Abstract
Contamination of food by Listeria monocytogenes is thought to occur most frequently in food-processing environments where cells persist due to their ability to attach to stainless steel and other surfaces. Once attached these cells may produce multicellular biofilms that are resistant to disinfection and from which cells can become detached and contaminate food products. Because there is a correlation between virulence and serotype (and thus phylogenetic division) of L. monocytogenes, it is important to determine if there is a link between biofilm formation and disease incidence for L. monocytogenes. Eighty L. monocytogenes isolates were screened for biofilm formation to determine if there is a robust relationship between biofilm formation, phylogenic division, and persistence in the environment. Statistically significant differences were detected between phylogenetic divisions. Increased biofilm formation was observed in Division II strains (serotypes 1/2a and 1/2c), which are not normally associated with food-borne outbreaks. Differences in biofilm formation were also detected between persistent and nonpersistent strains isolated from bulk milk samples, with persistent strains showing increased biofilm formation relative to nonpersistent strains. There were no significant differences detected among serotypes. Exopolysaccharide production correlated with cell adherence for high-biofilm-producing strains. Scanning electron microscopy showed that a high-biofilm-forming strain produced a dense, three-dimensional structure, whereas a low-biofilm-forming strain produced a thin, patchy biofilm. These data are consistent with data on persistent strains forming biofilms but do not support a consistent relationship between enhanced biofilm formation and disease incidence.
Listeria monocytogenes is a gram-positive bacterium capable of causing morbidity and mortality in both humans and animals. Due to the ubiquitous nature and hardy growth characteristics of this bacterium, L. monocytogenes is able to contaminate and thrive in the food-processing environment (11). In particular, the psychrotrophic nature of L. monocytogenes allows replication in refrigerated, ready-to-eat food products that have been contaminated during processing and packaging. Consequently, L. monocytogenes is frequently associated with food-borne disease outbreaks that are characterized by widespread distribution and relatively high mortality rates.
Although L. monocytogenes is clearly a pathogenic organism, not all infections result in serious illness and healthy carriers from high risk groups have been identified (17). Additionally, most outbreaks are caused by serotype 4b, even though serotype 1/2a is more frequently isolated from food and environmental samples (13). A pathogenicity island has been identified in L. monocytogenes (8, 32), and inactivation of these genes results in attenuation (1, 5, 7, 12, 29, 31). All strains of L. monocytogenes have these virulence-associated genes, and the sequences of many of these genes are conserved (14, 33). Nevertheless, not all strains appear equally capable of causing disease. Therefore, it is likely that a number of factors are involved in the ability of a strain to cause food-borne outbreaks, including differential ability to persist in environments where contamination of food products may occur.
Many bacteria are able to attach to and colonize environmental surfaces by producing a three-dimensional matrix of extracellular polymeric substances (EPS) called biofilm (10). Biofilms allow microorganisms to persist in the environment and resist desiccation, UV light, and treatment with antimicrobial and sanitizing agents. In the case of L. monocytogenes, Djordjevic et al. (9) reported a possible relationship between phylogeny and the ability to form biofilms. On average, strains associated with Division I (serovars 1/2b and 4b) produced more biofilm than did strains from Division II (serovars 1/2a and 1/2c). In contrast, others (18, 21) have shown that serotype 1/2c is a better biofilm former than are 4b strains. There is also disagreement regarding the relationship between persistence and the ability to form biofilms (9, 21). Furthermore, Kalmokoff et al. (16) argue that L. monocytogenes does not form a classic biofilm but simply adheres to surfaces. These differences might be explained by the strains used in the studies, sample sizes, and assay formats. Nevertheless, we were intrigued by the hypothesis that the ability to form biofilms might be conserved within phylogenetic lineages, because this would be consistent with conservation of other phenotypic traits, such as serotype. Furthermore, we wanted to further examine the hypothesis that persistent strains of L. monocytogenes are better biofilm formers than are nonpersistent strains. This pattern would be a logical explanation for cases where a single isolate is repeatedly isolated from a given environment (e.g., bulk milk tank) whereas other isolates may be found rarely.
MATERIALS AND METHODS
Bacterial strains and subtyping.
Bacterial strains and sources are listed in Table 1. L. monocytogenes isolates were subtyped by using serotyping, pulsed-filed gel electrophoresis (PFGE), and microarray analysis as previously described (3, 6, 19). Persistent strains were defined by repeated isolation from bulk milk samples obtained from the same dairy, and strain identity was established by ApaI and AscI PFGE restriction enzyme digest patterns (19). Strains characterized as nonpersistent were sporadically isolated from bulk milk tanks.
TABLE 1.
