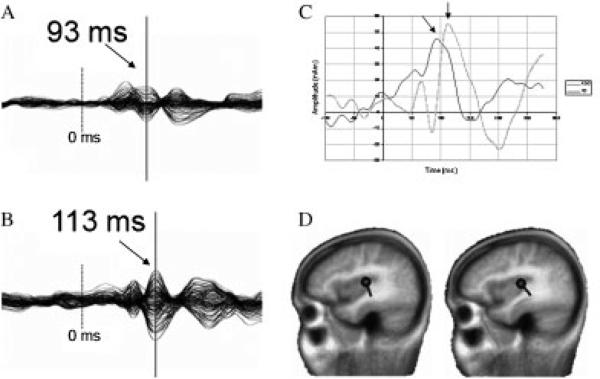
Figure 1.
(A) Right-hemisphere M100 STG sensor waveforms for a typically developing and (B) age-matched ASD participant. Note similar sensor waveform morphology, but temporal shift (~20 msec) in participant with ASD. Dashed vertical line indicates stimulus onset (0 msec). Arrows indicate M100 peak. (C) Right-hemisphere source waveforms derived from BESA standard source model applied to the sensor data shown in (A) and (B). Note the similarity in M100 peak latency between the sensor and source waveforms. (D) Sagittal brain image displays STG dipole differentially oriented at peak M100 amplitude for both subjects.
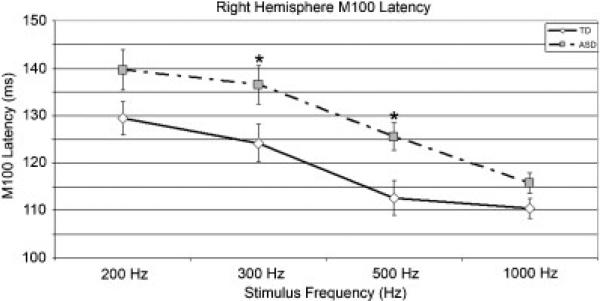
Figure 2.
Right-hemisphere M100 average latencies (error bars represent standard error of the mean) as a function of frequency for TD and ASD children. Although the right-hemisphere Group × Frequency interaction term was not significant, the ‘*’’ shows significant t-tests results (P < 0.05) at 300 and 500 Hz to allow comparison with other studies. Latency delay in children with ASD is most evident at 500 Hz.
The significance of left- and right-hemisphere ANOVA results were unchanged after removing the children with ASD receiving medications, a finding consistent with several previous studies observing no 100 msec latency (or amplitude) changes in subjects administered methylphenidate [e.g., Korostenskaja, Kicic, & Kahkonen, 2008; Verbaten et al., 1994]. Furthermore, as previously indicated, eliminating the six children with the greatest head motion did not change the significance of the repeated measures ANOVA (left: n.s., right: P = 0.02).
Regression Models
As group differences were observed primarily only for latency in the right hemisphere, regression analyses investigated only right-hemisphere M100 latency. To limit the number of regressions, regressions were performed only where the largest group latency difference was observed (500 Hz, see Fig. 2 and Table II).
CELF-4 predicting 500 Hz M100 latency
The full regression model (CELF-4, Group, interaction) accounted for a marginally significant 17% of the variance in the right-hemisphere 500 Hz M100 latency (P = 0.07). Added first, CELF-4 did not account for significant variance, indicating no relationship between language ability and M100 latency. Added second, the Group main effect added 15% of the variance (P = 0.01), demonstrating that group latency differences remained even after removing M100 latency variance associated with language ability. The CELF-4 × Group interaction was not significant. Eliminating children with excessive head motion, or those children taking medications, did not change any finding.
PRI predicting 500 Hz M100 latency
The full regression model (PRI, Group, interaction) accounted for considerable variance in right-hemisphere 500 Hz M100 latency (29%, P < 0.01). Added first, PRI did not account for significant variance, indicating no relationship between cognitive ability and M100 latency. Added second, Group added 28% of the variance (P < 0.01), indicating the group (ASD vs. TD) differences remained even after removing M100 latency variance associated with cognitive ability. The PRI IQ × Group interaction was not significant.
