Abstract
To determine whether levels of blood lead during gestation and infancy that are below the CDC action of level of 10 μg/dL affect infant growth, we studied 211 disadvantaged mother-infant pairs from Albany, NY. Mothers’ lead levels were low (2nd trimester x=2.8 μg/dL) as were infants’ (x= 3.3 μg/dL at 6 months; 6.4 μg/dL at 12 months). Multiple linear regression analyses showed that 2nd trimester lead levels were related to reduced head circumference at 6 and 12 months. Infants of mothers with 2nd trimester lead at or above the median (≥ 3 ug/dL) exhibited negative associations between blood lead and head circumference at 6 and 12 months, and with weight-for-age, weight-for-length and upper arm circumference at 6 months, but those below the median did not. Infants’ 6 month lead level was related to head circumference at 12 months in the total sample, and in the sub-sample of infants whose blood lead was above the infants’ 6 month blood lead median. Infants also were grouped by changes in their relative blood lead status, i.e., above vs. below the median, from 2nd trimester to 12 months of age. Infants whose lead levels changed from above to below the median were larger than infants whose lead levels went from below to above the median. The results suggest that lead may affect some dimensions of infant growth at levels below 10 ug/dL, but effects of lead levels less than 3 ug/dL are not evident in this sample.
Keywords: lead, children, growth, development, height, weight, head circumference
Introduction
Lead is a heavy metal that is entirely unnecessary for any physiological process, and is severely toxic at high doses (Centers for Disease Control and Prevention, 1988). Its harmful properties, especially among children, have been a concern and steps have been taken to reduce children’s lead levels. In the U.S., average blood lead levels of children have decreased markedly since 1980 (Brody et al., 1994; Pirkle et al., 1994), but children who live in impoverished communities and older, deteriorating housing still are at disproportionately high risk for elevated lead levels (Brown et al., 2000; Centers for Disease Control and Prevention, 1991; Centers for Disease Control and Prevention, 2001; LaFlash et al., 2000; Litaker et al., 2000). Concern about biological effects of lead among those children remains high and there now is concern about possible effects of the low levels of lead that characterize most children in the US and other countries (Brown and Rhoads, 2008).
Young children are particularly vulnerable to the detrimental effects of many environmental pollutants, particularly lead, due to their physiological immaturity, rate of growth and maturation, and greater exposure through physical contact and ingestion of lead-containing dust (Bearer, 2000; McCabe, 1979). Adverse effects of lead poisoning on growth were recognized several years ago (Johnson and Tenuta, 1979) when lead levels in the U.S. population were higher than they are now. Whether low lead levels impact child health and well-being is not so clear. One measure of child well-being and a traditional measure of general toxicity is physical growth. Growth is extremely rapid during the prenatal period, and in infancy. On average length doubles from birth to 12 months of age, while birth weight doubles by 5 months of age and triples by 12 months (Tanner, 1990). Head growth also is extremely rapid and reflects brain growth (Tanner, 1990). Such high rates of somatic growth during infancy are not found at any point later in life. Studies of rapidly growing dimensions allow observation of subtle impacts such as might be found from low levels of lead.
Studies of growth among children with low levels of lead have not been consistent. Several studies have reported associations between lead exposure and reduced prenatal growth (Bellinger et al., 1991; Bornschein et al., 1987; Gonzalez-Cossio et al., 1997; Schell and Stark, 1999). Lead levels have also been associated with reductions in postnatal height, weight, and/or head circumference growth (Ballew et al., 1999; Frisancho and Ryan, 1991; Kafourou et al., 1997; Rothenberg et al., 1999; Schwartz et al., 1986; Shukla et al., 1991). However, several studies have failed to observe significant effects of blood lead on growth (Huzior-Balajewicz et al., 2001), including two longitudinal studies (Greene and Ernhart, 1991; Sachs and Moel, 1989). A particularly difficult aspect of all analyses of growth and lead is controlling for the many other factors that affect physical growth such as maternal and paternal size, diet, smoking, birth order, as well as numerous socio-demographic factors, etc. and doing so with serial measurements to reflect changes in these variables, especially in diet. This problem is particularly acute in large samples where the cost of collecting such detailed data is prohibitive.
The goal of the current investigation is to determine the relationship between growth at 6 and 12 months, and prenatal and early childhood lead levels while also considering the most salient other factors that may affect growth. We take advantage of the longitudinal design to model diet and lead levels serially, and include controls for socio-demographic factors and maternal size. Growth parameters considered here are length-for-age, weight-for-age, weight-for-length, head circumference-for-age, and upper arm circumference-for-age. Of particular interest is the possible effect of very low levels of lead on growth.
Methods
The Albany Pregnancy Infancy Lead Study (APILS) is a prospective longitudinal study examining changes in lead levels through pregnancy, nutrient-lead interactions, and the effects of prenatal and early postnatal lead burden on infant growth and cognitive development (Schell et al., 2003; Schell et al., 2004). The study was conducted in two phases, the first from 1986 to 1992 that was planned as a pilot study, and the second from 1992 to 1998 (funded by NIEHS). These two phases followed similar protocols and data are combined for the current analysis. Any differences in protocols are described below, and are considered in the analysis and interpretation of findings. The Institutional Review Boards of the New York State Department of Health, Albany Medical Center (AMC), and the University at Albany reviewed and approved all data collection methods and procedures for each phase. All participating women gave their informed consent prior to data collection.
Sample recruitment and eligibility criteria.
APILS participants were mother-child pairs drawn from a population at risk for lead exposure due to poverty and residence in an urban center characterized by old, dilapidated housing adjacent to vehicular and industrial emissions. Women receiving first-time prenatal care at either the Albany County Department of Health (ACDH) Clinic or the AMC Obstetrics Clinic were invited to participate in the study. In order to be eligible for the study, each participant had to 1) be a current resident of Albany county; 2) be less than 24 weeks pregnant; 3) receive prenatal care at ACDH or AMC clinics for at least two trimesters of their pregnancy; 4) meet the eligibility requirements for the Women, Infants and Children program (WIC; < 185% of poverty level); 5) intend to deliver her infant at AMC and also receive subsequent pediatric care at ACDH or AMC; and 6) allow a cord blood sample to be collected at delivery. Women were not eligible to participate in the study if 1) their pregnancy was not a singleton pregnancy; 2) their pregnancies were considered high risk (women with high risk pregnancies were referred for specialized care); 3) they were unable to complete all interviews in English (English fluency was necessary to meet other study goals that included standardized assessment of maternal cognitive performance); or 4) they already had a child in the study.