L. monocytogenes strains used in this study
| Isolate | Serotype | Divisiona | Persistenceb | Sourcec | Isolate | Serotype | Divisiona | Persistenceb | Sourcec | |
|---|---|---|---|---|---|---|---|---|---|---|
| 15C18 | 1/2b | I | FDA | |||||||
| 2492 | 1/2b | I | FDA | |||||||
| 1159 | 1/2b | I | WASDOH | |||||||
| ILSI-7d | 1/2b | I | ILSI | |||||||
| 1164 | 1/2b | I | WASDOH | |||||||
| 9916B | 1/2b | I | USDA | |||||||
| ILSI-dd | 1/2b | I | ILSI | |||||||
| 9900104 | 1/2b | I | WASDOH | |||||||
| 9900101 | 1/2b | I | WASDOH | |||||||
| ILSI-6d | 1/2b | I | ILSI | |||||||
| 2475 | 1/2b | I | FDA | |||||||
| M33027A | 4b | I | NP | USDA | ||||||
| M13565A | 4b | I | NP | USDA | ||||||
| M11056A | 4b | I | NP | USDA | ||||||
| M35402A | 4b | I | NP | USDA | ||||||
| 1161 | 4b | I | WASDOH | |||||||
| V013368 | 4b | I | WADDL | |||||||
| ILSI-24d | 4b | I | ILSI | |||||||
| 3515 | 4b | I | FDA | |||||||
| 2172 | 4b | I | WASDOH | |||||||
| 3365 | 4b | I | FDA | |||||||
| 3276 | 4b | I | FDA | |||||||
| F2365d | 4b | I | CDC | |||||||
| F5070 | 4b | I | CDC | |||||||
| 1329 | 4b | I | WASDOH | |||||||
| 3655 | 4b | I | FDA | |||||||
| G1092 | 4b | I | CDC | |||||||
| 9900094 | 4b | I | WASDOH | |||||||
| 8807-X2 | 4b | I | WADDL | |||||||
| 2150 | 4b | I | WASDOH | |||||||
| 2140 | 4b | I | WASDOH | |||||||
| M35584A | 4c | NP | USDA | |||||||
| 3280 | 4c | FDA | ||||||||
| M36467A | 4c | USDA | ||||||||
| M32771C | 1/2a | II | NP | USDA | ||||||
| M36407A | 1/2a | II | NP | USDA | ||||||
| M36897A | 1/2a | II | NP | USDA | ||||||
| M37010C | 1/2a | II | NP | USDA | ||||||
| M36582B | 1/2a | II | NP | USDA | ||||||
| M37952A | 1/2a | II | NP | USDA | ||||||
| M36509A | 1/2a | II | NP | USDA | ||||||
| M34058E | 1/2a | II | NP | USDA | ||||||
| M36046A | 1/2a | II | NP | USDA | ||||||
| M10867C | 1/2a | II | NP | USDA | ||||||
| M12716B | 1/2a | II | P | USDA | ||||||
| M32490G | 1/2a | II | P | USDA | ||||||
| M35568E | 1/2a | II | P | USDA | ||||||
| M10777A | 1/2a | II | P | USDA | ||||||
| M32490E | 1/2a | P | USDA | |||||||
| M12716E | 1/2a | II | P | USDA | ||||||
| M13235A | 1/2a | II | P | USDA | ||||||
| M35303A | 1/2a | II | P | USDA | ||||||
| M10868A | 1/2a | II | P | USDA | ||||||
| M36012A | 1/2a | II | P | USDA | ||||||
| M39503A | 1/2a | II | P | USDA | ||||||
| 575 | 1/2a | II | USDA | |||||||
| 1165 | 1/2a | II | WASDOH | |||||||
| 2388 | 1/2a | II | FDA | |||||||
| 1166 | 1/2a | II | WASDOH | |||||||
| 750 | 1/2a | II | WASDOH | |||||||
| 1445 | 1/2a | II | WASDOH | |||||||
| 15cO3 | 1/2a | II | FDA | |||||||
| 1157 | 1/2a | II | WASDOH | |||||||
| 841 | 1/2a | II | WASDOH | |||||||
| CI-136 | 1/2a | II | USDA | |||||||
| ILSI-11 | 1/2a | II | ILSI | |||||||
| 3313 | 1/2a | II | FDA | |||||||
| ILSI-35 | 1/2a | II | ILSI | |||||||
| 1163 | 1/2a | II | WASDOH | |||||||
| 9900096 | 1/2c | II | WASDOH | |||||||
| ILSI-17 | 1/2c | II | ILSI | |||||||
| H9333 | 1/2c | II | CDC | |||||||
| H7973 | 1/2c | II | CDC | |||||||
| L028 | 1/2c | II | USDA | |||||||
| H9066 | 1/2c | II | CDC | |||||||
| G3321 | 1/2c | II | CDC | |||||||
| 3326 | 1/2c | II | FDA | |||||||
| H9067 | 1/2c | II | CDC | |||||||
| G1127 | 3a | CDC | ||||||||
| 15a90 | 3a | FDA |
Phylogenetic divisions are as defined by Piffaretti et al. (24).
