Abstract
Noncardiac chest pain (NCCP) is very common, affecting up to 25% of the adult population in the United States. Treatment for NCCP has markedly evolved in the past decade and is presently focused on gastroesophageal reflux disease (GERD) and visceral hypersensitivity. Aggressive treatment with proton pump inhibitors has become the standard of care for GERD-related NCCP. Pain modulators such as tricyclics, trazodone, and selective serotonin reuptake inhibitors are considered the mainstay of therapy for non-GERD-related NCCP Other therapeutic modalities such as botulinum toxin injections and hypnotherapy have demonstrated promise in small clinical trials.
Keywords: Noncardiac chest pain, proton pump inhibitors, tricyclics
Noncardiac chest pain (NCCP) is defined as recurring angina-like substernal chest pain of noncardiac origin. The prevalence of NCCP varies from 14–33% in different population-based studies.1,2 Due to the nature of the symptoms, which are indistinguishable from those of ischemic heart disease, a thorough evaluation by a cardiologist is often necessary. NCCP is associated with reports of poor quality of life, regardless of diagnostic and therapeutic interventions. Consequently, NCCP patients frequently utilize healthcare resources, resulting in the increase of direct, as well as indirect, costs.3
Although NCCP affects both genders, women with NCCP tend to consult healthcare providers and present to the hospital emergency department more often than men.2 In the United States, African Americans are less likely to report chest pain symptoms than whites.4 In comparison with chest pain patients of cardiac origin, NCCP patients are younger, consume greater amounts of alcohol, smoke more often, and are more likely to suffer from anxiety.5 Several studies have shown that the prevalence of NCCP decreases with age.6,7
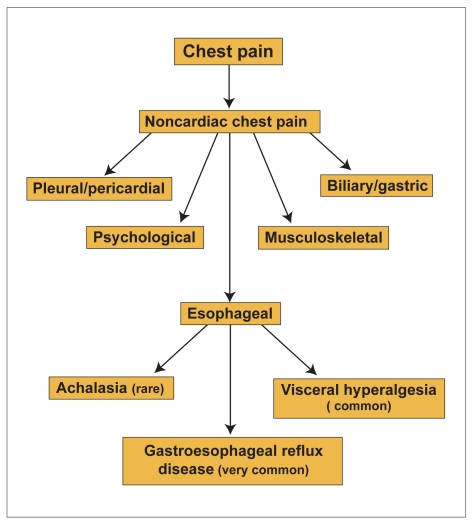
Esophageal factors that have been proposed as contributors of NCCP include gastroesophageal reflux disease (GERD), esophageal motility disorders, abnormal mechanophysical properties of the esophagus, and visceral hypersensitivity (Figure 1).
Figure 1.
Differential diagnosis of noncardiac chest pain.
GERD has been reported to be the most common esophageal cause of NCCP. Several studies have demonstrated that the prevalence of GERD ranges from 21–60% of patients with NCCP.8 The mechanism by which acid reflux causes heartburn in some patients and chest pain in other patients remains poorly understood, and is complicated by the fact that a subset of patients with NCCP complain of both heartburn and chest pain symptoms. The prevalence of erosive esophagitis among patients with GERD-related NCCP varies from 10–70% in different studies; this wide range of prevalence is likely due to the differences of the patient populations being evaluated. Studies have also shown that up to 50% of patients presenting with NCCP have abnormal acid exposure.9 A positive symptom index may be used in clinical practice to demonstrate the relationship between chest pain symptoms and acid-reflux events, despite its limited value in NCCP.10
Patients with non-GERD-related NCCP are commonly evaluated for esophageal dysmotility. These patients have a variety of esophageal motor disorders, including diffuse esophageal spasm (DES), nutcracker esophagus, achalasia, long-duration contractions, multipeaked waves, and hypertensive lower esophageal sphincter (LES). However, esophageal manometry has a very limited value in evaluating patients with NCCP, because the majority of patients (70%) demonstrate normal esophageal motor activity.11 Patients who have non-GERD-related NCCP (except achalasia) are considered to have functional chest pain of presumed esophageal origin.12 This group of patients includes those with normal or abnormal esophageal motility. The mechanism for pain in this group of patients still remains to be elucidated. A variety of underlying mechanisms have been proposed, including central and peripheral hypersensitivity, esophageal mechanophysical abnormalities, and sustained contractions of the esophageal longitudinal muscle.13,14
The treatment for NCCP is tailored to the potential underlying mechanism. In GERD patients, high-dose proton pump inhibitors (PPIs), combined with lifestyle modifications, provide the highest rate of symptom resolution. In non-GERD-related NCCP patients, pain modulators are the cornerstone of treatment.
GERD-related NCCP
Lifestyle Modifications
Lifestyle modifications include elevation of the head of the bed, weight loss, cessation of smoking, and the avoidance of alcohol, coffee, fresh citrus juice, and other associated food products, as well as avoidance of medications that can exacerbate reflux, such as narcotics, benzodiazepines, and calcium channel blockers.15,16 Although these lifestyle modifications are commonly advocated as first-line treatment in GERD patients, there is no evidence to support their efficacy in GERD-related NCCP. Regardless, enthusiasm about lifestyle modifications is very high among physicians, and thus it is highly likely that GERD-related NCCP subjects will be instructed to follow them.
