Abstract
Although epidemiologic data link biomarkers of cardiovascular risk with incident and prevalent coronary artery disease, exact anatomic relationships between biomarkers and coronary atherosclerosis as measured by coronary CT angiography remain unclear. Patients with acute chest pain who ultimately had no evidence of acute coronary syndrome underwent contrast-enhanced 64-slice coronary CT angiography to determine presence, extent and composition of coronary atherosclerotic plaque. We determined the differences in levels of blood biomarkers measured at the time of the CT scan between different CT-based atherosclerotic plaque groups. Among 313 patients (mean age: 51.6 ± 11 years, 62% male) high-sensitivity C-reactive protein (hs-CRP) and matrix metalloproteinase-2 were associated with the extent of calcified plaque (P = 0.03 and P<0.001), while hs-CRP and apolipoprotein A1 were associated with the extent of non-calcified plaque (P = 0.03 and P = 0.004; respectively). Despite a generally lower risk profile, subjects with exclusively non-calcified plaque had significantly higher levels of hs-CRP and oxidized low-density lipoprotein (P = 0.01 and P = 0.03; respectively) and lower levels of adiponectin (P = 0.03) when compared to subjects with calcified plaque (n = 130, 42%). Biomarkers reflecting inflammation, vascular remodeling, oxidation, and lipoprotein metabolism maybe associated with different patterns of coronary atherosclerosis as quantified by coronary CT angiography.
Keywords: Biomarkers, Atherosclerosis, Cardiac CT, Imaging, Coronary artery disease
Introduction
A large number of blood biomarkers involved in inflammation, oxidation, and lipid metabolism have been demonstrated to play an important role in atherogenesis [1]. Several of those biomarkers such as high-sensitivity C-reactive protein (hsCRP), tumor necrosis factor alpha (TNF), and oxidized low density lipoprotein (ox-LDL) have also been shown to be independent predictors of cardiovascular events [2].
With advances in imaging of coronary atherosclerosis comes the opportunity to better understand the link(s) between biomarkers predictive of disease presence and complication with the anatomic diagnosis of coronary atherosclerosis. Similarly, as various forms of coronary atherosclerosis, including the presence and extent of coronary artery calcification (CAC) variably predicts cardiovascular events independent of traditional risk profile [3], an opportunity to better understand plaque biology (as detected with computed tomography imaging) using biomarkers is also present. Moreover, with advances in contrast-enhanced computed tomography angiography (CTA), an opportunity to not only evaluate CAC but also detect and quantify non-calcified plaque components is now possible.
As it remained unclear which systemic biomarkers covering different areas of atherogenesis are most closely associated with the extent of atherosclerosis and composition of coronary atherosclerotic plaque, we decided to examine this question in detail, measuring a wide array of biomarkers in patients with detailed CTA examination. Our hypothesis was that there is a correlation between systemic and local markers of atherosclerosis.
Methods
Study sample
This analysis was performed in patients from the “Rule Out Myocardial Infarction Using Computer Assisted Tomography” (ROMICAT) study, designed to assess the clinical utility of coronary CTA to triage subjects with acute chest pain in the emergency department [4]. We included consecutive adult subjects presenting to the ED with acute chest pain but without diagnostic electrocardiographic (ECG) changes or positive initial cardiac biomarkers (troponin and CK-MB) but excluded subjects who developed an acute coronary syndrome (ACS, as defined by ST-elevation myocardial infarction [MI], non-ST-elevation MI, or unstable angina) and subjects with inflammatory diseases for this study. All patients underwent coronary CTA and blood sampling before hospital admission. The institutional review board of the Massachusetts General Hospital approved the study. All participants provided written informed consent.
Blood sampling
Blood samples were collected within 1 h prior to the coronary CTA scan and stored at −70°C until analysis. We selected a representative set of serum biomarkers covering aspects of atherogenesis: inflammation, oxidation, and lipid metabolism (Table 2). All measurements were performed in an independent laboratory (Biomarker Laboratory at the Department of Cardiology, University of Ulm, Germany) blinded to the patients clinical and CT findings. Concentrations of hs-CRP, lipoprotein (Lp)(a), and Apo A1 were measured nephelometrically on a BN II analyzer (Dade-Behring, Marburg, Germany). Enzyme-linked immunosorbent assays (ELISA) from R&D Systems (Wiesbaden; Germany) were used to measure TNF-α, MMP-2, and adiponectin. An ELISA was also used to determine levels of MPO (Immundiagnostik, Bensheim, Germany), Lp-PLA2 (PLAC, Diadexus, Inc. South San Francisco, CA), and ox-LDL (Mercodia, Uppsala, Sweden). Inter-assay coefficients of variation were<12% for ELISAs, and<5% for nephelometry and for chemoluminescence.
Table 2.