Interviews.
Participants were interviewed once in each trimester following recruitment, and at 3, 6, 9 and 12 months during infancy. Socio-demographic data (age, education, employment, self-identified race/ethnicity) were collected by interviews with mothers upon entry into the study. An education index was calculated to assess educational attainment independently of age as in this sample many younger mothers had low educational attainment because they were young but not undereducated. (Schell et al., 2003; Schell et al., 2004). For mothers younger than 19 years (the age norm for completing secondary school), the education index equals (years of education + 6) / age; for mothers 19 years of age and older, the education index equals years of education + 6) / 18.
During each pediatric clinic visit at 3, 6, 9, and 12 months, the infant’s diet was assessed using 24-hour diet recall as reported by the mother or primary care-taker. To compute the intake of 37 macronutrients, vitamins and minerals, Nutritionist IV software (Version 2.0, Salem, Oregon) was used. Three- and 6-month nutrient intakes were used in analyses, because bivariate testing indicated stronger effects on growth than 9- and 12-month intakes. To stabilize estimates, 3- and 6-month nutrient intakes were averaged. Infants’ breastfed at both 3 and 6 months (n=23) were excluded because accurate nutrient intakes could not be calculated. For infants who were breastfed at the 3-month visit, but not at the 6-month visit (n=17), the sample average was used for the 3-month data point when computing 3- and 6-month averages.
Anthropometric measurements.
Anthropometric assessments of children were made at 3, 6, 9, and 12-month follow-up visits in both phases 1 and 2 of APILS. Standard anthropometric techniques (Cameron, 1986; Lohman et al., 1988) were utilized to measure infants’ weight (kg), crown-heel length (mm), upper arm circumference (cm), and head circumference (cm) in triplicate by two APILS staff members. The Principal Investigator (LMS) trained APILS staff members in anthropometric techniques.
Blood sampling and analysis.
In both phases, a trained phlebotomist drew blood of participating mothers and their infants using a lead-free venous blood collection kit. Mothers’ blood was drawn at each prenatal study visit (32cc total) and at delivery (16cc). Infants’ blood was drawn at delivery (3cc cord or venous), and at 6-month (6cc) and 12-month (16cc) follow-up visits at the pediatric clinic.
Phase 1 and phase 2 blood lead analyses have previously been described (Schell et al., 1997; Schell et al., 2000). Briefly, phase 1 blood lead analyses were carried out at Bender Hygienic Laboratory in Albany, NY (New York State Department of Health certified) using the anodic stripping voltametry method. Phase 2 blood lead analyses were carried out in the Wadsworth Center’s Lead Poisoning/Trace Elements Laboratory (New York State Department of Health reference laboratory) using a Perkin Elmer (Norwalk, CT) Model 4100ZL atomic absorption spectrometer equipped with a transversely-heated electrothermal atomizer (THGA) with longitudinal Zeeman background correction. This analytical method for blood lead has been fully validated and described in the literature (Parsons and Slavin, 1993).
The phase 1 analytical method had a method detection limit (MDL) of 5 μg/dL, and values below the MDL were reported as < 5 μg/dL in all blood specimens analyzed at points of blood collection from mothers and infants. Phase 1 cases with values below the MDL were not included in the present analysis. The precision of the phase 2 analytical method allowed results to be reported to within ± one tenth of a microgram per deciliter (±0.1 μg/dL), and for statistical analyses reported here, blood lead values were reported with this degree of precision. The MDL for the AAS method is approximately 1 μg/dL, (based on 3 x SD) (Parsons and Slavin, 1993). For clinical purposes, values below the MDL are reported as < 1 μg/dL.
The sample.
Of 633 women ever enrolled in APILS, 218 dropped out of the study prior to their child’s 3-month visit for a variety of reasons, including malformations at birth (n=3), pregnancy loss or termination (n=59), transferred care or moved (n=127), or refused to continue participating (n=29). Sample sizes are further reduced at specific ages due to, 1) exclusion of phase 1 lead levels below the laboratory MDL, 2) missed follow-up visits, and 3) missing lead measurements. Those who dropped out of the study prior to the 3-month visit compared to those enrolled at 3 months did not differ in mother’s second trimester lead levels, age, height, age-adjusted education, or children’s lead levels at birth, birth weight, or gestation length (t <1.96; p>0.05); only maternal second trimester cigarette smoking appeared somewhat different (p=0.083) with drop-outs smoking 2.6 cigarettes per day vs. 3.5 for those remaining.
Statistical Analysis.
Blood lead concentrations are log transformed due to non-normal distribution. Anthropometric data were age-standardized to permit combining individuals’ whose measurements were made at slightly different ages as pediatric appointments targeted specific ages (6 and 12 months), but often did not occur exactly on schedule. Epi Info™, Version 3.3 software was utilized to calculate length-for-age, weight-for-age, weight-for-length, and head circumference-for-age percentiles and z scores (3, 6, 9, 12 months), as well as upper arm circumference-for-age z scores (9, 12 months). Epi Info percentiles and z scores for length-for-age, weight-for-age, weight-for-length, and head circumference-for-age are calculated with reference to the 2000 Centers for Disease Control and Prevention (CDC) sex-specific reference curves (Kuczmarski et al., 2000). Upper arm circumference-for-age is calculated with reference to World Health Organization (WHO) data (de Onis et al., 1997; Mei et al., 1997). Percentiles at all points of measurement are utilized for descriptive purposes, while z scores at 6 and 12 months only are utilized in analyses of lead effects.
