Abstract
Background: Infusion reactions have been associated with infliximab therapy, but no study has assessed how physicians treat and manage this common adverse event. Goals: To determine how gastroenterologists manage infusion reactions, identify prophylactic pretreatment protocols, and determine infliximab treatment persistence in the presence of infusion reactions. Method: This retrospective multicenter chart review analyzed data from adults younger than 90 years at the time of their first infliximab infusion from 9 academic or community-based gastroenterology practices. Infusion reaction rates were compared using a Chi-square test with Yates' correction. Kaplan-Meier methods assessed infliximab treatment persistency. Results: Among 6,468 infusions with known infusion reaction status administered to 447 patients, 3.5% (226/6,468) of infusions resulted in an infusion reaction, and less than 0.1% (2/6,468) were associated with a serious infusion reaction. Among all patients, 19.7% (88/447) experienced at least 1 infusion reaction, whereas 0.4% (2/447) experienced a serious infusion reaction. Patients receiving concomitant immunosuppressives had fewer infusion reactions compared to patients not receiving them (57/322 patients, 17.7% vs 31/125 patients, 24.8%; P=.118). The cumulative proportion of patients continuing infliximab therapy at 2, 4, and 5 years was 73%, 58%, and 54%, respectively. Conclusions: The incidence of serious infusion reactions was low. In the overall experience observed in this clinical practice retrospective cohort, no conclusions can be drawn regarding the effectiveness of specific infusion reaction prophylactic measures. In spite of infusion reactions, the long-term infliximab treatment persistence rate was high.
Keywords: Infliximab, infusion reaction, prophylaxis, treatment persistency
Crohn's disease (CD) is associated with long-term morbidity, increased mortality, poor quality of life, and increased use of healthcare resources. Management of this chronic disease often includes the use of medication during both asymptomatic and symptomatic stages.1 Pharmacologic options for disease control include aminosalicylates, immunosuppressives, antibiotics, corticosteroids, and biologic agents. Infliximab, a monoclonal antibody targeted against tumor necrosis factor (TNF)-α, is effective for inducing and maintaining disease remission in patients with moderate-to-severe CD.2–4
Findings derived from clinical experiences with infliximab have been published; however, these reports are limited to specific experiences at particular centers over short periods of time.5–9 As such, the long-term persistence of infliximab use in patients with CD is not well defined. In reports of studies that included assessments of medication compliance in inflammatory bowel disease in general, rates of nonadherence ranged from approximately 20% for short-term treatment to approximately 50% for long-term therapy.10–13 These nonadherence rates are similar to those reported for other chronic diseases.14 In addition, although infusion reactions associated with infliximab have been reported in the literature, few reports on the management of infusions reactions are available. Cheifetz and colleagues reported that infusion reactions (acute or delayed) occurred with 6.1% of infusions, affecting 9.7% of CD patients treated with infliximab.15 Treatment with a combination of acetaminophen, antihistamines, corticosteroids, and/or epinephrine resulted in rapid resolution of all acute reactions (reported to occur with 5% of infusions). The researchers established a protocol for the management of acute reactions and implemented a prophylaxis regimen in the patients who had experienced prior reactions. In this study, our aim was to further understand the clinical spectrum of infusion reaction management in light of the general patterns of infliximab use (including dose adjustments and persistency of use) in CD patients across several treatment centers in the United States for up to a 5-year period.
A qualitative survey of methods used to treat infusion reactions in patients treated with infliximab at high-prescribing gastroenterology and rheumatology centers was conducted between November 2003 and February 2004. From the outcome of this informal survey, a formal and quantitative medical record abstraction study was designed to collect and analyze data from rheumatoid arthritis and CD patients treated at these centers. Here, we report the results from CD patients treated with infliximab.
Materials and Methods
Study Design
This quantitative study was a multicenter retrospective chart review of patients with CD treated at academic and community-based gastroenterology practices with extensive in-office infusion experience. Recruitment of centers was based on patient volume, the ability of the site staff to review medical records and to complete medical record abstraction forms, and the availability of information pertaining to infliximab pretreatment methods. After site recruitment was conducted from January 2005 through September 2005, each study site was asked to identify a patient who received their first infliximab infusion before December 31, 2003, and then to collect data on 50 consecutive eligible patients receiving their first infliximab infusion before this index patient. The research protocol was approved by the central or local institutional review board at each participating gastroenterology practice, and data were collected in accordance with the Health Insurance Portability and Accountability Act (HIPAA).
Physicians or their designees were compensated for the administrative cost of completing patient record abstraction forms, but patients were not compensated for their participation. Centocor, the manufacturer of infliximab, sponsored the study and conducted post-hoc analyses, although all data were collected, managed, and analyzed by Galt Associates (now Cerner Galt), an independent research organization.
Study Participants
Patients who were younger than 90 years of age at the time of their first infusion and who had initiated therapy on or before December 31, 2003, at a study center were eligible for this study. Data were collected from the initial infusion until the date of data abstraction, treatment discontinuation, or loss of follow-up. Patients whose infliximab treatment occurred at a center other than a study site (eg, intermittent treatment in a hospital infusion center or treatment during travel) were included only from their initial infusion up to their last successive treatment at that study center. Patients being treated with infliximab under an experimental protocol were excluded.