L. monocytogenes isolates characterized as persistent (P) or nonpersistent (NP) as described by Muraoka et al. (19).
Sample sources are as follows: United States Department of Agriculture, Pullman, Wash. (USDA); Washington State Department of Health, Shoreline, Wash. (WASDOH); Centers for Disease Control and Prevention, Atlanta, Ga. (CDC); U.S. Food and Drug Administration, Bothel, Wash. (FDA); International Life Sciences Institute, L. monocytogenes strains collection (http://www.foodscience.cornell.edu/wiedmann/listeriadbase.htm) (ILSI); WADDL, Washington Animal Disease Diagnostic Laboratory.
Strains common to this study and to that of Djordjevic et al. (9).
Microtiter plate assay.
Isolates were recovered from −80°C glycerol stocks onto tryptic soy agar and were stored at 4°C. Isolated colonies were used to inoculate 3 ml of tryptic soy broth enriched with 0.6% yeast extract (TSBYE) in sterile 15- by 100-mm glass culture tubes and were incubated for 24 h at 37°C. PVCmicrotiter plates (Becton Dickinson, Franklin Lakes, N.J.) were sterilized by incubation in 150 μl of 70% ethanol for 30 min. The ethanol was aseptically removed by pipetting and was air dried for 30 min at 37°C. One milliliter of a 1:40 dilution of each overnight culture was prepared in freshly made Modified Welshimer's broth (MWB) (25) and was vortexed for 5 s. One hundred microliters of this dilution was then used to inoculate eight separate wells of a presterilized polyvinyl chloride (PVC) microtiter plate, and eight wells of MWB media were included as a control. To minimize evaporative loss and edge effects, the outermost rows and columns of each plate were filled with 150 μl of sterile water. The edges of the plate were then sealed with parafilm, and the plates were incubated for 40 h at 30°C. After 40 h the liquid from each of the wells was removed, and unattached cells were removed by rinsing three times in 150 μl of sterile water. Plates were then dried in an inverted position for 30 min. Biofilms were stained by adding 50 μl of a 0.1% crystal violet solution (in sterile water) to each well and incubating for 45 min at room temperature. Unbound dye was removed by rinsing three times in 150 μl of sterile water. The crystal violet was solubilized by adding 200 μl of 95% ethanol and incubating at 4°C for 30 min. The contents of each well (100 μl) were then transferred to a sterile polystyrene microtiter plate, and the optical density at 595 nm (OD595) of each well was measured in a microplate reader (Spectramax Plus 384; Molecular Devices, Sunnyvale, Calif.).
Ruthenium red staining of EPS on glass.
Biofilms were grown on glass slides and were stained with ruthenium red by using modifications of the method described by Prouty et al. (26). Briefly, overnight cultures of a strong biofilm-forming isolate (strain M39503A) were prepared as described above and were diluted 1:40 in MWB, and 35 μl of the dilution was added to each well of a 12-well Teflon-masked slide (Erie Scientific, Portsmouth, N.H.). The slides were incubated in a sterile humidity chamber for 40 h at 30°C and were rinsed gently in 100 mM cacodylate buffer to remove unbound cells. Biofilms were then fixed overnight at 4°C in 2% gluteraldehyde in 100 mM cacodylate buffer and were stained by incubating in a solution of 0.1% ruthenium red (Spectrum Chemical, New Brunswick, N.J.), 0.1% crystal violet, and 0.5% gluteraldehyde in 100 mM cacodylate buffer for 1 h at room temperature. Unbound dye was rinsed away by using 100 mM cacodylate buffer, and biofilms were visualized by using a Zeiss Axioskop 2 plus microscope.
Ruthenium red microplate assay.
Overnight cultures of each isolate were prepared as described above, diluted 1:40 in freshly prepared MWB media, and vortexed for 5 s. Cells (100 μl) were then transferred to seven wells of a presterilized PVC microtiter plate, and 100 μl of sterile MWB was added to the outer well of each row of the microtiter plate as a blank. The plates were incubated for 40 h at 30°C, and unattached cells were removed aseptically by pipetting. An aqueous solution of ruthenium red (0.1%, 150 μl) was added to each well of the plate, and biofilms were stained for 45 min at room temperature. The liquid from each well was then carefully transferred to a polystyrene microplate and the OD450 was measured in a microplate reader (Spectramax plus 384; Molecular Devices). The amount of dye bound by the biofilm in each well was determined by subtracting the OD450 of the well from the average of the 12 blank wells.
Scanning electron microscopic (SEM) analysis of biofilms on stainless steel coupons.
