Abstract
Leukocyte migration across the endothelial lining is a critical step in the body’s response to infection and inflammation. The homophilic interaction between endothelial PECAM and leukocyte PECAM is essential for this process. The molecular events that are triggered in the endothelial cell by PECAM engagement have been well characterised; however, the function of leukocyte PECAM remains to be elucidated. To study this, we first blocked leukocyte transmigration using anti-PECAM antibody and then specifically activated leukocyte PECAM. This was sufficient to overcome the block and promote transmigration, suggesting an active signaling role for leukocyte PECAM. Consistent with this, we found that ligation of leukocyte PECAM induces phosphorylation of two tyrosine residues on its cytoplasmic tail. By performing RNAi-rescue experiments, we demonstrate that these phosphorylation events are indispensable for transendothelial migration. Finally, we show that leukocyte PECAM translocates to a detergent resistant membrane (DRM) during transmigration. PECAM localised in DRMs displays reduced phosphorylation and does not support transmigration. Together, these data support a model whereby engagement of leukocyte PECAM induces its transient tyrosine phosphorylation and induction of downstream signals that drive transmigration. These signals are then down regulated following PECAMs translocation to DRMs.
INTRODUCTION
Trafficking of leukocytes from the blood stream to sites of inflammation is a critical step in the immune response (1). This process is tightly regulated by a number of proteins to ensure migration to the proper location at the appropriate time. Platelet endothelial adhesion molecule (PECAM) is a transmembrane protein belonging to the immunoglobulin superfamily (2). It is constitutively expressed in both endothelial cells and leukocytes and plays a critical role in the process of leukocyte transendothelial migration (3–5). Specifically, it is the homophilic interaction between endothelial PECAM and leukocyte PECAM that is required for transmigration. To date, most mechanistic studies of the role of PECAM in transmigration have focused on the endothelial side (6–8).
Leukocyte PECAM also plays a critical role during transmigration(5). However, its precise role is not well understood. It may serve primarily as an adhesion molecule, or, like its endothelial counterpart, actively engage in signal transduction events that drive transmigration. In order to test this hypothesis, we have used antibody cross-linking methods to manipulate the timing of leukocyte PECAM activation during transmigration. Here, we provide evidence that PECAM ligation leads to the activation of leukocyte signaling pathways that are critical during transmigration.
The cytoplasmic tail of PECAM contains tyrosine residues at positions 663 and 686, which constitute immuno-receptor tyrosine-based inhibitory motifs (ITIM) (9, 10). Src kinases have been shown to phosphorylate these residues (11, 12), which then serve as docking sites for SH2 domain-containing proteins such as SHP1, SHP2, PLCγ and SHIP (13–15). It is through these phosphorylation events and subsequent protein interactions, that PECAM can mediate downstream signaling. While the requirement for endothelial PECAM phosphorylation has been well studied with respect to endothelial junction function (16, 17), and leukocyte transmigration (6), the role of leukocyte PECAM phosphorylation in the transmigration process remains uncharacterized. In the present study we generated a leukocyte cell line in which endogenous PECAM was depleted and replaced with either wild type or non-phosphorylatable Y663F/Y686F mutants. Using this approach, we demonstrate that the phosphorylation of leukocyte PECAM is also required for transmigration.
Several immuno-receptors, including TCR, BCR and Fcγ receptors, initiate signal transduction by associating with specialized lipid regions in the membrane (18–20), termed detergent resistant membranes (DRM) (21). PECAM has previously been shown to associate with DRMs in platelets (22), but this association has not been studied in leukocytes. We present evidence that leukocyte PECAM moves into DRMs during transmigration where is displays reduced phosphorylation. Forcing PECAM into DRMs decreases transmigration. The data are consistent with a model in which homophilic interaction of PECAM induces signaling through PECAM phosphorylation that is necessary for transmigration. The activation of PECAM is then terminated by movement of PECAM into DRMs.
MATERIALS AND METHODS
Antibodies and reagents
Monoclonal mouse-anti-human hec7 (anti-PECAM) (ref 24) and hec2 (anti-CD99) (ref. 26) were produced from hybridomas. Polyclonal rabbit anti-human PECAM 177 and 301 were generated in house. The non-blocking mouse anti-PECAM mAb P1.1 was a kind gift from Dr Peter Newman (Blood Center of Wisconsin). Anti-phosphotyrosine 4G10 was purchased from Millipore. F(ab’)2 goat anti mouse and goat anti rabbit IgG were purchased from Jackson Immunological. Rabbit anti mouse IgG-HRP and swine anti rabbit IgG-HRP were purchased from Dako. Src kinase inhibitor PP2 was purchased from Calbiochem. Methyl-beta-cyclodextrin (MβCDX) was purchased from Sigma- Aldrich.
Cell culture and differentiation
U937L cells (kindly donated by Dr F. William Luscinskas) were maintained in RPMI, 10% FBS, L-glutamine and penicillin/streptomycin and differentiated to a more monocyte lineage with 1mM dibuteryl cyclic AMP (dbcAMP) (Sigma-Aldrich) stimulation for 3 days. These cells have previously been demonstrated to transmigrate HUVEC monolayers in a similar manner to primary monocytes (23).
HUVEC were isolated from fresh umbilical cords, as previously described (24), and grown in medium 199 (M199, Invitrogen) supplemented with 20% adult human serum and 100U/ml penicillin–streptomycin at 37°C in a humidified atmosphere of 5% CO2. Experiments were done routinely with cells at passage two plated on thick hydrated type I collagen gels in 96-well culture plates.
Peripheral blood mononuclear cell (PBMC) and monocyte isolation
PBMCs were isolated from healthy volunteers by density gradient centrifugation in Ficoll-Paque. Monocytes were isolated from the PBMC fraction, using a MACS monocyte isolation kit and magnetic depletion columns according to the manufacturer’s instructions (Miltenyi). This yielded >90% monocytes, as determined by flow cytometry following labeling with FITC-labeled anti-CD14.
Generation of U937L PECAM knockdown cell line
A GFP tagged lentiviral shRNA construct against human PECAM (PEC02) and a non-silencing control (NSC) construct were generously donated by Dr Peter Newman, and have been previously described (25). U937L cells were infected with lentiviral constructs at a MOI (multiplicity of infection) of 100 for 5 hours at 37°C. Cells, designated U937L PEC02 or U937L NSC, were washed and re-plated in a 12 well plate in 1ml normal culture media. After 3 days, strongly GFP positive cells were selected using a Becton-Dickinson FACS Vantage cell sorter.
Lentiviral PECAM rescue construct and transduction of U937L cells
Full length human PECAM in the lentviral plasmid pWPT (kindly donated by Dr Peter Newman) was used as a template to make PECAM rescue constructs. 6 silent mutations within the PECAM siRNA target region were introduced using site-directed mutagenesis (Stratagene) along with tyrosine and phenylalanine mutations at positions 663 ands 686, as described in supplemental materials. pWPT PECAM constructs were mixed with the packaging plasmid pCMV R8.2 and envelope vector pVSV-G and co-transfected into 293FT cells. Lentiviral particles were purified and U937L PEC02 cells were transduced to achieve expression levels close to those of endogenous PECAM as verified by flow cytometry.
Western blotting
Cells were lysed for 10 minutes at 4°C in a non-denaturing lysis buffer A, 1% Triton X100, 50mM Tris-HCl, 150mM NaCl, 1mM EDTA, 10mM NaVO4, 1mM PMSF and protease inhibitor cocktail (Sigma-Aldrich). Samples were then spun at 14,000rpm for 10 minutes at 4°C. Supernatants (triton soluble fraction) were collected and the remaining pellets (triton insoluble) were resuspended in sample buffer and boiled for 5 minutes, passed through a 26G needle five times and sonicated for 5 minutes before storing at −20°C. The samples were run on a 4–12% gradient Tris-Glycine SDS PAGE gel (Invitrogen) and transferred to a polyvinyldifluoride membrane. The membrane was blocked and then incubated overnight at 4°C with primary. Blots were incubated with horseradish peroxidase conjugated secondary antibodies and proteins were detected using enhanced chemiluminescence (Amersham).