Data Collection
The medical staff at each participating center reviewed and abstracted the predefined data points required for each eligible infliximab-treated patient. Participating study centers returned the completed chart abstraction forms in postage-paid envelopes to Galt, although they were required to keep a photocopy of the completed abstraction form for each patient. Data from the chart abstraction forms were reviewed by Galt staff or their designees, who contacted research sites to obtain any missing data or for data clarification. Patient data were double-entered, and data discrepancies were identified and resolved against the source abstraction form.
The collected data included the geographic region of the treating physician's practice, patient demographics (age at first infusion, gender, race), and disease information (treatment indications for infliximab, allergy history, concomitant medications). For each patient, the collected data for each infliximab infusion included infusion number (first, second, third, etc), information pertaining to changes in concomitant medications, infliximab dose, initial infusion rate (mL/min), duration of infusion (hrs), medication administered before or during an infusion to control infusion reactions (including over-the-counter or prescription prophylaxis taken by the patient before the infusion), and the outcome of the infusion (completed, stopped and restarted, stopped and not restarted). Also documented was the overall infliximab treatment outcome (eg, still receiving infliximab, discontinued infliximab therapy for what reason, switched to another anti-TNF-α product).
Infusion-related adverse events (ie, those occurring during the infusion or within 1 hour postinfusion) were documented as one or more of the following symptoms: infusion syndrome, flushing, headache, urticaria, nausea/vomiting, pruritus, chest pain, dyspnea, hypertension, hypotension, chills, allergic reaction, anaphylaxis, rash, tachycardia, angioedema, abdominal pain, back pain, bronchospasm, face edema, fever, or throat tightness. Physicians classified the intensity of all infusion reaction symptoms as mild (an event characterized by “awareness of symptoms which were easily tolerated”), moderate (an event in which “sufficient discomfort was present to cause interference with usual activity”), or severe (an event characterized by “extreme distress that caused significant impairment of function or incapacitation”).
Although this was a retrospective medical record study for which no intervention, procedure, or change in healthcare was dictated by research protocol, any serious infusion reaction or other serious adverse event (ie, a newly identified malignancy or serious infection) identified by the chart abstraction process was reported to Centocor within 24 hours of identification, regardless of the perceived relationship to infliximab.
Statistical Methods
Descriptive statistics were used to characterize continuous variables, and dichotomous endpoints were described using counts and percents. Where appropriate, proportions were compared using the Chi-square test with Yates' correction.16 Infusion reactions were described using both total patient count and total infusion count as denominators, allowing crude estimates of the risk of an infusion reaction per patient and per infusion in temporal sequence. Odds ratios and 95% confidence intervals were calculated17 for the risk of infusion reactions, with or without concomitant immunosuppressive therapy per patient and per infusion.
Persistency data were analyzed using survival analysis methods. In this study, the persistency rate was defined as the cumulative proportion of patients remaining on treatment at a given time point throughout the follow-up period. The Kaplan-Meier method18 was used to assess persistency rates. Data for patients who left the study while on treatment or who were lost to follow-up were included up to the last available data time point, at which time they were censored. Cox proportional hazards regression19 was used to assess the effects of various demographic parameters (age, gender, race) on infliximab treatment persistency. Also included in the model were variables indicating whether patients were receiving immunosuppressives and whether they were pretreated at all infusions.
The level of exposure (number of infliximab vials used per year) was calculated as follows:
For each infusion, the number of vials was calculated as the total dose in mg divided by 100 (because each vial contains 100 mg). Fractions of vials were rounded up to the next whole number.
The number of vials per year was the sum of the vials used over a full 12-month period. Partial vials were excluded from the calculations.
Results
A total of 447 CD patient charts from 9 gastroenterology centers were reviewed, and the overall cohort of patients had a total follow-up of 1,013 patient-years. The median (interquartile range) years of follow-up was 2.2 (1.1–3.2) years per patient (Table 1). The mean age was 40.9 years, the majority (88.4%) of patients were Caucasian, and there was a slight predominance of women (56.2%) in the study. Seventy-two percent of patients received concomitant immunosuppressive therapy (eg, azathioprine, 6-mercaptopurine, or methotrexate). The total number of infliximab infusions in up to 5 years of follow-up was 6,469 (although infusion reaction information was unknown for one infusion). Of the 447 CD patient charts reviewed, 142 patients (31.8%) discontinued infliximab therapy, with the occurrence of an adverse event (8.5%) as the most common reason (Table 2). Smaller numbers of patients discontinued therapy because of a lack of efficacy (6.5%) or because of infusion reactions (4.3%). In addition, 63 patients (14.1%) either moved or were lost to follow-up, with their continued infliximab-treatment status unknown.
Table 1.
Characteristics of Patients With Crohn's Disease Evaluated in Study
| Number of patients | 447 |
| Number of infusions | 6,469 |
| Duration of follow-up (yrs) | |
| Mean (SD) | 2.3 (1.5) |
| Median (IQ range) | 2.2 (1.1–3.2) |
| Age at first infusion (yrs), mean (SD) | 40.9 (14.7) |
| Gender, n (%) | |
| Men | 196 (43.9) |
| Women | 251 (56.2) |
| Race, n (%) | |
| Caucasian | 395 (88.4) |
| African American | 34 (7.6) |
| Hispanic | 7 (1.6) |
| Asian | 1 (0.2) |
| Other | 5 (1.1) |
| Unknown | 5 (1.1) |
Table 2.