Multivariable models were created to determine the relationship between growth during the first year and prenatal lead levels (mother’s second trimester lead level) when controlling for confounders. Multivariate model covariates were chosen based alpha levels (p<0.1) of correlations (continuous variables) or t-tests (categorical variables) with lead levels and/or anthropometric measures. Candidate covariates were: infant’s sex, birthweight (kg), gestational age (weeks), and breastfeeding (coded as any vs none), as well as mother’s age (in years, or dichotomized as <19 years or ≥ 19 years), marital status (single or married), self-reported race (Black or other), height (cm), employment status (employed or not in the previous 6 months), prepregnancy BMI (kg/m2), parity (primiparous or multiparous), second trimester cigarette smoking (average # cigarettes/day), and education index. Intakes of kilocalories, protein, carbohydrates, fat, iron, calcium, zinc, and vitamins C and D were also tested and met the criteria for inclusion in regression models. Due to the high degree of intercorrelation among these dietary variables (r = 0.249 - 0.967), a principal components analysis was conducted with these 3- and 6-month average intakes. Scores from a single component (72% of the variance) with high positive loadings for all nutrients were used in regression analysis. All calculations were performed using SPSS, Version 13.0 (Chicago, Illinois). All p-values reported here are from two-tailed (non-directional) tests. In order to reduce the total number of models tested while maximizing sample size, we focused upon mothers’ second trimester lead level, the measurement with the largest number of cases.
Of covariates considered, only mother’s age in years and breastfeeding were not related to any growth z score or lead level (results of preliminary testing to identify covariates are not shown). Additionally, prepregnancy BMI was not included in the model due to the number of missing values. Both birthweight and gestation length were related to infant anthropometry in bivariate testing, and to each other (r=0.460, p<0.001). Only birthweight was included in the model because it was significantly correlated (p<0.05) with every growth z score, whereas gestation length was correlated only with length-for-age, weight-for-age, and head circumference-for-age, but not with weight-for-length or upper arm circumference-for-age. The model controls for infant’s sex, birthweight, early nutrition, and mother’s age <19, marital status, employment, self-reported race, height, parity, second trimester cigarette smoking, and education index.
In order to closely examine possible effects of very low lead levels, analyses were conducted on the entire sample and the sample stratified into two groups based on median lead levels. Analyses to determine effects of mother’s second trimester lead levels (< 3 vs. ≥ 3 μg/dL) as well as children’s 6-month (< 3 vs. ≥ 3 μg/dL) and 12-month (< 6 vs. ≥ 6 μg/dL) lead levels were run with the sample split at the median to the nearest whole number. Median lead level sample splits were also utilized to construct categorical lead change variables, from 2nd trimester to both 6 and 12 months measurements, with lead at any given point in time categorized as low or high based on its position above or below the median split.
Results
Socio-demographic characteristics and lead levels of mother-infant pairs are described in Table 1. On average mothers were in their early twenties with 26.7% younger than 19 years. Of mothers 19 years of age or older, 81.5% had completed high school. The majority of mothers did not smoke cigarettes during their second trimester (61.3%). Roughly half identified themselves as Black. The pattern for mothers’ and infants’ lead levels in the current study is in accord with previously published analyses of this sample (Schell et al., 1997), and shows the common increases associated with age and development. Average infant lead levels began close to their mothers’ average at birth, then more than tripled from delivery to 12 months. The rate of infants’ lead levels above the CDC action limit of 10 increased from 3.1% at delivery, to 11.6% at 6 months, to 20.7% at 12 months. At both 6 and 12 months, the averages for length-for-age and weight-for-age are at approximately the 50th percentile, while weight-for-length and head circumference-for-age are at approximately the 60th percentile. The average for upper arm circumference-for-age roughly corresponds to the 60th percentile. Z-scores describing the growth of APILS infants relative to sex-specific CDC 2000 (length-for-age, weight-for-age, weight-for-length, and head circumference-for-age) and WHO (upper arm circumference-for-age) reference standards are presented in Table 1.
Table 1.
Sociodemographic characteristics and lead levels of APILS mother-infant pairs participating in the study at the 3-month visit and whose phase 1 lead levels were above the laboratory MDL, Albany, NY, 1986-1998.
| N | Mean1 | SD | Min. | Max. | |
|---|---|---|---|---|---|
| Mothers | |||||
| Age (years) | 244 | 22.7 | 5.27 | 13.2 | 37.7 |
| Prepregnancy BMI (kg/m2) | 237 | 25.0 | 6.65 | 15.1 | 53.1 |
| Mother’s Height (cm) | 244 | 163.6 | 7.39 | 132.1 | 185.4 |
| Completed Education (years) | 243 | 11.3 | 1.80 | 6 | 21 |
| Education Index2 | 243 | 1.0 | 0.09 | 0.7 | 1.5 |
| Second Trimester Smoking (#/day) | 243 | 3.6 | 6.60 | 0 | 40 |
| 2nd Trimester Blood Lead (μg/dL) | 244 | 2.8 | 2.63 | 0.4 | 13 |
| 3rd Trimester Blood Lead (μg/dL) | 201 | 2.6 | 2.23 | 0.3 | 10 |