Histamine-2 Receptor Antagonists (H2RAs)
The efficacy of H2RAs for controlling symptoms in patients with GERD-related NCCP has been shown to range from 42–52%.17 In one study, cimetidine (unknown dose) and antacids were shown to be effective in only 42% of patients with GERD-related NCCP who were followed for a period of 2–3 years.18 It is widely accepted that a potent antireflux treatment that provides long-term and consistent antisecretory effects is needed in patients with GERD-related NCCP. Additionally, from our experience, stepping down GERD-related NCCP patients from PPI to H2RA therapy has been an unrewarding strategy.
PPIs
Omeprazole (Prilosec, AstraZeneca) 20 mg twice daily or placebo was administered over a period of 8 weeks to GERD-related NCCP patients in the only doubleblind, placebo-controlled trial that has been performed.19 Patients who received omeprazole had a significant reduction in both the number of days with chest pain and in their chest pain severity scores compared to the patients who received placebo. Thus far, most of the studies assessing the efficacy of PPIs in NCCP primarily utilized omeprazole. However, it is highly likely that all other PPIs would demonstrate similar efficacy. In fact, a recent openlabel study with esomeprazole (Nexium, AstraZeneca) administered 60 mg once daily demonstrated complete resolution of symptoms in 57.1% of subjects with either NCCP or laryngeal manifestations of GERD.20
Patients with GERD-related NCCP should be treated with at least double the standard dose of PPI until symptoms remit, followed by dose tapering to determine the lowest PPI dose that can control the symptoms. As with other extraesophageal manifestations of GERD, NCCP patients may require more than 2 months of therapy for optimal symptom control. Long-term maintenance PPI treatment has been shown to be highly effective.21 Borzecki and colleagues22 developed a decision tree to compare empiric treatments for NCCP patients with H2RAs or standard-dose PPI for 8 weeks with initial investigations (upper endoscopy or upper gastrointestinal series). Empiric treatment was more cost-effective in the initial investigation strategy, with a cost of $849 per patient versus $2,187 per patient.
Surgical Treatment
The role of antireflux surgery in GERD-related NCCP remains to be further elucidated. Several studies have demonstrated a significant improvement in symptoms following laparoscopic fundoplication in patients with GERD-related NCCP. For instance, Patti and associates reported improvement in chest pain symptoms following laparoscopic fundoplication in 85% of patients with GERD-related NCCP.23 In addition, Farrell and coworkers reported that 90% of NCCP patients who underwent antireflux surgery experienced improvement in chest pain and 50% reported complete symptom resolution.24 In contrast, So and colleagues reported that after laparoscopic fundoplication, relief of atypical GERD symptoms (eg, chest pain) was less satisfactory than relief of typical GERD symptoms (eg, heartburn).25 In their study, the authors evaluated symptom improvement with a questionnaire given 3 months and 12 months after antireflux surgery. Overall, heartburn was relieved in 93% of patients, whereas only 48% of patients reported relief of chest pain symptoms.
Non-GERD-related NCCP
The treatment of non-GERD-related NCCP is primarily based on esophageal pain modulation. An important development in this field was the recognition that NCCP patients with spastic esophageal motor disorders (except achalasia), as documented by esophageal manometry, are more likely to respond to pain modulators than to muscle relaxants. Presently, there are very few well-designed, randomized, double-blind, placebo-controlled trials that assess the value of pain modulators in patients with non-GERD-related NCCP.
Muscle Relaxants
Nitrates
Nitroglycerin and long-acting nitrates have been shown to cause relaxation of gastrointestinal smooth muscles by stimulating cyclic guanosine monophosphate (GMP)-dependent pathways. Several open-label studies have reported that nitrates improve symptoms and esophageal motility patterns in patients with chest pain and esophageal dysmotility. Several investigators reported symptomatic improvement in patients with DES, accompanied by normalization of the motility pattern on esophageal manometry during treatment with nitrates.26,27 In one study, 5 patients with DES experienced a 4-year clinical and manometrical remission.28 However, other studies have failed to demonstrate similar efficacy.29,30 Long-acting nitrates in doses of 10–20 mg two to three times daily, as well as short-acting, sublingual nitrates for acute episodes of chest pain in NCCP patients, were used in these studies.
Overall, studies that evaluated the value of nitrates in NCCP have been limited by small numbers of patients and inconsistent results in regard to drug efficacy. A placebo-controlled trial that excludes patients with GERD has yet to be performed.
Calcium Channel Blockers
Calcium plays an important role in esophageal muscle contraction. Consequently, the role of calcium channel blocking agents in patients with NCCP and esophageal spastic motility disorders has been evaluated by many investigators.
