Abstract
Injury to articular cartilage leads to degenerative changes resulting in a loss of mechanical and biochemical properties. In engineered cartilage, the injury response of developing constructs is unclear.
Objective
To characterize the cellular response of tissue-engineered constructs cultured in chemically-defined medium after mechanical insult, either by compression-induced cracking, or by cutting, as a function of construct maturity.
Methods
Primary immature bovine articular chondrocytes (4-6 weeks) were encapsulated in agarose hydrogel (2%, 30 million cells/mL) and cultured in chemically-defined medium supplemented with TGF-β3 (10ng/mL, first two weeks). At early (5 days) and late (35 days) times in culture, subsets of constructs were exposed to mechanical overload to produce a crack in the tissue or were exposed to a sharp wound with a perpendicular cut. Constructs were returned to culture and allowed to recover in static conditions. Mechanical and biochemical properties were evaluated at two-week intervals to day 70, and cellular viability was assessed at two-week intervals to day 85.
Results
Constructs injured early in culture recovered their mechanical stiffness back to control values, regardless of the mode of injury. Later in culture, when constructs exhibited properties similar to those of native cartilage, compression-induced cracking catastrophically damaged the bulk matrix of the tissue and resulted in permanent mechanical failure with persistent cell death. No such detrimental outcomes were observed with cutting. Biochemical content was similar across all groups irrespective of mode or time of injury.
Conclusions
Unlike native cartilage, engineered cartilage constructs exhibit a reparative capacity when the bulk integrity of the developing tissue is preserved after injury.
Keywords: articular cartilage, engineered cartilage, injury, chondrocytes, apoptosis
INTRODUCTION
Articular cartilage exhibits a poor intrinsic healing response subsequent to injury [1] that engenders a need for cell-based therapies for repair. As the growing promise of engineered cartilage grafts is realized in clinical practice, it will be important to understand the engineered construct’s response to the joint loading environment after implantation, including potential injurious loading.
Injury to cartilage can occur with traumatic loading of the joint (traumatic injury) as well as in surgical procedures that include graft harvesting (iatrogenic injury). In native cartilage, the mechanotransduction resulting from injury can induce chondrocyte death as early as a few hours after, and up to 7 days, post injury [2]. These downstream effects can produce structural damage and osteoarthritic-like changes including a loss of mechanical properties, increased collagen degradation, and reduced proteoglycan synthesis [3, 4].
To develop strategies that mitigate tissue injury and promote repair, culture injury models have been used to study the response of cartilage to trauma by taking advantage of the more controllable loading environment afforded by in vitro systems [5, 6]. In addition, cartilage injury to sharp and blunt trauma with scalpels and tissue trephines has been studied as a model system of iatrogenic injury [1, 7, 8].
The current investigation aims to complement these explant injury studies by providing an initial characterization of the in vitro injury response of engineered cartilage. The use of a tissue engineering model as the surrogate tissue permits injury to be studied on constructs spanning a range of mechanical and biochemical properties that reflect tissue culture maturation. Elucidation of potential failure mechanisms of engineered tissues that may occur after implantation in the defect site may be important for optimization of implantation guidelines and tissue construct design criteria.
MATERIALS AND METHODS
Experimental Design
To understand the influence of tissue maturity at the time of applied injury on cellular response and extracellular matrix remodeling, we have developed a trauma model to induce chondrocyte death in engineered cartilage constructs of increasing culture age, in response to controlled mechanical overloading (compression-induced cracking), or sharp cutting. The cutting injury served as a method of controlling the location and extent of injury, relative to the greater uncertainty in the extent and location of cracking.
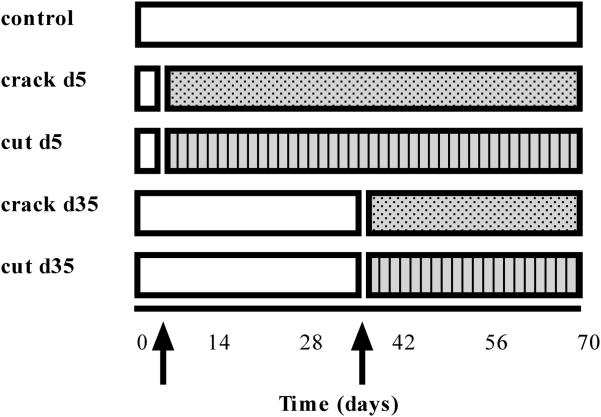
The timeline of the studies is detailed in Figure 1. There are two variables in the experiments: (1) the day on which trauma is imparted to the developing construct, and (2) the type of injury imposed (‘crack’ or ‘cut’). Specifically, subsets of constructs were exposed to trauma, after which they were returned to culture conditions to study their subsequent response.
Figure 1.
Timeline of experimental setup for different groups exposed to trauma. Arrows indicate time in culture when injury was imposed.
Tissue Isolation and Cell Culture
Articular cartilage was harvested from bovine carpo-metacarpal (CMC) joints of freshly slaughtered 2-4-week-old calves. Four to six joints were used for each experiment and cells were pooled from all joints. Cartilage was digested in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) with collagenase type V (Sigma, St. Louis, MO) for 11 hours at 37°C with shaking. Cell suspensions were filtered through a 70μm porous mesh and sedimented in a bench-top centrifuge for 15 min at 1500g. Viable cells were counted with a hemacytometer and trypan blue. Cell suspensions (60 × 106 cells/mL) were mixed in equal parts with 4% low-gelling agarose (type VII, Sigma) at 37°C to yield a final cell concentration of 30 × 106 cells/mL in 2% agarose. The chondrocyte/agarose mixture was cast into slabs and cored using a sterile disposable punch (Miltex) to final dimensions of 4mm diameter and 2.34 mm thick.