Reasons for Discontinuation of Infliximab Therapy Among Crohn's Disease Patients Evaluated in Study*
| Reason, n (%)† | n=447 |
|---|---|
| Moved/lost to follow-up | 63 (14.1) |
| Total number of discontinuations | 142 (31.8) |
| Adverse event | 38 (8.5) |
| Infusion reaction | 19 (4.3) |
| Delayed reaction | 4 (0.9) |
| Other adverse events | 15 (3.4) |
| Lack of efficacy | 29 (6.5) |
| Loss/change of insurance | 17 (3.8) |
| Surgery | 16 (3.6) |
| Discontinued by patient's choice | 14 (3.1) |
| Disease remission | 13 (2.9) |
| Switched therapy | 6 (1.3) |
| Other medical issues | 5 (1.1) |
| Unknown | 4 (0.9) |
Table also includes the number of patients who moved or were lost to follow-up.
Proportions based on the total number of patients.
Infusion Reactions and Pretreatment Protocols
In this study, infusion reactions and serious infusion reactions occurred in 3.5% (226/6,468) and less than 0.1% (2/6,468) of infusions, respectively. When assessed on a per patient basis, infusion reactions and serious infusion reactions occurred in 19.7% (88/447) and 0.4% (2/447) of patients, respectively.
The most commonly used pretreatment medications were acetaminophen (3,298/3,625 infusions, 91.0%), standard antihistamine (eg, diphenhydramine) (2,090/3,625 infusions, 57.7%), nonsedating antihistamine (eg, loratadine) (1,330/3,625 infusions, 36.7%), and H2-antagonists (834/3,625 infusions, 23.0%). Systemic corticosteroids (615/3,625 infusions, 17.0%), other medications (46/3,625 infusions, 1.3%), and narcotic analgesics (9/3,625 infusions, 0.2%) were also used, but less frequently.
The most frequently used pretreatment protocols (ie, a single medication or combination of medications used to pretreat at least 100 infusions) were:
acetaminophen, nonsedating antihistamines
acetaminophen, standard antihistamines
acetaminophen, standard antihistamines, H2-antagonists
acetaminophen, standard antihistamines, corticosteroids
acetaminophen
acetaminophen, standard antihistamines, H2-antagonists, corticosteroids
The incidence of infusion reactions for these frequently used pretreatment protocols among all infusions is summarized in Table 3.
Table 3.
Incidence of Infusion Reactions by Pretreatment Protocol
| Pretreatment Protocol | Number of infusions, n(%)*† | Infusions with an infusion reaction, n(%)†‡ |
|---|---|---|
| Total | 6,468 (100.0) | 226 (3.5) |
| Total not pretreated | 2,844 (44.4) | 58 (2.0) |
| Total pretreated | 3,624 (56.0) | 168 (4.6) |
| Acetaminophen, nonsedating antihistamines | 1,098 (30.3) | 10 (0.9) |
| Acetaminophen, standard antihistamines | 878 (24.2) | 22 (2.5) |
| Acetaminophen, standard antihistamines, H2-antagonists | 645 (17.8) | 15 (2.3) |
| Acetaminophen, standard antihistamines, corticosteroids | 256 (7.1) | 28 (10.9) |
| Acetaminophen | 168 (4.6) | 1 (0.6) |
| Acetaminophen, standard antihistamines, H2-antagonists, corticosteroids | 110 (3.0) | 35 (31.8) |
| All other regimens that include acetaminophen | 142 (3.9) | 27 (19.0) |
| All other pretreatment regimens | 327 (9.0) | 30 (9.2) |
Pretreated/not pretreated proportions based on total number of infusions in the column. Pretreatment regimen proportions based on number of pretreated infusions in the column.
One infusion had an unknown infusion reaction status.
Proportions based on the total number of infusions in the row.
The incidence of infusion reactions in patients treated prophylactically before their first infliximab infusion, although not statistically significant, was lower than that in patients not pretreated before their first infliximab infusion (2.6% vs 3.9%, respectively; P=.752; Table 4). On the other hand, among all infusions, prophylactically pretreated infusions were significantly more likely to be associated with an infusion reaction as compared with infusions not pretreated (4.6% vs 2.0%, respectively; P <.001; Table 5). The rates of infusion reactions by study site are summarized in Table 6.
Table 4.
Incidence of Infusion Reactions at First Infusion by Pretreatment Status
| n | Patients with infusion reactions, n (%) | P* | Patients with serious infusion reactions, n (%) | |
|---|---|---|---|---|
| Pretreated | 190 | 5 (2.6) | .752 | 0 (0.0) |
| Not pretreated | 257 | 10 (3.9) | 0 (0.0) | |
| Total | 447 | 15 (3.4) | 0 (0.0) |
P based on Yates' Chi-square test.
Table 5.