Nifedipine, in an oral dose of 10–30 mg three times daily, has been shown to decrease the amplitude and duration of esophageal contractions in patients with nutcracker esophagus after only 2 weeks.31 Unfortunately, the effect of the drug disappeared after 6 weeks of treatment with the complete recurrence of symptoms. Davies and associates used a placebo-controlled trial to assess the efficacy of nifedipine in the prevention of symptomatic episodes of esophageal spasm in 8 NCCP patients over a period of 6 weeks.32 The authors were unable to find statistically significant differences in symptom improvement between the two therapeutic arms. In contrast, symptom improvement was noted in 20 NCCP patients with various esophageal motility disorders, including hypertensive LES, nutcracker esophagus, DES, and vigorous achalasia, who were treated with nifedipine 10 mg three times daily.33 Nifedipine was also found to significantly decrease LES resting pressure, with a direct correlation to the plasma levels of the drug.34
Diltiazem, in doses of 60–90 mg four times daily for 8 weeks, significantly improved mean chest pain scores and esophageal motility studies in patients with nutcracker esophagus when compared to placebo.35,36 However, in a study evaluating 8 patients with DES, the effect of diltiazem in relieving chest pain was not different from the effect of placebo, probably due to the small number of patients who participated in the study.37
Other calcium channel blockers have been evaluated in patients with primary esophageal motor disorders including verapamil, fendiline, nimodipine (Nimotop, Bayer), and nisoldipine (Sular, Sciele), with various effects on LES resting pressure and esophageal amplitude contractions.34 Regardless, calcium channel blockers appear to have a transient esophageal motor effect that translates to a short-lived improvement in symptoms, compounded by a variety of side effects such as hypotension, bradycardia, and pedal edema.
Phosphodiesterase Type 5 (PDE5) Inhibitors
Sildenafil (Viagra, Pfizer) is a potent selective inhibitor of cyclic GMP-specific PDE5, which inactivates the nitric oxide-stimulated GMP. Intracellular accumulation of the latter induces smooth muscle relaxation. The drug has been shown to improve esophageal motility in patients with nutcracker esophagus or hypertensive LES by lowering LES resting pressure, reducing distal esophageal amplitude contractions, and prolonging the duration of LES relaxation.38,39 However, thus far, there have been no studies that specifically address NCCP patients, so the value of this compound in NCCP remains unknown. Additionally, the usage of this compound in NCCP will likely be limited by its cost and side effects.
Other Muscle Relaxant Agents
The antispasmodic cimetropium bromide has been shown to be efficacious in 8 NCCP patients with nutcracker esophagus when taken intravenously, but clinical data regarding the efficacy of an oral formulation are still lacking. Hydralazine, an antihypertensive compound that directly dilates peripheral vessels, was shown to improve chest pain and dysphagia by decreasing the amplitude and duration of esophageal amplitude contractions in a study evaluating only 5 patients.30
Overall, evidence to support the therapeutic benefit of anticholinergic agents for the treatment of NCCP remains very limited. Furthermore, there is no evidence to suggest that a selective or nonselective anticholinergic agent would improve symptoms in patients with esophageal spastic motor disorder.
Pain Modulators
Visceral hyperalgesia is thought to be the primary underlying mechanism of patients with non-GERD-related NCCP, regardless of the presence or absence of esophageal motor disorder. Consequently, drugs that can alter esophageal pain perception have become the mainstay of therapy in these patients.
Several drugs have been shown to have a pain modulatory effect or a visceral analgesic effect, thus alleviating chest pain symptoms. These drugs include tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), theophylline, and trazodone.
TCAs
Several studies have demonstrated that antidepressants have a visceral analgesic effect,40 but they also appear to inhibit calcium channels and thus may have an additional muscle relaxant-like effect.41 TCAs have both central neuromodulatory and peripheral visceral analgesic effects. Several clinical trials have found favorable TCA-related effects on esophageal pain perception in both healthy subjects and subjects with NCCP.
Imipramine, administered at a dose of 75 mg daily, was shown to significantly increase the pain threshold of healthy men during intraesophageal balloon distension as compared to baseline.42 In another study, 60 patients with NCCP and normal coronary angiography were randomized to receive clonidine (0.2 mg daily), imipramine (50 mg nightly), or placebo for a period of 3 weeks. Patients who received imipramine had a significant (52%) reduction in the frequency of chest pain episodes, independent of cardiac, esophageal, or psychiatric test results, suggesting that imipramine has a visceral analgesic effect on chest pain.43 In contrast, amitriptyline failed to show an effect on both perception and esophageal compliance in subjects undergoing balloon distention protocol.44
Because of their anticholinergic side effects, TCAs are commonly administered at nighttime. Doses are initially low and then slowly titrated to a maximum of 50 mg daily. The incremental increase in dosing should be based on symptom improvement and development of side effects.
Trazodone
Trazodone, given 100–150 mg daily for 6 weeks, showed a significant improvement in the symptoms of patients with NCCP and esophageal dysmotility as compared to placebo.45 However, esophageal motility abnormalities remained unchanged. A small, open-label study reported symptom control and improved esophageal motility in patients with NCCP and DES following treatment with both trazodone and clomipramine.46
SSRIs
A randomized trial assessing the effect of sertaline in patients with NCCP demonstrated a significant reduction in pain scores, regardless of concomitant improvement in psychological scores.47 In addition, a recent study demonstrated that citalopram, given 20 mg intravenously in a single dose, reduced chemical and mechanical esophageal hypersensitivity without altering esophageal motility.48
Octreotide
Octreotide, a synthetic analog of somatostatin, has been shown to increase rectal and sigmoid perception thresholds for pain in irritable bowel syndrome (IBS) subjects, as well as healthy subjects.49,50 It has been postulated that the effect of octreotide is mediated by the activation of somatostatin receptors at the spinal cord and/or the supraspinal level.
Octreotide, administered 100 mg subcutaneously, was found to significantly increase perception thresholds for pain as compared to placebo in healthy subjects undergoing intraesopahgeal balloon distention.51 Unfortunately, due to cost and the lack of an oral formulation, octreotide is rarely utilized for NCCP in clinical practice.