Constructs were cultured in hgDMEM supplemented with 1X PSF, 0.1μM dexamethasone, 50μg/mL ascorbate 2-phosphate, 40μg/mL L-proline, 100μg/mL sodium pyruvate, and 1X ITS+ premix (insulin, human transferrin, and selenous acid, Becton Dickinson, Franklin Lakes, NJ). Medium was further supplemented with 10ng/mL TGF-β3 (Invitrogen, Carlsbad, CA) for the first 14 days of culture. Culture media was changed every other day.
Injury
On days 5 and 35 of culture, constructs were subjected to one of two injury modes: compression-induced cracking or cutting (Figure 2).
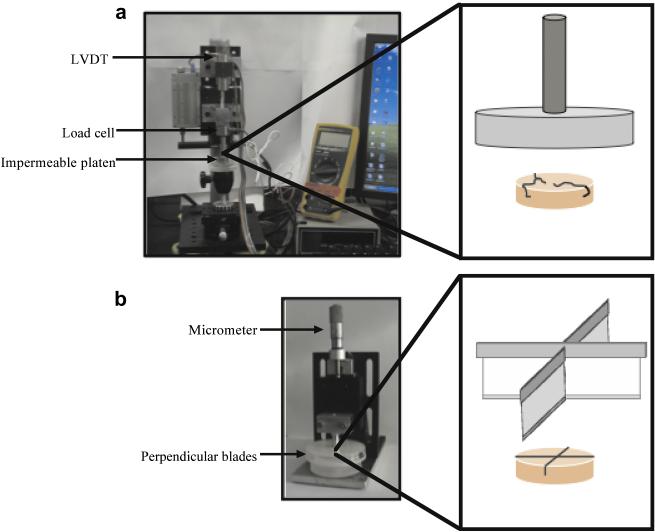
Figure 2.
Experimental setup of the (a) cracking and (b) cutting setups with a blown up schematic of the injurious device. Compression was applied with an impermeable platen until cracking was achieved. For the cutting setup, blades were cut into the construct to a depth of 50%.
Briefly, to produce cracking, constructs were loaded using a computer-controlled custom device and ramped to failure at a constant strain rate of 0.3% sec−1. Mechanical failure was identified by a drop in applied load on a force-displacement plot and confirmed by gross visual inspection of each construct to ensure that constructs had sustained a fissure throughout the sample. For the cutting injury, two orthogonal cuts were made in constructs by pushing a razor blade to 50% of the construct’s original thickness. Razor blades were pushed straight down to prevent the application of additional shear forces, and were replaced after every five constructs to prevent dulling of the blade. Afterwards, samples (including controls manipulated similarly to injured constructs, without actual trauma) were returned to culture in freshly supplemented media and allowed to recover in culture to day 70 for mechanical testing, or to day 85 for viability staining.
Cell Viability Assessment
Assessment of cell viability was performed using the Live/Dead cytotoxicity assay (Molecular Probes, Eugene, OR) at immediate- (1 day), short- (3, 7 days), and long- (14, 28, 50 days) time points post trauma. The assay is based on calcein AM permeating the membranes of cells with intact cell membranes, producing a green fluorescence (live), while ethidium homodimer permeates the nuclei of dead cells (with compromised cell membranes) to produce a red fluorescence (dead). After each viability assay, constructs were aseptically returned to culture media to allow for assessment of cell death in the same sample at later timepoints. Live and dead images were taken separately on an inverted confocal fluorescent microscope (Olympus Imaging America, Inc., Center Valley, PA) and overlaid to produce a composite image of the region (Adobe Systems Inc., San Jose, CA).
To quantify cell viability over time for an injured sample, live and dead images were processed separately for automated counting of fluorescing particles (ImageJ, NIH). The fraction of live cells present in an image was determined by calculating the area fraction of particles in a centered region of an 8-bit, thresholded image. Live cells were characterized as those particles with sizes ranging from 0-2500 pixel2 (approx. cell size for the given image magnification). These sizes were previously determined using other sample images by calculating average cell body sizes for multiple images. Data was collected from the ImageJ program, and the fraction of live cells reported by normalizing to respective controls.
Mechanical Testing
Samples were tested in unconfined compression to assess equilibrium modulus (EY) and dynamic modulus (G*) using a custom computer-controlled system [9]. An initial 0.02N tare load was applied, followed by compression to 10% strain, at a strain rate of 0.05% sec−1. After stress-relaxation was achieved, a 2% peak-to-peak strain was superimposed at 0.1Hz. EY was measured from the stress-relaxation equilibrium response, and G* from the slope of the stress-strain response under dynamic loading.
Biochemical Content
Full constructs were used for biochemical analysis to ensure that any biochemical changes from the effect of the injury (crack or cut) would be captured. Construct swelling was quantified by measuring the gross water content of the constructs [10]. Samples were dried and digested in proteinase-K buffer overnight at 56°C, as described previously [11]. An aliquot was analyzed for glycosaminoglycan (GAG) content via the 1,9-dimethylmethylene blue dye-binding assay [12]. A further aliquot was hydrolyzed in 12N HCl at 110°C for 16 hours, dried, and resuspended in assay buffer [11]. Orthohydroxyproline (OHP) content was determined using a colorimetric assay via a reaction with chloramine T and dimethylaminobenzaldehyde [13], scaled for microplates. Overall collagen content was calculated by assuming a 1:7.64 OHP-to-collagen mass ratio [13]. dsDNA content was also assessed by the Picogreen assay according to the manufacturer’s standard protocols. Each biochemical constituent was normalized to either tissue wet or dry weight.
Statistics
Statistics were performed using two-way ANOVA with Tukey’s HSD post-hoc tests (Statistica, Tulsa, OK), with α=0.05 and statistical significance set at p≤0.05 to compare groups across day and treatment. All data are reported as the mean ± 95% confidence interval of 4-5 samples per time point and group.