Measured concentration of blood biomarkers in 313 patients undergoing coronary CT angiography stratified by atherosclerotic plaque group
| Variables | Overall cohort | Subjects with |
P value | ||
|---|---|---|---|---|---|
| N | 313 | No plaque | Exclusively non-calcified plaque | Any calcified plaque | |
| 168 | 15 | 130 | |||
| hs-CRP (mg/L) | 1.35 [0.59; 2.88] | 1.05 [0.4; 2.4] | 2.68 [1.4; 8.4] | 1.59 [0.8; 3.1] | < 0.0001 |
| TNF-α (pg/mL) | 1.05 [0.67; 1.86] | 1.0 [0.6; 1.8] | 1.56 [0.7; 2.5] | 1.05 [0.7; 1.9] | 0.37 |
| MMP-2 (ng/mL) | 161.0 [139.0; 186.0] | 1.57 [135; 176] | 156 [138; 172] | 171 [147; 199] | 0.1 |
| Oxidized LDL (U/mL) | 67.7 [54.9; 85.4] | 68.05 [54.5; 86.3] | 76.1 [67.4; 91.5] | 65.6 [53.4; 84.5] | 0.01 |
| MPO (U/ML) | 19.7 [13.6; 35.1] | 18.7 [13.1; 32.4] | 36.0 [12.8; 57.2] | 21.1 [13.8; 36.1] | 0.21 |
| Lp-PLA2 (ng/mL) | 171.0 [142.0; 201.0] | 174 [144; 205] | 166 [124; 205] | 166 [140; 196] | 0.42 |
| ApoA1 (g/L) | 1.52 [1.28; 1.77] | 1.57 [1.3; 1.8] | 1.36 [1.1; 1,5] | 1.48 [1.3; 1.8] | 0.07 |
| ApoB (g/L) | 0.91 [0.77; 1.09] | 0.92 [0.8; 1.1] | 1.1 [0.8; 1.2] | 0.89 [0.7; 1.1] | 0.21 |
| Adiponectin (μg/mL) | 4.76 [2.70; 7.52] | 3.36 [2.2; 5.0] | 4.6 [2.9; 7.5] | 5.2 [2.7; 8.3] | 0.06 |
| LP(a) (ng/mL) | 0.12 [0.04; 0.30] | 0.09 [0.03; 0.33] | 0.12 [0.04; 0.3] | 0.12 [0.04; 0.3] | 0.83 |
Data are provided as medians with [interquartile range]
hs-CRP high-sensitivity C-reactive protein, TNF-α tumor necrosis factor alpha, MMP-2 matrix metalloproteinase 2, oxidized LDL oxidized low-density lipoprotein, MPO myeloperoxidase, Lp-PLA2 lipoprotein-associated phospolipase A2, ApoA1 apolipoprotein A1, ApoB apolipoprotein B, Lp(a) lipoprotein (a)
Coronary CT angiography
All subjects underwent ECG-gated contrast enhanced 64-slice coronary CTA (Sensation 64, Siemens Medical Solutions, Forchheim, Germany) with image acquisition during an inspiratory breath-hold. Pre-scan sublingual nitroglycerin and intravenous beta-blockers were given for all subjects with a heart rate greater than 60 beats per minute and no contraindications present.
CT scan parameters included: 32 × 0.6 mm slice collimation, gantry rotation time of 330 ms, tube voltage of 120 kV, effective tube current of 850–950 mAs, and ECG correlated tube current modulation (when appropriate). The average radiation dose of 14.8 ± 3.8 mSv was estimated based on the measured dose length product (DLP) multiplied by the conversion factor 0.017 mSv mGy−1 cm−1. A total of 64 overlapping 0.6 slices per rotation were acquired with the use of a focal spot periodically moving in the longitudinal direction. Contrast agent (Iodhexodol 320 g/cm3, Visipaque, General Electrics Healthcare, Princeton, NJ) was injected intravenously at a rate of 5 mL per second (mean volume 78 ± 11 mL) with use of a test bolus.
Overlapping transaxial images were reconstructed using a medium sharp convolution kernel (B25f) with an image matrix of 512 × 512 pixels, slice thickness and increment of 0.75/0.4 mm using an ECG gated half-scan algorithm with a resulting temporal resolution 165 ms in the center of rotation. Image reconstruction was retrospectively gated to the ECG. The position of the reconstruction window within the cardiac cycle was individually optimized to minimize motion artifacts.
Coronary CTA analysis
On average, three data sets per subject were reconstructed. Reconstructed CTA data sets of all subjects were transferred to an offline workstation (Leonardo, Siemens Medical Solutions, Forchheim, Germany) and the reconstruction with the highest image quality was used for further analysis. CTA data sets were interpreted for the presence, extent, and composition of coronary atherosclerotic plaque on a segmental level using the modified American Heart Association classification (with 17-coronary segments) [5].
Non-calcified plaque (NCAP) was defined as any clearly discernible structure that could be assigned to the coronary artery wall in at least two independent image planes and had a CT density less than 130 HU but greater than the surrounding connective tissue. Calcified atherosclerotic plaque was defined as any structure with a density of 130 HU or more that could be visualized separately from the contrast-enhanced coronary lumen (because its density was above the contrast-enhanced lumen), that could be assigned to the coronary artery wall, and that could be identified in at least two independent planes. Mixed coronary atherosclerotic plaque was defined as the presence of non-calcified and calcified plaque, either because it was “embedded” within non-calcified plaque or adjacent to each other within a coronary segment. All plaque components were assessed on a per segment basis. This approach has previously been validated [6] and shown to have excellent inter-observer agreement on a per subject and per segment level (Cohen’s κ = 0.92 and 0.81, respectively) [7].