Theophylline
Theophylline, a xanthine derivative, has been shown to inhibit adenosine-induced angina-like chest pain and adenosine-induced pain in other regions of the body.52 A study using esophageal balloon distention protocol and impedance demonstrated that intravenous theophylline increased thresholds for sensation and pain in 75% of patients with functional chest pain.53 Similar results were documented in functional chest pain patients receiving oral theophylline for a period of 3 months. In another study, the same authors showed that oral doses of theophylline 200 mg twice daily was more effective than placebo in preventing chest pain in 19 patients with functional chest pain.54
Benzodiazepines
Alprazolam has been shown in a study to ameliorate chest pain at a mean dose of 4.3 mg daily in patients with NCCP and panic disorder.55 In this study, 15 out of 20 patients reported at least a 50% reduction in panic attack episodes and a corresponding decline in the frequency of chest pain episodes. Clonazepam, given 1–4 mg daily, was also shown to be effective in the treatment of patients with NCCP and panic disorder.56 The treatment of a functional disorder such as NCCP with benzodiazepines has been greatly discouraged, primarily due to the likelihood of developing addiction and, subsequently, dependence on this class of drugs.
5-Hydroxytryptamine (5-HT) Antagonists and Agonists
5-HT, also called serotonin, is a neurotransmitter present in the central nervous system, enteric neurons, and extrinsic afferents of the gut. It is involved in the visceral perception and motor activity processes in the gastrointestinal tract.57 Ondansetron, a 5-HT3 antagonist that is used as an antiemetic, has been shown to increase esophageal perception thresholds for pain in patients with NCCP.57 The selective 5-HT4 receptor agonist tegaserod (Zelnorm, Novartis) has been recently demonstrated to reduce both chemoreceptor sensitivity to acid and mechanoreceptor sensitivity to balloon distention in patients with functional heartburn.58 Presently, there are no studies assessing the value of tegaserod in patients with non-GERD-related NCCP.
Endoscopic Treatment for NCCP
Botulinum Toxin
Botulinum toxin (BoTox, Allergan) interacts selectively with cholinergic neurons to inhibit the release of acetylcholine at the presynaptic terminals. Botulinum toxin injection into the LES has been used in several uncontrolled trials that included patients with NCCP and documented esophageal spastic motility disorder. Injecting botulinum toxin into the LES in a small, uncontrolled study resulted in 50% reduction of chest pain episodes in 72% of the subjects for a mean duration of 7.3 months.59
Surgery and Endoscopic Treatment for NCCP
Laparoscopic fundoplication relieves heartburn and acid regurgitation in most patients with GERD, but its effect on chest pain is less clear. DeMeester and associates18 identified a temporal correlation between chest pain and acid reflux events in 12 of 23 patients with NCCP. Chest pain resolved in all 12 patients treated either by surgery (8 patients) or acid-reducing agents (4 patients). Patti and coworkers23 reviewed patients who complained of chest pain in addition to heartburn and acid regurgitation. Overall, chest pain improved in 85% of these patients after undergoing laparoscopic fundoplication for GERD. Improvement in chest pain increased to 96% in patients whose chest pain correlated with GERD most of the time. Farrell and colleagues24 evaluated the effectiveness of antireflux surgery for patients with atypical manifestations of GERD. Chest pain improved in 90% of patients after laparoscopic fundoplication, with symptom resolution in 50% of patients. Although surgical studies demonstrated a high success rate of antireflux surgery in GERD-related NCCP patients, the patients included were carefully selected.
Several endoscopic techniques have been designed to bolster the antireflux barrier at the gastroesophageal junction.60 There are three basic types of endoscopic treatments: suturing, radiofrequency, and injection.61 No studies to date have specifically evaluated patients with GERD-related NCCP. Consequently, these endoscopic methods are considered experimental and should not be routinely performed in patients with confirmed GERD-related NCCP. The value of endoscopic therapy in GERD-related NCCP patients should be studied in the future, primarily because several recent anecdotal reports have suggested a good symptomatic response.
Psychological Treatment
Psychological comorbidity, mainly depression and anxiety, is common in patients with NCCP. Psychotherapy may be helpful in the treatment of patients with NCCP, particularly those who also have hypochondriasis, anxiety, or panic disorder.
Several studies have demonstrated that patients with NCCP who are treated with cognitive-behavioral therapy report significant improvement in quality of life and reduction in chest pain symptoms. Additionally, cognitive-behavioral therapy has been successfully used for the treatment of NCCP patients without an existing panic disorder.62 A study evaluating patients who were treated with cognitive-behavioral therapy reported that 48% of these patients remained pain free at 12-month follow-up, as compared to only 13% of the patients in the nonintervention group. Other psychological interventions that have been suggested to be effective in patients with NCCP include reassurance, education, relaxation techniques, breathing training, and biofeedback. Biofeedback was assessed in a study that compared it to primary care visits only in patients with NCCP.63 Patients in the biofeedback group demonstrated a significantly lower symptom frequency and severity. However, a large group of patients assigned to the biofeedback arm (52%) did not complete the study.
Hypnotherapy
Hypnotherapy has been recently evaluated in the treatment of NCCP patients. Jones and colleagues64 reported an 80% improvement in symptoms, with a significant reduction in pain intensity, among patients who were receiving 12 sessions of hypnotherapy, compared to only a 23% symptom improvement in the control group. The study concluded that hypnotherapy appears to have a role in treating NCCP and that further studies are needed.