RESULTS
Gross Cracking Response as a Function of Tissue Culture Maturity
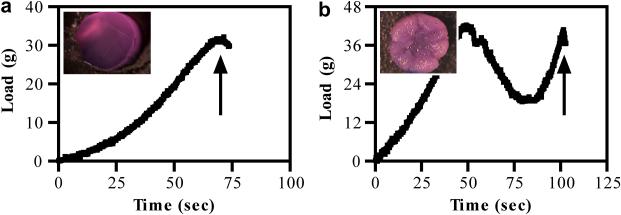
Using a computer-controlled system to impart strain, force-displacement profiles were generated to study the gross failure response of the constructs subjected to cracking, under controlled deformation. At early culture time points, when constructs were immature, load increased steadily to 36.1 ± 0.85% strain before cracking the construct (Figure 3a). In comparison, compression-induced cracking of more mature constructs produced catastrophic damage, where compaction of the extracellular matrix produced bulk tissue compression (Figure 3b, inset) before crack initiation at 50.3 ± 0.13% strain (Figure 3b).
Figure 3.
Representative load-time curves generated during mechanical overloading for constructs injured (a) early in culture on day 5 or (b) later in culture on day 35. Arrows indicate point of construct failure. Insets: stereoscopic images of constructs after trauma at each representative timepoint.
Cell Viability and Proliferation Post Injury
Cellular viability and subsequent response after insult, as assessed by the Live/Dead cytotoxicity assay, was found to vary with mode of trauma as well as culture maturity at time of injury. For cut constructs, exposed to trauma on days 5 and 35, the area fraction of live cells within the same region of each construct increased significantly at 50 days post injury (d5 injury: 31.1 ± 2.81%, d35 injury: 59.1 ± 4.40%, Table 1, p<0.001, n=5/group) by the end of culture.
Table 1.
Normalized area fraction of live cells in a centralized portion of cut constructs compared to control samples at different time points after early (d5) and late (d35) injury. d5 injury: 31.1 ± 2.81%, d35 injury: 59.1 ± 4.40%, P < 0.001, n = 5/group
| ds injury | d35 injury | |
|---|---|---|
| 1 day post cut | 1.02 | 0.44 |
| 3 days post cut | 1.05 | 0.54 |
| 50 days post cut | 1.34 | 0.70 |
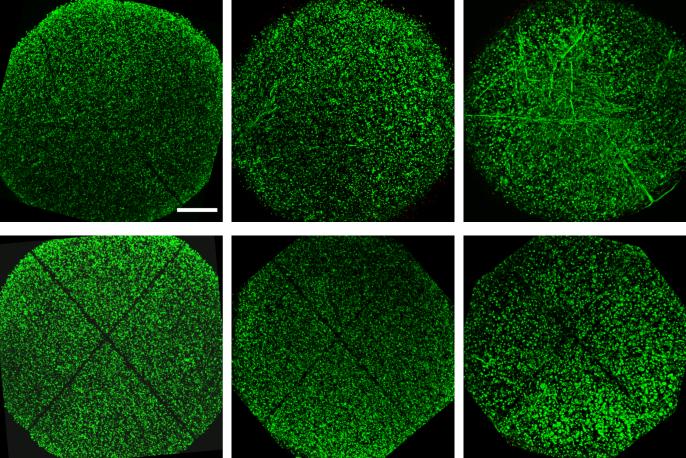
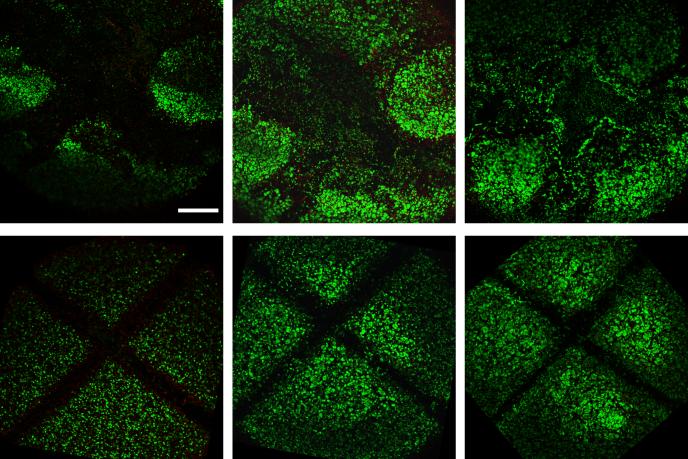
At early times in culture, immediately following induced trauma on day 5, both cracked and cut constructs exhibited little cellular damage, with the only loss of cells contained to the immediate vicinity of the site of injury (Figure 4a,d). Over time, as early as 14 days post injury, cellular infiltration from neighboring regions into the injured space was observed, depending on the type of injury: Cellular infiltration and elongation along the site of injury was observed for constructs exposed to compression-induced cracking (Figure 4b). By 28 days after injury, injured sites for cracked constructs were fully infiltrated with neighboring cells (Figure 4c). For cut constructs, however, neighboring cells did not infiltrate the void space, leaving a clearly demarcated region of cell loss throughout the culture period (Figure 4e,f).
Figure 4.
Representative superimposed live and dead images of immature cracked (top) and cut (bottom) constructs at 1 day (a,d), 14 days (b,e), and 28 days (c,f) after injury. Scalebar: 0.5mm. With time in culture, neighboring cells infiltrate into the cracked region of constructs. For constructs injured with a scalpel cut, cells did not infiltrate the cut region, leaving a void space (represented by black space), which was still present at 28 days after injury. Dead cells are visible upon further magnification of the region only immediately (1 day) after injury.
Following a longer time in culture, the response of the engineered cartilage to injury was immediately noticeable. Constructs catastrophically damaged on day 35 by compression-induced cracking exhibited a large loss of cell viability in the immediate and peripheral regions to the site of injury; these regions were not subsequently filled with neighboring cells over the remaining time in culture (Figure 5a-c). For constructs cut at this later time point, a larger void space demarcated the region of injury and these constructs responded similarly to constructs cut earlier in culture (Figure 5d-f).
Figure 5.