Age predicting 500 Hz M100 latency
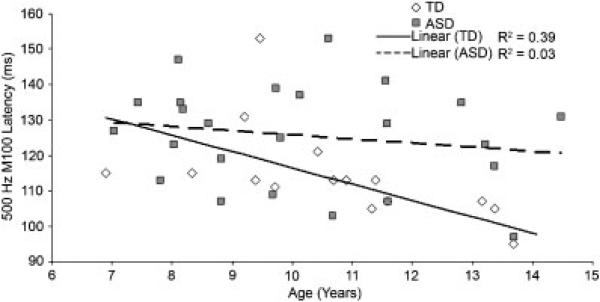
The full regression model (age, Group, interaction) accounted for considerable variance in right-hemisphere 500 Hz M100 latency (32%, P < 0.01). Added first, age accounted for significant variance (15%, P < 0.05). Added second, Group added 12% of the variance (P < 0.05). The Age Group interaction was marginally significant (5%, P = 0.12). Given an a priori prediction of a significant association between age and M100 latency only in the control group, zero-order correlations in each group were examined although the Age Group interaction was not significant. As hypothesized, and shown in Figure 3, an association between age and M100 latency was observed in controls (R2 = 0.39, P < 0.01) but not children with ASD (R2 = 0.03, ns). Significance of age and M100 latency results were unchanged after removing the six children with ASD receiving medications.
Figure 3.
Scatterplot of right-hemisphere 500 Hz M100 response latencies and age for TD and ASD children. Age was significantly associated with M100 latency only in controls.
ROC analyses
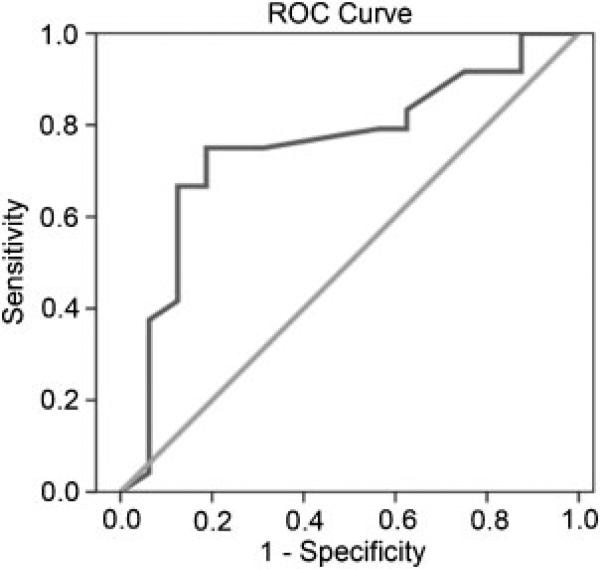
A classification model determined the latency value that best distinguished the ASD and control groups. In particular, ROC analyses on right-hemisphere M100 latency determined the sensitivity and specificity of the M100 latency measure. As shown in Figure 4 (a graphical plot of the sensitivity vs. 1-specificity for a binary classifier system as its discrimination threshold is varied), focusing on the right-hemisphere 500 Hz condition, a cut-off threshold of 116 msec provided a sensitivity of 75%, a specificity of 81%, and a positive predictive value (PPV) of 86% for ASD.
Figure 4.
ROC analysis of M100 response latency to a 500 Hz tone stimulus in the right hemisphere: sensitivity 75% and specificity of 81%. Positive predictive value (PPV) is 86%.
Discussion
This study reports significant differences in M100 evoked response latency in children with ASD compared to TD controls. These differences were manifest primarily in the right hemisphere, evident at all frequencies (although maximal at 500 Hz), and M100 latency classified subjects with ASD with high sensitivity, specificity, and PPV.