Future Therapy for NCCP
New therapeutic modalities are unlikely to be specifically developed for NCCP. However, successful pain modulators developed for IBS and/or functional dyspepsia will most likely be evaluated also in patients with non-GERD-related NCCP, regardless of the presence or absence of esophageal dysmotility. IBS and functional dyspepsia are highly prevalent disorders and thus may attract more interest in the pharmaceutical industry. Many of the compounds under investigation target peripheral receptors of neurotransmitters that are thought to play an important role in pain perception. Potential targets that are currently under consideration include vanilloid receptor ion channels, acid-sensing ion channels, sensory neuron-specific Na+ channels, P2X purinoceptors, cholecystokinin receptors, bradykinin and prostaglandin receptors, glutamate receptors, tachykinin and calcitonin gene-related peptide receptors, as well as peripheral opioid and cannabinoid receptors.65 The peripheral opioid receptor agonists are of high interest, because they may offer visceral analgesic effect without crossing the blood-brain barrier and thus affecting the central nervous system.
Spinal afferents, which play a role in visceral nociception, express tachykinins, a family of biologically active peptides that includes substance P, neurokinins A and B, and neuropeptide K. Tachykinin antagonists may confer a visceral analgesic effect that can be used in non-GERD-related NCCP patients. Neurokinin-1, -2, and -3 receptor antagonists were evaluated only in preclinical trials. Cholecystokinin receptor antagonists such as loxiglumide may alter visceral pain perception.66 However, studies in NCCP are still unavailable.
For GERD-related NCCP, acid-pump antagonists or potassium channel blockers are characterized by rapid onset of action, predictable dose response effect, profound acid suppression, and lack of dependency on meals. Thus, this class of drugs may improve the efficacy of the current PPIs in the treatment of GERD-related NCCP.
Summary
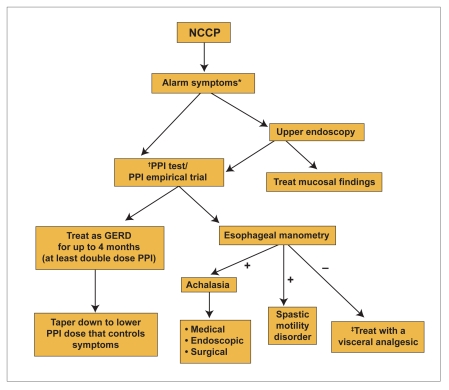
Treatment with a double-dose PPI for a period of 2–4 months should be considered in those with GERD-related NCCP (Figure 2). In patients with nutcracker esophagus, GERD should be first excluded by initiating treatment with a potent antireflux medication.67 In patients with non-GERD-related NCCP, smooth muscle relaxants have demonstrated very limited efficacy in ameliorating symptoms. In contrast, pain modulators such as tricyclic antidepressants, trazodone, and SSRIs have become the mainstay of treatment, regardless of the presence or absence of esophageal dysmotility (except achalesia). Psychological comorbidity is very common in patients with NCCP and thus should not be overlooked. Pharmacologic or nonpharmacologic approaches have been used with varied success. Future therapy for NCCP will likely include new pain modulators and possibly more potent antireflux medications.
Figure 2.
Diagnostic and treatment algorithm for noncardiac chest pain (NCCP).
*Dysphagia, odynophagia, weight loss, anorexia, etc.
† There are available data for omeprazole, lansoprazole (Prevacid, TAP), and rabeprazole (Aciphex, Eisai).
‡ Tricyclic antidepressants, trazodone, and selective serotonin reuptake inhibitors.
- GERD
- gastroesophageal reflux disease
- PPI
- proton pump inhibitor
References
- 1.Wong WM, Lam KF, Cheng C, et al. Population based study of noncardiac chest pain in southern Chinese: prevalence, psychosocial factors and health care utilization. World J Gastroenterol. 2004;10:707–712. doi: 10.3748/wjg.v10.i5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eslick GD, Jones MP, Talley NJ. Non-cardiac chest pain: prevalence, risk factors, impact and consulting-a population-based study. Aliment Pharmacol Ther. 2003;17:1115–1124. doi: 10.1046/j.1365-2036.2003.01557.x. [DOI] [PubMed] [Google Scholar]
- 3.Eslick GD, Coulshed DS, Talley NJ. Review article: the burden of illness of non-cardiac chest pain. Aliment Pharmacol Ther. 2002;16:1217–1223. doi: 10.1046/j.1365-2036.2002.01296.x. [DOI] [PubMed] [Google Scholar]
- 4.Klingler D, Green-Weir R, Nerenz D, et al. Perceptions of chest pain differ by race. Am Heart J. 2002;144:51–59. doi: 10.1067/mhj.2002.122169. [DOI] [PubMed] [Google Scholar]
- 5.Tew R, Guthrie E, Creed FH, et al. A long-term follow-up study of patients with ischemic heart disease versus patients with nonspecific chest pain. J Psychosom Res. 1995;39:977–985. doi: 10.1016/0022-3999(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 6.Chiocca J, Olmos J, Salis G, et al. Prevalence, clinical spectrum and atypical symptoms of gastrooesophageal reflux in Argentina: a nationwide population-based study. Aliment Pharmacol Ther. 2005;22:331–342. doi: 10.1111/j.1365-2036.2005.02565.x. [DOI] [PubMed] [Google Scholar]
- 7.Locke GR, 3rd, Talley NJ, Fett SL, et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 8.Liuzzo JP, Ambrose JA. Chest pain from gastroesophageal reflux disease in patients with coronary artery disease. Cardiol Rev. 2005;13:167–173. doi: 10.1097/01.crd.0000148844.13702.ce. [DOI] [PubMed] [Google Scholar]
- 9.Fass R, Naliboff B, Higa L, et al. Differential effect of long-term esophageal acid exposure on mechanosensitivity and chemosensitivity in humans. Gastroenterology. 1998;115:1363–1373. doi: 10.1016/s0016-5085(98)70014-9. [DOI] [PubMed] [Google Scholar]
- 10.Dekel R, Martinez-Hawthorne SD, Guillen RJ, et al. Evaluation of symptom index in identifying gastroesophageal reflux disease-related noncardiac chest pain. J Clin Gastroenterol. 2004;38:24–29. doi: 10.1097/00004836-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Dekel R, Pearson T, Wendel C, et al. Assessment of oesophageal motor function in patients with dysphagia or chest pain - the Clinical Outcomes Research Initiative experience. Aliment Pharmacol Ther. 2003;18:1083–1089. doi: 10.1046/j.1365-2036.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- 12.Drossman D, Corazziari R, Talley N, et al. Rome II: The Functional Gastrointestinal Disorders. McLean: Degnon Associates; 2000.