Representative superimposed live and dead images of mature cracked (top) and cut (bottom) constructs at 1 day (a,d), 28 days (b,e), and 50 days (c,f) after injury. Scalebar: 0.5mm. Loss of cell viability (represented by black/void space) is present in the immediate and peripheral regions to the site of injury (crack or cut), which is not recovered with time in culture. Dead cells (nuclei of dead cells are stained red) are visible upon further magnification of the region at 1 day (for cut samples) and up to 28 days (for cracked samples) after injury.
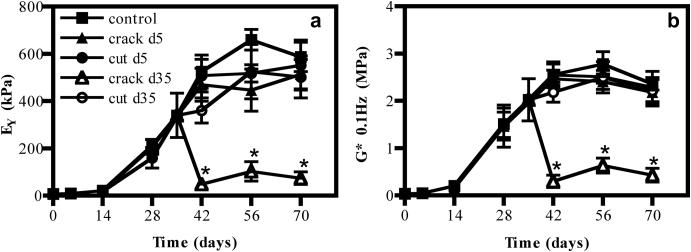
Recovery of Mechanical Response
Mechanical properties of constructs injured on day 5 of culture recovered to uninjured control values by day 14, despite the overload-cracking or cutting injury imposed on the nascent tissue (pEY>0.999 pG>0.999, Figure 6). After recovery, at the end of the culture period, constructs achieved properties similar to the Young’s modulus of native bovine juvenile tissue (EY: 587 ± 43.9 kPa, G*: 2.4 ± 0.1 MPa, control; EY: 513 ± 43.8 kPa, G*: 2.2 ± 0.2 MPa, crack, pEY=0.794, pG*=0.998; EY: 552 ± 74.9 kPa, G*: 2.3 ± 0.2 MPa, cut, pEY>0.999, pG*>0.999).
Figure 6.
(a) Young’s modulus (EY) and (b) Dynamic modulus (G*) of constructs with time in culture. *p<0.05 vs. control, n=5/group. Crack d35 vs. control, EY: pday42<0.001, pday56<0.001, pday70<0.001. Crack d35 vs. control, G*: pday42<0.001, pday56<0.001, pday70<0.001.
When injured on day 35, however, the response of constructs depended on the mode of injury, with overloaded constructs exhibiting an immediate drop in the compressive moduli at day 42 and thereafter continually weakening, never recovering mechanical integrity even by the end of culture (EY: 73.9 ± 20.9 kPa, G*: 0.4 ± 0.1 MPa, pEY<0.001, pG*<0.001, Figure 6). Cut constructs, however, were not significantly different from uninjured samples by the end of the culture period (EY: 502 ± 70.1 kPa, G*: 2.2 ± 0.2 MPa, pEY=0.521, pG*>0.999, Figure 6).
Biochemical Composition After Injury
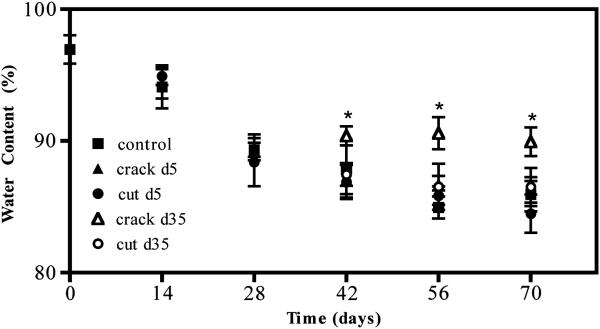
Water content and biochemical composition were measured at each time point following injury. Water content for injured constructs remained similar to uninjured control samples except for those exposed to compression-induced cracking on day 35 (pday42=0.020, pday56<0.001, pday70<0.001, Figure 7). For all time points, biochemical composition was normalized to construct wet weight. In cases when significant tissue swelling was noted (days 42-70), biochemical content was also normalized to construct dry weight (Figure 8).
Figure 7.
Water content of engineered constructs over time. *p<0.05 vs. control, n=5/group. pday42=0.020, pday56<0.001, pday70<0.001.
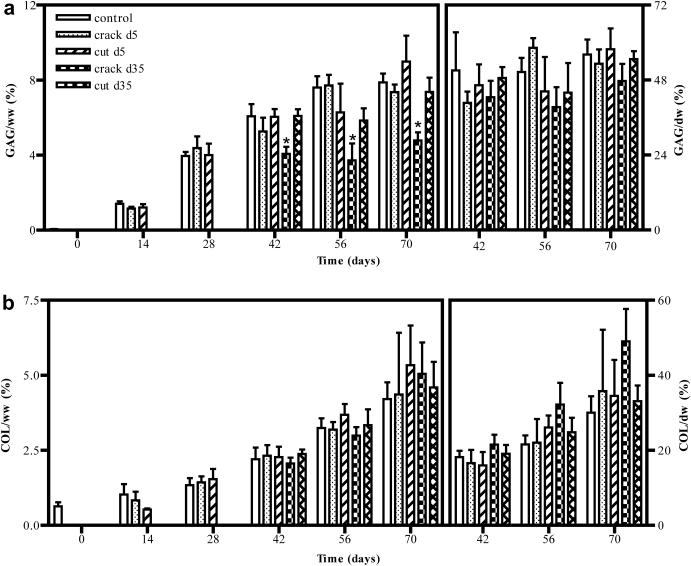
Figure 8.
(a) GAG and (b) collagen content of control and injured constructs over time normalized to both construct wet weight (left) and dry weight (right). *p<0.05 vs. control, n=5/group. Crack d35 vs. control, GAG: pday42 <0.001, pday56 <0.001, pday70 <0.001.