Covariates and risk factor assessment
Presence of cardiovascular risk factors was established from measurements obtained during the index hospitalization. Hypertension was defined as systolic blood pressure of at least 140 mmHg or diastolic blood pressure of at least 90 mmHg or current antihypertensive treatment. Diabetes mellitus was defined as a fasting plasma glucose ≥126 mg/dL or treatment with a hypoglycemic agent. Hyperlipidemia was defined as total cholesterol of ≥200 mg/dL or treatment with a lipid lowering agent. Subjects were classified as smokers if they had smoked at least one cigarette per day in the year prior to the study. Body-mass index (BMI) was defined as weight (kilograms) divided by the height (meters) squared. Family history (Fx) of coronary artery disease (CAD) was defined as having a first-degree female (<65 years) or male (<55 years) relative with a documented history of MI or sudden cardiac death. Subjects were classified as high-risk (>20%), intermediate (10–20%), or low-risk (<10%) according to their Framingham Risk Score (FRS) [8].
Statistical analysis
Descriptive characteristics for all variables (demographics/risk factors, blood biomarkers, and plaque findings) are expressed as means ± SD for normally distributed continuous variables, medians with interquartile range (IQR) for non-normally distributed continuous variables, and percentages for categorical variables. Differences regarding demographics, cardiovascular risk factors and drug medication between subjects with no plaque, with exclusively non-calcified plaque, and with calcified plaque were tested using Kruskal–Wallis test for continuous variables and Fisher’s Exact test for categorical variables. We performed two non-parametric exploratory analyses. First we compared median concentrations of each biomarker in subjects with exclusively non-calcified to subjects with no plaque or with calcified plaque using Wilcoxon Rank Sum test. Biomarkers with significant differences were included in multivariate analysis using quantile regression. A simplex algorithm as described by Barrodale and Roberts [9] has been used to estimate adjusted median, upper and lower quantiles for a 52 year old male subject with none of the included cardiovascular risk factors or drug treatments. The 95% confidence intervals (95% CI) for the differences in biomarkers concentrations between the above described subgroups (no plaque, exclusively non-calcified plaque, calcified plaque) were computed by inverting the rank score test. If the 95% CI excluded zero, the difference was considered as significant with a P<0.05. Two different models were fitted. One adjusted for age, gender, and number of cardiovascular risk factors. The second model included additionally all potential confounders, which were different with P<0.10 between patients in univariate analysis.
Second, we estimated the association between levels of biomarkers and the extent of plaque (overall plaque burden, burden of non-calcified plaque, and burden calcified plaque) using Spearman’s rank correlation. In multivariate analysis, these correlations were adjusted using partial Spearman’s rank correlation similar to the approach described above.
All analyses were performed using SAS (Version 9.1, SAS Institute Inc., Cary, NC, USA) and a two sided p-value of <0.05 was considered statistically significant.
Results
From 412 subjects initially enrolled in the study, we excluded 99 subjects due to the occurrence of an ACS during index hospitalization (n = 38), other inflammatory processes (n = 30), prior history of CAD (n = 17), incomplete CTA scanning (n = 10), and four subjects who did not consent to a blood draw (n = 4). Thus, our study cohort consisted of 313 patients (mean age: 51.6 ± 11 years, 62% male; Table 1).
Table 1.
Main characteristics of 313 patients who underwent blood sampling for measurement of a selected set of blood biomarkers and 64-slice cardiac computed tomography for the assessment of coronary atherosclerotic plaque burden
| Variables | Overall cohort | Subjects with |
P-value | ||
|---|---|---|---|---|---|
| N | 313 | No plaque | Exclusively non-calcified plaque | Any calcified plaque | |
| 168 | 15 | 130 | |||
| Age, years | 51.6 ± 11.27 | 46.8 ± 9.0 | 48.7 ± 5.0 | 58.2 ± 11.2 | < 0.0001 |
| Gender male | 194 (62.0%) | 96 (57.1%) | 11 (73.3%) | 87 (66.9%) | 0.15 |
| Cardiovascular risk factors | |||||
| Number of risk factors | 1.4 ± 1.2 | 1.1 ± 1.0 | 1.3 ± 1.1 | 1.9 ± 1.2 | < 0.0001 |
| Diabetes mellitus | 33 (10.5%) | 12 (7.1%) | 2 (13.3%) | 19 (14.6%) | 0.08 |
| Family history of CAD | 72 (23.0%) | 36 (21.4%) | 3 (20.0%) | 33 (25.4%) | 0.72 |
| Hypertension | 114 (36.4%) | 41 (24.4%) | 4 (26.7%) | 69 (53.1%) | < 0.0001 |
| Hyperlipidemia | 105 (33.7%) | 34 (20.1%) | 8 (53.3%) | 63 (48.5%) | < 0.0001 |
| Obesity (BMI ≥ 30) | 105 (33.7%) | 51 (30.5%) | 8 (53.3%) | 46 (35.4%) | 0.16 |
| Smoking | 151 (48.2%) | 67 (39.9%) | 6 (40.4%) | 78 (60.0%) | 0.002 |
| Medication | |||||
| Statin use | 80 (25.6%) | 26 (15.5%) | 4 (26.7%) | 50 (38.5%) | < 0.0001 |
| Beta blocker use | 61 (19.5%) | 24 (14.3%) | 2 (13.3%) | 35 (26.9%) | 0.02 |
| ACE inhibitor use | 46 (14.7%) | 15 (8.9%) | 2 (13.3%) | 29 (22.3%) | 0.004 |
Data are provided as mean ± standard deviation or n (%). The P-value compared the distribution of the variables across subjects with no plaque, exclusively non-calcified plaque and any calcified plaque
CAD coronary artery disease, FRS Framingham risk score
Atherosclerotic plaque burden
Based on coronary CTA, 145 (46%) subjects had any coronary atherosclerotic plaque and 168 (54%) subjects had no plaque; 5.1% had a significant coronary stenosis (n = 16). Among subjects with CAD, 15 (10%) had exclusively non-calcified plaque and 130 (90%) had any calcified plaque. Baseline demographics and cardiovascular risk factors and levels of blood biomarkers stratified by atherosclerotic plaque group are detailed in Table 1 and 2; respectively. In patients with CAD, 4.25 ± 3.49 (range 1–16) segments contained any plaque. On average, non-calcified and calcified plaque were detected in 1.76 ± 2.15 (range 1–14) and 3.71 ± 3.49 (range 1–15) coronary segments; respectively.