- 13.Balaban DH, Yamamoto Y, Liu J, et al. Sustained esophageal contraction: a marker of esophageal chest pain identified by intraluminal ultrasonography. Gastroenterology. 1999;116:29–37. doi: 10.1016/s0016-5085(99)70225-8. [DOI] [PubMed] [Google Scholar]
- 14.Hobson AR, Furlong PL, Sarkar S, et al. Neurophysiologic assessment of esophageal sensory processing in noncardiac chest pain. Gastroenterology. 2006;130:80–88. doi: 10.1053/j.gastro.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Fass R, Bautista J, Janarthanan S. Treatment of gastroesophageal reflux disease. Clin Cornerstone. 2003;5:18–29. doi: 10.1016/s1098-3597(03)90096-2. [DOI] [PubMed] [Google Scholar]
- 16.Kitchin LI, Castell DO. Rationale and efficacy of conservative therapy for gastroesophageal reflux disease. Arch Intern Med. 1991;151:448–454. [PubMed] [Google Scholar]
- 17.Fang J, Bjorkman D. A critical approach to noncardiac chest pain: pathophysiology, diagnosis, and treatment. Am J Gastroenterol. 2001;96:958–968. doi: 10.1111/j.1572-0241.2001.03678.x. [DOI] [PubMed] [Google Scholar]
- 18.DeMeester T, O'Sullivan G, Bermudez G, et al. Esophageal function in patients with angina-type chest pain and normal coronary angiograms. Ann Surg. 1982;196:488–498. doi: 10.1097/00000658-198210000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Achem SR, Kolts BE, MacMath T, et al. Effects of omeprazole versus placebo in treatment of noncardiac chest pain and gastroesophageal reflux. Dig Dis Sci. 1997;42:2138–2145. doi: 10.1023/a:1018843223263. [DOI] [PubMed] [Google Scholar]
- 20.Louis E, Jorissen P, Bastens B, et al. Atypical symptoms of GORD in Belgium: epidemiological features, current management and open label treatment with 40 mg esomeprazole for one month. Acta Gastroenterol Belg. 2006;69:203–208. [PubMed] [Google Scholar]
- 21.Fass R, Malagon I, Schmulson M. Chest pain of esophageal origin. Curr Opin Gastroenterol. 2001;17:376–380. doi: 10.1097/00001574-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Borzecki AM, Pedrosa MC, Prashker MJ. Should noncardiac chest pain be treated empirically? A cost-effectiveness analysis. Arch Intern Med. 2000;160:844–852. doi: 10.1001/archinte.160.6.844. [DOI] [PubMed] [Google Scholar]
- 23.Patti MG, Molena D, Fisichella PM, et al. Gastroesophageal reflux disease (GERD) and chest pain. Results of laparoscopic antireflux surgery. Surg Endosc. 2002;16:563–566. doi: 10.1007/s00464-001-8220-9. [DOI] [PubMed] [Google Scholar]
- 24.Farrell TM, Richardson WS, Trus TL, et al. Response of atypical symptoms of gastro-oesophageal reflux to antireflux surgery. Br J Surg. 2001;88:1649–1652. doi: 10.1046/j.0007-1323.2001.01949.x. [DOI] [PubMed] [Google Scholar]
- 25.So JB, Zeitels SM, Rattner DW. Outcomes of atypical symptoms attributed to gastroesophageal reflux treated by laparoscopic fundoplication. Surgery. 1998;124:28–32. [PubMed] [Google Scholar]
- 26.Orlando R, Bozymski E. Clinical and manometric effects of nitroglycerin in diffuse esophageal spasm. N Engl J Med. 1973;289:23–25. doi: 10.1056/NEJM197307052890106. [DOI] [PubMed] [Google Scholar]
- 27.Millaire A, Ducloux G, Marquand A, et al. Clinical effects and effects on esophageal motility. Arch Mal Coeur Vaiss. 1989;82:63–68. [PubMed] [Google Scholar]
- 28.Swamy N. Esophageal spasm: clinical and manometric response to nitroglycerine and long acting nitrites. Gastroenterology. 1977;72:23–27. [PubMed] [Google Scholar]
- 29.Kikendall J, Mellow M. Effect of sublingual nitroglycerin and long-acting nitrate preparations on esophageal motility. Gastroenterology. 1980;79:703–706. [PubMed] [Google Scholar]
- 30.Mellow M. Effect of isosorbide and hydralazine in painful primary esophageal motility disorders. Gastroenterology. 1982;83:364–370. [PubMed] [Google Scholar]
- 31.Richter J, Dalton C, Buice R, et al. Nifedipine: a potent inhibitor of contractions in the body of the human esophagus. Studies in healthy volunteers and patients with the nutcracker esophagus. Gastroenterology. 1985;89:549–554. doi: 10.1016/0016-5085(85)90450-0. [DOI] [PubMed] [Google Scholar]