Regardless of culture maturity at the time of injury, constructs retained their biochemical constituents immediately following insult, as well as throughout the culture period. Specifically, constructs injured early in culture were able to recover their biochemical content with time in culture relative to uninjured controls (GAG: 7.9 ± 0.3% ww, collagen: 4.2 ± 0.4% ww, control day 70; GAG: 7.4 ± 0.3% ww, collagen: 4.4 ± 1.5 % ww, crack d5, pGAG>0.999, pCOL>0.999; GAG: 9.0 ± 1.0% ww, collagen: 5.3 ± 0.9% ww, cut d5, pGAG = 0.208, pCOL=0.150, Figure 8). When injured later in culture (d35), cut constructs similarly retained their biochemical makeup (GAG: 7.4 ± 0.5% ww, collagen: 4.6 ± 0.4% ww, pGAG>0.999, pCOL=0.546, Figure 8). Mature constructs cracked on day 35 exhibited an apparent loss in GAG content (4.8 ± 0.3% ww, p<0.001, Figure 8), however, when normalized to dry weight to account for tissue swelling and increased water content, GAG content was comparable to uninjured controls (56 ± 3.4% dw, control; 48 ± 3.8% dw, crack d35, p=0.556, Figure 8). Collagen content was consistent for control and injured groups regardless of normalization method.
DISCUSSION
Currently, the predominant approach to clinical applications of cartilage tissue engineering is to design a delivery system and scaffold that promote tissue growth in situ (e.g., [14-19]). However, based on our understanding of the biomechanics of diarthrodial joints, we believe that the in situ environment is too harsh to allow a fledgling engineered tissue construct to develop a functional cartilage matrix that can withstand the native mechanical and chemical loading conditions. In particular, as the biomechanical environment is dictated by several factors including contact geometry, size of defect, and degree of load sharing, the extent of functional tissue elaboration needed to ensure the construct’s survival after implantation into a joint defect remains unclear. As a first step toward predicting and characterizing the response to this harsh mechanical environment, engineered cartilage constructs of different culture maturity were exposed to either compression-induced cracking or controlled cutting.
While Young’s modulus and GAG are at native levels in 8 weeks or less, the dynamic modulus (a functional measure that reflects tissue attributes such as radial tensile properties) and collagen levels remain significantly lower (native juvenile bovine: G*: ~40MPa, %Collagen/ww: ~10%, [20]) thereby signifying a major difference between our engineered cartilage and the native tissue. The ability to achieve native collagen levels, presumably needed to recapitulate the normal structure-function relationships of articular cartilage, continues to represent a significant challenge to the field of cartilage tissue engineering. In the current study, mature (day 35) engineered cartilage constructs exhibiting physiologic values of Young’s modulus demonstrated a poor healing capacity following a cracking injury, in analogy to the known behavior of native cartilage [3, 21, 22]. In contrast, cracking of immature (day 5) constructs was followed by a complete recovery to control values by the end of the culture period.
This maturation-dependent response may be attributed to the advanced development of extracellular matrix (ECM) in the day 35 constructs. At an early stage of culture, chondrocytes embedded in the agarose hydrogel are not mechanically tethered to each other, due to the absence of a continuous collagen fibrillar matrix. The deformation applied to the construct is transduced to the cells only via the agarose hydrogel. In fact, chondrocytes deform less than the surrounding agarose because of the formation of a local pericellular matrix (PCM), which occurs around day 4 in culture [23], making them stiff inclusions in a soft matrix. Therefore, when cracking occurs, it is primarily a failure of the agarose hydrogel, which is known to have a limited resistance to tensile stresses, exhibiting a relatively brittle behavior [24]. The chondrocytes are only subjected to a transient deformation mitigated by their pericellular matrix, and are thus able to survive the agarose cracking event (Figure 4) and continue to thrive by producing a functional matrix, as evident from the observed results (Figures 6 to 8).
However, as the cell-elaborated matrix begins to coalesce into tissue islands that eventually form a more contiguous ECM, the construct deformation may now be transduced to the chondrocytes via pulling of the collagen fibers [25], and the cracking event may rip at the cells via integrin attachments. Therefore, cracking of more mature constructs leads to greater loss of cells (Figure 5) and poor recovery of mechanical integrity (Figure 6). The role of the ECM in mediating the deformation of mature constructs is directly evident in the load response at the gross level (Figure 3b). The biphasic load response noted in Figure 3b may be attributed to initial cracking of the less dense ECM inside the construct [26], followed by subsequent cracking of the denser collagenous matrix known to grow on the outer surface of the construct. Similarly, the ability of collagen fibrils to resist radial and circumferential tension is manifested in the later onset of failure in mature versus immature constructs (~50% versus ~36% axial compression). Furthermore, due to the increased compressive stiffness of the GAG-laden mature constructs, chondrocytes are no longer able to resist the deformation of their surrounding matrix, so that the crack-inducing loading event may cause significantly greater cell deformation, and possibly cell death, than in immature constructs.
In contrast, sharp cutting does not exhibit the same long-term detrimental effects as cracking, analogous to native cartilage’s response to partial-thickness cutting [27]. The response of immature constructs to cutting is similar to the cracking event: the cut primarily affects the agarose hydrogel and, other than cells that come in direct contact with the blade, chondrocytes remain unaffected by the cutting event (Figure 4d). Thus, the biosynthetic capacity of the surrounding cells is maintained and constructs are able to grow a functional matrix over time. In mature constructs, the blade now cuts through an elaborated ECM, causing more significant pulling of the connected network of collagen fibers and producing cell death beyond the immediate path of the blade (Figure 5d). However, cell death is significantly less widespread than with cracking, such that cell death is no longer observed at later time point (Figures 5b versus 5e). Since the chasms produced by the blade are not as wide as the cracks, a collagen network is apparently able to bridge these gaps over time, as evident from the measured functional properties (Figure 6).