Immunoprecipitation
Protein G Dynabeads (Invitrogen), 20ul, were first derivatized with 10ug rabbit polyclonal anti-PECAM, 301Ab, in lysis buffer. After four washes, triton soluble supernatants were added and rotated for 3 hours at 4°C. Samples were washed four times and proteins eluted by addition of sample buffer followed by boiling for 5 minutes. To immunoprecipitate from triton insoluble fractions, pellets obtained after triton lysis were resuspended in a denaturing lysis buffer (1% SDS, 50mM Tris-HCl, 10mM NaVO4), and then passed through a 26G needle five times, boiled for 5 minutes and sonicated for 5 minutes. Samples were then diluted 1:10 with non-denaturing lysis buffer before being added to Protein G beads as above.
Isolation of detergent resistant membranes by sucrose density gradient ultracentrifugation
Differentiated U937L cells (1×107) were stimulated and lysed with 0.5ml lysis buffer A, see above, on ice for 10 minutes. Total lysates were passed through a 26G needle five times before being mixed with an equal volume of 80% sucrose in buffer A, containing 0.2% Triton-X100. This was then overlaid with 7ml 35% sucrose in buffer A and 3ml 5% sucrose in buffer A. After ultracentrifugation for 20 hours at 40,000 rpm in a Sorvall TH-641 rotor, 11-1ml fractions were collected starting from the top of the gradient and analysed by western blotting.
Transendothelial migration assay
This assay was performed and quantified as previously described(5). In brief, PBMCs or differentiated U937L cells were preincubated or without 10μg/ml anti-PECAM antibodies on ice for 15 minutes washed and resuspended in M199 + 0.1% HSA at 2×106/ml. 100μl cells were added to each replicate well of confluent monolayers of HUVECs grown on hydrated collagen gels and incubated for 1 hr at 37°C in a CO2 incubator. To induce surface leukocyte PECAM cross-linking, F(ab’)2 secondary antibodies, 50μg/ml final concentration, were added to the desired wells. The percentage of transmigration in 10 different fields of view was calculated by dividing the number of leukocytes below the HUVEC monolayer by the total number of leukocytes in the field.
Fluorescent microscopy on Triton-extracted samples
Confluent HUVEC monolayers were grown on fibronectin coated glass-bottomed dishes and pre-labeled with 20μg/ml Alexafluor 488 conjugated anti-VE cadherin for 20 minutes at 37°C. PBMC were pre-labeled with AlexaFluor 546 conjugated non-blocking anti-PECAM (P1.1) on ice before washing and resuspension in M199 + 0.1% HSA at 2×106/ml. 100μl PBMC were added to monolayers of HUVECs for 10 minutes at 37°C. Dishes were either fixed in 2% paraformaldehyde or treated with cold Triton X100 extraction buffer (0.5% Triton, 138 mM KCl, 3 mM MgCl2, 2 mM EGTA, 0.32 M sucrose, 10 mM MES, protease inhibitors, 1mM PMSF, pH 6.1) for 20 minutes on ice before fixation. Dishes were imaged using a Deltavision microscope. Analysis of fluorescent intensity was carried out using Metamorph software.
Statistics
The Students t test was used to evaluate statistical significance in the data presented, using Prism (Graphpad).
RESULTS
Cross-linking monocyte PECAM promotes transendothelial migration
Leukocyte transendothelial migration can be inhibited using anti-PECAM neutralizing antibodies (4, 5) that bind to the homophilic interaction domain preventing leukocyte PECAM interaction with endothelial cell PECAM. If these neutralizing antibodies are subsequently clustered, by cross-linking with a secondary antibody, PECAM signaling can be artificially activated. This system allows us to mimic the concentrated PECAM ligation that occurs in time and space on a leukocyte during transmigration, and to temporally control PECAM activation in the treated cells.
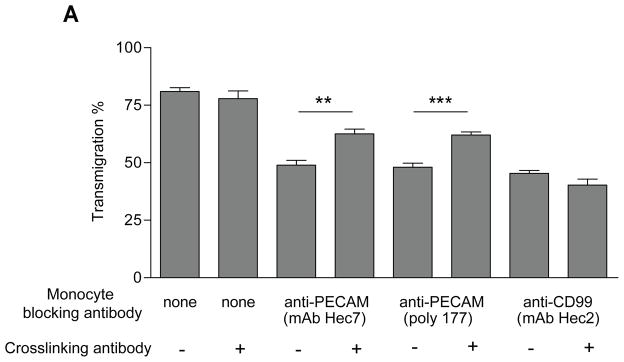
To analyse the role of leukocyte PECAM, we added primary monocytes, treated with a blocking anti-PECAM IgG antibody, to endothelial monolayers for 30 min. This allowed sufficient time for monocytes to migrate to endothelial junctions, where they are arrested (Fig. 1). Clustering of leukocyte PECAM to induce its activation was then performed by the addition of a F(ab’)2 secondary cross-linking antibody after 30 minutes., Figure 1 shows that blocking monocyte PECAM with monoclonal antibody hec7 inhibited transmigration. Strikingly, cross-linking PECAM on these arrested cells, after 30 minutes, reversed this blockade and significantly promoted transmigration. Similar results were seen using the blocking polyclonal anti-PECAM antibody, 177. (The partial nature of the recovery is explained by the biology of the system, as elucidated by the experiments that follow [see Discussion.]) However, antibodies directed against CD99, a protein important in transmigration at a step distal to that regulated by PECAM, and expressed on both leukocytes and endothelial cells (26), failed to promote transmigration after cross-linking, demonstrating specificity. In our hands this method of antibody cross-linking does not result in non-specific activation of Fcγ receptors as we see similar results using F(ab’)2 antibodies, and effects were not inhibited by blocking Fcγ receptors (data not shown). These data suggest that the role of leukocyte PECAM extends beyond its function as an adhesion receptor and that activation of this molecule may be required during transmigration. We found no evidence to suggest that PECAM activation mediates the release of soluble factors that aide the transmigration event (Figure S1), although we cannot formally exclude the possibility of extremely localized short-lived factors.
Figure 1. Cross-linking leukocyte PECAM reverses an anti-PECAM block in transmigration.
Human monocyte transmigration through unstimulated HUVEC monolayers grown on collagen gels. Monocytes were prelabelled on ice with blocking antibodies against PECAM (hec7, 177) and CD99 (hec2) at 20μg/ml, before being washed free of unbound antibody and added to HUVEC monolayers for 60 minutes. PECAM was activated by the addition of cross-linking secondary antibodies (50μg/ml) 30 minutes into the assay where indicated. Data represent mean ± SEM from 7 separate experiments. ** P<0.003, *** P<0.001.