Incidence of Infusion Reactions for All Infusions by Pretreatment Status
| n | Infusions with infusion reactions, n (%) | P* | Infusions with serious infusion reactions, n (%) | |
|---|---|---|---|---|
| Pretreated† | 3,624 | 168 (4.6) | <.001 | 2 (0.1) |
| Not pretreated | 2,844 | 58 (2.0) | 0 (0.0) | |
| Total† | 6,468 | 226 (3.5) | 2 (<0.1) |
P based on Yates' Chi-square test.
One infusion had an unknown infusion reaction status.
Table 6.
Incidence of Infusion Reactions by Study Site
| Gastroenterology site | Number of patients, n | Number of infusions, n | Pretreated first infusions, n (%) | Pretreated any infusions, n (%) | Infusion reactions at first infusion, n (%) | Infusion reactions at any infusion, n (%) | Patients on immunosuppressives, n (%) | Most common pretreatment protocol* |
|---|---|---|---|---|---|---|---|---|
| 1 | 60 | 934 | 8 (13.3) | 92 (9.9) | 0 | 13 (1.4) | 44 (73.3) | 4 |
| 2 | 37 | 589 | 31 (83.8) | 578 (98.1) | 2 (5.4) | 9 (1.5) | 22 (59.5) | 1 |
| 3 | 50 | 1,017 | 3 (6.0) | 674 (66.3) | 3 (6.0) | 52 (5.1) | 31 (62.0) | 2 |
| 4 | 34 | 581 | 30 (88.2) | 494 (85.0) | 0 | 2 (0.3) | 26 (76.5) | 2 |
| 5 | 44 | 543 | 44 (100.0) | 543 (100.0) | 0 | 3 (0.6) | 38 (86.4) | 1 |
| 6 | 64 | 701 | 63 (98.4) | 689 (98.3) | 2 (3.1) | 40 (5.7) | 38 (59.4) | 3 |
| 7 | 45 | 434 | 3 (6.7) | 97 (22.4) | 0 | 19 (4.4) | 40 (88.9) | 4 |
| 8 | 60 | 865 | 1 (1.7) | 235 (27.2) | 4 (6.7) | 67 (7.7) | 41 (68.3) | 4 |
| 9 | 42 | 600 | 2 (4.8) | 134 (22.3) | 4 (9.5) | 18 (3.0) | 32 (76.2) | 5 |
| Other sites | 11 | 204 | 5 (45.5) | 88 (43.1) | 0 | 3 (1.5) | 10 (90.9) | NA |
| Total | 447 | 6,468t | 190 (42.5) | 3,624 (56.0) | 15 (3.4) | 226 (3.5) | 322 (72.0) | NA |
Most common pretreatment protocol used at each site: 1: acetaminophen, nonsedating antihistamine; 2: acetaminophen, standard antihistamine; 3: acetaminophen, standard antihistamine, H2-antagonist; 4: acetaminophen, standard antihistamine, corticosteroid; 5: acetaminophen; NA= not applicable.
One infusion had unknown infusion reaction status.
Concomitant Immunosuppressive Therapy
The incidence of infusion reactions did not differ significantly when comparing patients not receiving concomitant immunosuppressives with patients receiving concomitant immunosuppressives (24.8%, 31/125 patients vs 17.7%, 57/322 patients; P=.118; Table 7). However, a trend was observed: When analyzed on a per-infusion basis, significantly more infusion reactions occurred with infusions in patients not receiving concomitant immunosuppressives than in patients who were receiving concomitant immunosuppressives (5.6%, 135/2,432 infusions vs 2.3%, 91/4,036 infusions, respectively, P<.001).
Table 7.
Incidence of Infusion Reactions in Patients Treated With or Without Immunosuppressive* Therapy
| n | Infusion reactions, n (%) | OR (95% CI)†P value | |
|---|---|---|---|
| Patients | |||
| With immunosuppressives | 322 | 57 (17.7) | .65 (.40–1.07) |
| Without immunosuppressives | 125 | 31 (24.8) | .118 |
| Total | 447 | 88 (19.7) | |
| Infusions | |||
| With immunosuppressives | 4,036 | 91 (2.3) | .39 (.30–.52) |
| Without immunosuppressives | 2,432 | 135 (5.6) | <.001 |
| Total‡ | 6,468 | 226 (3.3) | |
Immunosuppressives include methotrexate, azathioprine, and 6-mercaptopurine.
P based on Yates' Chi square test.
One infusion had unknown infusion reaction status.
- OR
odds ratio
- CI
confidence interval
.
Persistency of Infliximab Therapy
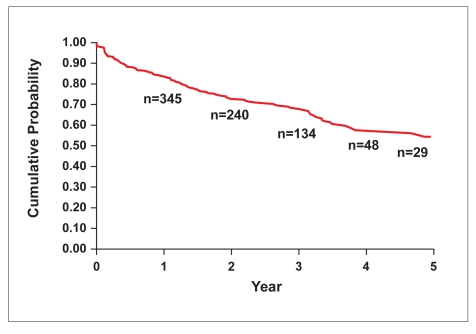
The cumulative probability of a CD patient continuing infliximab therapy at 2, 4, and 5 years was 73%, 58% and 54%, respectively (Table 8, Figure 1).
Table 8.