The lack of pervasive and continuing cell death after injury observed in this study may also reflect the unique situation in which core biochemical constituents are maintained both immediately and long-term after imposition of trauma. In both modes of injury, overloading or cutting, transient indications of cell death were localized around crack and lesion sites. In extreme loading conditions, articular cartilage can exhibit fissuring after mechanical insult [28], with cell death in the proximity of tissue matrix cracks [29]. With time in culture, however, cell death is consistently seen to emanate away from the site of injury as paracrine factors are released from necrotic cells (dying from cell injury) and subsequently influence neighboring cells to undergo programmed cell death, a consequence of apoptosis [1]. Similarly, in the most extreme loading condition of this study, constructs exposed to catastrophic damage exhibited prolonged cell death almost 4 weeks after injury (Figure 5b), suggesting that the mode of cell death is no longer necrotic, which occurs within hours or days [2], but rather in a manner consistent with apoptosis. However, in contrast to native cartilage, with further time in culture, cell death in overloaded constructs was mitigated, and further signs of continual cell death were not observed (Figure 5c). In comparison, for constructs injured earlier in culture or less severely, patterns of delayed cell loss after injury were not observed, suggesting that cells either were not continually dying or new cells from cell division were repopulating injured regions and masking cell loss, as indicated by the comparable DNA content across all groups. This possibility is supported by analysis of the fraction of live cells in the center region of cut constructs which found that cells proliferated at a greater rate than uninjured control constructs and may have offset the loss in cell number stemming from the initial cell death after injury.
Explant studies have previously shown that chondrocytes close to an injury site tend to react to the imposed trauma by either dying or proliferating [30], although it is unclear what triggers one path over the other. The findings of this study suggest that while cells in engineered cartilage are immediately mechanically compromised after injury, the alternative downstream pathways of cell death or proliferation depend on the culture maturity of the construct. For constructs injured early in culture or injured less severely, rather than entering apoptotic pathways, the cells proliferate in the regions adjacent to the lesion. For engineered constructs exposed to catastrophic matrix damage, some cells enter apoptotic pathways and exhibit delayed death, but interestingly, the system seems to only reside in this state transiently with little effects on the biochemical composition of the tissue.
However, while we did not observe biochemical content degradation or continual cell death, constructs cracked late in culture were unable to recover their mechanical properties and intrinsically repair themselves, similar to that which is seen with cartilage explants in vitro and suggested to happen in vivo. The observed mechanical failure suggests immediate catastrophic structural damage of the construct rather than subsequent degradation cascades. This proposed mechanism of failure is further supported by the different responses seen with compression-induced cracking and cutting. Like cartilage explants exposed to scalpel injuries [31, 32], cut constructs lack the traumatic structural changes associated with other modes of injury such as compression [5, 33-35], indentation [2, 36], and trephine punches [1, 32]. As such, it may be that localized structural damage from the blade is insufficient to cause bulk structural damage of the construct that is necessary for mechanical failure.
It is important to note, however, that this response to mechanical overload as well as cutting likely reflects the conditions of the experimental setup, specifically the chosen scaffold system and the cell type used, which together define the nature of the cell-matrix interactions that develop in culture. There exist several possible mechanisms that may underscore differences in the injury response of engineered cartilage to literature reports of cartilage explant injury. First, while our engineered tissues recapitulate many of the structure-function relationships of native cartilage [10], the collagen content remains significantly lower than native levels, in contrast to GAG levels that reach native content. Collagen content is likely to affect cell-matrix coupling. Additionally, variations in engineered extracellular matrix organization may lead to differences in how dead cells and apoptotic bodies are entrapped. The avascular nature of cartilage and lack of mononuclear phagocytes in vivo prohibit the removal of apoptotic bodies, which subsequently become lodged in surrounding lacunae [37]. As such, for cartilage explants injured in vitro, the inherently dense extracellular matrix may act to keep apoptotic bodies bound to the tissue. The prolonged presence of these bodies has been suggested to perpetuate a degradative cascade, as is seen in osteoarthritis [38, 39]. In contrast, the less dense matrix of engineered cartilage may facilitate the removal of dead cells and apoptotic bodies; therefore, injured constructs, even in the most severe cases, do not exhibit continual cell death at 50 days post injury (Figure 5c).
We note that constructs having Young’s modulus of approximately 300 kPa failing at 40g of force experienced a compressive stress of 0.03MPa, much lower than what is reported in vitro for cartilage [40], but with perhaps similar failure strain levels [41, 42]. We attribute this disparity as a consequence of the low collagen content of our engineered cartilage as well as the unconfined compression experimental injury setup, which may exacerbate the latter by permitting free radial tissue expansion during axial compressive loading. Whether the current tissues could survive undamaged in a living joint is a complex question and would depend on the location of the focal defect on the joint being treated and its dimensions relative to intact articulating surface, as the surrounding tissue (around the defect) would shoulder a portion of the joint loads depending on the contact geometry. Tissues grown in our laboratory with native Young’s modulus have survived in focal defects (4 mm diameter) created in the canine stifle joint (trochlear groove) for 12 weeks [43]. As the tissue construct gets increasingly larger, such as for replacing an entire articular surface, such as for a patella [44], the loading demands would increase as the contribution from surrounding tissues diminishes.
Furthermore, unlike native cartilage, the engineered cartilage consists of a mixed population of chondrocytes isolated from full-thickness cartilage. As such, the normal zonal (cellular, biochemical, structural and mechanical) organization of cartilage is not recapitulated. Yet, it is known that zonal cell-to-cell differences may predispose certain chondrocytes to exhibit strain-induced loss of viability [45]. In the future, the injury response of constructs with specific zonal cell populations and/or with stratified engineered hydrogel layers may need to be examined [46]. To the best of our knowledge, this study represents the first attempt to model and characterize injury and the subsequent response of engineered cartilage constructs under a controlled loading environment. Unlike native cartilage explants, engineered cartilage possesses the ability to heal and repair with further time in culture as long as the bulk structural makeup of the construct is left intact. The results of this study begin to characterize the conditions under which mechanical failure occurs and provide greater insight to the behavior and response of engineered cartilage tissue grafts to mechanical insult.
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Institute of Arthritis, Musculoskeletal and Skin Diseases of the U.S. National Institutes of Health (AR46568, CTH) and by a National Science Foundation Graduate Fellowship (ART).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST The authors certify that there is no conflict of interest related to the work presented in this manuscript.