Stainless steel coupons (1 mm diameter, stainless steel 304 finish no. 4) were prepared as described previously (15) and were sterilized by autoclaving at 121°C for 15 min. Coupons were then placed into separate wells of a sterile polystyrene 24-well microtiter plate (Costar, Corning, N.Y.) containing 1 ml of a 1:40 dilution of overnight culture (in MWB) for each isolate and were incubated for 40 h at 30°C. Subsequently the sterile coupons were rinsed by gentle immersion in 100 mM cacodylate buffer and were fixed overnight at 4°C in a 2% gluteraldehyde, 0.1% ruthenium red solution in 100 mM cacodylate buffer. Biofilms were then gently rinsed by immersion in 100 mM cacodylate buffer to remove unbound dye and were dehydrated in serial dilutions of 30, 50, 60, 70, 90, and 95% ethanol for 10 min each, followed by three 10-min rinses in 100% ethanol. Samples were then critical-point dried (Samdri PVT-3B; Tsousimis Research Co, Rockville, Md.) and were immediately sputter coated with gold (Technics Hummer V; Technics, San Jose, Calif.). Biofilms were visualized by using a Hitachi S-570 scanning electron microscope (Hitachi, Mountain View, Calif.).
SEM analysis of biofilms on PVC coupons.
Overnight cultures were grown in TSBYE as described above and were diluted 1:40 in MWB. One milliliter of each dilution was added to a single well of a 24-well microtiter plate (Costar) containing a 5- by 5-mm PVC coupon (cut from a lid of a 96-well microtiter plate; Becton Dickinson) that was presterilized in 70% ethanol for 20 min. The plate was incubated for 40 h at 30°C and then was rinsed and fixed as described above. After fixing, coupons were rinsed twice in sterile H2O to remove residual buffer and were dehydrated by being placed in liquid nitrogen for 5 min followed by freeze drying for 24 h in an Emitech K750X freeze drier (Empdirect, Houston, Tex.). Samples were gold sputter coated and visualized as described above.
Statistical analysis.
NCSS 2000 (NCSS Statistical Software, Kaysville, Utah) was used for statistical analysis. Parametric tests (e.g., Student's t test) were performed when data were normally distributed as assessed by Omnibus normality of residuals test. Nonparametric statistical tests (e.g., Mann-Whitney or Krustal-Wallis) were used for data that were not normally distributed.
RESULTS
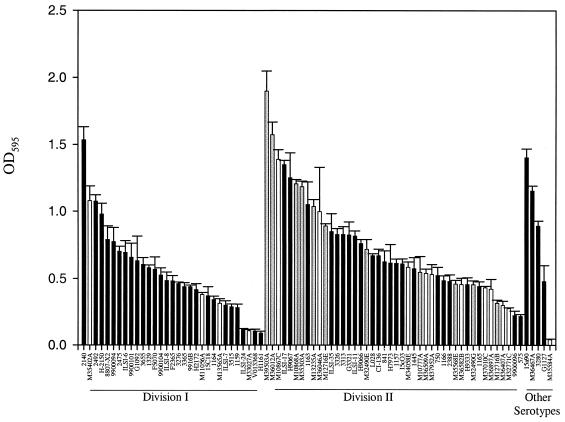
Eighty L. monocytogenes strains were assayed for biofilm formation by using a microtiter plate assay. As reported in other studies (9, 18, 21, 22), there was significant interstrain variability in biofilm formation (Fig. 1). Significant differences were evident between phylogenetic divisions of L. monocytogenes, but in contrast to a previous report (9), Division II strains were significantly better biofilm formers than were Division I strains (Division I average, 0.54; Division II average, 0.73; P = 0.02, Mann-Whitney test) (Fig. 1). Variation in biofilm formation at the level of serotypes was also detected; however, it was not statistically significant (Table 2). Serotypes 3a, 1/2c, and 1/2a had the highest average intensity values, but the range of absorption values was high and no statistically significant differences were observed among serotypes (P = 0.11, Kruskal-Wallis one-way analysis of variance). Persistent strains were significantly better biofilm formers than were strains obtained sporadically from bulk milk tanks (persistent strain average, 0.93; sporadic strain average, 0.55; P = 0.027, two-sample Student's t test) (Fig. 1).
FIG. 1.
L. monocytogenes biofilm formation measured by microtiter plate assay (crystal violet destaining). Bars represent average OD595 values and standard errors. Persistent strains are represented by gray bars, nonpersistent strains are represented by white bars, and all other strains have black bars. Division I consists of serotypes 1/2b and 4b, Division II consists of serotypes 1/2a and 1/2c, and strains belonging to serotypes 4c and 3a are listed as Other Serotypes.
TABLE 2.