Blood biomarkers stratified by presence and composition of coronary atherosclerotic plaque
Subjects with exclusively non-calcified plaque had significantly higher levels of hs-CRP (2.68 [1.44, 8.42] vs. 1.05 [0.41, 2.40], P = 0.0005; Fig. 1a), and a trend towards higher concentrations of ox-LDL (76.1 [67.4, 91.5] vs. 68.1 [54.5, 86.3], P = 0.07; Fig. 1b) as compared to subjects without plaque. Further, such patients had lower levels of Adiponectin (3.36 [2.16, 4.95] vs. 4.62 [2.90, 7.57]; P = 0.03; Fig. 1c) as compared to subjects without plaque. However, there was no difference in age, gender, or the number of cardiovascular risk factors between subjects with non-calcified plaque and subjects without plaque (48.7 ± 5 vs. 46.7 ± 9 years, P = 0.27; 73 vs. 57% male, P = 0.28; and 0.83 vs. 0.9; P = 0.7; respectively).
Fig. 1.
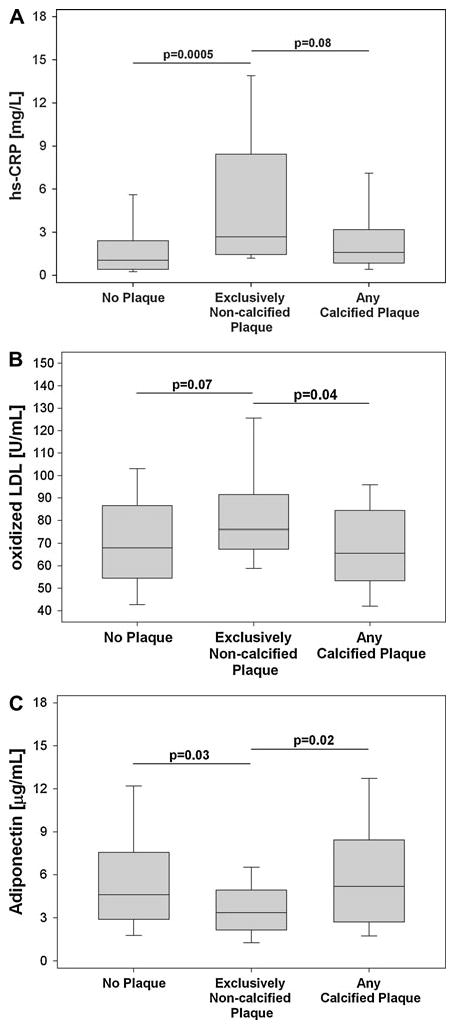
Box-and Whisker Plots showing differences in levels of a high-sensitivity C-reactive protein (hs-CRP), b oxidized low density lipoprotein (oxidized LDL), and c Adiponectin between subjects without plaque, subjects with non-calcified plaque, and subjects with calcified plaque. The Whisker was defined as 1.5-times of the interquartile range, if all outliers were within this range, the Whisker stopped at the minimal/maximal point
In comparison to subjects with calcified plaque, subjects with exclusively non-calcified plaque had a trend towards higher levels of hs-CRP (2.68 [1.44, 8.42] vs. 2.04 [0.95, 3.58]; P = 0.08; Fig. 1a), higher ox-LDL (76.1 [67.4, 91.5] vs. 63.7 [47.1, 83.7]; P = 0.04; Fig. 1b) and lower levels of adiponectin (3.36 [2.16, 4.95] vs. 5.55 [3.30, 9.77], P = 0.02; Fig. 1c). Notably, subjects with non-calcified plaque were significantly younger and had fewer cardiovascular risk factors than subjects with calcified plaque (48.7 ± 5 vs. 58.2 ± 11 years, P<0.001 and 0.9 vs. 1.7 cardiovascular risk factors, P = 0.03; respectively) and did not differ with respect to gender (73 vs. 67% males, P = 0.77; respectively).
No significant differences were found for levels of hs-CRP, ox-LDL, or Adiponectin between subjects with mixed plaque as compared to subjects with exclusively calcified plaque (all P>0.30).
In multivariate analysis subjects with exclusively non-calcified plaque had significant higher levels of hs-CRP and lower levels of Adiponectin, as compared with subjects with no plaque or calcified plaque after adjustment age, gender, and number of cardiovascular risk factors (all P<0.05). This association remained significant after adjustment for all potential confounders (Table 3). In contrast, after adjustment, the association between exclusively non-calcified plaque and higher levels of ox-LDL was attenuated (Table 3).
Table 3.