Supporting Hypothesis 1, a Group main effect indicated right-hemisphere M100 latency prolongation at all frequencies in children with ASD (Fig. 2). Present findings thus indicate that a primary feature of auditory perception in ASD is an encoding deficit manifested as a delayed M100 response. As noted in the introduction, in several studies [Oram Cardy et al., 2004; Tecchio et al., 2003], control and autism 100 msec latency differences were not observed. In other studies individuals with autism were found to have an earlier 100 msec response [Ferri et al., 2003]. An examination of the data in these studies suggests somewhat less variability in 100 msec latency findings than a cursory examination of the results indicates. For example, although Lincoln et al. [1995] did not observe a delayed N1 in subjects with autism, their Table II, Table III, and Figure 3 show longer 100 msec latencies in the autism group, suggesting that a lack of 100 msec latency group differences may be due to a somewhat small sample size (N = 10 children with autism, 10 controls). It is also worth noting whereas in Lincoln et al. [1995] the lowest tone frequency was 1,000 Hz, in the present study, examination of group differences at each frequency showed significant right-hemisphere differences only at 300 and 500 Hz (see Fig. 2 and Table II). The 100–1,000 Hz spectral range encompasses the first formant (F1) position of most vowel sounds [Roberts et al., 2000]. As such, distinguishing between different frequencies within this range may be of great importance for communication. The present findings suggest that the latency delay in ASD may be most evident when individuals with autism are presented auditory information within a specific range of frequencies (although in the present study the significant main effect indicates that the direction of the right-hemisphere latency finding is the same at each frequency).
A somewhat different set of issues may account for differences between the present findings and those reported in Oades et al. [1988]. Although Oades et al. [1988] noted earlier 100 msec responses in autism, the N1 latencies they report in their Table III are somewhat late, with Cz mean latency values in controls of 178 msec (500 Hz tones) and 184 msec (1,000 Hz tones). In the present study, the 1,000 Hz control mean latency value in the left hemisphere was 120 msec (SD = 18) and in the right hemisphere 110 msec (SD = 7.9). M100 latencies in the present study are consistent with latencies reported in other studies examining 100 msec auditory activity in similarly aged subjects [e.g., see Ferri et al., 2003; Lincoln et al., 1995; Tonnquist-Uhlen et al., 1995]. Oades et al. [1988], discussing the late N1 observed in their study, suggested that activity from other components (N2b and Nc) may have contributed to their N1 latency measure. In addition, although their N1 measures were obtained at Fz and Pz, the authors noted a more varied distribution of amplitude maxima for all ERP components in the participants with autism. The above suggests that group differences in the orientation of the N1 generators, or group differences in the brain regions contributing to N1, could have contributed to the Oades et al. findings. Such concerns underscore the need to examine brain space activity rather than the multiply determined sensor activity [for a detailed discussion of these issues see Edgar et al., 2003]. Finally, differences in subject populations may also account for study differences, with the Oades et al. autism group including several subjects with MR, and the present study excluding subjects with MR.
Such considerations, however, may not account for the earlier N1 responses Ferri et al. [2003] observed in children with autism and concomitant MR, with a ~17 msec earlier N1 Cz response in autism than controls. In the present study, a nonsignificant earlier response in autism than controls was observed for the 1,000 Hz left-hemisphere M100. Such results perhaps suggest either a normal or an abnormally early left-hemisphere M100 response in ASD, and a delayed right-hemisphere M100 response in ASD. The Ferri et al. [2003] EEG findings may reflect a greater contribution of left-hemisphere activity to the Cz response in their group of ASD subjects. Such considerations again underscore the need to separately examine left- and right-hemisphere activity.
Support for the hypothesis of a decreased dynamic range between the 200 and 1,000 Hz tones in children with ASD was not obtained (Hypothesis 2). In each hemisphere and for M50 and M100, an earlier response to high frequency than low frequency tones was observed in both groups, a finding that replicated previous studies examining typically developing subjects [Roberts & Poeppel, 1996; Roberts et al., 2000].
Although in the right hemisphere the M100 Age × Group interaction was not significant, zero-order correlations in each group did show an association in controls (R2 = 0.39) but not children with ASD (R2 = 0.03). Thus, supporting Hypothesis 3, only TD children showed the expected association between M100 latency and age. Present findings replicate Gage et al. [2003b], and indicate auditory cortex maturational abnormalities in ASD. An examination of 500 Hz latency and age scatterplot (Fig. 3) suggests this finding in ASD is due to a failure in older subjects with ASD to achieve full maturational development of the auditory system. This suggests that resolvable right-hemisphere latency delays in ASD will be more frequently observed in older than younger children with ASD.