Historical Persistency Rates Reported in the Literature for Common Medications Used to Treat Chronic Disorders*
| Chronic disorder Medication | Persistency rate† (%) | ||||
|---|---|---|---|---|---|
| 6 mo | 1 yr | 2 yrs | 4 yrs | 5 yrs | |
| Crohn's disease | |||||
| Infliximab | >99 | >99 | 73 | 58 | 54 |
| Hypertension | |||||
| All antihypertensives | — | 5132 | — | — | — |
| Angiotensin II receptor blocker | — | 6733/6234 | — | 5133 | — |
| Angiotensin-converting enzyme inhibitor | — | 6133/6034 | — | 4733 | — |
| Calcium channel blocker | — | 5433/3534 | — | 4133 | — |
| Beta-blockers | — | 4633/3534 | — | 3533 | — |
| Diuretics | — | 2133/3334 | — | 1633 | — |
| Type 2 diabetes mellitus | |||||
| Oral hypoglycemic agents/insulin | 3935 | 20–5835 | 7035 | — | — |
| Osteoporosis | |||||
| Bisphosphonate, o.d. | — | 32–3736 | — | — | — |
| Bisphosphonate, o.w. | — | 44–5536 | — | — | — |
| Alendronate, o.w. | 3737 | — | — | — | — |
| Ibandronate, o.m. | 5737 | — | — | — | — |
| Dementia of Alzheimer's type | |||||
| Donepezil | — | 6238 | — | — | — |
| Rivastigmine | — | 4038 | — | — | — |
| Galantamine | — | 3338 | — | — | — |
Infliximab results from the current study are presented for comparison.
Persistency rate was defined as the cumulative proportion of patients remaining on treatment at a given time point throughout the follow-up period.
Figure 1.
Persistency of infliximab therapy in patients with Crohn's disease. Year 2=0.73; Year 4=0.58; Year 5=0.54.
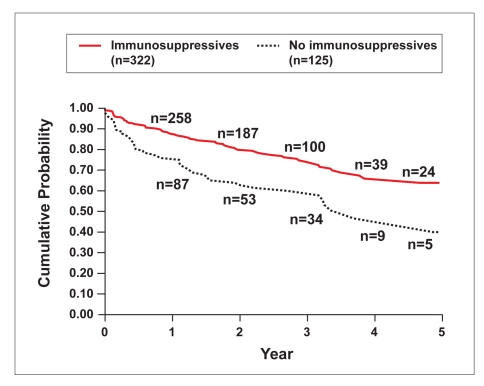
Using regression analysis, concomitant immunosuppressive therapy and gender were significantly associated with infliximab treatment persistency (P=.015 and P=.037, respectively; Table 9). Using a univariate model, concomitant immunosuppressive therapy remained significant (P=.009; Figure 2). Based on Kaplan-Meier estimates, persistency estimates at 2, 4, and 5 years for patients treated with immunosuppressives versus those not treated with immunosuppressives were 77% vs 63%, 64% vs 47%, and 60% vs 47%, respectively.
Table 9.
Association of Demographic and Clinical Characteristics with Infliximab Treatment Persistency
| Variable | P* | Hazard ratio | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Immunosuppressives | .015 | 0.60 | 0.40 | 0.91 |
| Gender | .037 | 1.47 | 1.02 | 2.11 |
| Race† | .077 | 0.64 | 0.39 | 1.05 |
| Pretreatment | .238 | 1.24 | 0.87 | 1.76 |
| Age | .541 | 1.00 | 0.99 | 1.02 |
P based on Cox's proportional hazard regression analysis.
Caucasian versus other.
- CI
confidence interval
.
Figure 2.
Persistency of infliximab therapy by immunosuppressive use in patients with Crohn's disease. Immunosuppressives include azathioprine, 6-mercaptopurine, and methotrexate.
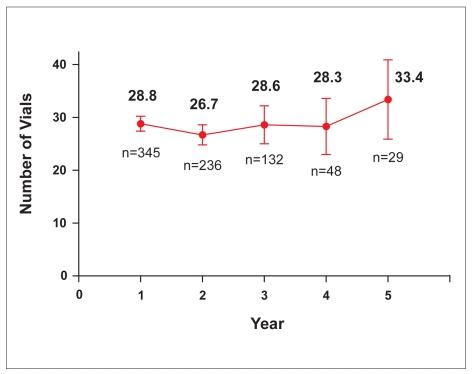
The total number of vials per patient-year of treatment remained consistent from Year 1 through Year 4, although a slight increase in the number of vials used was seen at Year 5 (Figure 3).
Figure 3.
Total vials (mean, 95% confidence interval) used by patient per year of treatment. Only complete years are included.