| Delivery Blood Lead (μg/dL) | 184 | 2.8 | 2.37 | 0.6 | 15 |
| Infants | |||||
| Gestation Age (weeks) | 244 | 39.3 | 1.91 | 32 | 45 |
| Birthweight (g) | 244 | 3.3 | 0.60 | 1.3 | 5.2 |
| Kilocalorie Intake3 | 244 | 796.9 | 205.06 | 380.5 | 1501.5 |
| Protein Intake (g)3 | 244 | 18.5 | 6.29 | 9.1 | 63.1 |
| Fat Intake (g)3 | 244 | 38.6 | 10.10 | 19.5 | 70.2 |
| Carbohydrate Intake (g)3 | 244 | 94.8 | 27.55 | 41.9 | 235.5 |
| Calcium Intake (mg)3 | 244 | 661.0 | 242.49 | 242.4 | 2335.0 |
| Iron Intake (mg)3 | 244 | 15.4 | 6.43 | 1.5 | 44.9 |
| Zinc Intake (mg)3 | 244 | 5.6 | 1.44 | 2.7 | 10.2 |
| Vitamin C Intake (mg)3 | 244 | 85.9 | 33.16 | 24.9 | 226.3 |
| Vitamin D Intake (mg)3 | 244 | 10.4 | 2.80 | 4.8 | 19.6 |
| Delivery Blood Lead (μg/dL)4 | 187 | 2.3 | 2.65 | 0.1 | 13 |
| 6 Month Blood Lead (μg/dL) | 204 | 3.2 | 3.32 | 0.3 | 24 |
| 12 Month Blood Lead (μg/dL) | 196 | 6.3 | 4.84 | 0.7 | 28 |
| Length-for-Age z score – 6 Month Visit | 242 | 0.06 | 0.962 | −2.86 | 2.86 |
| Length-for-Age z score – 12 Month Visit | 215 | 0.06 | 0.962 | −2.86 | 2.86 |
| Weight-for-Age z score – 6 Month Visit | 242 | 0.13 | 1.162 | −3.09 | 3.72 |
| Weight-for-Age z score – 12 Month Visit | 215 | 0.03 | 1.209 | −2.90 | 3.83 |
| Weight-for-Length z score – 6 Month Visit | 242 | 0.31 | 1.205 | −3.88 | 3.67 |
| Weight-for-Length z score – 12 Month Visit | 215 | 0.54 | 1.128 | −2.59 | 3.80 |
| Head Circumference-for-Age z score – 6 Month Visit | 242 | 0.30 | 1.050 | −2.72 | 3.75 |
| Head Circumference-for-Age z score – 12 Month Visit | 215 | 0.50 | 1.182 | −2.92 | 4.43 |
| Upper Arm Circumference-for-Age z score – 12 Month Visit | 215 | 0.50 | 1.174 | −2.98 | 3.94 |
| Count | % | |
|---|---|---|
| Prior parity | ||
| Nulliparous | 114 | 46.7 |
| Multiparous | 130 | 53.3 |
| Marital Status | ||
| Single | 206 | 84.4 |
| Married | 38 | 15.6 |
| Employment for prior 6 months | ||
| Employed | 125 | 51.2 |
| Not employed | 117 | 48.0 |
| Race | ||
| Black | 122 | 50.0 |
| All other | 122 | 50.0 |
| Mother’s Age | ||
| Less than 19 years | 65 | 26.6 |
| Greater than or equal to 19 years | 179 | 73.4 |
| Breastfeeding at 3 months | ||
| No | 227 | 93.0 |
| Yes | 17 | 7.0 |
| Child’s Sex | ||
| Male | 128 | 52.5 |
| Female | 116 | 47.5 |
Geometric mean reported for all blood lead levels.
The education index was calculated as follows, if mother was younger than 19 = (years of education + 6) / age, and if mother was 19 years of age and older = (years of education + 6) / 18.
Nutrient intakes are averages of 3 and 6 month intakes.
Infant delivery lead was either cord (n=160) or venous (n=27), as available.
Multivariate regression analyses controlling for infant’s sex, birth weight, and nutrition, and mother’s age, marital status, employment, self-reported race, height, parity, second trimester cigarette smoking, and age-adjusted education indicated that second trimester lead levels are negatively associated with head circumference-for-age z scores in the total sample (Table 2). No other measures were significantly related to prenatal lead level in the total sample. The regression models were re-run with the sample split at the median of mother’s second trimester lead level (<3 μg/dL, ≥ 3 μg/dL). In the high lead group, negative associations between second trimester lead level and several growth z scores are seen (weight-for-age at 6 months, weight-for-length at 6 months, head circumference-for-age at 6 and 12 months, and upper arm circumference at 12 months). No statistically significant relationships were observed in the lower lead group.
Table 2.
Associations between log transformed mother’s second trimester blood lead levels and infant’s growth z scores at 6 and 12 month study visits when controlling for covariates1 in multivariate regression models: total sample (n=238, 211 at 6 and 12 months respectively), and stratified by second trimester blood lead levels less than 3 μg/dL (n=133, 114 at 6 and 12 months respectively) and greater than or equal to 3 μg/dL (n=105, 97 at 6 and 12 months respectively).
| Total Sample | Lower Lead (<3 μg/dL) | Higher Lead (≥3 μg/dL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Unstd. B | SE | p | Unstd. B | SE | p | Unstd. B | SE | p | |
| Length-for-age | |||||||||
| 6 months | 0.149 | 0.076 | 0.05 | 0.243 | 0.172 | 0.16 | 0.457 | 0.271 | 0.10 |
| 12 months | 0.073 | 0.083 | 0.38 | 0.217 | 0.195 | 0.27 | −0.076 | 0.301 | 0.80 |
| Weight-for-age | |||||||||
| 6 months | 0.013 | 0.098 | 0.89 | 0.109 | 0.223 | 0.63 | − 0.771 | 0.344 | 0.03 |
| 12 months | 0.124 | 0.107 | 0.25 | 0.089 | 0.246 | 0.72 | −0.475 | 0.403 | 0.24 |
| Weight-for-length | |||||||||
| 6 months | −0.158 | 0.111 | 0.16 | −0.120 | 0.244 | 0.62 | − 1.461 | 0.404 | <0.01 |
| 12 months | 0.084 | 0.111 | 0.45 | −0.052 | 0.251 | 0.84 | −0.594 | 0.416 | 0.16 |
| Head circumference-for-age | |||||||||
| 6 months | − 0.242 | 0.094 | 0.01 | 0.003 | 0.226 | 0.99 | − 0.846 | 0.338 | 0.01 |
| 12 months | − 0.220 | 0.109 | <0.05 | 0.073 | 0.266 | 0.79 | − 1.163 | 0.376 | 0.00 |
| Upper arm circumference-for-age | |||||||||
| 12 months | −0.132 | 0.114 | 0.25 | 0.198 | 0.250 | 0.43 | − 1.063 | 0.436 | 0.02 |
Regression models control for infant’s sex, birth weight (kg), and nutrition (principal component scores), and mother’s age (19 years or ≥ 19 years), marital status (single or married), employment (yes or no), self-reported race (Black or other), height (cm), parity (primiparous or multiparous), second trimester cigarette smoking (average # cigarettes/day), education index (if younger than 19 = years of education + 6) / age, if 19 years of age and older = years of education + 6) / 18).