As a delayed M100 was observed in LI children with ASD as well as in children with ASD without LI, support for the hypothesis of an association between M50 or M100 latency and LI was not obtained (Hypothesis 4). As such, present results suggest that M100 latency shifts are a marker of impaired brain function in ASD per se and not a representation of LI. Oram Cardy et al. [2008] observed that right-hemisphere M50 latency was associated with language ability (CELF-4 scores), and also that right-hemisphere M50 latency differentiated language impaired and nonlanguage impaired ASD groups. In the present study, CELF-4 scores did not predict M100 latency (observed either as a main effect or in the Group × CELF-4 interaction). In addition, after removing variance in M100 latency accounted for by CELF-4, M100 group latency differences remained significant. In Oram Cardy et al. [2008], associations between M50 latency and CELF-4 scores were examined for the total sample, a sample which included subjects with SLI (N = 5) and not ASD. In addition, whereas in the present study only 9 of the 25 subjects had ASD with LI, in the Oram Cardy et al. study 14 of the ASD subjects had LI and only 8 of the ASD subjects had no LI. Thus, the Oram Cardy findings may reflect associations dominated by language-impaired groups.
Future Directions
The present findings indicate a delayed 100 msec response in ASD and implicate a failure in subjects with ASD to achieve typical maturational development of the auditory system. The variability in 100 msec latency findings across studies may not be as great as a cursory review of the literature suggests. Combined with the results of this study, a review of the literature in this area indicates that to better understand latency delays in ASD future studies should: (1) when possible, separately examine left- and right-hemisphere activity, (2) present stimuli at multiple frequencies, preferably examining activity in response to stimuli with frequencies between 200 and 1,000 Hz, and (3) provide as much demographic and clinical information on the patient and control samples as possible (e.g., age, IQ, concomitant diagnoses, language ability) so that when study differences are observed they can be better understood. Finally, studies are needed to determine whether the delayed latency is specific to the auditory system. Examining visual evoked responses in centroparietal areas, Sokhadze et al. [2009] observed prolonged 100 msec latencies in ASD compared to controls.
Research examining electrophysiological activity in psychiatric populations increasingly focuses on identifying endophenotypes. For use of M100 latency as an ASD endophenotype, specificity is required. The literature does suggest that a 100 msec latency abnormality may be unique to ASD. As an example, although other studies have reported abnormal 50 and 100 msec responses in a variety of disorders [e.g., schizophrenia, depression, post-traumatic stress disorder; see Edgar et al., 2007, for examples], the observation of abnormal latency (but not amplitude) may be specific to ASD. Indeed, while certainly not accounting for the tremendous heterogeneity of phenotype observed in ASD, it is encouraging that the auditory evoked field response to a single (500 Hz) tone can be used as a diagnostic biomarker with 75% sensitivity, 81% specificity, and 86% PPV. Further improvements to sensitivity and specificity are likely to follow from incorporation of later electrophysiological responses to more sophisticated stimulus paradigms.
Acknowledgments
The authors gratefully acknowledge the contributions of all faculty, staff, and students of the Penn/CHOP Center for Autism Research. Dr. Roberts thanks to the Oberkircher Family for the Oberkircher Family Endowed Chair in Pediatric Radiology.
Grant sponsor: NIH; Grant number: R01DC008871; Grant sponsors: The Nancy Lurie Marks Family Foundation (NLMFF); Autism Speaks; The Pennsylvania Department of Health.
Footnotes
Disclaimer: No author declares a conflict of interest. This study was supported in part by NIH grant R01DC008871 (T.R.) and T32NS007413 from NINDS (GS), a grant from the Nancy Lurie Marks Family Foundation (NLMFF), and Autism Speaks. This research has been funded (in part) by a grant from the Pennsylvania Department of Health. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. Dr. Roberts gratefully acknowledges the Oberkircher Family for the Oberkircher Family Chair in Pediatric Radiology at Children's Hospital of Philadelphia.