Estimated concentrations of biomarkers stratified by atherosclerotic plaque types
| hs-CRP [mg/L] |
oxidized LDL [U/mL] |
Adiponectin [μg/mL] |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | P value | Median | IQR | P value | Median | IQR | P value | |
| Adjusted for age, gender, CVRF | |||||||||
| No plaque | 0.75 | 0.25–1.41 | < 0.05 | 67.32 | 53.86–87.92 | NS | 4.83 | 3.26–6.41 | < 0.05 |
| Exclusively non-calcified plaque | 1.91 | 1.36–7.90 | -ref- | 75.15 | 62.08–89.93 | -ref- | 3.54 | 2.46–3.94 | -ref- |
| Any calcified plaque | 0.90 | 0.61–1.66 | < 0.05 | 64.41 | 51.52–85.03 | NS | 5.49 | 3.10–7.66 | < 0.05 |
| Adjusted for age, gender, diabetes, HTN, HLP, smoking, medicationa | |||||||||
| No plaque | 0.78 | 0.32–1.76 | < 0.05 | 69.76 | 54.35–84.90 | NS | 4.70 | 2.91–6.68 | < 0.05 |
| Exclusively non-calcified plaque | 1.92 | 1.33–8.52 | -ref- | 71.55 | 63.49–85.08 | -ref- | 3.52 | 2.45–4.43 | -ref- |
| Any calcified plaque | 0.96 | 0.58–1.76 | < 0.05 | 63.40 | 48.36–77.67 | NS | 5.30 | 3.00–8.19 | < 0.05 |
Levels were adjusted in two different models, the first included age, gender and number of cardiovascular risk factors (CVRF), the second included all potential confounder. The presented median, upper and lower quartile corresponds to a 52 year old male subject with none of the included cardiovascular risk factors or drug treatments
HTN hypertension, HLP hyperlipidemia, hs-CRP high-sensitivity C-reactive protein, oxidized LDL oxidized low-density lipoprotein
Medication includes Statin, Beta-Blocker, ACE inhibitor use
Association between blood biomarkers and the extent of calcified and non-calcified plaque burden
Levels of hs-CRP and MMP-2 were positively and levels of ApoA1 were negatively correlated with the overall extent of coronary atherosclerotic burden (P = 0.001, P = 0.003 and P = 0.02; respectively; Table 4). While levels of hs-CRP were positively correlated with the extent of both, non-calcified and calcified plaque (all P = 0.03; Table 3), lower levels of ApoA1 were only correlated with non-calcified plaque (r = −0.17, P = 0.004). MMP-2 was strongly correlated to the extent of calcified plaque and demonstrated a trend towards an association to the extent of non-calcified plaque (P = 0.06). Adjusting for all potential confounders (Table 4), the correlations of hs-CRP and MMP-2 with overall extent of plaque remained significant.
Table 4.
Association between a selected set of blood markers and the extent of any, calcified and non-calcified atherosclerotic plaque burden
| Extent of any plaque |
Extent of non-calcified plaque |
Extent of calcified plaque |
||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Univariate, un-adjusted analysis | ||||||
| Hs-CRP | 0.19 | 0.001 | 0.12 | 0.03 | 0.12 | 0.03 |
| MMP-2 | 0.17 | 0.003 | 0.07 | 0.06 | 0.20 | < 0.001 |
| ApoA1 | −0.13 | 0.02 | −0.17 | 0.004 | −0.08 | 0.15 |
| Adjusted for age, gender, CVRF | ||||||
| Hs-CRP | 0.11 | 0.06 | 0.07 | 0.27 | 0.02 | 0.75 |
| MMP-2 | 0.13 | 0.03 | 0.05 | 0.37 | 0.16 | 0.007 |
| ApoA1 | −0.05 | 0.39 | −0.08 | 0.17 | 0.009 | 0.88 |
| Adjusted for age, gender, diabetes, HTN, HLP, smoking, medicationa | ||||||
| Hs-CRP | 0.12 | 0.04 | 0.08 | 0.19 | 0.03 | 0.65 |
| MMP-2 | 0.14 | 0.02 | 0.05 | 0.40 | 0.17 | 0.005 |
| ApoA1 | −0.04 | 0.49 | −0.06 | 0.29 | 0.02 | 0.79 |
CVRF number of cardiovascular risk factors, HTN hypertension, HLP hyperlipidemia, hs-CRP high-sensitivity C-reactive protein, MMP-2 matrix metalloproteinase-2, ApoA1 apolipoprotein A1
Medication includes Statin, Beta-Blocker, ACE Inhibitor use
Discussion
Coronary risk is predicted both by blood biomarkers as well as the local extent of atherosclerosis as measured by CT. However, mechanistically, it remains yet unclear why biomarkers predict risk for incident and prevalent coronary atherosclerosis events; furthermore, the link between many biomarkers and the anatomic diagnosis of coronary atherosclerosis remains tenuous. Moreover, with various forms of coronary atherosclerosis present—calcified, non-calcified, fibrous, lipid rich—the association with risk remains similarly yet explained.
In this study we determined the association between blood biomarkers of athero-and thrombogenesis with the presence, extent and composition of coronary atherosclerotic plaque as detected by coronary CTA. Overall, our results suggest that among these markers, hs-CRP, MMP-2, and ApoA1 may be associated with the extent of calcified and/or non-calcified plaque. However, more importantly, we found that a small group of subjects with exclusively non-calcified plaque may have significantly higher levels of hs-CRP and ox-LDL and lower levels of adiponectin than subjects without plaque or calcified plaque, despite that the latter group was older and had a more unfavorable cardiac risk profile.