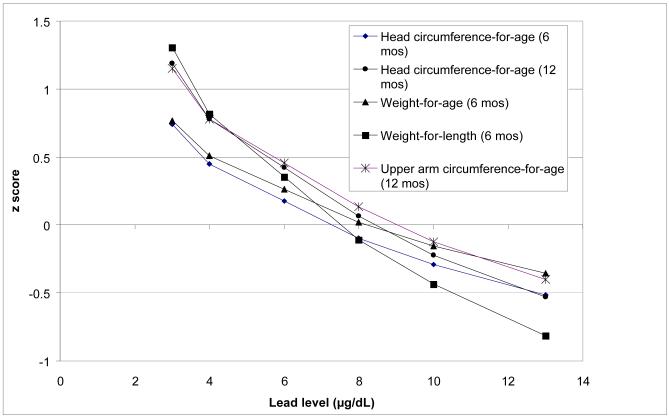
Figure 1 shows for the half of the sample with lead levels greater than 3 μg/dL, the size of significant effects of mother’s second trimester lead levels on 6- and 12-month growth z scores. Z scores are estimated from multivariate regression analyses (Table 2) and calculated for actual lead levels observed in this study: the lowest lead level, highest, the geometric mean (6 μg/dL), and the geometric mean +/− 1 SD (4 μg/dL, 8 μg/dL), and 10 μg/dL. At lower lead levels, size measures are associated with higher percentiles: weight-for-length, upper arm circumference-for-age, and 12-month head circumference-for-age z scores approximate the 79th percentile at 4 μg/dL and the 88-90th percentiles at 3 μg/dL. Size measures cluster around a z score of 0 (approximates the 50th percentile) at lead levels of 8 μg/dL and drop to approximately the 31st percentile (z score of −0.5) at the highest lead levels of 10-13 μg/dL.
Figure 1.
Estimated growth z scores of APILS infants across mothers’ second trimester lead levels (only significant associations are shown). Calculations are based upon regression models presented in Table 3 for the >3 μg/dL group only, and hold all other variables constant at their respective means.
Due to the longitudinal character of the study, postnatal lead levels of infants were available for analysis in addition to prenatal maternal lead levels. Multivariate regression analyses were used to determine the relationship between 6-month lead levels and size at 12 months. Six-month lead level was negatively associated with head circumference-for-age at 12 months in the total sample (b=−0.250; p=0.027, n= 176), and not with other measures of size. With the sample split into low and high groups based on the 6-month lead level (<3 μg/dL ≥3 μg/dL), there were no significant associations in the low lead group with 12 month size measures (n=84), and in the high lead group the association of 6 month lead with head circumference at 12 months was suggestive (b = −0.450; p=0.107, n= 92) of a relationship.
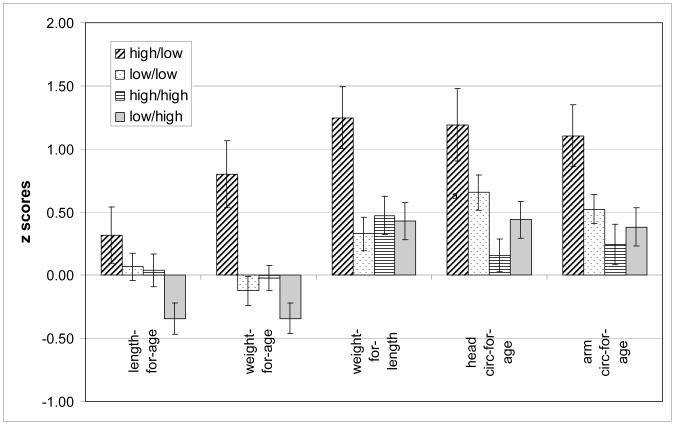
We also considered the effect of changes in lead levels and constructed categorical variables to characterize changes from second trimester lead to 12-month lead. (Substantial changes in lead levels are not expected by 6 months of age.) We split the sample at the median lead level (3 μg/dL for 2nd trimester blood lead and 6 μg/dL for 12-month lead level). Four lead categories were defined: low prenatal/low 12 month (n= 77), low prenatal/high 12 month (n=44), high prenatal /low 12 month (n=18), and high prenatal /high 12 month (n=64). Significant differences were observed in all second trimester to12-month lead group comparisons (F test significance of p<0.05) with the exception of 6-month length-for-age. Figure 2 shows means (+/− SE) for growth measures by second trimester to 12-month lead variables. The high to low lead group has the largest values of every growth measure, while the low to high lead group has the smallest length-for-age and weight-for-age values. The high to high group is smallest for weight-for-length, head circumference-for-age and upper arm circumference-for-age.
Figure 2.
Mean growth z scores at 12 months by change in maternal second trimester lead level to infant’s 12 months level.
As the bivariate testing indicated significant effects, multivariate regression analysis was utilized to correct for the same covariates as were used in other analyses of lead effects, with the high to low lead group as the reference category (table 4). As in the bivariate testing, significant size differences were observed between the high to low group and other groups. By 12 months of age the high to low group had significantly larger weights-for-age and weight for length compared to the low to high and low to low groups. Also at 12 months, the high to low group had a larger head-circumference-for-age than the low to high. No other comparisons were significantly different.
Conclusions
We observed significant, negative associations between prenatal blood lead levels and postnatal weight, weight-for-length, head circumference, and upper arm circumference during the first year of life in both bivariate and multivariate analyses controlling for dietary, social and biological covariates. The results of this study are consistent with previous cross-sectional and longitudinal studies of samples with higher lead levels that have reported reductions in children’s weight (Bithoney, 1986; Habercam et al., 1974; Little et al., 1990; Sanin et al., 2001; Schwartz et al., 1986), head circumference (Ballew et al., 1999; Kafourou et al., 1997; Little et al., 1990; Rothenberg et al., 1993; Rothenberg et al., 1999), and upper arm circumference (Lauwers et al., 1986) with increasing lead levels. Of the few studies to have investigated BMI and lead levels in children, two found no significant associations (Ballew et al., 1999; Huzior-Balajewicz et al., 2001), while Kim and colleagues (1995) reported a positive association in Boston children. However, the Boston study focused on chronic lead exposure as measured in dentin lead at 7 years and assessed BMI at 7 years and the change in BMI from 7 to 20 years. Because of the difference in how lead was measured and when BMI was assessed, the Boston study may not be directly comparable to the current analysis. Furthermore, it is possible that a different effect of lead might be observed at different developmental periods.