REFERENCES
- Berg P, Scherg M. A multiple source approach to the correction of eye artifacts. Electroencephalography and Clinical Neurophysiology. 1994;90:229–241. doi: 10.1016/0013-4694(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Bomba MD, Pang EW. Cortical auditory evoked potentials in autism: a review. International Journal of Psychophysiology. 2004;53:161–169. doi: 10.1016/j.ijpsycho.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Bruneau N, Roux S, Adrien JL, Barthelemy C. Auditory associative cortex dysfunction in children with autism: evidence from late auditory evoked potentials (N1 wave-T complex). Clinical Neurophysiology. 1999;110:1927–1934. doi: 10.1016/s1388-2457(99)00149-2. [DOI] [PubMed] [Google Scholar]
- Constantino J, Gruber CP. Social Responsiveness Scale. Western Psychological Services; Los Angeles, CA: 2005. [Google Scholar]
- Dawson G, Finley C, Phillips S, Galpert L. Hemispheric specialization and the language abilities of autistic children. Child Development. 1986;57:1440–1453. [PubMed] [Google Scholar]
- Dunn M, Vaughan H, Kreuzer J, Kurtzberg D. Electrophysiologic correlates of semantic classification in autistic and normal children. Developmental Neuropsychology. 1999;16:79–99. [Google Scholar]
- Edgar JC, Huang MX, Weisend MP, Sherwood A, Miller GA, et al. Interpreting abnormality: an EEG and MEG study of P50 and the auditory paired-stimulus paradigm. Biological Psychology. 2003;65:1–20. doi: 10.1016/s0301-0511(03)00094-2. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Keller J, Heller W, Miller GA. Psychophysiology in research on Psycopathology. In: Tassinary LG, Cacioppo JT, Bernston GG, editors. Handbook of Psychophysiology. 3rd ed. Cambridge University Press; New York: 2007. [Google Scholar]
- Eggermont JJ, Ponton CW, Don M, Waring MD, Kwong B. Maturational delays in cortical evoked potentials in cochlear implant users. Acta Otolaryngologica. 1997;117:161–163. doi: 10.3109/00016489709117760. [DOI] [PubMed] [Google Scholar]
- Ferri R, Elia M, Agarwal N, Lanuzza B, Musumeci SA, Pennisi G. The mismatch negativity and the P3a components of the auditory event-related potentials in autistic low-functioning subjects. Clinical Neurophysiology. 2003;114:1671–1680. doi: 10.1016/s1388-2457(03)00153-6. [DOI] [PubMed] [Google Scholar]
- Gage NM, Siegel B, Callen M, Roberts TP. Cortical sound processing in children with autism disorder: an MEG investigation. Neuroreport. 2003a;14:2047–2051. doi: 10.1097/00001756-200311140-00008. [DOI] [PubMed] [Google Scholar]
- Gage NM, Siegel B, Roberts TP. Cortical auditory system maturational abnormalities in children with autism disorder: an MEG investigation. Brain research. Developmental Brain Research. 2003b;144:201–209. doi: 10.1016/s0165-3806(03)00172-x. [DOI] [PubMed] [Google Scholar]
- Hari R. The neuromagnetic method in the study of the human auditory cortex. In: Grandori F, Hoke M, Romani G, editors. Auditory evoked magnetic fields and potentials. Advances in audiology. Vol. 6. Karger; Basel, Switzerland: 1990. pp. 222–282. [Google Scholar]
- Huotilainen M, Winkler I, Alho K, Escera C, Virtanen J, et al. Combined mapping of human auditory EEG and MEG responses. Electroencephalography and Clinical Neurophysiology. 1998;108:370–379. doi: 10.1016/s0168-5597(98)00017-3. [DOI] [PubMed] [Google Scholar]
- Jeste SS, Nelson CA., 3rd Event related potentials in the understanding of autism spectrum disorders: an analytical review. Journal of Autism and Developmental Disorders. 2009;39:495–510. doi: 10.1007/s10803-008-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsa RE, Clontz KB. Long latency auditory event-related potentials from children with auditory processing disorders. Ear and Hearing. 1990;11:222–232. doi: 10.1097/00003446-199006000-00010. [DOI] [PubMed] [Google Scholar]
- Kemner C, Verbaten MN, Cuperus JM, Camfferman G, van Engeland H. Auditory event-related brain potentials in autistic children and three different control groups. Biological Psychiatry. 1995;38:150–165. doi: 10.1016/0006-3223(94)00247-Z. [DOI] [PubMed] [Google Scholar]