Leukocyte PECAM is phosphorylated upon cross-linking
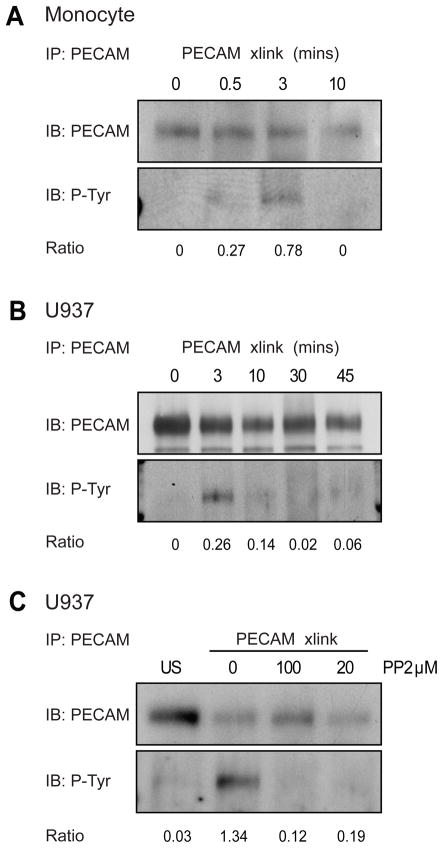
Next we investigated the possibility that leukocyte PECAM promotes transmigration by engaging in intracellular signaling transduction. PECAM contains several tyrosine residues that may become phosphorylated upon activation, the best characterized of these are at positions 663 and 686 which reside within ITIM like domains. PECAM is not phosphorylated in resting monocytes, but becomes rapidly and transiently tyrosine phosphorylated upon cross-linking, Figure 2A. Using dbcAMP differentiated U937L cells as a monocyte model, we were able to see the same pattern of transient PECAM phosphorylation after cross-linking, Figure 2B. The phosphorylation of PECAM was inhibited using the src kinase inhibitor PP2, Figure 2C. We next set out to determine whether the phosphorylation events triggered by leukocyte PECAM clustering were involved in the transmigration process.
Figure 2. Leukocyte PECAM becomes tyrosine phosphorylated after cross-linking.
(A) Human monocytes or (B) differentiated U937L cells, were stimulated by PECAM cross-linking for the indicated times and lysates were immunoprecipitated (IP) with anti-PECAM antibodies. Samples were resolved on SDS-PAGE gels and analysed by western blotting (IB) using anti-phosphotyrosine and PECAM antibodies. Band intensities were measured via densitometry, and the ratio of phosphorylated PECAM to total PECAM is shown below. (C) Differentiated U937L were left either untreated or pretreated with the Src kinase inhibitor PP2 (20 or 100μM, 30 minutes) before PECAM cross-linking for 3 minutes. Lysates were analysed by western blotting (performed as in A and B). Results shown are representative of 3 separate experiments.
Phosphorylation of tyrosine 663/686 on leukocyte PECAM is required for transendothelial migration
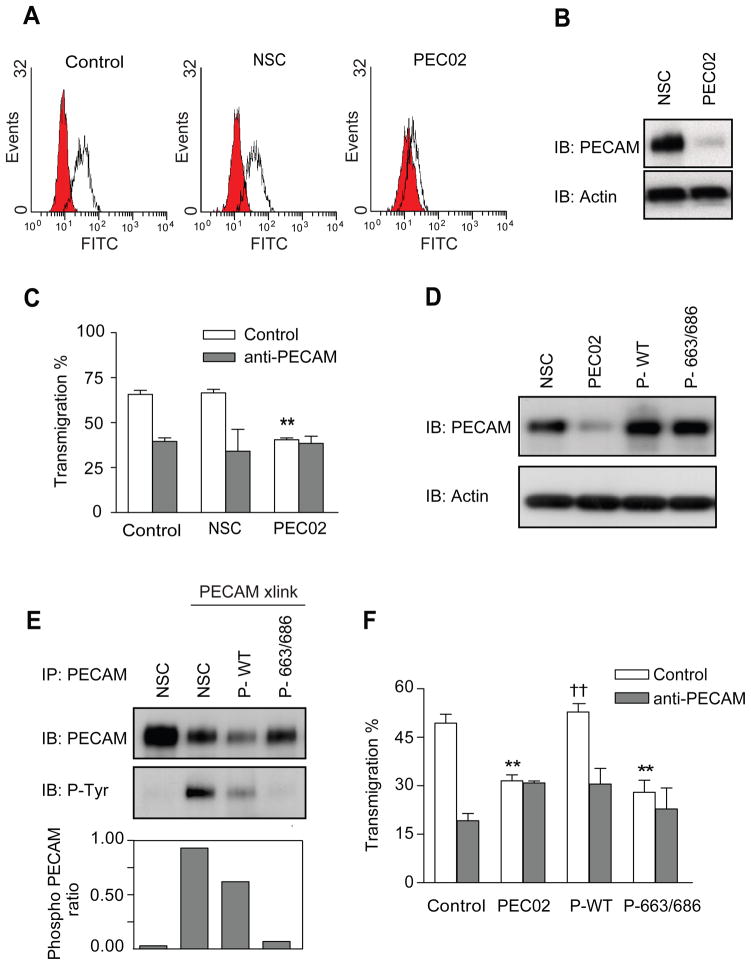
In order to test whether leukocyte PECAM phosphorylation is involved in transendothelial migration, we generated monocytic U937L cell lines expressing either wild type or mutant forms of PECAM lacking the 663 and 686 tyrosine phosphorylation sites. To make sure that the cells expressed the same levels of total PECAM, we first depleted endogenous PECAM using a lentiviral shRNA. Levels of PECAM knockdown were confirmed by flow cytometry and western blotting, Figure 3A and B. The stable cell line PEC02, presents a robust PECAM knockdown of around 70–80%, a scrambled control shRNA was used in a similar way to generate the control cell line NSC. PEC02 cells displayed significantly reduced transmigration and showed no further reduction in the presence of anti-PECAM antibodies (Figure 3C). This data clearly shows that U937L cell transmigration is dependent upon leukocyte PECAM.
Figure 3. Tyrosine phosphorylation of leukocyte PECAM is required for transendothelial migration.
Control or stable U937L cells expressing either a control shRNA or an shRNA targeting PECAM (named NSC or PEC02 respectively) were analysed for PECAM expression by (A) flow cytometry and (B) western blotting. (C) Differentiated control and NSC U937L cells transmigrate across IL-1β treated HUVEC monolayers (200pg/ml, 4hours) to similar levels that are blocked in the presence of anti-PECAM antibodies. PEC02 cells show a reduced level of transmigration, which is not further inhibited by anti-PECAM antibodies. Data shown are mean ± SEM from 3 experiments, ** P<0.01. (D) Western blot analysis of lysates from NSC, PEC02 and PEC02 cells transduced to re-express either wild type (P-WT) or Y663/686F (P-663/686) forms of PECAM. Immuno-blotting was performed against PECAM and actin. (E) Mutating tyrosine sites 663 and 686 to phenylalanine reduces PECAM phosphorylation after crosslinking. Following PECAM cross-linking for 3 minutes, lysates from all the U937L cell lines were immunoprecipitated with anti-PECAM and probed for anti-phosphotyrosine. Ratios of phospho-PECAM in the cell lines are presented below the blots. (F) Differentiated U937L cell lines were allowed to transmigrate across IL-1β treated HUVEC monolayers for 1 hour, in the presence or absence of anti-PECAM antibodies. PEC02 cells re-expressing wild type (P-WT) PECAM, showed rescue of transmigration to levels similar to those on control U937L cells, †† P<0.02. Cells expressing the 663 and 686 mutant form of PECAM (P-663/686) remained inhibited in their transmigration, ** P<0.03, experiments show the mean ± SEM from 3 experiments.
U937L cell lines were generated re-expressing either wild type (P-WT) or double Y663F and Y686F (P-663/686) mutations. Figure 3D shows western blot data confirming the re-expression of PECAM, at close to endogenous levels. The phosphorylation status of re-expressed PECAM was tested by immunoprecipitation and anti-phosphotyrosine western blots. Figure 3E demonstrates that the double mutant has reduced PECAM phosphorylation after cross-linking compared to control U937L or P-WT cells, indicating that these represent major phosphorylation sites following leukocyte PECAM activation.
U937L cells re-expressing wild type PECAM (P-WT) regained the ability to transmigrate to levels similar to those of control cells and displayed sensitivity to a PECAM blocking antibody, Figure 3F. Strikingly, however, re-expression of the Y663/686F mutant was unable to rescue the defect in transmigration caused by depletion of the endogenous protein (Figure 3F). We found no difference in the level of adhesion or ability to polarize between the different cell lines (data not shown). From these results we conclude that the tyrosine phosphorylation within the ITIM domains of leukocyte PECAM plays a critical role in transendothelial migration.