- 32.Davies H, Lewis M, Rhodes J, et al. Trial of nifedipine for prevention of oesophageal spasm. Digestion. 1987;36:81–83. doi: 10.1159/000199403. [DOI] [PubMed] [Google Scholar]
- 33.Nasrallah S, Tommaso C, Singleton R, et al. Primary esophageal motor disorders: clinical response to nifedipine. South Med J. 1985;78:312–315. doi: 10.1097/00007611-198503000-00019. [DOI] [PubMed] [Google Scholar]
- 34.Konrad-Danlhoff I, Baunack A, Ramsch K, et al. Effect of the calcium antagonists nifedipine, nirendipine, nimodipine, and nisoldipine on esophageal motility in man. Eur J Clin Pharmacol. 1991;41:313–316. doi: 10.1007/BF00314958. [DOI] [PubMed] [Google Scholar]
- 35.Richter J, Spurling T, Cordova C, et al. Effects of oral calcium blocker, diltiazem, on esophageal contractions. Studies in volunteers and patients with nutcracker esophagus. Dig Dis Sci. 1984;29:649–656. doi: 10.1007/BF01347298. [DOI] [PubMed] [Google Scholar]
- 36.Cattau E, Jr, Castell D, Johnson D, et al. Diltiazem therapy for symptoms associated with nutcracker esophagus. Am J Gastroenterol. 1991;86:272–276. [PubMed] [Google Scholar]
- 37.Drenth J, Bos L, Engels L. Efficacy of diltiazem in the treatment of diffuse oesophageal spasm. Aliment Pharmacol Ther. 1990;4:411–416. doi: 10.1111/j.1365-2036.1990.tb00487.x. [DOI] [PubMed] [Google Scholar]
- 38.Lee JI, Park H, Kim JH, et al. The effect of sildenafil on esophageal motor function in healthy subjects and patients with nutcracker esophagus. Neurogastroenterol Motil. 2003;15:617–623. doi: 10.1046/j.1350-1925.2003.00450.x. [DOI] [PubMed] [Google Scholar]
- 39.Bortolotti M, Pandolfo N, Giovannini M, et al. Effect of sildenafil on hypertensive lower esophageal sphincter. Eur J Clin Invest. 2002;32:682–685. doi: 10.1046/j.1365-2362.2002.01059.x. [DOI] [PubMed] [Google Scholar]
- 40.Egbunike I, Chaffee B. Antidepressants in the management of chronic pain syndromes. Pharmacotherapy. 1990;10:262–270. [PubMed] [Google Scholar]
- 41.Becker B, Morel N, Vanbellinghen A, et al. Blockade of calcium entry in smooth muscle cells by the antidepressant imipramine. Biochem Pharmacol. 2004;68:833–842. doi: 10.1016/j.bcp.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 42.Peghini P, Katz P, Castell D. Imipramine decreases oesophageal pain perception in human male volunteers. Gut. 1998;42:807–813. doi: 10.1136/gut.42.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cannon RO, 3rd, Quyyumi AA, Mincemoyer R, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med. 1994;330:1411–1417. doi: 10.1056/NEJM199405193302003. [DOI] [PubMed] [Google Scholar]
- 44.Gorelick A, Koshy S, Hooper F, et al. Differential effects of amitriptyline on perception of somatic and visceral stimulation in healthy humans. Am J Physiol. 1998;275(3 pt 1):G460–466. doi: 10.1152/ajpgi.1998.275.3.G460. [DOI] [PubMed] [Google Scholar]
- 45.Clouse RE, Lustman PJ, Eckert TC, et al. Low-dose trazodone for symptomatic patients with esophageal contraction abnormalities. A double-blind, placebo-controlled trial. Gastroenterology. 1987;92:1027–1036. doi: 10.1016/0016-5085(87)90979-6. [DOI] [PubMed] [Google Scholar]
- 46.Handa M, Mine K, Yamamoto H, et al. Antidepressant treatment of patients with diffuse esophageal spasm: a psychosomatic approach. J Clin Gastroenterol. 1999;28:228–232. doi: 10.1097/00004836-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Varia I, Logue E, O'Connor C, et al. Randomized trial of sertaline in patients with unexplained chest pain of noncardiac origin. Am Heart J. 2000;140:367–372. doi: 10.1067/mhj.2000.108514. [DOI] [PubMed] [Google Scholar]
- 48.Broekaert D, Fischler B, Sifrim D, et al. Influence of citalopram, a selective serotonin, reuptake inhibitor, on oesophageal hypersensitivity: a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2006;23:365–370. doi: 10.1111/j.1365-2036.2006.02772.x. [DOI] [PubMed] [Google Scholar]
- 49.Bradette M, Delvaux M, Staumont G, et al. Octreotide increases thresholds of colonic visceral perception in IBS patients without modifying muscle tone. Dig Dis Sci. 1994;39:1171–1178. doi: 10.1007/BF02093780. [DOI] [PubMed] [Google Scholar]