Average OD for six serotypes of L. monocytogenes based on a crystal violet destaining biofilm assay
| Serotype | Assay results
|
|||
|---|---|---|---|---|
| Mean OD | SD | Range | Sample size | |
| 1/2a | 0.713 | 0.381 | 1.90-0.22 | 35 |
| 1/2b | 0.534 | 0.236 | 1.08-0.28 | 11 |
| 1/2c | 0.774 | 0.354 | 1.35-0.22 | 9 |
| 3a | 0.939 | 0.653 | 1.40-0.48 | 2 |
| 4b | 0.537 | 0.366 | 1.53-0.09 | 20 |
| 4c | 0.695 | 0.580 | 1.15-0.04 | 3 |
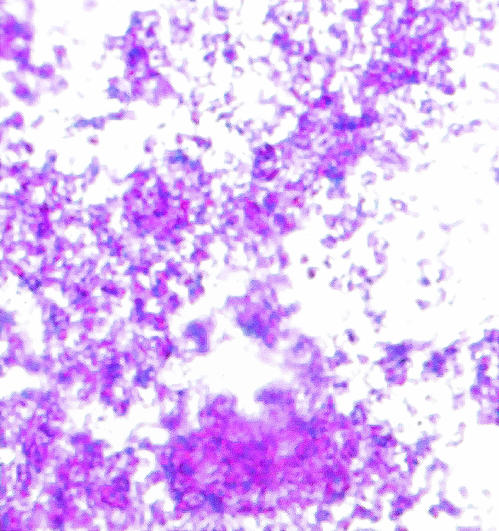
There is still some question as to whether or not L. monocytogenes forms a classic biofilm or if observed differences using the crystal violet assay merely reflect differential adherence to the substrate (16). L. monocytogenes is not known to produce capsules (30), and therefore we hypothesized that if we could stain the cells with a carbohydrate-binding dye we could measure actual EPS production. Consequently, we developed a microtiter plate assay using ruthenium red, which is a carbohydrate-binding dye. All L. monocytogenes tested in this study clearly stained by this method, which is consistent with production of biofilm. This observation was verified by staining cell-associated EPS on glass slides (Fig. 2). To quantify EPS produced by each strain, the amount of dye bound to the cell layer was measured. Because ruthenium red is not soluble once bound to carbohydrates, the amount of bound dye was estimated by measuring the amount of unbound dye and comparing this to the amount of dye initially added to the well. The uptake of ruthenium red was measured for the 10 strains that had the highest adherence to PVC (as determined by crystal violet uptake in the microtiter plate assay) and the 10 strains with the lowest cell adherence in the microtiter assay. Although the mean for the high-adherence group was greater than the mean for the low-adherence group (0.129 and 0.118, respectively), the difference was not significant (P = 0.28, two-tailed Student's t test) and there was no correlation between crystal violet and ruthenium red uptake (Spearman Rank correlation coefficient, 0.45). Nevertheless, the four strains with the highest ruthenium red uptake also showed the highest adherence, and enhanced staining by ruthenium red was obvious on visual inspection. Therefore, we hypothesized that indirect measurement of ruthenium red in this format lacks sensitivity when low numbers of adherent cells are present. To test this hypothesis, the correlation of crystal violet and ruthenium red uptake was measured separately for the high-cell-adherence group and the low-cell-adherence group. The correlation coefficient was significant for the high-cell-adherence group (Pearson correlation coefficient, 0.89) but not for the low-cell-adherence group (Spearman Rank correlation coefficient, −0.04).
FIG. 2.
Ruthenium red carbohydrate stain of L. monocytogenes strain M39503A on glass (magnification, ×100). Crystal violet (purple) was used to stain bacterial cells, and ruthenium red (pink) was used to stain extracellular carbohydrate. The presence of pink stain between cells is consistent with an EPS matrix.
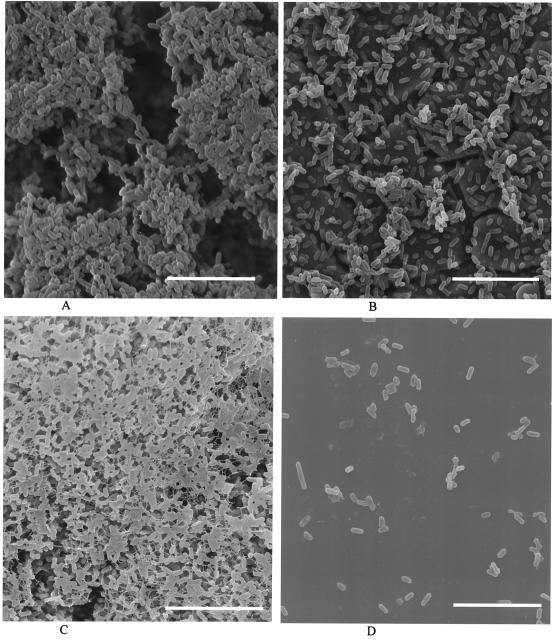
SEM was used to examine a high biofilm former (strain M39503A) and a low biofilm former (strain M35584A) grown on stainless steel and PVC. The high-biofilm-forming strain was originally classified as a persistent strain from bulk milk samples, and it clearly produced a dense, three-dimensional composite of cells with well-distributed channels and pores on both stainless steel and PVC (Fig. 3). Conversely, the low-biofilm-forming strain (nonpersistent from bulk milk) produced only sparse aggregates of cells on stainless steel and predominantly single attached cells on PVC (Fig. 3).