Our results confirm earlier studies that have suggested that selected blood biomarkers of atherogenesis including inflammation, oxidation, and lipid metabolism to moderately correlate with morphologic measures of cardiovascular risk, including CAC and the degree of stenosis in invasive coronary angiography [10–14], intima-media thickness and ankle-brachial index [15], visceral abdominal and pericardial fat [16, 17]. This observation emphasizes that levels of blood biomarkers such as hs-CRP may represent systemic but not local atherothrombogenic potential [18]. It is further interesting that biomarkers associated with plaque burden do not point to a single component of atherogenesis linked to CAD burden, rather markers associated with the extent of calcified (hs-CRP and MMP-2) and non-calcified (hs-CRP and ApoA1) represent all contributing pathways to atherothrombosis [19, 20]. However, all of these associations were attenuated when adjusted for confounders. To this end, our results are in line with prior studies, which used coronary calcification or coronary angiography as surrogate markers of CAD [10–14, 20]. One explanation maybe that as a systemic marker, levels of blood biomarkers may reflect diffuse atherosclerosis involving all vascular beds while coronary atherosclerosis may not represent overall plaque burden due to its local nature [18]. This is supported by data showing stronger correlations between blood biomarker levels and subclinical extra-coronary atherosclerosis (i.e. carotid intima-media thickness or ankle brachial index) [21, 22].
We did not systematically study the association of mixed plaque, a plaque type containing calcified and non-calcified plaque components, which has shown some associations with plaque progression and future events [23, 24]. However, it was a predefined aim of the study to assess different stages of atherosclerosis as detected by CT including absence, early stage as non-calcified, and calcified plaque. Moreover, it is known from histology studies that the calcified plaque most often does have a non-calcified component [25].
An interesting and provocative finding of our study is that subjects with exclusively non-calcified plaque had generally higher levels of ox-LDL and hs-CRP and lower levels of adiponectin. This observation, although limited by the relatively small number of patients, is important as non-calcified plaque preceedes calcified plaque in the natural hisory of atherosclerosis [26], has been found highly prevalent in culprit lesions in patients with ACS [27, 28] as well as in patients with myocardial infarction but without significant epicardial stenosis. In contrast, calcified coronary plaque is thought to represent more advanced stage of the natural history of disease (and may be less prone to ACS) [25].
Research on mechanisms of atherosclerotic plaque formation has identified more than 240 biomarkers associated with CAD [1]. Both hs-CRP and ox-LDL are among the more established blood biomarkers that have been linked to the initiation and formation of early atherosclerosis [29] and cardiovascular events [30–32]. In animal models, intimal accumulation of ox-LDL occurs prior to the development of coronary plaques [33] as ox-LDL is taken up by macrophages via the scavenger receptors, promoting foam cell formation and fatty plaque development [34]. Further, in humans increased ox-LDL is strongly associated with endothelial-dependent vasomotor function [35] and can be histologically found in lipid-rich plaque [36]. Similarly, CRP appears to serve not only as a marker of the inflammatory process of atherosclerosis but also as an active partaker in all stages of atherogenesis, since it is present in atherosclerotic lesions but not in the normal vessel wall [37]. Latest research suggests that hs-CRP is an independent predictor of cardiovascular event risk [1].
Previously, Hausleiter et al. found marginal differences in levels of CRP in patients with non-calcified plaque when compared to those without plaque (1.7 vs. 1.0 mg/L, P = 0.045) [38]. Our results extend these observations and we specifically demonstrate that hs-CRP significantly elevated in patients with exclusively non-calcified plaque when compared to patients with calcified plaque, despite that these patients were older and had a less favorable cardiovascular risk profile. These findings support the central role of inflammation in initiation and formation of early atherosclerosis and generate the hypothesis that detection of non-calcified plaque may improve cardiovascular risk predicition beyond traditional risk assessment and calcified plaque detection. Clearly, long-term follow-up studies in larger populations are needed to establish a role of non-calcified plaque detection by coronary CTA in cardiovascular risk predicition.
Limitations
We acknowledge the cross-sectional nature of our study design, which precludes establishing causality. Our findings, especially in subjects with exclusively non-calcified, plaque are significantly limited by sample size, which resulted in statistical trends in some biomarker analyses. However, this is the largest study on the association of blood biomarkers and coronary plaque to date and it has become evident that subjects with exclusively non-calcified plaque present a minority (between 5 and 10%) in subjects without known CAD. Thus, our finding in subjects with exclusively non-calcified plaque is hypothesis generating and requires further confirmation in future studies. Our sample was drawn from a population of patients presenting with acute chest pain, which may limit the generalizability of our findings to asymptomatic patients. However, we excluded subjects with history of CAD and subjects with ACS from the analysis and thus all subject had acute chest pain without known origin. Also, we did not quantitatively assess the amount of plaque as prior research suggested high inter-observer variability of these measures.
Acknowledgments
The authors would like to thank Gerlinde Trischler for excellent technical assistance of the blood biomarker measurements. This work was supported by the NIH (R01 HL080053), and in part supported by Siemens Medical Solutions and General Electrics Healthcare. Dr. Januzzi is supported in part by the Balson Scholar Fund. Dr. Schlett is supported in part by the German National Merit Foundation and the ERP Scholarship.
Footnotes
Conflict of interest Dr. Januzzi has received grant support from Roche Diagnostics, Siemens Diagnostics, and Critical Diagnostics.
Contributor Information
Fabian Bamberg, Email: fbamberg@med.lmu.de, Massachusetts General Hospital, Cardiac MR PET CT Program, Harvard Medical School, Boston, MA, USA. Department of Clinical Radiology, Ludwig-Maximilians University, Klinikum Grosshadern, Marchioninistrasse 15, 81377 Munich, Germany.