Leukocyte PECAM resists detergent extraction after cross-linking
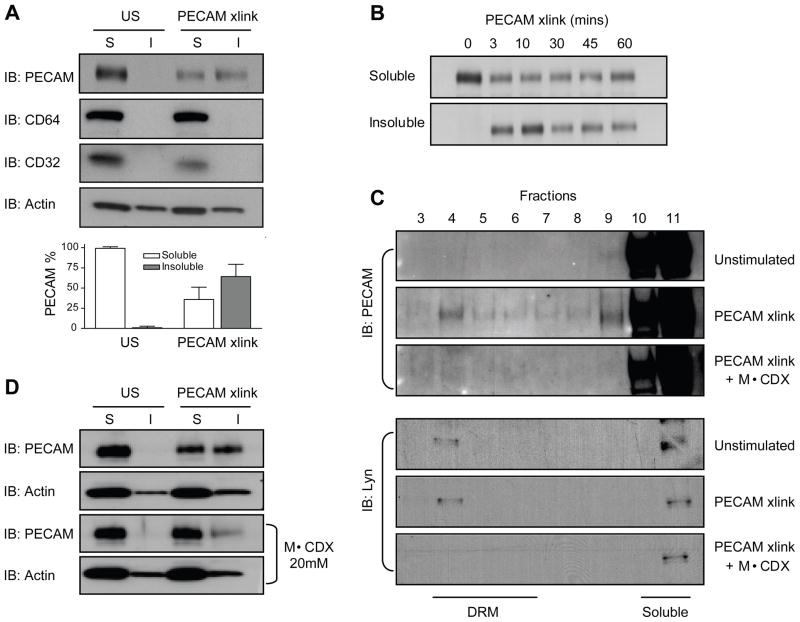
Coupled with the observation of leukocyte PECAM phosphorylation after cross-linking, a change in its detergent solubility was also detected. Figure 4A, shows that U937L PECAM is completely solubilized in 1% cold Triton X100 lysis buffer under resting conditions. However, following 3 minutes of PECAM cross-linking, approximately 50% of PECAM is found in the insoluble pellet. This movement is specific to PECAM, as other receptors, CD64 and CD32a, which are known to associate with Triton X100 insoluble membranes upon activation (insert references?), remain in the soluble fraction after PECAM cross-linking, Figure 4A.
Figure 4. PECAM translocates to DRMs after cross-linking.
(A) Differentiated U937L cells were stimulated by PECAM cross-linking for 3 minutes followed by lysis in cold Triton buffer. Soluble proteins in the supernatant (S) and insoluble proteins in the pellet (I) were analysed by western blotting using antibodies against PECAM, CD64, CD32 and actin. Quantification of levels of PECAM from each fraction is shown below, data are from 7 separate experiments. (B) Soluble and insoluble fractions from U937L cells stimulated for increasing times by PECAM cross-linking were probed for PECAM. (C) U937L cells were left untreated or pretreated with 20μM MβCDX for 45min before undergoing PECAM cross-linking for 3 minutes. Lysates were run over a sucrose density gradient and fractions probed for PECAM or Lyn kinase by western blotting. (D) Purified human monocytes with or without MβCDX pretreatment were stimulated by PECAM cross-linking for 3 minutes followed by lysis in cold Triton buffer. Soluble and insoluble fractions were analysed by western blotting using antibodies against PECAM.
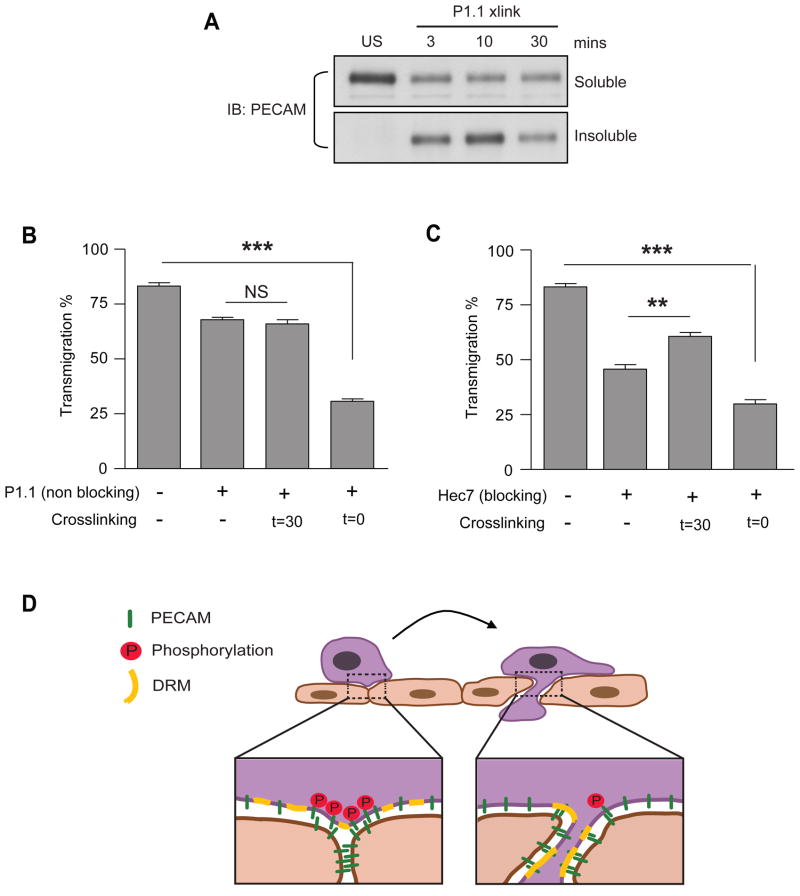
Resistance to cold triton extraction could be due to partitioning into detergent resistant microdomains (DRMs) or association with the cytoskeleton. We found that U937L PECAM could be efficiently solubilized using warm 1% Triton X100 lysis buffer even after cross-linking, a property that would be inconsistent with cytoskeletal association (data not shown). Similarly, PECAM insolubility was not due to cytoskeleton association, as Cytochalasin D had no effect on cross-linking induced PECAM movement to DRMs (data not shown). These results are therefore consistent with PECAM partitioning into DRMs. Furthermore, we found that the movement to an insoluble fraction is both rapid and prolonged. PECAM translocated to DRMs within 3 minutes and remained there for up to 60 minutes after cross-linking, Figure 4B.
PECAM is recruited to low-density DRMs after its cross-linking
To further test the hypothesis that PECAM was recruited to DRMs after cross-linking, we employed a more definitive technique. DRMs can be purified by density ultracentrifugation due to their high lipid content. After cross-linking PECAM, differentiated U937L cells were subjected to sucrose gradient centrifugation. Figure 4C shows that, in unstimulated cells, PECAM was found in the high-density fractions of the gradient representing the soluble protein. After its cross-linking, movement of a portion of PECAM to lower-density fractions was observed. A known DRM component, lyn kinase (27), was also enriched in the same light-density fraction, and remained the same before and after PECAM cross-linking.
Depletion of membrane cholesterol, by methyl-β-cyclodextrin (MβCDX), has previously been shown to disrupt DRMs (28). Pre-treating cells with 20mM MβCDX for 45 minutes inhibited PECAM translocation to DRMs upon cross-linking, Figure 4C. Lyn kinase also moved to a cold-Triton soluble fraction after MβCDX treatment, verifying the disruption of DRMs. Similar results of cholesterol dependent movement of PECAM to a cold-Triton insoluble fraction were seen using purified human monocytes, Figure 4D. These cells remained viable after MβCDX treatment and showed clear signs of cholesterol depletion including cell rounding and loss of polarized morphology (data not shown). The movement of PECAM to DRMs is independent of tyrosine phosphorylation, as PP2 did not stop PECAM movement to DRMs after cross-linking. PECAM from the P-663/686 cell line also translocates to DRMs after crosslinking, Figure S2.