- Korostenskaja M, Kicic D, Kahkonen S. The effect of methylphenidate on auditory information processing in healthy volunteers: a combined EEG/MEG study. Psychopharmacology (Berl) 2008;197:475–486. doi: 10.1007/s00213-007-1065-8. [DOI] [PubMed] [Google Scholar]
- Korpilahti P, Jansson-Verkasalo E, Mattila ML, Kuusikko S, Suominen K, et al. Processing of affective speech prosody is impaired in Asperger syndrome. Journal of Autism and Developmental Disorders. 2007;37:1539–1549. doi: 10.1007/s10803-006-0271-2. [DOI] [PubMed] [Google Scholar]
- Krug D, Arick JR. Krug Asperger's disorder index. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- Lee YS, Lueders H, Dinner DS, Lesser RP, Hahn J, Klem G. Recording of auditory evoked potentials in man using chronic subdural electrodes. Brain. 1984;107:115–131. doi: 10.1093/brain/107.1.115. [DOI] [PubMed] [Google Scholar]
- Liegeois-Chauvel C, Musolino A, Badier JM, Marquis P, Chauvel P. Evoked potentials recorded from the auditory cortex in man: evaluation and topography of the middle latency components. Electroencephalography and Clinical Neurophysiology. 1994;92:204–214. doi: 10.1016/0168-5597(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Lincoln AJ, Courchesne E, Harms L, Allen M. Sensory modulation of auditory stimuli in children with autism and receptive developmental language disorder: event-related brain potential evidence. Journal of Autism and Developmental Disorders. 1995;25:521–539. doi: 10.1007/BF02178298. [DOI] [PubMed] [Google Scholar]
- Lins OG, Picton TW, Berg P, Scherg M. Ocular artifacts in recording EEGs and event-related potentials. II: source dipoles and source components. Brain Topography. 1993;6:65–78. doi: 10.1007/BF01234128. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Makela JP, Hamalainen M, Hari R, McEvoy L. Whole-head mapping of middle-latency auditory evoked magnetic fields. Electroencephalography and Clinical Neurophysiology. 1994;92:414–421. doi: 10.1016/0168-5597(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Martineau J, Garreau B, Barthelemy C, Lelord G. Evoked potentials and P300 during sensory conditioning in autistic children. Annals of the New York Academy of Sciences. 1984;425:362–369. doi: 10.1111/j.1749-6632.1984.tb23557.x. [DOI] [PubMed] [Google Scholar]
- Novick B, Vaughan HG, Jr., Kurtzberg D, Simson R. An electrophysiologic indication of auditory processing defects in autism. Psychiatry Research. 1980;3:107–114. doi: 10.1016/0165-1781(80)90052-9. [DOI] [PubMed] [Google Scholar]
- Oades RD, Walker MK, Geffen LB, Stern LM. Event-related potentials in autistic and healthy children on an auditory choice reaction time task. International Journal of Psychophysiology. 1988;6:25–37. doi: 10.1016/0167-8760(88)90032-3. [DOI] [PubMed] [Google Scholar]
- Oram Cardy JE, Ferrari P, Flagg EJ, Roberts W, Roberts TP. Prominence of M50 auditory evoked response over M100 in childhood and autism. Neuroreport. 2004;15:1867–1870. doi: 10.1097/00001756-200408260-00006. [DOI] [PubMed] [Google Scholar]
- Oram Cardy JE, Flagg EJ, Roberts W, Roberts TP. Auditory evoked fields predict language ability and impairment in children. International Journal of Psychophysiology. 2008;68:170–175. doi: 10.1016/j.ijpsycho.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Paetau R, Ahonen A, Salonen O, Sams M. Auditory evoked magnetic fields to tones and pseudowords in healthy children and adults. Journal of Clinical Neurophysiology. 1995;12:177–185. doi: 10.1097/00004691-199503000-00008. [DOI] [PubMed] [Google Scholar]
- Pelizzone M, Hari R, Makela JP, Huttunen J, Ahlfors S, Hamalainen M. Cortical origin of middle-latency auditory evoked responses in man. Neuroscience Letters. 1987;82:303–307. doi: 10.1016/0304-3940(87)90273-4. [DOI] [PubMed] [Google Scholar]
- Picton TW, Alain C, Woods DL, John MS, Scherg M, et al. Intracerebral sources of human auditory-evoked potentials. Audiology and Neurootology. 1999;4:64–79. doi: 10.1159/000013823. [DOI] [PubMed] [Google Scholar]