Discussion
The objective of this multicenter retrospective chart review of infliximab-treated CD patients treated at academic and community-based gastroenterology practices was to assess the effect of prophylactic pretreatment protocols on infusion reactions. The impact of infusion reactions on infliximab treatment persistency was also assessed. Our patient demography (mean age of 41 years at first infusion, white majority, and slight predominance of women) was similar to the demographics of the general Crohn's disease population.20,21 Seventy-two percent of the patients were receiving concomitant immunosuppressive therapy, which is consistent with the typical CD patient treated with infliximab.22
Infliximab therapy has been associated with infusion reactions that manifest as fever or chills, cardiopulmonary reactions (chest pain, hypertension, hypotension, dyspnea), pruritus, or urticaria.22 Empiric pretreatment protocols include algorithms for prophylactic medication in patients receiving their first or subsequent infusions with no history of infusion-related adverse event, as well as for patients with a history of infusion reactions. In patients with no history of infusion reactions, prophylaxis may include oral administration of 25–50 mg diphenhydramine, with or without 250 mg acetaminophen. Pretreatment for patients with a history of infusion reactions may include any combination of the following medications: 25–50 mg diphenhydramine orally or intravenously, 250 mg acetaminophen orally and/or prednisone 40 mg orally or the equivalent intravenously.
Infusion reactions and serious infusion reactions in this retrospective patient cohort occurred in 19.7% (88/447) and 0.4% (2/447) of patients, respectively. These rates are consistent with those reported in the product labeling for infliximab.22 In this study, 46 unique prophylactic protocols were identified from the 9 gastroenterology sites. Acetaminophen, antihistamines, and H2-antagonists were most commonly used in pretreatment protocols. No study site used a standardized pretreatment protocol across all patients, and the use of prophylaxis pretreatment was determined empirically on a case-by-case basis. Despite this variability and the apparent lack of a pretreatment protocol, the risk of infusion reactions was low.
Among the 6,469 infusions administered over 5 years, infusions in patients who were pretreated were significantly more likely to be associated with an infusion reaction compared to infusions in patients who were not pretreated (4.6% vs 2.0%, respectively; P<.001). This finding suggests bias by indication, which is a common occurrence in nonrandomized studies,23–25 as patients with a high risk of infusion reaction typically receive pre treatment before an infliximab infusion. In particular, this might include patients who had previously had an infusion reaction. Similar results were found by Wasserman and colleagues,26 who reported a significantly (P<.05) greater proportion of infusions with infusion reactions in rheumatoid arthritis patients who were prophylactically pretreated with diphenhydramine before an infliximab infusion, regardless of the reason for pretreatment, compared to those who were not pretreated.
Significantly fewer infusion reactions occurred during infusions given to patients receiving concomitant immunosuppressive therapy when compared to patients not receiving concomitant immunosuppressive therapy (2.3%, 91/4,036 infusions vs 5.6%, 135/2,432 infusions; P<.001). This result is consistent with findings reported by Hanauer and colleagues,27 who found that infusions in patients who received concomitant immunosuppressives (6-mercaptopurine, azathioprine, methotrexate) were associated with a significantly lower incidence of infusion reactions compared to infusions in patients not receiving concomitant immunosuppressives (3%, 38/1,174 infusions vs 6%, 171/2,666 infusions; P<.001).
Based on Cox's proportional hazards regression analysis, both concomitant immunosuppressives and gender were significantly associated with patients remaining on infliximab therapy each year, through 5 years of follow-up (P=.015 and P=.037, respectively). The association of immunosuppressive therapy with remaining on therapy may be due to the fact that patients receiving immunosuppressives have fewer infusion reactions than those not taking immunosuppressives. Patients also may experience enhanced efficacy with combination therapy versus infliximab monotherapy. It is also possible that patients receiving both infliximab and immunosuppressives have more severe disease and their physicians are more reluctant to consider discontinuing combination therapy. The observed gender effect may be explained by the higher rate of discontinuation due to adverse events in women (6.0%) compared to men (2.5%); however, further interpretation of this result is limited by the lack of information on the number and specific type of adverse events experienced by these patients.
Patients who become positive for antibodies to infliximab are reportedly more likely to have an infusion reaction than patients who are negative for these antibodies. It is now recognized that a 3-dose induction regimen followed by maintenance therapy compared to a single dose followed by episodic treatment is associated with reduced antibody formation and greater clinical benefit. The incidence of antibody formation is reduced with the use of immunosuppressives, especially for patients receiving episodic treatment.27 Antibodies to infliximab are not routinely measured in actual clinical practice; thus, these relationships could not be explored in this study.
The cumulative probability of a CD patient continuing infliximab therapy at 2, 4, and 5 years of follow-up was 73%, 58%, and 54%, respectively. These results are comparable to the 4-year infliximab treatment persistency rates (62%) recently reported for patients with rheumatoid arthritis.28 Our results may be conservative because a number of patients discontinued therapy due to remission of disease. Although information on treatment persistency of inflammatory bowel disease medications is lacking, we can compare persistency rates of infliximab treatment and treatment compliance rates of other inflammatory bowel disease medications. Recent studies have reported that only approximately 40% of ulcerative colitis patients receiving maintenance 5-aminosalicylic acid therapy were compliant with their treatment regimen over a 6-month period, even though noncompliance increases their risk of clinical relapse.13,29,30 Additionally, it is notable that the persistency rates found for CD patients treated with infliximab compare favorably with persistency rates of other therapies used to treat chronic diseases (Table 8). For example, infliximab persistency rates compare favorably with antihypertensive therapies. In the longrun, infliximab persistency rates remained favorable. Our infliximab persistency results are supported by the findings of Kane and Dixon, who reported a low nonadherence rate for infliximab infusions (48 “no show” appointments/1,185 scheduled infusion appointments, 4%) between June 1, 2002, and October 30, 2003, at the University of Chicago.31
It is notable that the total number of infliximab vials used per patient-year of treatment remained consistent through 4 years of follow-up. Although the number of evaluable patients at the fifth year of follow-up was small, it is possible that the slight increase in the average number of vials per patient-year of treatment may be attributed to the use of infliximab as episodic treatment in patients with acute luminal or fistulizing CD prior to June 2002. Between June 2002 and April 2003, the use of infliximab was expanded to include a 3-dose induction regimen and an every-8-week maintenance regimen for both luminal and fistulizing CD.