Leukocyte PECAM is found in DRMs during transendothelial migration
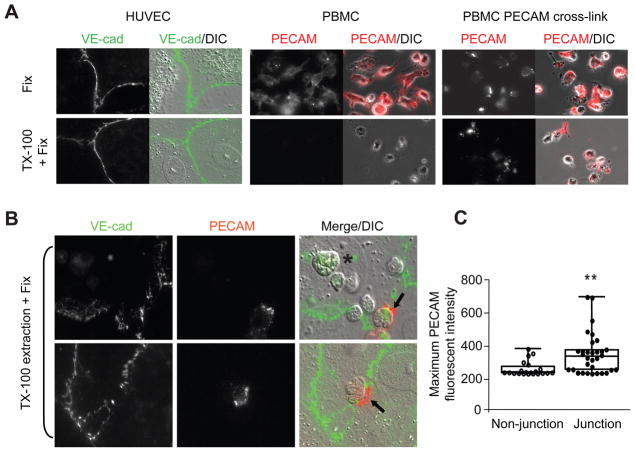
We next asked whether movement of PECAM into DRMs actually occurs during leukocyte transmigration. To do this we designed an assay to detect Triton X100 insoluble proteins using detergent-extraction and fluorescence microscopy. In this assay, monocytes were pre-labeled with an AlexaFluor 546 conjugated anti-PECAM antibody (P1.1) which does not block transmigration. Endothelial cells were preincubated with a non-blocking AlexFluor 488 conjugated anti-VEcadherin, to mark junctions. Figure 5A shows that PECAM of unstimulated monocytes adhered to glass is completely removed from cells by cold-Triton X100 extraction, while VE-cadherin remains visible at endothelial cell junctions (due to its association with the actin cytoskeleton). Consistent with biochemical approaches described above, leukocyte PECAM became resistant to cold-Triton extraction and was still visible following antibody mediated cross-linking, Figure 5A.
Figure 5. Leukocyte PECAM moves to a Triton insoluble fraction during transmigration.
(A) HUVEC monolayers labeled with AlexaFluor488 conjugated anti-VE-cadherin and human monocytes adhered to glass labeled with non-blocking AlexaFluor546 anti-PECAM with or without addition of secondary cross-linking antibodies. Samples were fixed in 2% paraformaldehyde or incubated with a Triton X100 extraction buffer at 4°C for 30minutes before fixation and imaged by wide-field microscopy. (B) Cells were labeled as in A and monocytes were added to endothelial monolayers for 10 minutes at 37°C. Cells were then processed and fixed as above. Leukocytes were seen adhered away from endothelial junctions (asterisks) and directly over junctions (arrows). Representative images are shown. (C) The maximal fluorescent intensity of PECAM found on leukocytes at junction and non-junction positions was analysed for 40 cells. Graphs show individual data points with mean fluorescent intensity, **P<0.007.
Using a similar approach, pre-labeled monocytes were added onto pre-labeled HUVEC monolayers for 10 minutes at 37°C followed by cold-Triton extraction and fixation, in order to analyse leukocyte PECAM behaviour during transmigration. We then compared the levels of PECAM on monocytes in the process of transmigration, as assessed by their progression through VE-cadherin stained junctions. Some monocytes present at endothelial junctions exhibited PECAM staining even after Triton X100 extraction (arrow), while cells away from the junction showed no PECAM staining (asterisk), Figure 5B. The maximum fluorescent intensity of PECAM was quantified for >50 monocytes whose position was classed as junctional or non-junctional. A greater proportion of monocytes found at endothelial junctions displayed a significantly greater PECAM staining, Figure 5C. These data suggest that leukocyte PECAM can move to DRMs upon homophilic ligation with endothelial PECAM.
Tyrosine phosphorylated leukocyte PECAM is enriched in non-DRM regions
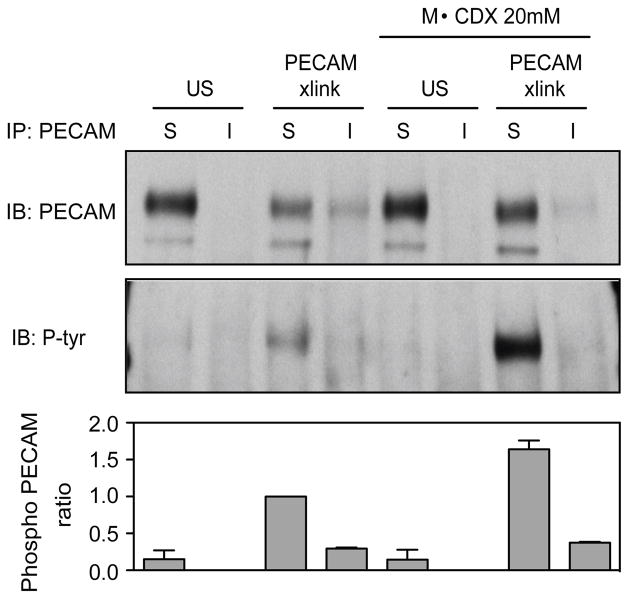
As the phosphorylation state of PECAM was not important in its movement to DRMs (Figure S2), we investigated whether the inclusion of PECAM in DRMs had an effect on PECAM phosphorylation. Unexpectedly, we found that phosphorylated PECAM in U937L cells was more abundant in the triton soluble fraction than the insoluble fraction, Figure 6. The levels of PECAM protein appear lower in insoluble fractions; however, this is a reflection of the efficiency of immunoprecipitation from insoluble fractions compared to soluble fractions rather than a difference in total protein. Thus, the PECAM to Phospho-PECAM ratio was used to compare the fractions. Consistent with this result, disruption of DRMs by MβCDX treatment resulted in a hyper-phosphorylation of PECAM. These data suggest that PECAM is phosphorylated in non-DRM regions, while PECAM in DRMs either undergoes less phosphorylation or is actively de-phosphorylated.
Figure 6. PECAM phosphorylation is reduced in DRMs.
U937L cells were left untreated or pretreated with 20μM MβCDX for 45min before undergoing PECAM cross- linking for 3 minutes. Soluble and insoluble fractions were immunoprecipitated (IP) with anti-PECAM antibodies and resolved on SDS-PAGE gels and analysed by western blotting (IB) using anti-phosphotyrosine and PECAM antibodies. Band intensities were measured via densitometry, and the ratio of phosphorylated PECAM to total PECAM is shown below from two separate experiments. Blots shown are representative of 3 separate experiments.
Leukocyte PECAM movement to DRMs negatively regulates transendothelial migration
We have demonstrated that leukocyte PECAM phosphorylation is important for transmigration and also that leukocyte PECAM in DRMs is less phosphorylated after cross-linking. We next sought to examine whether movement of leukocyte PECAM to DRMs affected transendothelial migration. Transmigration experiments using MβCDX to disrupt DRMs could not be performed as cholesterol depletion affects the ability of leukocytes to adhere, polarize and migrate which would all impair the assay (29–31). Instead of inhibiting PECAM movement we thus chose to force and lock PECAM into DRMs. To achieve this without affecting PECAM’s ability to undergo homophilic interactions, we exploited a non-blocking anti-PECAM antibody (P1.1), which does not inhibit transmigration, but when cross-linked translocates PECAM to DRMs and retains it there (Figure 7A) without effecting its homophilic adhesion properties (Figure S3). Forcing leukocyte PECAM into DRMs through P1.1 cross-linking, prior to their addition to HUVEC (t=0), resulted in profound inhibition of transmigration. Meanwhile, P1.1 on its own or cross-linked after 30 minutes (t=30) had little effect, Figure 7B. Similarly, cross-linking PECAM with the blocking antibody hec7 at t=0 inhibited transmigration, while cross-linking at t=30 overcomes the block, Figure 1 and Figure 7C. These data suggest that the timing of leukocyte PECAM activation and subsequent translocation to DRMs is important for transmigration. Forcing PECAM into DRMs before monocytes are able to get to endothelial junctions (i.e., at t = 0) inhibits transendothelial migration. Together, these data suggest that the retention of leukocyte PECAM in DRMs leads to a decrease in its phosphorylation and a subsequent inhibition of transmigration.