- Reite M, Teale P, Zimmerman J, Davis K, Whalen J. Source location of a 50 msec latency auditory evoked field component. Electroencephalography and Clinical Neurophysiology. 1988;70:490–498. doi: 10.1016/0013-4694(88)90147-2. [DOI] [PubMed] [Google Scholar]
- Roberts TP, Ferrari P, Stufflebeam SM, Poeppel D. Latency of the auditory evoked neuromagnetic field components: stimulus dependence and insights toward perception. Journal of Clinical Neurophysiology. 2000;17:114–129. doi: 10.1097/00004691-200003000-00002. [DOI] [PubMed] [Google Scholar]
- Roberts TP, Poeppel D. Latency of auditory evoked M100 as a function of tone frequency. Neuroreport. 1996;7:1138–1140. doi: 10.1097/00001756-199604260-00007. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lloyd C. SCQ: Social Communication Questionnaire. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- Scherg M. Fundamentals of dipole source potential analysis. In: Gandori MHGLR, editor. Auditory evoked magnetic fields and electric potentials. Advances in audiology. 6th ed. Karger; Basel, Switzerland: 1990. pp. 40–69. [Google Scholar]
- Scherg M, Berg P. New concepts of brain source imaging and localization. Electroencephalography and Clinical Neurophysiology. 1996;46:127–137. [PubMed] [Google Scholar]
- Scherg M, Ebersole JS. Brain source imaging of focal and multifocal epileptiform EEG activity. Neurophysiologie Clinique. 1994;24:51–60. doi: 10.1016/s0987-7053(05)80405-8. [DOI] [PubMed] [Google Scholar]
- Scherg M, Ille N, Bornfleth H, Berg P. Advanced tools for digital EEG review: virtual source montages, whole-head mapping, correlation, and phase analysis. Journal of Clinical Neurophysiology. 2002;19:91–112. doi: 10.1097/00004691-200203000-00001. [DOI] [PubMed] [Google Scholar]
- Scherg M, von Cramon D. A new interpretation of the generators of BAEP waves I–V: results of a spatio-temporal dipole model. Electroencephalography and Clinical Neurophysiology. 1985;62:290–299. doi: 10.1016/0168-5597(85)90006-1. [DOI] [PubMed] [Google Scholar]
- Semel EM, Wiig EH, Secord W. Clinical evaluation of language fundamentals (CELF-4) The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Seri S, Cerquiglini A, Pisani F, Curatolo P. Autism in tuberous sclerosis: evoked potential evidence for a deficit in auditory sensory processing. Clinical Neurophysiology. 1999;110:1825–1830. doi: 10.1016/s1388-2457(99)00137-6. [DOI] [PubMed] [Google Scholar]
- Sokhadze E, Baruth J, Tasman A, Sears L, Mathai G, et al. Event-related potential study of novelty processing abnormalities in autism. Applied Psychophysiology and Biofeedback. 2009;34:37–51. doi: 10.1007/s10484-009-9074-5. [DOI] [PubMed] [Google Scholar]
- Tecchio F, Benassi F, Zappasodi F, Gialloreti LE, Palermo M, et al. Auditory sensory processing in autism: a magnetoencephalographic study. Biological Psychiatry. 2003;54:647–654. doi: 10.1016/s0006-3223(03)00295-6. [DOI] [PubMed] [Google Scholar]
- Tonnquist-Uhlen I, Borg E, Spens KE. Topography of auditory evoked long-latency potentials in normal children, with particular reference to the N1 component. Electroencephalography and Clinical Neurophysiology. 1995;95:34–41. doi: 10.1016/0013-4694(95)00044-y. [DOI] [PubMed] [Google Scholar]
- Verbaten MN, Overtoom CC, Koelega HS, Swaab-Barneveld H, van der Gaag RJ, et al. Methylphenidate influences on both early and late ERP waves of ADHD children in a continuous performance test. Journal of Abnormal Child Psychology. 1994;22:561–578. doi: 10.1007/BF02168938. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for children. 3rd ed. The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Yoshiura T, Ueno S, Iramina K, Masuda K. Source localization of middle latency auditory evoked magnetic fields. Brain Research. 1995;703:139–144. doi: 10.1016/0006-8993(95)01075-0. [DOI] [PubMed] [Google Scholar]
- Yvert B, Crouzeix A, Bertrand O, Seither-Preisler A, Pantev C. Multiple supratemporal sources of magnetic and electric auditory evoked middle latency components in humans. Cerebral Cortex. 2001;11:411–423. doi: 10.1093/cercor/11.5.411. [DOI] [PubMed] [Google Scholar]