- 50.Schwetz I, Naliboff B, Munakata J, et al. Anti-hyperalgesic effect of octreotide in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2004;19:123–131. doi: 10.1111/j.1365-2036.2004.01774.x. [DOI] [PubMed] [Google Scholar]
- 51.Johnston B, Shils J, Leite L, et al. Effects of octreotide on esophageal visceral perception and cerebral evoked potentials induced by balloon distension. Am J Gastroenterol. 1994;94:65–70. doi: 10.1111/j.1572-0241.1999.00772.x. [DOI] [PubMed] [Google Scholar]
- 52.Crea F, Pupita G, Galassi A, et al. Role of adenosine in pathogenesis of anginal pain. Circulation. 1990;81:164–172. doi: 10.1161/01.cir.81.1.164. [DOI] [PubMed] [Google Scholar]
- 53.Rao SS, Mudipalli RS, Mujica V, et al. An open-label trial of theophylline for functional chest pain. Dig Dis Sci. 2002;47:2763–2768. doi: 10.1023/a:1021017524660. [DOI] [PubMed] [Google Scholar]
- 54.Rao SS, Mudipalli RS, Remes-Troche JM, et al. Theophylline improves esophageal chest pain-a randomized, placebo-controlled study. Am J Gastroenterol. 2007 Feb 21; doi: 10.1111/j.1572-0241.2007.01112.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 55.Beitman B, Basha I, Trombka L, et al. Pharmacotherapeutic treatment of panic disorder in patients presenting with chest pain. J Fam Pract. 1989;28:177–180. [PubMed] [Google Scholar]
- 56.Wulsin L, Maddock R, Beitman B, et al. Clonazepam treatment of panic disorder in patients with recurrent chest pain and normal coronary arteries. Int J Psychiatry Med. 1999;29:97–105. doi: 10.2190/X6N2-8HYG-7LLJ-X6U2. [DOI] [PubMed] [Google Scholar]
- 57.Tack J, Sarnelli G. Serotonergic modulation of visceral sensation: upper gastrointestinal tract. Gut. 2002;51(suppl 1):i77–i80. doi: 10.1136/gut.51.suppl_1.i77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez-Stanley S, Zubaidi S, Proskin HM, et al. Effect of tegaserod on esophageal pain threshold, regurgitation, and symptom relief in patients with functional heartburn and mechanical sensitivity. Clin Gastroenterol Hepatol. 2006;4:442–450. doi: 10.1016/j.cgh.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 59.Miller L, Pullela S, Parkman H, et al. Treatment of chest pain in patients with noncardiac, nonreflux, nonachalasia spastic esophageal motor disorders using botulinum toxin injection into the gastroesophageal junction. Am J Gastroenterol. 2002;97:1640–1646. doi: 10.1111/j.1572-0241.2002.05821.x. [DOI] [PubMed] [Google Scholar]
- 60.Moss S, Armstrong D, Arnold R, et al. GERD 2003: a consensus on the way ahead. Digestion. 2002;67:111–117. doi: 10.1159/000071290. [DOI] [PubMed] [Google Scholar]
- 61.Waring J. Surgical and endoscopic treatment of gastroesophageal reflux disease. Gastroenterol Clin North Am. 2002;31(suppl):S89–S109. doi: 10.1016/s0889-8553(02)00044-4. [DOI] [PubMed] [Google Scholar]
- 62.van Peski-Oosterbaan A, Spinhoven P, van Rood Y, et al. Cognitive-behavioral therapy for noncardiac chest pain: a randomized trial. Am J Med. 1999;106:424–429. doi: 10.1016/s0002-9343(99)00049-2. [DOI] [PubMed] [Google Scholar]
- 63.Ryan M, Gervirtz R. Biofeedback-based psychophysiological treatment in a primary care setting: an initial feasibility study. Appl Psychophysiol Biofeedback. 2004;29:79–93. doi: 10.1023/b:apbi.0000026635.03016.ef. [DOI] [PubMed] [Google Scholar]
- 64.Jones H, Cooper P, Miller V, et al. Treatment of noncardiac chest pain: a controlled trial of hypnotherapy. Gut. 2006;55:1403–1408. doi: 10.1136/gut.2005.086694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holzer P. Gastrointestinal afferents as targets of novel drugs for the treatment of functional bowel disorders and visceral pain. Eur J Pharmacol. 2001;429:177–93. doi: 10.1016/s0014-2999(01)01319-x. [DOI] [PubMed] [Google Scholar]
- 66.Scarpignato C, Pelosini I. Management of irritable bowel syndrome: novel approaches to the pharmacology of gut motility. Can J Gastroenterol. 1999;13(suppl A):50A–65A. doi: 10.1155/1999/183697. [DOI] [PubMed] [Google Scholar]
- 67.Achem SR, Kolts BE, Wears R, et al. Chest pain associated with nutcracker esophagus: a preliminary study of the role of gastroesophageal reflux. Am J Gastroenterol. 1993;88:187–192. [PubMed] [Google Scholar]