FIG. 3.
SEM images of strain M39503A (high biofilm former) on stainless steel (A) and PVC (C) and strain M35584A (low biofilm former) on stainless steel (B) and PVC (D). The cracks visible under cells in panel B are artifacts in the stainless steel surface. Scale bars, 8.6 μm.
DISCUSSION
Numerous molecular analyses have classified L. monocytogenes strains into two major phylogenetic divisions (2, 3, 4, 6, 20, 24, 27), with Division I consisting of serotypes 4b and 1/2b and Division II consisting of serotypes 1/2a and 1/2c. A third division has also been described, although the division composition is not well defined (28, 34). Djordjevic et al. (9) reported that Division I strains were significantly better at forming biofilms than strains belonging to Division II, and this suggested a possible relationship between biofilm production and the phylogenetic division most closely associated with food-borne outbreaks. They also found no differences in the ability of different serotypes to produce biofilms. Our data were consistent with a correlation between phylogeny and biofilm formation, but our findings were opposite of the previous study. We also found no relationship between biofilms and serotypes, but sample sizes for some serotypes were small for both the present study and for Djordjevic et al. (9). Conflicting conclusions might arise due to differences in methodology, samples sizes, and specific strains used in the analyses.
Our panel of L. monocytogenes isolates included strains from several different studies with eight strains common to Djordjevic et al. (9) (Table 1). Although our microtiter plate assay was similar to that described in previous publications (9, 23), we made several modifications to increase assay reproducibility. For example, we found that decreasing the concentration of crystal violet from 1.0% to 0.1% allowed comparable saturation of attached cells while minimizing dye precipitation that contributed to higher variance. To determine if methodology explained the differences in phylogenetic correlation with biofilms, five of the eight Djordjevic et al. (9) isolates were analyzed by using both our modified microtiter assay protocol and the unmodified protocol described by Djordjevic et al. (9). The average OD values from the two methods were not statistically different (P = 0.3). Although this comparison was based on a small number of isolates, the results suggest that differences between the two studies are not due to methodology. Instead, discrepancies probably reflect differences in sample sizes and the specific strains used in each study.
Differences in biofilm formation were also detected between persistent and nonpersistent strains isolated from bulk milk samples (P = 0.027) (Fig. 3). Although these data agree with observations made by Lunden at al. (18), Djordjevic et al. (9) found no difference between persistent and nonpersistent strains. It is important to note that the definition of strain persistence may vary between studies, and a classification scheme such as this is going to be heavily affected by sampling error. That is, because there may be a low probability of detecting persistent strains during every site visit, strains defined as nonpersistent may include both nonpersistent and persistent strains and therefore statistical power will be low unless large sample sizes are evaluated.
It is unclear if the crystal violet microtiter assay measures three-dimensional biofilm formation or simply the number of cells adhered to the test surface. Kalmokoff et al. (16) reported that L. monocytogenes strains appear to attach to stainless steel but do not actually form biofilm. To investigate the actual production of EPS, a carbohydrate-binding dye was used to stain the attached cells (Fig. 2). All L. monocytogenes strains stained with ruthenium red, indicating that they were producing some type of extracellular carbohydrate consistent with biofilm matrix (26). The strains showing the highest cell adherence with the crystal violet microtiter assay also had the most intense staining with the ruthenium red assay. Nevertheless, because this dye may also bind carbohydrates present on the cell surface but not associated with biofilm formation, these data are not conclusive. Therefore, we employed SEM imaging to examine the structure of biofilms for a high-biofilm former (persistent strain) and a low-biofilm former (nonpersistent strain) on stainless steel and PVC (Fig. 3). Although the low-biofilm-forming strain clearly adhered to the stainless steel surface, only patches of aggregated cells formed and the cracks in the stainless steel surface were clearly visible even after 40 h of growth. When grown on a PVC surface, the low-biofilm-forming strain adhered to the surface in low numbers and did not produce a biofilm. Conversely, the high-biofilm-forming strain formed a dense, three-dimensional network of cells with channels apparent among the layers of aggregated cells on both stainless steel and PVC. These results indicate that some L. monocytogenes strains are capable of conventional biofilm formation and validate the observation of high variance between strains as indicated by this and other studies. Additionally, the SEM data indicate that variance in biofilm formation depends on the substrate tested.