Figure 7. Activating PECAM prior to adhesion inhibits transmigration.
(A) Monocytes were stimulated by PECAM cross-linking using the monoclonal anti-PECAM antibody P1.1 for the indicated times. Triton X100 soluble and insoluble fractions were analysed by western blotting. (B,C) Human monocyte transmigration through unstimulated HUVEC monolayers grown on collagen gels. Monocytes were prelabelled on ice with (B) non-blocking antibody against PECAM (P1.1), or (C) blocking antibody against PECAM (Hec7) at 20μg/ml, before being washed free of unbound antibody and added to HUVEC monolayers for 60 minutes. Cross-linking secondary antibodies (50μg/ml) were added at the start of the assay (t=0) or after 30 minutes (t=30) where indicated. Data represent mean ± SEM from 3 separate experiments, ** P<0.02, *** P<0.002. (D) Proposed model of leukocyte PECAM regulation during transendothelial migration. Leukocytes migrate to endothelial cell junctions where they homophilically engage PECAM. The subsequent phosphorylation of leukocyte PECAM drives as yet unknown mechanisms required for the continuation and completion of the transmigration process. After PECAM engagement, its phosphorylation and signaling in the leukocyte membrane is terminated by translocation to DRMs.
DISCUSSION
PECAM has previously been implicated in signal transduction mediated by the phosphorylation of its cytoplasmic tail and association with other signaling complexes (32). Since PECAM is so fundamental to the process of leukocyte transmigration, it would seem likely that it acts as a signaling molecule in this setting too. While much work has been undertaken to elucidate the role of endothelial PECAM, far less has been done to explore the function of leukocyte PECAM during transmigration. Our present study reveals a key role for the tyrosine phosphorylation of leukocyte PECAM in transmigration. Furthermore, we describe a novel mechanism regulating the phosphorylation of leukocyte PECAM through its association with specialized lipid domains (DRMs).
Many immune receptors have been shown to cluster on the cell surface after ligand binding and this clustering plays an important role in initiating downstream signaling events (33). There is a high, local enrichment of endothelial PECAM at junctions, and it seems likely that this may induce leukocyte PECAM to cluster during transmigration. We hypothesized that such a clustering of leukocyte PECAM might transmit signals required for the continuation and completion of transmigration. Antibody mediated cross-linking of surface receptors initiates signal transduction, although the actual mechanism of how this works remains unclear. In the present study, we utilized cross-linking of leukocyte PECAM in a novel manner to mimic its natural clustering and thus precisely control its activation during transmigration.
Blocking leukocytes with anti-PECAM antibodies inhibited homophilic engagement with endothelial cell PECAM and thus arrested cells at endothelial junctions, unable to transmigrate through the monolayer (5, 34). Importantly, we found that the subsequent activation of leukocyte PECAM, by the addition of secondary cross-linking antibodies, was sufficient to promote transmigration in a subpopulation of the arrested cells. We hypothesize that the partial nature of the recovery may reflect the fact that only leukocytes located at permissible sites (e.g. endothelial junctions) at the time of cross-linking would be prompted to transmigrate. For those cells not engaging endothelial junctions at the time, PECAM cross-linking would drive PECAM into DRMs where it would be held in a state of deactivation that would actually inhibit transmigration. Indeed, cross-linking PECAM on leukocytes before they get to endothelial junctions inhibits their transmigration, Figure 7. These data confirm that leukocyte PECAM is critical during transmigration and suggest that, in addition to its accepted role as an adhesion molecule, it plays an active signaling function.
As phosphorylation was a consequence of PECAM ligation we examined its role in transmigration using dibutryl cAMP differentiated U937L cells in which we depleted endogenous PECAM and re-expressed phospho-mutant constructs. We found that depletion of endogenous PECAM led to a block in transmigration that could be rescued by re-expression of the wild-type protein. Strikingly however, U937L cells re-expressing PECAM with tyrosine-phenylalanine mutations at positions 663 and 686 displayed a sustained inhibition of transmigration. Further work will be required to determine the relative contributions of phosphorylation at the Y663 and Y686 sites during transmigration; however, our data provide clear evidence for the importance of phosphorylation within the ITIM domains of leukocyte PECAM.
Previous studies suggest that a downstream consequence of PECAM phosphorylation at sites 663 and 686 is the recruitment and activation of SH2 domain-containing phosphatases SHP1 and SHP2 (13–15). Other signaling proteins, such as PLCγ and SHIP, have also been proposed to complex with phosphorylated PECAM, although this remains controversial (35, 36), and indirect association of PI3K and Grb2 with PECAM have been reported (15, 37). PECAM is often cited as a negative regulator of signaling (38–40), but SHP2 is capable of transmitting both stimulatory and inhibitory signals (41, 42). The fact that we see an increase in transmigration upon PECAM ligation suggests that, in our system PECAM acts as a stimulatory molecule. Consistent with this possibility, we and other groups have reported integrin activation downstream of leukocyte PECAM ligation (34, 43, 44). The identification and characterization of signals initiated downstream of leukocyte PECAM phosphorylation are currently under investigation. It is tempting to speculate that phosphorylation of leukocyte PECAM at endothelial junctions may activate a sub-population of integrins to provide an anchor point while the cell passes through the endothelial monolayer. Integrin affinity changes have been monitored during leukocyte migration over endothelial substrates (45), but as yet there are no studies looking at their activation status during transmigration.
The association of immune receptors with specialized domains within the plasma membrane has been implicated in their ability to transduce signals (33). The clustering of receptors into these specialized lipid domains brings them into close proximity with and exposes them to other proteins such as kinases, phosphatases and scaffolding proteins that act to initiate and amplify signal transduction. These domains are often rich in cholesterol and sphingolipids, which reduces their solubility in some non-ionic detergents (21, 46), and are often termed detergent resistant membranes (DRMs). We provide evidence that in resting monocytes, PECAM is found almost exclusively in a Triton X100 soluble fraction, but that a portion of it becomes associated with DRMs after cross-linking. We examined PECAM movement to DRMs both by analyzing its solubility in different lysis buffers and by sucrose gradient analysis. Using both techniques we obtained clear evidence for this activation induced translocation, although the percentage of PECAM shifted differed. This disparity is most likely due to differences in the methods used to obtain samples and the efficiency of solubilization during the procedures. Although we cannot accurately define the exact amount of PECAM that moves to the DRM, we were able to efficiently manipulate the translocation and analyse its functional implications.
Most important, we found that leukocyte PECAM associates with DRMs during actual transmigration (Fig 5). PECAM on leukocytes adherent to endothelial monolayers demonstrated greater detergent resistance when located at endothelial junctions compared to positions away from junctions. PECAM ligation and clustering would likely occur at junctions due to the local enrichment of endothelial PECAM. Amplification of leukocyte PECAM clustering in this region could be achieved by the active enrichment of endothelial PECAM from the lateral border-recycling compartment that occurs in transmigration (7, 47). Ours is the first direct evidence to suggest that leukocyte proteins partition into DRMs during the transmigration process. It would be of interest to see if other leukocyte proteins known to be involved in transmigration also partition into DRMs.