Acetaminophen alone as infusion prophylaxis was associated with the lowest proportion of infusion reactions. Acetaminophen and antihistamines (standard or nonsedating), with or without H2-antagonists, also appeared to be associated with a low proportion of infusion reactions. Including corticosteroids in the pretreatment protocol did not appear to reduce the rate of infusion reactions. However, these data are weakened by potential selection bias in this study because patients were not randomized to different premedication protocols. This experience suggests the need for a prospective study to establish a standardized protocol for optimal infusion reaction prophylaxis. Nonetheless, despite the variety of pretreatment protocols with or without concurrent immunosuppression across study sites and the large number of infusions given without pretreatment, the overall rate of serious infusion reactions was low.
Footnotes
Dr. Mayer is a consultant for Centocor, Inc., and received research funding in conjunction with the conduct of this study from Centocor, Inc. Drs. Keshavarzian, Salzberg, Garone, Finkelstein, Cappa, Brand, Hain, and Cominelli received research funding in conjunction with the conduct of this study from Centocor, Inc. Drs. Campbell and Stang, Mr. Watson, and Ms. Lane are consultants for Centocor, Inc. Drs. Zelinger and Diamond and Mr. Hegedus are employees of Centocor, Inc. Editorial and writing support was provided by Mr. James Barrett, an employee of Centocor, Inc.
References
- 1.Sewitch JM, Abrahamowicz A, Barkun A, et al. Patient nonadherence to medication in inflammatory bowel disease. Am J Gastroenterol. 2003;98:1535–1544. doi: 10.1111/j.1572-0241.2003.07522.x. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer S, Feagan B, Lichtenstein G, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 3.Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology. 2004;126:402–413. doi: 10.1053/j.gastro.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 5.Colombel JF, Loftus EV, Jr, Termaine WJ, et al. The safety profile of infliximab in patients with Crohn's disease: the Mayo Clinic experience in 500 patients. Gastroenterology. 2004;126:19–31. doi: 10.1053/j.gastro.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 6.Ljung T, Karlen P, Schmidt D, et al. Infliximab in inflammatory bowel disease: Clinical outcome in a population based cohort from Stockholm County. Gut. 2004;53:849–853. doi: 10.1136/gut.2003.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrell RJ, Shah SA, Lodhavia PJ, et al. Clinical experience with infliximab therapy in 100 patients with Crohn's disease. Am J Gastroenterol. 2000;95:3490–3497. doi: 10.1111/j.1572-0241.2000.03366.x. [DOI] [PubMed] [Google Scholar]
- 8.Seiderer J, Göke B, Ochsenkühn T. Safety aspects of infliximab in inflammatory bowel disease patients. A retrospective cohort study in 100 patients of a German University Hospital. Digestion. 2004;70:3–9. doi: 10.1159/000080075. [DOI] [PubMed] [Google Scholar]
- 9.Sample C, Bailey RJ, Todoruk D. Clinical experience with infliximab for Crohn's disease: the first 100 patients in Edmonton, Alberta. Can J Gastroenterol. 2002;16:165–170. doi: 10.1155/2002/379307. [DOI] [PubMed] [Google Scholar]
- 10.Farup PG, Hovde O, Halvorsen FA, et al. Mesalazine suppositories versus hydrocortisone foam in patients with distal ulcerative colitis. A comparison of the efficacy and practicality of two topical treatment regimens. Scand J Gastroenterol. 1995;30:164–170. doi: 10.3109/00365529509093256. [DOI] [PubMed] [Google Scholar]
- 11.Riley SA, Mani V, Goodman MJ, Lucas S. Why do patients with ulcerative colitis relapse? Gut. 1990;30:179–183. doi: 10.1136/gut.31.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Hees PA, van Tongeren JH. Compliance to therapy in patients on maintenance dose of sulfasalazine. J Clin Gastroenterol. 1982;4:333–336. doi: 10.1097/00004836-198208000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Kane SV, Cohen RD, Aikens JE, et al. Prevalence of nonadherence with maintenance mesalamine in quiescent ulcerative colitis. Am J Gastroenterol. 2001;96:2929–2933. doi: 10.1111/j.1572-0241.2001.04683.x. [DOI] [PubMed] [Google Scholar]
- 14.DiMatteo MR. Adherence to treatment. In: Feldman MD, Christensen JF, editors. Behavioral Medicine: A primary care handbook. New York: Appleton & Lange; 1997. pp. 136–140. [Google Scholar]
- 15.Cheifetz A, Smedly M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98:1315–1324. doi: 10.1111/j.1572-0241.2003.07457.x. [DOI] [PubMed] [Google Scholar]
- 16.Yates F. Contingency table involving small numbers and the ξ2 test. JR Stat Soc. 1934;(suppl 1):217–235. [Google Scholar]