AUTHOR CONTRIBUTIONS All authors contributed to the conception and design of the study, collection and analysis of data, drafting and revising the manuscript, and gave final approval of this submitted work.
REFERENCES
- 1.Tew SR, Kwan AP, Hann A, Thomson BM, Archer CW. The reactions of articular cartilage to experimental wounding: role of apoptosis. Arthritis Rheum. 2000;43:215–225. doi: 10.1002/1529-0131(200001)43:1<215::AID-ANR26>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 2.Chen C-T, Burton-Wurster N, Borden C, Hueffer K, Bloom SE, Lust G. Chondrocyte necrosis and apoptosis in impact damaged articular cartilage. J. Orthop. Res. 2001;19:703–711. doi: 10.1016/S0736-0266(00)00066-8. [DOI] [PubMed] [Google Scholar]
- 3.Quinn TM, Allen RG, Schalet BJ, Perumbuli P, Hunziker EB. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: effects of strain rate and peak stress. J. Orthop. Res. 2001;19:242–249. doi: 10.1016/S0736-0266(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 4.Chen C-T, Burton-Wurster N, Lust G, Bank RA, Tekoppele JM. Compositional and metabolic changes in damaged cartilage are peak-stress, stress-rate, and loading-duration dependent. J. Orthop. Res. 1999;17:870–879. doi: 10.1002/jor.1100170612. [DOI] [PubMed] [Google Scholar]
- 5.Loening AM, James IE, Levenston ME, Badger AM, Frank EH, Kurz B, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch. Biochem. Biophys. 2000;381:205–212. doi: 10.1006/abbi.2000.1988. [DOI] [PubMed] [Google Scholar]
- 6.Patwari P, Fay J, Cook MN, Badger AM, Kerin AJ, Lark MW, et al. In vitro models for investigation of the effects of acute mechanical injury on cartilage. Clin Orthop Relat Res. 2001:S61–71. doi: 10.1097/00003086-200110001-00007. [DOI] [PubMed] [Google Scholar]
- 7.Costouros JG, Kim HT. Preventing chondrocyte programmed cell death caused by iatrogenic injury. Knee. 2007;14:107–111. doi: 10.1016/j.knee.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Amin AK, Huntley JS, Bush PG, Simpson AH, Hall AC. Osmolarity influences chondrocyte death in wounded articular cartilage. The Journal of bone and joint surgery American volume. 2008;90:1531–1542. doi: 10.2106/JBJS.G.00857. [DOI] [PubMed] [Google Scholar]
- 9.Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927–934. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 10.Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF B3. Osteoarthritis Cartilage. 2007;15:1025–1033. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly TA, Ng K, Wang CC, Ateshian G, Hung C. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. Journal of biomechanics. 2006;39:1489–1497. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 13.Stegeman H, Stalder K. Determination of hydroxyproline. Clin. Chim. Acta. 1967;19:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 14.Orr TE, Patel AM, Wong B, Hatzigiannis GP, Minas T, Spector M. Attachment of periosteal grafts to articular cartilage with fibrin sealent. J. Biomed. Mater. Res. 1999;44:308–313. doi: 10.1002/(sici)1097-4636(19990305)44:3<308::aid-jbm9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Caplan AI, Goto T, Wakitani S, Pineda SJ, Haynesworth SE, Goldbery VM. Cell-based technologies for cartilage repair. In: Finerman GAM, Noyes FR, editors. Biology and Biomechanics of the Traumatized Synovial Joint: The Knee as a Model. AAOS; Rosemont: 1993. pp. 111–122. [Google Scholar]
- 16.Kawamura S, Wakitani S, Kimura T, Maeda A, Caplan AI, Shino K, et al. Articular cartilage repair. Rabbit experiments with a collagen gel-biomatrix and chondrocytes cultured in it. Acta. Orthop. Scand. 1998;69:56–62. doi: 10.3109/17453679809002358. [DOI] [PubMed] [Google Scholar]
- 17.Wakitani S, Goto T, Young RG, Mansour JM, Goldberg VM, Caplan AI. Repair of large full-thickness articular cartilage defects with allograft articular chondrocytes embedded in a collagen gel. Tissue Eng. 1998;4:429–444. doi: 10.1089/ten.1998.4.429. [DOI] [PubMed] [Google Scholar]
- 18.Caplan AI, Elyaderani M, Mochizuki Y, Wakitani S, Goldberg VM. Principles of cartilage repair and regeneration. Clin. Orthop. 1997;342:254–269. [PubMed] [Google Scholar]
- 19.Gao J, Dennis JE, Solchaga LA, Goldberg VM, Caplan AI. Repair of osteochondral defect with tissue-engineered two-phase composite material of injectable calcium phosphae and hyaluronan sponge. Tissue Eng. 2002;5:827–837. doi: 10.1089/10763270260424187. [DOI] [PubMed] [Google Scholar]
- 20.Bian L, Lima EG, Angione SL, Ng KW, Williams DY, Xu D, et al. Mechanical and biochemical characterization of cartilage explants in serum-free culture. J Biomech. 2008;41:1153–1159. doi: 10.1016/j.jbiomech.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kühn K, D’Lima DD, Hashimoto S, Lotz M. Cell death in cartilage. Osteoarthr Cartil. 2004;12:1–16. doi: 10.1016/j.joca.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Lewis JL, Deloria LB, Oven-Tiesma M, Thompson RC, Jr., Ericson M, Oegema TR., Jr. Cell death after cartilage impact occurs around matrix cracks. J. Orthop. Res. 2003;21:881–887. doi: 10.1016/S0736-0266(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 23.Lee DA, Bader DL. The development and characterization of an in vitro system to study strain-induced cell deformation in isolated chondrocytes. In Vitro Cell Dev Biol Anim. 1995;31:828–835. doi: 10.1007/BF02634565. [DOI] [PubMed] [Google Scholar]