In conclusion, biofilm formation correlated with phylogenetic division but not serotype. By using the crystal violet microtiter assay it was possible to differentiate strong- and weak-biofilm-forming L. monocytogenes strains where persistent strains produced higher assay values. Nevertheless, all strains tested positive for extracellular polysaccharides, with the highest biofilm formers producing noticeably more EPS. SEM analysis revealed dramatic structural differences in biofilm formation between persistent and nonpersistent strains of L. monocytogenes.
Acknowledgments
We gratefully acknowledge the excellent technical assistance provided by Edward Kuhn, Stacey LaFrentz, Edith Orozco, and the staff at the Washington State University Scanning Electron Microscopy Center. L. monocytogenes isolates were kindly provided by Peggy Hayes and Louis Graves (Centers for Disease Control and Prevention, Atlanta, Ga.), Jinxin Hu (Washington State Department of Health, Olympia, Wash.), Karen Jinneman (U.S. Food and Drug Adminisration, Bothel, Wash.), and Lisa Gorski (U.S. Department of Agriculture—Agricultural Research Service, Albany, Calif.).
Funding was provided by USDA-Agricultural Research Service CWU 5348-32000-017-00D; the Agricultural Animal Health Program (College of Veterinary Medicine, Washington State University); and the National Science Foundation through a Faculty Early Career Development award to Frank Loge (BES-0092312) and an Integrative Graduate Education and Research Training grant (NSF grant DGE-9972817) to Washington State University.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the supporting organizations.
REFERENCES
- 1.Barry, R. A., H. G. Bouwer, D. A. Portnoy, and D. J. Hinrichs. 1992. Pathogenicity and immunogenicity of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect. Immun. 60:1625-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bibb, W. F., B. G. Gellin, R. Weaver, B. Schwartz, B. D. Plikaytis, M. W. Reeves, R. W. Pinner, and C. V. Broome. 1990. Analysis of clinical and food-borne isolates of Listeria monocytogenes in the United States by multilocus enzyme electrophoresis and application of the method to epidemiologic investigations. Appl. Environ. Microbiol. 56:2133-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borucki, M. K., M. J. Krug, W. T. Muraoka, and D. R. Call. 2003. Discrimination among Listeria monocytogenes isolates using a mixed genome DNA microarray. Vet. Microbiol. 92:351-362. [DOI] [PubMed] [Google Scholar]
- 4.Brosch, R., J. Chen, and J. B. Luchansky. 1994. Pulsed-field fingerprinting of listeriae: identification of genomic divisions for Listeria monocytogenes and their correlation with serovar. Appl. Environ. Microbiol. 60:2584-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brundage, R. A., G. A. Smith, A. Camilli, J. A. Theriot, and D. A. Portnoy. 1993. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc. Natl. Acad. Sci. USA 90:11890-11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Call, D. R., M. K. Borucki, and T. E. Besser. 2003. Mixed-genome microarrays reveal multiple serotype and lineage-specific differences among strains of Listeria monocytogenes. J. Clin. Microbiol. 41:632-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty, T., T. Hain, and E. Domann. 2000. Genome organization and the evolution of the virulence gene locus in Listeria species. Int. J. Med. Microbiol. 290:167-174. [DOI] [PubMed] [Google Scholar]
- 9.Djordjevic, D., M. Wiedmann, and L. A. McLandsborough. 2002. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 68:2950-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnelly, C. W. 2001. Listeria monocytogenes: a continuing challenge. Nutr. Rev. 59:183-194. [DOI] [PubMed] [Google Scholar]
- 12.Freitag, N. E., L. Rong, and D. A. Portnoy. 1993. Regulation of the prfA transcriptional activator of Listeria monocytogenes: multiple promoter elements contribute to intracellular growth and cell-to-cell spread. Infect. Immun. 61:2537-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilot, P., A. Genicot, and P. Andre. 1996. Serotyping and esterase typing for analysis of Listeria monocytogenes populations recovered from foodstuffs and from human patients with listeriosis in Belgium. J. Clin. Microbiol. 34:1007-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graves, L. M., B. Swaminathan, and S. Hunter. 1999. Subtyping Listeria monocytogenes, p. 279-298. In E. T. Ryser and E. H. Marth (ed.), Listeria, listeriosis, and food safety. Marcel Dekker, New York, N.Y.