Quynh A. Truong, Massachusetts General Hospital, Cardiac MR PET CT Program, Harvard Medical School, Boston, MA, USA
Wolfgang Koenig, Department of Internal Medicine Cardiology, University of Ulm Medical Center, Ulm, Germany.
Christopher L. Schlett, Massachusetts General Hospital, Cardiac MR PET CT Program, Harvard Medical School, Boston, MA, USA
Khurram Nasir, Massachusetts General Hospital, Cardiac MR PET CT Program, Harvard Medical School, Boston, MA, USA.
Javed Butler, Massachusetts General Hospital, Cardiac MR PET CT Program, Harvard Medical School, Boston, MA, USA.
Emily Kurtz, Massachusetts General Hospital, Cardiac MR PET CT Program, Harvard Medical School, Boston, MA, USA.
Konstantin Nikolaou, Department of Clinical Radiology, Ludwig-Maximilians University, Klinikum Grosshadern, Marchioninistrasse 15, 81377 Munich, Germany.
Udo Hoffmann, Massachusetts General Hospital, Cardiac MR PET CT Program, Harvard Medical School, Boston, MA, USA.
James L. Januzzi, Jr, Division of Cardiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
References
- 1.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113(19):2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 2.Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol. 2007;27(1):15–26. doi: 10.1161/01.ATV.0000251503.35795.4f. [DOI] [PubMed] [Google Scholar]
- 3.Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JA, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114 (16):1761–1791. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, Truong QA, Cury RC, Abbara S, Shapiro MD, Moloo J, Butler J, Ferencik M, Lee H, Jang IK, Parry BA, Brown DF, Udelson JE, Achenbach S, Brady TJ, Nagurney JT. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53(18):1642–1650. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease. Report of the ad hoc committee for grading of coronary artery disease, council on cardiovascular surgery, American Heart Association. Circulation. 1975;51(4 Suppl):5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 6.Achenbach S, Moselewski F, Ropers D, Ferencik M, Hoffmann U, MacNeill B, Pohle K, Baum U, Anders K, Jang I-K, Daniel WG, Brady TJ. Detection of calcified and non-calcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109(1):14–17. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann U, Nagurney JT, Bamberg F, Pena A, Ferencik M, Chae CU, Cury RC, Butler J, Abbara S, Brown DF, Manini A, Nichols JH, Achenbach S, Brady TJ. Coronary multidetector computed tomography in the assessment of patients with acute chest pain. Circulation. 2006;114(21):2251–2260. doi: 10.1161/CIRCULATIONAHA.106.634808. [DOI] [PubMed] [Google Scholar]
- 8.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 9.Barrodale I, Roberts FDK. An improved algorithm for discrete l 1 linear approximation. SIAM J Num Anal. 1973;10:839–848. [Google Scholar]
- 10.Khera A, de Lemos JA, Peshock RM, Lo HS, Stanek HG, Murphy SA, Wians FH, Jr, Grundy SM, McGuire DK. Relationship between C-reactive protein and subclinical atherosclerosis: the Dallas Heart Study. Circulation. 2006;113(1):38–43. doi: 10.1161/CIRCULATIONAHA.105.575241. [DOI] [PubMed] [Google Scholar]
- 11.Redberg RF, Rifai N, Gee L, Ridker PM. Lack of association of C-reactive protein and coronary calcium by electron beam computed tomography in postmenopausal women: implications for coronary artery disease screening. J Am Coll Cardiol. 2000;36(1):39–43. doi: 10.1016/s0735-1097(00)00680-x. [DOI] [PubMed] [Google Scholar]
- 12.Hunt ME, O’Malley PG, Vernalis MN, Feuerstein IM, Taylor AJ. C-reactive protein is not associated with the presence or extent of calcified subclinical atherosclerosis. Am Heart J. 2001;141(2):206–210. doi: 10.1067/mhj.2001.112488. [DOI] [PubMed] [Google Scholar]
- 13.Wang TJ, Larson MG, Levy D, Benjamin EJ, Kupka MJ, Manning WJ, Clouse ME, D’Agostino RB, Wilson PW, O’Donnell CJ. C-reactive protein is associated with subclinical epicardial coronary calcification in men and women: the Framingham Heart Study. Circulation. 2002;106(10):1189–1191. doi: 10.1161/01.cir.0000032135.98011.c4. [DOI] [PubMed] [Google Scholar]
- 14.Sukhija R, Fahdi I, Garza L, Fink L, Scott M, Aude W, Pacheco R, Bursac Z, Grant A, Mehta JL. Inflammatory markers, angiographic severity of coronary artery disease, and patient outcome. Am J Cardiol. 2007;99(7):879–884. doi: 10.1016/j.amjcard.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 15.McDermott MM, Ferrucci L, Guralnik JM, Tian L, Green D, Liu K, Tan J, Liao Y, Pearce WH, Schneider JR, Ridker P, Rifai N, Hoff F, Criqui MH. Elevated levels of inflammation, d-dimer, and homocysteine are associated with adverse calf muscle characteristics and reduced calf strength in peripheral arterial disease. J Am Coll Cardiol. 2007;50(9):897–905. doi: 10.1016/j.jacc.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Jr, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O’Donnell CJ, Benjamin EJ, Fox CS. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116(11):1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 17.Djaberi R, Schuijf JD, van Werkhoven JM, Nucifora G, Jukema JW, Bax JJ. Relation of epicardial adipose tissue to coronary atherosclerosis. Am J Cardiol. 2008;102(12):1602–1607. doi: 10.1016/j.amjcard.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Khoury Z, Schwartz R, Gottlieb S, Chenzbraun A, Stern S, Keren A. Relation of coronary artery disease to atherosclerotic disease in the aorta, carotid, and femoral arteries evaluated by ultrasound. Am J Cardiol. 1997;80(11):1429–1433. doi: 10.1016/s0002-9149(97)00701-7. [DOI] [PubMed] [Google Scholar]