- 17.Breslow NE, Day NE. Statistical Methods in Cancer Research. Vol. 1. Lyon, France: International Agency for Research on Cancer; 1980. [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.Cox DR. Regression models and life tables (with discussion) J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 20.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic options. Inflamm Bowel Dis. 2006;12(suppl 1):S3–S9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 21.Loftus EV, Schoenfeld P, Sandborn WJ. The epidemiology and natural history of Crohn's disease in population-based cohorts from North America: a systematic review. Aliment Pharmacol Ther. 2002;16:51–60. doi: 10.1046/j.1365-2036.2002.01140.x. [DOI] [PubMed] [Google Scholar]
- 22.Malvern, Pa: Centocor; 2006. Remicade (infliximab) for IV injection [package insert] [Google Scholar]
- 23.Lee GH, Benner D, Regidor DL, et al. Impact of kidney bone disease and its management on survival of patients on dialysis. J Renal Nutr. 2007;17:38–44. doi: 10.1053/j.jrn.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Rigidor DL, McAllister CJ, et al. Time-dependent associations between iron and mortality in hemodialysis patients. J Am Soc Nephrol. 2005;16:3070–3080. doi: 10.1681/ASN.2005040423. [DOI] [PubMed] [Google Scholar]
- 25.Schols AMWJ, Wesseling G, Kester ADM, et al. Dose dependent increased mortality in COPD patients treated with oral Glucocorticoids. Eur Respir J. 2001;17:337–342. doi: 10.1183/09031936.01.17303370. [DOI] [PubMed] [Google Scholar]
- 26.Wasserman MJ, Weber DA, Guthrie JA, et al. Infusion-related reactions to infliximab in patients with rheumatoid arthritis in a clinical practice setting: relationship to dose, antihistamine pretreatment, and infusion number. J Rheumatol. 2004;31:1912–1917. [PubMed] [Google Scholar]
- 27.Hanauer SB, Wagner CL, Bala M, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn's disease. Clin Gastroenterol Hepatol. 2004;2:542–553. doi: 10.1016/s1542-3565(04)00238-1. [DOI] [PubMed] [Google Scholar]
- 28.Cruyssen BV, Van Looy S, Wyns B, et al. Four-year follow-up of infliximab therapy in rheumatoid arthritis patients with long-standing refractory disease: attrition and long-term evolution of disease activity. Arthritis Res Ther. 2006;8:R112. doi: 10.1186/ar2001. (doi: 10.1186/ar2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone MA, Mayberry JF, Baker R. Prevalence and management of inflammatory bowel disease: a cross-sectional study from central England. Gastroenterol Hepatool. 2003;15:1275–1280. doi: 10.1097/00042737-200312000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Kane S, Huo D, Aikens J, et al. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med. 2003;114:39–43. doi: 10.1016/s0002-9343(02)01383-9. [DOI] [PubMed] [Google Scholar]
- 31.Kane S, Dixon L. Adherence rates with infliximab therapy in Crohn's disease. Aliment Pharmacol Ther. 2006;24:1099–1103. doi: 10.1111/j.1365-2036.2006.03092.x. [DOI] [PubMed] [Google Scholar]
- 32.Morgan SG, Yan L. Persistence with hypertension treatment among community-dwelling BC seniors. Can J Clin Pharmacol. 2004;11:e267–e273. [PubMed] [Google Scholar]
- 33.Conlin PR, Gerth WC, Fox J, et al. Four-year persistence patterns among patients initiating therapy with the angiotensin II receptor antagonist losartan versus other antihypertensive drug classes. Clin Therap. 2003;23:1999–2010. doi: 10.1016/s0149-2918(01)80152-1. [DOI] [PubMed] [Google Scholar]
- 34.Erkens JA, Panneman MMJ, Klungel OH, et al. Differences in antihypertensive drug persistence associated with drug class and gender: a PHARMO study. Pharmacooepidemiool Drug Saf. 2005;14:795–803. doi: 10.1002/pds.1156. [DOI] [PubMed] [Google Scholar]
- 35.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27:1218–1224. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- 36.Gold DT, Simon JA, Amonkar MM, et al. Treatment persistence is improved with one-weekly versus once-daily bisphosphonate therapy, but remains suboptimal. Presented at the Sixth International Symposium on Osteoporosis, Washington DC, April 6, 2005. Poster.
- 37.Cooper A, Drake J, Brankin E, et al. Treatment persistence with oncemonthly ibandronate and patient support vs. one-weekly alendronate: results from the PERSIST study. Int J Clin Prac. 2006;60:896–905. doi: 10.1111/j.1742-1241.2006.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sicras A, Rejas-Gutierrez J. Drug-cholinesterase-inhibitors persistence patterns in treated patients with dementia of Alzheimer type: retrospective comparative analysis of donepezil, rivastigmine and galantamine [in Spanish] Rev Neurol. 2004;39:312–316. [PubMed] [Google Scholar]