- 15.Hood, S., and E. A. Zottola. 1997. Growth media and surface conditioning influence the adherence of Pseudomonas fragi, Salmonella typhimurium, and Listeria monocytogenes cells to stainless steel. J. Food Prot. 60:1034-1037. [DOI] [PubMed] [Google Scholar]
- 16.Kalmokoff, M. L., J. W. Austin, X. D. Wan, G. Sanders, S. Banerjee, and J. M. Farber. 2001. Adsorption, attachment and biofilm formation among isolates of Listeria monocytogenes using model conditions. J. Appl. Microbiol. 91:725-734. [DOI] [PubMed] [Google Scholar]
- 17.Lamont, R. J., and R. Postlethwaite. 1986. Carriage of Listeria monocytogenes and related species in pregnant and non-pregnant women in Aberdeen, Scotland. J. Infect. 13:187-193. [DOI] [PubMed] [Google Scholar]
- 18.Lunden, J. M., M. K. Miettinen, T. J. Autio, and H. J. Korkeala. 2000. Persistent Listeria monocytogenes strains show enhanced adherence to food contact surface after short contact times. J. Food Prot. 63:1204-1207. [DOI] [PubMed] [Google Scholar]
- 19.Muraoka, W., C. Gay, D. Knowles, and M. Borucki. 2003. Prevalence of Listeria monocytogenes subtypes in bulk milk of the pacific northwest. J. Food Prot. 66:1413-1419. [DOI] [PubMed] [Google Scholar]
- 20.Norrung, B., and N. Skovgaard. 1993. Application of multilocus enzyme electrophoresis in studies of the epidemiology of Listeria monocytogenes in Denmark. Appl. Environ. Microbiol. 59:2817-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norwood, D. E., and A. Gilmour. 1999. Adherence of Listeria monocytogenes strains to stainless steel coupons. J. Appl. Microbiol. 86:576-582. [DOI] [PubMed] [Google Scholar]
- 22.Norwood, D. E., and A. Gilmour. 2001. The differential adherence capabilities of two Listeria monocytogenes strains in monoculture and multispecies biofilms as a function of temperature. Lett. Appl. Microbiol. 33:320-324. [DOI] [PubMed] [Google Scholar]
- 23.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 449-461. [DOI] [PubMed]
- 24.Piffaretti, J. C., H. Kressebuch, M. Aeschbacher, J. Bille, E. Bannerman, J. M. Musser, R. K. Selander, and J. Rocourt. 1989. Genetic characterization of clones of the bacterium Listeria monocytogenes causing epidemic disease. Proc. Natl. Acad. Sci. USA 86:3818-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Premaratne, R. J., W. J. Lin, and E. A. Johnson. 1991. Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl. Environ. Microbiol. 57:3046-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prouty, A. M., W. H. Schwesinger, and J. S. Gunn. 2002. Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect. Immun. 70:2640-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasmussen, O. F., T. Beck, J. E. Olsen, L. Dons, and L. Rossen. 1991. Listeria monocytogenes isolates can be classified into two major types according to the sequence of the listeriolysin gene. Infect. Immun. 59:3945-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen, O. F., P. Skouboe, L. Dons, L. Rossen, and J. E. Olsen. 1995. Listeria monocytogenes exists in at least three evolutionary lines: evidence from flagellin, invasive associated protein and listeriolysin O genes. Microbiology 141:2053-2061. [DOI] [PubMed] [Google Scholar]
- 29.Schluter, D., E. Domann, C. Buck, T. Hain, H. Hof, T. Chakraborty, and M. Deckert-Schluter. 1998. Phosphatidylcholine-specific phospholipase C from Listeria monocytogenes is an important virulence factor in murine cerebral listeriosis. Infect. Immun. 66:5930-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seeliger, H. P., and D. Jones. 2003. Listeria, p. 1235-1245. In P. H. Sneath (ed.), Bergey's manual of systematic bacteriology. Williams & Wilkins, Baltimore, Md.
- 31.Suarez, M., B. Gonzalez-Zorn, Y. Vega, I. Chico-Calero, and J. A. Vazquez-Boland. 2001. A role for ActA in epithelial cell invasion by Listeria monocytogenes. Cell Microbiol. 3:853-864. [DOI] [PubMed] [Google Scholar]
- 32.Vazquez-Boland, J. A., G. Dominguez-Bernal, B. Gonzalez-Zorn, J. Kreft, and W. Goebel. 2001. Pathogenicity islands and virulence evolution in Listeria. Microbes Infect. 3:571-584. [DOI] [PubMed] [Google Scholar]
- 33.Vines, A., and B. Swaminathan. 1998. Identification and characterization of nucleotide sequence differences in three virulence-associated genes of Listeria monocytogenes strains representing clinically important serotypes. Curr. Microbiol. 36:309-318. [DOI] [PubMed] [Google Scholar]
- 34.Wiedmann, M., J. L. Bruce, C. Keating, A. E. Johnson, P. L. McDonough, and C. A. Batt. 1997. Ribotypes and virulence gene polymorphisms suggest three distinct Listeria monocytogenes lineages with differences in pathogenic potential. Infect. Immun. 65:2707-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]