- 24.Huang AH, Yeger-McKeever M, Stein A, Mauck R. Tensile properties of engineered cartilage formed from chondrocyte- and MSC-laden hydrogels. Osteoarthr Cartil. 2008 doi: 10.1016/j.joca.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinn TM, Schmid P, Hunziker EB, Grodzinsky AJ. Proteoglycan deposition around chondrocytes in agarose culture: construction of a physical and biological interface for mechanotransduction in cartilage. Biorheology. 2002;39:27–37. [PubMed] [Google Scholar]
- 26.Bian L, Angione SL, Ng KW, Lima EG, Williams DY, Mao DQ, et al. Influence of decreasing nutrient path length on the development of engineered cartilage. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meachim G. The Effect of Scarification on Articular Cartilage. Journal of Bone and Joint Surgery. 1963;45B:150–161. [Google Scholar]
- 28.Atkinson TS, Haut RC, Altiero NJ. Impact-induced fissuring of articular cartilage: an investigation of failure criteria. J. Biomech. Eng. 1998;120:181–187. doi: 10.1115/1.2798300. [DOI] [PubMed] [Google Scholar]
- 29.Lewis JL, Deloria LB, Oyen-Tiesma M, Thompson RC, Jr., Ericson M, Oegema TR., Jr. Cell death after cartilage impact occurs around matrix cracks. J Orthop Res. 2003;21:881–887. doi: 10.1016/S0736-0266(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 30.Stockwell RA. Biology of cartilage cells. Cambridge University Press; Cambridge ; New York: 1979. [Google Scholar]
- 31.Amin AK, Huntley JS, Bush PG, Simpson AH, Hall AC. Chondrocyte death in mechanically injured articular cartilage--the influence of extracellular calcium. J Orthop Res. 2009;27:778–784. doi: 10.1002/jor.20809. [DOI] [PubMed] [Google Scholar]
- 32.Tew S, Redman S, Kwan A, Walker E, Khan I, Dowthwaite G, et al. Differences in repair responses between immature and mature cartilage. Clin Orthop Relat Res. 2001:S142–152. doi: 10.1097/00003086-200110001-00014. [DOI] [PubMed] [Google Scholar]
- 33.Wang CC-B, Guo XE, Sun D, Mow VC, Ateshian GA, Hung CT. The functional environment of chondrocytes within cartilage subjected to compressive loading: theoretical and experimental approach. Biorheology. 2002;39:39–45. [PubMed] [Google Scholar]
- 34.Torzilli PA, Deng XH, Ramcharan M. Effect of compressive strain on cell viability in statically loaded articular cartilage. Biomech. Model. Mechanobiol. 2006 doi: 10.1007/s10237-006-0030-5. [DOI] [PubMed] [Google Scholar]
- 35.Chahine NO, Wang CC, Hung CT, Ateshian GA. Anisotropic strain-dependent material properties of bovine articular cartilage in the transitional range from tension to compression. J Biomech. 2004;37:1251–1261. doi: 10.1016/j.jbiomech.2003.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levin A, Burton-Wurster N, Chen C-T, Lust G. Intercellular signaling as a cause of cell death in cyclically impacted cartilage explants. Osteoarthritis Cartilage. 2001;9:702–711. doi: 10.1053/joca.2001.0467. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto S, Ochs RL, Rosen F, Quach J, McCabe G, Solan J, et al. Chondrocyte-derived apoptotic bodies and calcification of articular cartilage. Proc Natl Acad Sci U S A. 1998;95:3094–3099. doi: 10.1073/pnas.95.6.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kouri JB, Aguilera JM, Reyes J, Lozoya KA, Gonzalez S. Apoptotic chondrocytes from osteoarthrotic human articular cartilage and abnormal calcification of subchondral bone. J Rheumatol. 2000;27:1005–1019. [PubMed] [Google Scholar]
- 39.Blanco FJ, Guitian R, Vazquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 40.Milentijevic D, Torzilli PA. Influence of strain magnitude on water loss and chondrocyte viability in impacted cartilage. Adv. Bioeng. 2001;BED-50:781–782. doi: 10.1115/1.1610021. [DOI] [PubMed] [Google Scholar]
- 41.Ewers BJ, Dvoracek-Driksna D, Orth MW, Haut RC. The extent of matrix damage and chondrocyte death in mechanically traumatized articular cartilage explants depends on rate of loading. J Orthop Res. 2001;19:779–784. doi: 10.1016/S0736-0266(01)00006-7. [DOI] [PubMed] [Google Scholar]
- 42.Flachsmann R, Broom ND, Hardy AE. Deformation and rupture of the articular surface under dynamic and static compression. J Orthop Res. 2001;19:1131–1139. doi: 10.1016/S0736-0266(01)00049-3. [DOI] [PubMed] [Google Scholar]
- 43.Ng KW, Lima EG, Bian L, O’Conor CJ, Jayabalan PS, Stoker AM, et al. Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: a canine model. Tissue Eng Part A. 16:1041–1051. doi: 10.1089/ten.tea.2009.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hung CT, Lima EG, Mauck RL, Takai E, LeRoux MA, Lu HH, et al. Anatomically shaped osteochondral constructs for articular cartilage repair. J. Biomech. 2003;36(12):1853–1864. doi: 10.1016/s0021-9290(03)00213-6. [DOI] [PubMed] [Google Scholar]
- 45.Chahine NO, Ateshian GA, Hung CT. The effect of finite compressive strain on chondrocyte viability in statically loaded bovine articular cartilage. Biomech. Model. Mechanobiol. 2007;6:103–111. doi: 10.1007/s10237-006-0041-2. [DOI] [PubMed] [Google Scholar]
- 46.Ng KW, Wang CC-B, Mauck RL, Kelly TN, Chahine NO, Costa KD, et al. A layered agarose approach to fabricate depth-dependent inhomogeneity in chondrocyte-seeded constructs. J. Orthop. Res. 2005;23:134–141. doi: 10.1016/j.orthres.2004.05.015. [DOI] [PubMed] [Google Scholar]