For most transmembrane receptors, movement into DRMs is associated with an increase in their signaling potential. This can be due to the exclusion of negative regulators from the DRM, such as phosphatases (48), or the enrichment of activating kinases (49, 50). Unexpectedly, we found that PECAM association with DRMs had a negative effect on its activation and signaling capacity; PECAM was less phosphorylated in the Triton insoluble fraction as compared to soluble fractions. Consistent with this we saw that disruption of DRMs by cholesterol depletion led to hyperphosphorylation of PECAM phosphorylation within the Triton soluble fraction. As the kinase and/or phosphatase involved in controlling leukocyte PECAM phosphorylation are not known, it is difficult to understand by what mechanism PECAM is negatively regulated in DRMs.
Since PECAM association with DRMs decreases its phosphorylation (Fig. 6), and our data (Fig. 3) define a key role for leukocyte PECAM phosphorylation in transmigration, we sought to determine if the movement of PECAM to DRMs affected transmigration. Experiments to disrupt DRMs via cholesterol depletion could not be carried out in the context of transmigration, as this disrupts many crucial upstream processes (i.e. adhesion, polarization, migration). Instead, we chose to force PECAM into DRMs by cross-linking with non-blocking anti-PECAM antibodies prior to incubation on the endothelial monolayer. This does not affect its ability to homophilically bind PECAM but could potentially affect its ability to initiate further signal transduction. Cross-linking PECAM on leukocytes this way, before they have adhered and migrated to endothelial junctions, significantly inhibits their ability to transmigrate (Fig. 7). We propose that this is due to cross-linked PECAM being shifted to DRMs where its phosphorylation capacity is lower. These data may seem at odds with that of Figure 1, where cross-linking PECAM on blocked leukocytes promoted transmigration. However, a critically important distinction is that PECAM activation was induced at different times in these experiments. When leukocyte PECAM is activated at the start of the assay, before leukocytes adhere or move to the junctions, there is no transmigration because PECAM has been sequestered in DRMs in a long-term, dephosphorylated, signaling-incompetent state. However, when cross-linking is performed when cells are poised at junctions ready to transmigrate, transmigration is promoted because the transient activation of PECAM can drive this process. Our current working model proposes that during normal transmigration, leukocyte PECAM is subject to transient activation, stimulated by engagement with locally enriched endothelial PECAM at the junction, which is critical to drive transmigration. This may be followed shortly afterwards by down regulation of PECAM signaling by sequestration and dephosphorylation in DRMs, Figure 7D. This may turn off the local signaling within the leukocyte at that point, allowing leukocyte deadhesion and progression of that portion of the leukocyte across the endothelial cell border. All this is likely to work in concert with PECAM mediated events triggered in the endothelial cell.
In summary, we provide evidence that the precise spatial and temporal control of leukocyte PECAM signaling is required for transmigration. Deregulation of leukocyte PECAM activation disrupts transmigration. Further work will be required to define the signaling events downstream of leukocyte PECAM and to establish whether dysregulation of this pathway may contribute to disease.
Supplementary Material
Acknowledgments
We thank Dr Peter Newman for the shRNA lentiviral against PECAM and full length human PECAM in pWPT vector, Dr Bill Lusinskas for U937L cells and Ron Liebman for HUVEC isolation and culture.
Supported by grants from the U.S. National Institutes of Health, R01 HL046849 and R37 HL064774 to W.A.M.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 2.Newman PJ, Berndt MC, Gorski J, White GC, 2nd, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- 3.Liao F, Ali J, Greene T, Muller WA. Soluble domain 1 of platelet-endothelial cell adhesion molecule (PECAM) is sufficient to block transendothelial migration in vitro and in vivo. J Exp Med. 1997;185:1349–1357. doi: 10.1084/jem.185.7.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao F, Huynh HK, Eiroa A, Greene T, Polizzi E, Muller WA. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J Exp Med. 1995;182:1337–1343. doi: 10.1084/jem.182.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dasgupta B, Dufour E, Mamdouh Z, Muller WA. A novel and critical role for tyrosine 663 in platelet endothelial cell adhesion molecule-1 trafficking and transendothelial migration. J Immunol. 2009;182:5041–5051. doi: 10.4049/jimmunol.0803192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 2003;421:748–753. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 8.Rattan V, Shen Y, Sultana C, Kumar D, Kalra VK. Glucose-induced transmigration of monocytes is linked to phosphorylation of PECAM-1 in cultured endothelial cells. Am J Physiol. 1996;271:E711–717. doi: 10.1152/ajpendo.1996.271.4.E711. [DOI] [PubMed] [Google Scholar]
- 9.Ilan N, Madri JA. PECAM-1: old friend, new partners. Curr Opin Cell Biol. 2003;15:515–524. doi: 10.1016/s0955-0674(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 10.Newman PJ. Switched at birth: a new family for PECAM-1. J Clin Invest. 1999;103:5–9. doi: 10.1172/JCI5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao MY, Huber M, Beauchemin N, Famiglietti J, Albelda SM, Veillette A. Regulation of mouse PECAM-1 tyrosine phosphorylation by the Src and Csk families of protein-tyrosine kinases. J Biol Chem. 1998;273:15765–15772. doi: 10.1074/jbc.273.25.15765. [DOI] [PubMed] [Google Scholar]
- 12.Lu TT, Barreuther M, Davis S, Madri JA. Platelet endothelial cell adhesion molecule-1 is phosphorylatable by c-Src, binds Src-Src homology 2 domain, and exhibits immunoreceptor tyrosine-based activation motif-like properties. J Biol Chem. 1997;272:14442–14446. doi: 10.1074/jbc.272.22.14442. [DOI] [PubMed] [Google Scholar]
- 13.Jackson DE, Kupcho KR, Newman PJ. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of platelet/endothelial cell adhesion molecule-1 (PECAM-1) that are required for the cellular association and activation of the protein-tyrosine phosphatase, SHP-2. J Biol Chem. 1997;272:24868–24875. doi: 10.1074/jbc.272.40.24868. [DOI] [PubMed] [Google Scholar]
- 14.Masuda M, Osawa M, Shigematsu H, Harada N, Fujiwara K. Platelet endothelial cell adhesion molecule-1 is a major SH-PTP2 binding protein in vascular endothelial cells. FEBS Lett. 1997;408:331–336. doi: 10.1016/s0014-5793(97)00457-2. [DOI] [PubMed] [Google Scholar]
- 15.Sagawa K, Kimura T, Swieter M, Siraganian RP. The protein-tyrosine phosphatase SHP-2 associates with tyrosine-phosphorylated adhesion molecule PECAM-1 (CD31) J Biol Chem. 1997;272:31086–31091. doi: 10.1074/jbc.272.49.31086. [DOI] [PubMed] [Google Scholar]
- 16.Kim CS, Wang T, Madri JA. Platelet endothelial cell adhesion molecule-1 expression modulates endothelial cell migration in vitro. Lab Invest. 1998;78:583–590. [PubMed] [Google Scholar]
- 17.Osawa M, Masuda M, Harada N, Lopes RB, Fujiwara K. Tyrosine phosphorylation of platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) in mechanically stimulated vascular endothelial cells. Eur J Cell Biol. 1997;72:229–237. [PubMed] [Google Scholar]
