Abstract
Saxitoxin (STX) is a potent natural sodium channel blocker and represents a significant health concern worldwide. We describe here the antagonistic effects of STX and veratridine (VTD), an Na+ channel activator, on three gram-negative bacteria and their application to an STX bioassay. STX reduced the total cellular levels of both Na+ and K+, as measured by flame photometry, whereas VTD increased the cellular concentrations relative to control ion fluxes in the cyanobacterium Cylindrospermopsis raciborskii AWT205. Endogenous STX production in toxic cyanobacterial strains of C. raciborskii and Anabaena circinalis prevented cell lysis induced by VTD stress. Microscopic cell counts showed that non-STX producing cyanobacteria displayed complete cell lysis and trichome fragmentation 5 to 8 h after addition of VTD and vanadate (VAN), an inhibitor of sodium pumps. The addition of STX, or its analogue neoSTX, prior to treatment with VTD plus VAN prevented complete lysis in non-STX-producing cyanobacteria. VTD also affected cyanobacterial metabolism, and the presence of exogenous STX in the sample also ameliorated this decrease in metabolic activity, as measured by the cellular conversion of tetrazolium into formazan. Reduced primary metabolism was also recorded as a decrease in the light emissions of Vibrio fischeri exposed to VTD. Addition of STX prior to VTD resulted in a rapid and dose-dependent response to the presence of the channel blocker, with samples exhibiting resistance to the VTD effect. Our findings demonstrate that STX and VTD influence bacterial Na+ and K+ fluxes in opposite ways, and these principles can be applied to the development of a prokaryote-based STX bioassay.
Paralytic shellfish poisoning (PSP) is a deadly affliction that results from the accidental consumption of some potent natural neurotoxins, typically via contaminated seafood. These molecules, of which the most potent representative is saxitoxin (STX), are highly selective blockers of Na+ channels in excitable cells, thereby affecting nerve impulse generation in animals (6). This syndrome can lead, in extreme cases, to death (31). STX and analogue compounds, known collectively as the PSP toxins, have been reported to occur in both marine and freshwater microorganisms. Three genera of marine dinoflagellates have been described to synthesize PSP toxins (15, 24, 28), a limited number of bacterial strains (10), and several species of freshwater filamentous cyanobacteria (1, 5, 26), including strains of Anabaena circinalis and Cylindrospermopsis raciborskii (17, 20). Occurrences of harmful algal blooms associated with PSP toxins represent a serious health concern worldwide and have been increasingly reported (18, 25, 31). All nonanalytical detection systems for these neurotoxins rely on bioassays based on whole animals or animal cell lines (14, 21), since much is known regarding the pharmacological effect of STX on eukaryotes. On the other hand, PSP toxins have rarely been studied according to their effect on prokaryotic cells (30). For economic, practical, and ethical reasons, alternatives to the standard animal biological tests are desired. Here we describe our findings, which represent the first investigation of the feasibility of a prokaryote-based bioassay for STX and its analogues.
In a previous study we reported that, in the STX-producing cyanobacterium C. raciborskii T3, STX accumulation was directly correlated to variations in intracellular Na+ levels (F. Pomati, C. Rossetti, G. Manarolla, and B. A. Neilan, unpublished data). These results suggested a possible role of this toxin in the maintenance of cyanobacterial homeostasis under Na+ stress. Questions regarding whether STX-producing cyanobacteria have a potential advantage over other nonneurotoxic strains under conditions of critical Na+ levels or whether STX interferes with bacterial Na+ fluxes as it does in eukaryotic cells arose from that study (Pomati et al., unpublished).
In the present study we first investigated the effect of STX and veratridine (VTD), a sodium channel activator that increases Na+ permeability in eukaryotic cells (8), on the cyanobacterial total Na+ and K+ cellular contents measured by flame photometry. C. raciborskii AWT205, a cyanobacterium known not to produce any neurotoxin, including STX (16), was exposed to STX and VTD. Further, by using VTD to induce intracellular Na+ stress, we assayed PSP toxins producing and nontoxic strains of C. raciborskii and A. circinalis in a “cyanolytic” test similar to those used with eukaryotic cells (21, 29). In the assay developed here cyanobacteria were stressed with VTD and o-vanadate (VAN), an inhibitor of bacterial ion pumps (ion translocating P-type ATPases) (9, 13). The detection of cell lysis was used as the endpoint. Two nonneurotoxic strains (C. raciborskii AWT205 and A. circinalis 271C) were then used in the same test to assay the presence of channel blockers such as lidocaine, amiloride, STX, and neoSTX. In order to investigate whether the demonstrated sensitivity of C. raciborskii AWT205 to VTD and STX was also related to changes in the cell's metabolic activity, the test was applied to a cell titer cytotoxicity assay. Subsequently, the method based on VTD-induced Na+ stress was used to assay the presence of STX with the commercially available and standardized toxicity test LUMIStox by using the bioluminescent bacterium Vibrio fischeri.