The longitudinal study design employed here allowed for the assessment of associations between growth measures across the first year and both prenatal and postnatal lead exposures. While both prenatal and postnatal lead levels were negatively associated with later weight, weight-for-length, head circumference, and upper arm circumference, these inverse relationships were most consistently observed in the high prenatal exposure group (having mothers’ second trimester lead levels ≥ 3 μg/dL). These results indicate the importance of both the timing and dose of exposure. It is worth noting that lead levels in the high lead group are relatively low (geometric mean=5.9 μg/dL, range 3-13 μg/dL), and most would not be considered cause for clinical concern. Other longitudinal studies have indicated the importance of the timing of exposure on growth effects of lead. In the Mexico City Prospective Lead Study, a cohort of children was followed from birth to 48 months. Head circumference at 6 months was negatively associated with prenatal maternal blood lead level (Rothenberg et al., 1993; Rothenberg et al., 1999), similar to the results reported here. In the Cincinnati cohort, children’s lead levels were associated with reductions in stature at 15 months only among children whose mothers’ prenatal lead levels exceeded the cohort median of 7.7 μg/dL (Shukla et al., 1989).
The plausibility of an effect of lead on human growth is well established by controlled laboratory studies of non-human animals that show reductions in growth with increasing lead exposure, particularly following in utero exposure (Ronis et al., 1996; Ronis et al., 1998b; Ronis et al., 1998a). Evidence for several mechanisms of effect exists, including alteration of hypothalamic-pituitary axis function. Growth hormone release in response to growth hormone releasing factor is decreased in lead treated rats (Berry, Jr. et al., 2002; Camoratto et al., 1993). This suggests that lead acts at the level of the pituitary to attenuate the release of growth hormone, rather than at the hypothalamic level. Furthermore, a study of children before and after chelation therapy found lead to be inversely correlated with growth hormone levels, and associated with decreased release of growth hormone in response to insulin and L-dopa provocation (Huseman et al., 1992).
Lead may also suppress growth indirectly by affecting appetite or directly through its effects on bone. Laboratory research has shown lead to depress the appetite (Hammond et al., 1989) and the appetite setpoint (Hammond et al., 1990) in rats. Lead may also directly affect bone by inhibiting osteocalcin secretion in osteoblasts (Long and Rosen, 1992) and/or by decreasing the width of growth plates in bone and disturbing mineralization (Hamilton and O’Flaherty, 1995).
While there were negative associations between lead and several growth measures, we did not observe any associations between blood lead levels and length after controlling for covariates in the total or in either subsample. These results for length do not replicate other studies that have reported adverse effects of lead on stature (Frisancho and Ryan, 1991; Habercam et al., 1974; Kafourou et al., 1997; Little et al., 1990; Schwartz et al., 1986; Shukla et al., 1987; Shukla et al., 1989; Shukla et al., 1991), including a recent analysis of NHANES III that reported a 1.6 cm reduction in stature for each 10 μ/dL of lead among non-Hispanic children 1-7 years of age (Ballew et al., 1999). The most likely reasons for our results for length is that there is no effect of lead at the levels observed or any effect of lead is too small to be detected given the small sample size. However, the effects on head circumference in the same infants suggest that the same low levels of lead may negatively impact this rapidly growing dimension.
Analysis of the groups defined by a change in lead levels from the prenatal period to the 12 month levels indicates that the strongest and most consistent contrasts appear between the high to low and the high to high groups. The consistently high group always has significantly lower values than the high to low group. The high to low group also is significantly heavier than the low to high group. These contrasts are consistent with the hypothesis that decreasing lead levels reflect a supportive environment for growth and consistently high lead levels and increasing lead levels do not. However, the group with consistently lower lead levels also had smaller growth parameters which is discordant with that hypothesis and cannot be explained with the available data. Weight and head circumference seem the most sensitive while length is unaffected in this sample. The use of extensive control variables suggests that the effects attributed to lead are not due to uncontrolled effects of nutritional, socio-demographic or economic factors that we measured, although at the low lead levels characterizing this sample, any unmeasured factors could be influential.
These conclusions should be tempered with the knowledge that larger samples should be examined before accepting lead levels below 10 ug/dL as significant influences on infant growth. Interpretation of the data used in this analysis is further limited by the use of two methods to measure lead. Strengths are the inclusion of a large number of relevant covariates to control for other influences on growth, the use of serial measurements spanning the prenatal and infant periods, and the presence of low levels of lead in the sample that make the results relevant for a large portion of the US population.
Table 3.
Differences in groups defined by changes in lead levels from mother’s 2nd trimester lead to infant’s 12 month lead level: contrasts reference the high prenatal to low postnatal group (regression model covariates are the same as in table 2).
| Weight-for-age | Weight-for-length | Head circumference-for-age | ||||
|---|---|---|---|---|---|---|
| b | p | b | p | b | p | |
| At 12 months | ||||||
|
| ||||||
| low to high | −0.618 | 0.047 | −0.555 | 0.077 | −0.489 | 0.117 |
| high to high | −0.480 | 0.099 | −0.600 | 0.042 | −0.975 | 0.001 |
| low to low | −0.609 | 0.035 | −0.848 | 0.004 | −0.502 | 0.083 |
Grant acknowledgement:
Support from NIEHS R01-ES – 05280;
Literature Cited
- Ballew C, Khan LK, Kaufmann R, Mokdad A, Miller DT, Gunter EW. Blood lead concentration and children’s anthropometric dimensions in the Third National Health and Nutrition Examination Survey (NHANES III) 1988-1994. J Pediatr. 1999;134:623–630. doi: 10.1016/s0022-3476(99)70250-7. [DOI] [PubMed] [Google Scholar]
- Bearer CF. The special and unique vulnerability of children to environmental hazards. Neurotox. 2000;21:925–934. [PubMed] [Google Scholar]
- Bellinger DC, Leviton A, Rabinowitz M, Allred E, Needleman HL, Schoenbaum S. Weight gain and maturity in fetuses exposed to low levels of lead. Environ Res. 1991;54:151–158. doi: 10.1016/s0013-9351(05)80097-0. [DOI] [PubMed] [Google Scholar]
- Berry WD, Jr., Moriarty CM, Lau Y-S. Lead attenuation of episodic growth hormone secretion in male rats. Int J Toxicol. 2002;21:93–98. doi: 10.1080/10915810252866060. [DOI] [PubMed] [Google Scholar]
- Bithoney WG. Elevated lead levels in children with nonorganic failure to thrive. Pediatrics. 1986;78:891–895. [PubMed] [Google Scholar]
- Bornschein RL, Succop PA, Dietrich KN, Krafft KM, Grote J, Mitchell T, Berger OG, Hammond P. Prenatal lead exposure and pregnancy outcomes in the Cincinnati Lead Study. In: Lindenburg SE, Hutchinson TP, editors. Heavy Metals in the Environment. CEP Consultants; Edinburgh: 1987. pp. p156–158. [Google Scholar]
- Brody DJ, Pirkle JL, Kramer RA, Flegal KA, Matte TD, Gunter EW, Paschal DC. Blood lead levels in the US population. Phase 1 of the Third National Health and Nutrition Examination Survey (NHANES III, 1988-1991) JAMA. 1994;272:277–283. doi: 10.1001/jama.272.4.277. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Rhoads GG. Guest Editorial Responding to Blood Lead Levels < 10 μg/dL. Environ Health Perspect. 2008;116:A60–A61. doi: 10.1289/ehp.10703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MJ, Shenassa E, Matte TD, Catlin SN. Children in Illinois with elevated blood lead levels, 1993-1998, and lead-related pediatric hospital admissions in Illinois, 1993-1997. Public Health Rep. 2000;115:532–536. doi: 10.1093/phr/115.6.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N. The methods of auxological anthropometry. In: Falkner F, Tanner JM, editors. Human Growth: Methodology; Ecological, Genetic, and Nutritional Effects on Growth. Plenum Press; New York: 1986. pp. p3–46. [Google Scholar]
- Camoratto AM, White LM, Lau Y-S, Ware GO, Berry WD, Moriarty CM. Effect of exposure to low level lead on growth and growth hormone release in rats. Toxicology. 1993;83:101–114. doi: 10.1016/0300-483x(93)90095-a. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Childhood lead poisoning -- United States: report to the Congress by the Agency for Toxic Substances and Disease Registry. MMWR. 1988;37:481–504. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . Preventing Lead Poisoning in Young Children. U.S. Department of Health and Human Services, Public Health Service; Atlanta: 1991. [Google Scholar]
- Centers for Disease Control and Prevention Trends in blood lead levels among children--Boston, Massachusetts, 1994-1999. MMWR. 2001;50:337–339. [PubMed] [Google Scholar]
- de Onis M, Yip R, Mei Z. The development of MUAC-for-age reference data recommended by a WHO Expert Committee. Bull World Health Org. 1997;75:11–18. [PMC free article] [PubMed] [Google Scholar]
- Frisancho AR, Ryan AS. Decreased stature associated with moderate blood lead concentrations in Mexican-American children. Am J Clin Nutr. 1991;54:516–519. doi: 10.1093/ajcn/54.3.516. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cossio T, Peterson KE, Sanin L-H, Fishbein E, Palazuelos E, Hernandez-Avila M, Hu H. Decrease in birth weight in relation to maternal bone-lead burden. Pediatrics. 1997;100:856–862. doi: 10.1542/peds.100.5.856. [DOI] [PubMed] [Google Scholar]
- Greene T, Ernhart CB. Prenatal and preschool age lead exposure: relationship with size. Neurotoxicol Teratol. 1991;13:417–127. doi: 10.1016/0892-0362(91)90091-a. [DOI] [PubMed] [Google Scholar]
- Habercam JW, Keil JE, Reigart JR, Croft HW. Lead content of human blood, hair, and deciduous teeth: correlation with environmental factors and growth. J Dent Res. 1974;53:1160–1163. doi: 10.1177/00220345740530051501. [DOI] [PubMed] [Google Scholar]
- Hamilton JD, O’Flaherty EJ. Influence of lead on mineralization during bone growth. Fundam Appl Toxicol. 1995;26:265–271. doi: 10.1006/faat.1995.1097. [DOI] [PubMed] [Google Scholar]
- Hammond PB, Chernausek SD, Succop PA, Shukla R, Bornschein RL. Mechanisms by which lead depresses linear and ponderal growth in weanling rats. Toxicol Appl Pharmacol. 1989;99:474–486. doi: 10.1016/0041-008x(89)90155-5. [DOI] [PubMed] [Google Scholar]
- Hammond PB, Minnema DJ, Shulka R. Lead exposure lowers the set point for food consumption and growth in weanling rats. Toxicol Appl Pharmacol. 1990;106:80–87. doi: 10.1016/0041-008x(90)90108-7. [DOI] [PubMed] [Google Scholar]
- Huseman CA, Varma MM, Angle CR. Neuroendocrine effects of toxic and low blood lead levels in children. Pediatrics. 1992;90:186–189. [PubMed] [Google Scholar]
- Huzior-Balajewicz A, Pietrzyk JJ, Schlegel-Zawadzka M, Piatkowska E, Zachwieja Z. The influence of lead and cadmium environmental pollution on anthropometric health factors in children. Przegl Lek. 2001;58:315–324. [PubMed] [Google Scholar]
- Johnson NE, Tenuta K. Diets and blood lead levels of children who practice pica. Environ Res. 1979;18:369–376. doi: 10.1016/0013-9351(79)90113-0. [DOI] [PubMed] [Google Scholar]
- Kafourou A, Touloumi G, Makropoulos V, Loutradi A, Papanagioutou A, Hatzakis A. Effects of lead on the somatic growth of children. Arch Environ Health. 1997;52:377–383. doi: 10.1080/00039899709602214. [DOI] [PubMed] [Google Scholar]
- Kim R, Hu H, Rotnitzky A, Bellinger DC, Needleman HL. A longitudinal study of chronic lead exposure and physical growth in Boston children. Environ Health Perspect. 1995;103:952–957. doi: 10.1289/ehp.95103952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. 314. U.S. Department of Health and Human Services; 2000. [PubMed] [Google Scholar]
- LaFlash S, Joosse-Coons M, Havlena J, Anderson HA. Wisconsin children at risk for lead poisoning. Wisc Med J. 2000;99:18–22. [PubMed] [Google Scholar]