- 18.Barabe F, Rollet-Labelle E, Gilbert C, Fernandes MJ, Naccache SN, Naccache PH. Early events in the activation of Fc gamma RIIA in human neutrophils: stimulated insolubilization, translocation to detergent-resistant domains, and degradation of Fc gamma RIIA. J Immunol. 2002;168:4042–4049. doi: 10.4049/jimmunol.168.8.4042. [DOI] [PubMed] [Google Scholar]
- 19.Cheng PC, Brown BK, Song W, Pierce SK. Translocation of the B cell antigen receptor into lipid rafts reveals a novel step in signaling. J Immunol. 2001;166:3693–3701. doi: 10.4049/jimmunol.166.6.3693. [DOI] [PubMed] [Google Scholar]
- 20.Rollet-Labelle E, Marois S, Barbeau K, Malawista SE, Naccache PH. Recruitment of the cross-linked opsonic receptor CD32A (FcgammaRIIA) to high-density detergent-resistant membrane domains in human neutrophils. Biochem J. 2004;381:919–928. doi: 10.1042/BJ20031808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 22.Lee FA, van Lier M, Relou IA, Foley L, Akkerman JW, Heijnen HF, Farndale RW. Lipid rafts facilitate the interaction of PECAM-1 with the glycoprotein VI-FcR gamma-chain complex in human platelets. J Biol Chem. 2006;281:39330–39338. doi: 10.1074/jbc.M607930200. [DOI] [PubMed] [Google Scholar]
- 23.Allport JR, Muller WA, Luscinskas FW. Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J Cell Biol. 2000;148:203–216. doi: 10.1083/jcb.148.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller WA, Ratti CM, McDonnell SL, Cohn ZA. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989;170:399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergom C, Goel R, Paddock C, Gao C, Newman DK, Matsuyama S, Newman PJ. The cell-adhesion and signaling molecule PECAM-1 is a molecular mediator of resistance to genotoxic chemotherapy. Cancer Biol Ther. 2006;5:1699–1707. doi: 10.4161/cbt.5.12.3467. [DOI] [PubMed] [Google Scholar]
- 26.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3:143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- 27.Kwiatkowska K, Frey J, Sobota A. Phosphorylation of FcgammaRIIA is required for the receptor-induced actin rearrangement and capping: the role of membrane rafts. J Cell Sci. 2003;116:537–550. doi: 10.1242/jcs.00254. [DOI] [PubMed] [Google Scholar]
- 28.Neufeld EB, Cooney AM, Pitha J, Dawidowicz EA, Dwyer NK, Pentchev PG, Blanchette-Mackie EJ. Intracellular trafficking of cholesterol monitored with a cyclodextrin. J Biol Chem. 1996;271:21604–21613. doi: 10.1074/jbc.271.35.21604. [DOI] [PubMed] [Google Scholar]
- 29.Gomez-Mouton C, Lacalle RA, Mira E, Jimenez-Baranda S, Barber DF, Carrera AC, Martinez AC, Manes S. Dynamic redistribution of raft domains as an organizing platform for signaling during cell chemotaxis. J Cell Biol. 2004;164:759–768. doi: 10.1083/jcb.200309101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manes S, Mira E, Gomez-Mouton C, Lacalle RA, Keller P, Labrador JP, Martinez AC. Membrane raft microdomains mediate front-rear polarity in migrating cells. EMBO J. 1999;18:6211–6220. doi: 10.1093/emboj/18.22.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierini LM, Eddy RJ, Fuortes M, Seveau S, Casulo C, Maxfield FR. Membrane lipid organization is critical for human neutrophil polarization. J Biol Chem. 2003;278:10831–10841. doi: 10.1074/jbc.M212386200. [DOI] [PubMed] [Google Scholar]
- 32.Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953–964. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]
- 33.Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK. Location is everything: lipid rafts and immune cell signaling. Annu Rev Immunol. 2003;21:457–481. doi: 10.1146/annurev.immunol.21.120601.141021. [DOI] [PubMed] [Google Scholar]
- 34.Berman ME, Xie Y, Muller WA. Roles of platelet/endothelial cell adhesion molecule-1 (PECAM-1, CD31) in natural killer cell transendothelial migration and beta 2 integrin activation. J Immunol. 1996;156:1515–1524. [PubMed] [Google Scholar]
- 35.Henshall TL, Jones KL, Wilkinson R, Jackson DE. Src homology 2 domain-containing protein-tyrosine phosphatases, SHP-1 and SHP-2, are required for platelet endothelial cell adhesion molecule-1/CD31-mediated inhibitory signaling. J Immunol. 2001;166:3098–3106. doi: 10.4049/jimmunol.166.5.3098. [DOI] [PubMed] [Google Scholar]
- 36.Pumphrey NJ, Taylor V, Freeman S, Douglas MR, Bradfield PF, Young SP, Lord JM, Wakelam MJ, Bird IN, Salmon M, Buckley CD. Differential association of cytoplasmic signalling molecules SHP-1, SHP-2, SHIP and phospholipase C-gamma1 with PECAM-1/CD31. FEBS Lett. 1999;450:77–83. doi: 10.1016/s0014-5793(99)00446-9. [DOI] [PubMed] [Google Scholar]
- 37.Pellegatta F, Chierchia SL, Zocchi MR. Functional association of platelet endothelial cell adhesion molecule-1 and phosphoinositide 3-kinase in human neutrophils. J Biol Chem. 1998;273:27768–27771. doi: 10.1074/jbc.273.43.27768. [DOI] [PubMed] [Google Scholar]
- 38.Jones KL, Hughan SC, Dopheide SM, Farndale RW, Jackson SP, Jackson DE. Platelet endothelial cell adhesion molecule-1 is a negative regulator of platelet-collagen interactions. Blood. 2001;98:1456–1463. doi: 10.1182/blood.v98.5.1456. [DOI] [PubMed] [Google Scholar]
- 39.Newton-Nash DK, Newman PJ. A new role for platelet-endothelial cell adhesion molecule-1 (CD31): inhibition of TCR-mediated signal transduction. J Immunol. 1999;163:682–688. [PubMed] [Google Scholar]
- 40.Rui Y, Liu X, Li N, Jiang Y, Chen G, Cao X, Wang J. PECAM-1 ligation negatively regulates TLR4 signaling in macrophages. J Immunol. 2007;179:7344–7351. doi: 10.4049/jimmunol.179.11.7344. [DOI] [PubMed] [Google Scholar]
- 41.Lacalle RA, Mira E, Gomez-Mouton C, Jimenez-Baranda S, Martinez AC, Manes S. Specific SHP-2 partitioning in raft domains triggers integrin-mediated signaling via Rho activation. J Cell Biol. 2002;157:277–289. doi: 10.1083/jcb.200109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qu CK. The SHP-2 tyrosine phosphatase: signaling mechanisms and biological functions. Cell Res. 2000;10:279–288. doi: 10.1038/sj.cr.7290055. [DOI] [PubMed] [Google Scholar]
- 43.Berman ME, Muller WA. Ligation of platelet/endothelial cell adhesion molecule 1 (PECAM-1/CD31) on monocytes and neutrophils increases binding capacity of leukocyte CR3 (CD11b/CD18) J Immunol. 1995;154:299–307. [PubMed] [Google Scholar]
- 44.Tanaka Y, Albelda SM, Horgan KJ, van Seventer GA, Shimizu Y, Newman W, Hallam J, Newman PJ, Buck CA, Shaw S. CD31 expressed on distinctive T cell subsets is a preferential amplifier of beta 1 integrin-mediated adhesion. J Exp Med. 1992;176:245–253. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green CE, Schaff UY, Sarantos MR, Lum AF, Staunton DE, Simon SI. Dynamic shifts in LFA-1 affinity regulate neutrophil rolling, arrest, and transmigration on inflamed endothelium. Blood. 2006;107:2101–2111. doi: 10.1182/blood-2005-06-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.London E, Brown DA. Insolubility of lipids in triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts) Biochim Biophys Acta. 2000;1508:182–195. doi: 10.1016/s0304-4157(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 47.Mamdouh Z, Kreitzer GE, Muller WA. Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. J Exp Med. 2008;205:951–966. doi: 10.1084/jem.20072328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodgers W, Rose JK. Exclusion of CD45 inhibits activity of p56lck associated with glycolipid-enriched membrane domains. J Cell Biol. 1996;135:1515–1523. doi: 10.1083/jcb.135.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montixi C, Langlet C, Bernard AM, Thimonier J, Dubois C, Wurbel MA, Chauvin JP, Pierres M, He HT. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 1998;17:5334–5348. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.