- 19.Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA. 2007;298(7):786–798. doi: 10.1001/jama.298.7.786. [DOI] [PubMed] [Google Scholar]
- 20.Reilly MP, Wolfe ML, Localio AR, Rader DJ. C-reactive protein and coronary artery calcification: the study of inherited risk of coronary atherosclerosis (SIRCA) Arterioscler Thromb Vasc Biol. 2003;23(10):1851–1856. doi: 10.1161/01.ATV.0000092327.60858.4A. [DOI] [PubMed] [Google Scholar]
- 21.Musicant SE, Taylor LM, Jr, Peters D, Schuff RA, Urankar R, Landry GJ, Moneta GL. Prospective evaluation of the relationship between C-reactive protein, D-dimer and progression of peripheral arterial disease. J Vasc Surg. 2006;43(4):772–780. doi: 10.1016/j.jvs.2005.12.051. (discussion 780) [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97(5):425–428. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 23.Schmid M, Achenbach S, Ropers D, Komatsu S, Ropers U, Daniel WG, Pflederer T. Assessment of changes in non-calcified atherosclerotic plaque volume in the left main and left anterior descending coronary arteries over time by 64-slice computed tomography. Am J Cardiol. 2008;101(5):579–584. doi: 10.1016/j.amjcard.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Pundziute G, Schuijf JD, Jukema JW, Boersma E, de Roos A, van der Wall EE, Bax JJ. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007;49(1):62–70. doi: 10.1016/j.jacc.2006.07.070. [DOI] [PubMed] [Google Scholar]
- 25.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, American Heart Association. Circulation. 1995;92(5):1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 26.Bamberg F, Dannemann N, Shapiro MD, Seneviratne SK, Ferencik M, Butler J, Koenig W, Nasir K, Cury RC, Tawakol A, Achenbach S, Brady TJ, Hoffmann U. Association between cardiovascular risk profiles and the presence and extent of different types of coronary atherosclerotic plaque as detected by multidetector computed tomography. Arterioscler Thromb Vasc Biol. 2008;28(3):568–574. doi: 10.1161/ATVBAHA.107.155010. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann U, Moselewski F, Nieman K, Jang IK, Ferencik M, Rahman AM, Cury RC, Abbara S, Joneidi-Jafari H, Achenbach S, Brady TJ. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol. 2006;47(8):1655–1662. doi: 10.1016/j.jacc.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 28.Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, Virmani R, Ozaki Y, Hishida H, Narula J. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50(4):319–326. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 29.Tsimikas S, Willerson JT, Ridker PM. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients. J Am Coll Cardiol. 2006;47(8 Suppl):C19–C31. doi: 10.1016/j.jacc.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 30.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 31.Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, Danesh J, Whincup PH. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006;114(7):623–629. doi: 10.1161/CIRCULATIONAHA.106.618918. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Mac-Fadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 33.Holvoet P, Theilmeier G, Shivalkar B, Flameng W, Collen D. LDL hypercholesterolemia is associated with accumulation of oxidized LDL, atherosclerotic plaque growth, and compensatory vessel enlargement in coronary arteries of miniature pigs. Arterioscler Thromb Vasc Biol. 1998;18(3):415–422. doi: 10.1161/01.atv.18.3.415. [DOI] [PubMed] [Google Scholar]
- 34.Kodama T, Freeman M, Rohrer L, Zabrecky J, Matsudaira P, Krieger M. Type I macrophage scavenger receptor contains alpha-helical and collagen-like coiled coils. Nature. 1990;343(6258):531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto T, Takashima H, Ohira N, Tarutani Y, Yasuda Y, Yamane T, Matsuo S, Horie M. Plasma level of oxidized low-density lipoprotein is an independent determinant of coronary macrovasomotor and microvasomotor responses induced by bradykinin. J Am Coll Cardiol. 2004;44(2):451–457. doi: 10.1016/j.jacc.2004.03.064. [DOI] [PubMed] [Google Scholar]
- 36.Nishi K, Itabe H, Uno M, Kitazato KT, Horiguchi H, Shinno K, Nagahiro S. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler Thromb Vasc Biol. 2002;22(10):1649–1654. doi: 10.1161/01.atv.0000033829.14012.18. [DOI] [PubMed] [Google Scholar]
- 37.Torzewski M, Rist C, Mortensen RF, Zwaka TP, Bienek M, Waltenberger J, Koenig W, Schmitz G, Hombach V, Torzewski J. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20(9):2094–2099. doi: 10.1161/01.atv.20.9.2094. [DOI] [PubMed] [Google Scholar]
- 38.Hausleiter J, Meyer T, Hadamitzky M, Kastrati A, Martinoff S, Schomig A. Prevalence of non-calcified coronary plaques by 64-slice computed tomography in patients with an intermediate risk for significant coronary artery disease. J Am Coll Cardiol. 2006;48(2):312–318. doi: 10.1016/j.jacc.2006.02.064. [DOI] [PubMed] [Google Scholar]