- Lauwers M-C, Hauspie RC, Susanne C, Verheyden J. Comparison of biometric data of children with high and low levels of lead in the blood. Am J Phys Anthropol. 1986;69:107–116. doi: 10.1002/ajpa.1330690112. [DOI] [PubMed] [Google Scholar]
- Litaker D, Kippes CM, Gallagher TE, O’Connor ME. Targeting lead screening: The Ohio Lead Risk Score. Pediatrics. 2000;106:E69. doi: 10.1542/peds.106.5.e69. [DOI] [PubMed] [Google Scholar]
- Little BB, Snell LM, Johnston WL, Knoll KA, Buschang PH. Blood lead levels and growth status of children. Am J Hum Biol. 1990;2:265–269. doi: 10.1002/ajhb.1310020308. [DOI] [PubMed] [Google Scholar]
- Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Human Kinetics Books; Champaign, IL: 1988. [Google Scholar]
- Long GJ, Rosen JF. Lead perturbs epidermal growth factor (EGF) modulation of intracellular calcium metabolism and collagen synthesis in clonal rat osteoblastic (ROS 17/2.8) cells. Toxicol Appl Pharmacol. 1992;114:63–70. doi: 10.1016/0041-008x(92)90097-c. [DOI] [PubMed] [Google Scholar]
- McCabe EB. Age and sensitivity to lead toxicity: a review. Environ Health Perspect. 1979;29:29–33. doi: 10.1289/ehp.792929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Z, Grummer-Strawn LM, de Onis M, Yip R. The development of a MUAC-for-height reference, including a comparison to other nutritional status screening indicators. Bull World Health Org. 1997;75:333–341. [PMC free article] [PubMed] [Google Scholar]
- Parsons P, Slavin W. A rapid zeeman graphite-furnace atomic absorption spectrometric method for the determination of lead in blood. Spectrochim Acta B. 1993;48B:925–939. [Google Scholar]
- Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, Matte TD. The decline in blood lead levels in the United States: the National Health and Nutrition Examination Surveys (NHANES) JAMA. 1994;272:284–291. [PubMed] [Google Scholar]
- Ronis MJ, Badger TM, Shema SJ, Roberson PK, Templer L, Ringer D, Thomas PE. Endocrine mechanisms underlying the growth effects of developmental lead exposure in the rat. J Toxicol Environ Health. 1998a;54:101–120. doi: 10.1080/009841098158944. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Badger TM, Shema SJ, Roberson PK, Shaikh F. Reproductive toxicity and growth effects in rats exposed to lead at different periods during development. Toxicol Appl Pharmacol. 1996;136:361–371. doi: 10.1006/taap.1996.0044. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Badger TM, Shema SJ, Roberson PK, Shaikh F. Effects on pubertal growth and repdoduction in rats exposed to lead perinatally or continuously throughout development. J Toxicol Environ Health A. 1998b;53:327–341. doi: 10.1080/009841098159312. [DOI] [PubMed] [Google Scholar]
- Rothenberg SJ, Schnaas L, Perez-Guerrero I, Peroni-Hernandez E, Mercado-Torres L, Gomez-Ruiz C, Zea F. Prenatal and postnatal blood lead level and head circumference in children to three years: preliminary results from the Mexico City Prospective Lead Study. J Expo Anal Environ Epidemiol. 1993;3:165–172. [PubMed] [Google Scholar]
- Rothenberg SJ, Schnaas L, Perroni E, Hernandez R, Martinez S, Hernandez C. Pre- and postnatal lead effect on head circumference: A case for critical periods. Neurotoxicol Teratol. 1999;21:1–11. doi: 10.1016/s0892-0362(98)00034-8. [DOI] [PubMed] [Google Scholar]
- Sachs HK, Moel DI. Height and weight following lead poisoning in childhood. Am J Dis Child. 1989;143:820–822. doi: 10.1001/archpedi.1989.02150190070023. [DOI] [PubMed] [Google Scholar]
- Sanin LH, Gonzalez-Cossio T, Romieu I, Peterson KE, Ruiz S, Palazuelos E, Hernandez-Avila M, Hu H. Effect of maternal lead burden on infant weight and weight gain at one month of age among breastfed infants. Pediatrics. 2001;107:1016–1023. doi: 10.1542/peds.107.5.1016. [DOI] [PubMed] [Google Scholar]
- Schell LM, Czerwinski S, Stark AD, Parsons PJ, Gomez M, Samelson R. Variation in blood lead and hematocrit levels during pregnancy in a socioeconomically disadvantaged population. Arch Environ Health. 2000;55:134–140. doi: 10.1080/00039890009603400. [DOI] [PubMed] [Google Scholar]
- Schell LM, Denham M, Stark AD, Gomez M, Ravenscroft J, Parsons PJ, Aydemir A, Samelson R. Maternal blood lead concentration, diet during pregnancy, and anthropometry predict neonatal blood lead in a socioeconomically disadvantaged population. Environ Health Perspect. 2003;111:195–200. doi: 10.1289/ehp.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell LM, Denham M, Stark AD, Ravenscroft J, Parsons P, Schulte E. Relationship between blood lead concentration and dietary intakes of infants from 3 to 12 months of age. Environ Res. 2004;96:264–273. doi: 10.1016/j.envres.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Schell LM, Stark AD. Pollution and child health. In: Schell LM, Ulijaszek SJ, editors. Urbanism, Health and Human Biology in Industrialised Countries. Cambridge University Press; Cambridge: 1999. pp. p136–157. [Google Scholar]
- Schell LM, Stark AD, Gomez MI, Grattan WA. Blood lead level by year and season among poor pregnant women. Arch Environ Health. 1997;52:286–291. doi: 10.1080/00039899709602200. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Angle CR, Pitcher H. Relationship between childhood blood lead levels and stature. Pediatrics. 1986;77:281–288. [PubMed] [Google Scholar]
- Shukla R, Bornschein RL, Dietrich KN, Buncher CR, Berger OG, Hammond PB, Succop PA. Fetal and infant lead exposure: effects on growth in stature. Pediatrics. 1989;84:604–612. [PubMed] [Google Scholar]
- Shukla R, Bornschein RL, Dietrich KN, Mitchell T, Grote J, Berger OG, Hammond PB, Succop PA. Effects of fetal and early postnatal lead exposure on child’s growth in stature-the Cincinnati Lead Study. In: Lindberg S, Hutchinson T, editors. Heavy Metals in the Environment. CEP Consultants; Edinburgh: 1987. pp. p210–212. [Google Scholar]
- Shukla R, Dietrich KN, Bornschein RL, Berger O, Hammond PB. Lead exposure and growth in the early preschool child: a follow-up report from the Cincinnati Lead Study. Pediatrics. 1991;88:886–892. [PubMed] [Google Scholar]
- Tanner JM. Physical Growth from Conception to Maturity. Harvard University Press; Cambridge, Massachusetts: 1990. Foetus into Man. [Google Scholar]