MATERIALS AND METHODS
Cyanobacterial strains and culture conditions.
C. raciborskii AWT205, a non-PSP-toxin producer (16), was obtained from Peter R. Hawkins (Australia Water Technologies, EnSight, West Ryde, New South Wales, Australia). C. raciborskii T3, an STX producer (20), was obtained from Sandra Azevedo (Federal University of Rio de Janeiro, Rio de Janeiro, Brazil). Strains AWT205 and T3 were maintained in ASM-1 medium (12). PSP toxin-producing strains of A. circinalis, 344B, 134C, 150A, and 131C and the nontoxic strains 271C, 306A, and 332H were obtained from the Australian Water Quality Centre (Adelaide, South Australia) and maintained in Jaworski's medium (17). All cyanobacterial cultures were grown in 250-ml glass flasks at a constant temperature of 26°C under continuous irradiance of cool white light at an intensity of 15 μmol of photons m−2 s−1. Cyanobacterial growth was monitored by recording the optical density at 750 nm with a Lambda 10 UV/VS spectrometer (Perkin-Elmer, Inc., Shalton, Conn.). Mid-exponential-phase cultures were chosen for the tests described below.
Chemicals.
All reagents and chemicals were obtained from Sigma-Aldrich (Dorset, United Kingdom). Lidocaine hydrochloride, amiloride, and VAN solutions (100 μM, 100 mM, and 10 mM, respectively) were prepared in Milli-Q water, stored at 4°C (protected from light), and diluted into the culture medium to obtain the desired concentration. VTD was dissolved to a final concentration 10 mM in acidic Milli-Q water (pH 2) and stored at −20°C. Certified standard solutions of PSP toxins (PSP-1C and STXdiHCl-C) were obtained from the Institute of Marine Bioscience, National Research Council of Canada, Halifax, Nova Scotia. PSP toxin standards were stored at −20°C with the stock solutions diluted in culture medium to obtain the final test concentrations.
Total cellular Na+ and K+ content and flame photometry.
To evaluate the effect of 1 μM STX and 100 μM VTD on total Na+ and K+ cellular levels, aliquots of the same culture (20 ml) of C. raciborskii AWT205 were adjusted to pH 8.1 by adding HEPES buffer to a final concentration of 10 mM. Samples (2 ml) were harvested before exposure (−5 min), immediately after exposure (0 min), and at 10, 30, and 60 min postexposure. Experimental replicates included negative controls (unexposed culture sample) and positive controls (10 mM NaCl). Aliquots of challenged AWT205 cultures were collected by centrifugation in 2-ml plastic tubes at 11,000 × g for 15 min. All sampled pellets were resuspended in 0.5 ml of diluent flame solution (3 mM lithium in MilliQ water) and analyzed for total Na+ and K+ cellular content by using a FLM3 flame photometer (Radiometer, Copenhagen, Denmark). All experiments were performed in quadruplicate. Control traces were subtracted from the tested samples for threshold correction, and the data were normalized by expressing the values as the percentile variation over samples at −5 min.
Cyanobacterial cell lysis test.
The cyanobacterial cell lysis assay was based on the same principles as the animal neuroblastoma and red blood cell culture assays described elsewhere (21, 29), except for the use of VAN to inhibit Na+/ K+ ATPase activity instead of ouabain since ouabain is known to be ineffective against algal Na+ pumps (11). Briefly, 96-well microtiter plates were used for the cyanolytic assay, in which 100 μl of the cyanobacterial cultures was inoculated and exposed to the agents. Controls consisted of untreated aliquots of cyanobacterial cultures and samples with 4 μl of either 10 mM VTD or 10 mM VAN. Toxic and nontoxic cyanobacteria were also assayed after the addition of a combination of 4 μl of 10 mM VTD and 4 μl of 10 mM VAN. The final concentration of VTD and VAN, 400 μM, was chosen based on previous studies (13, 29). For the treatment of C. raciborskii AWT205 and A. circinalis 271C with STX and neoSTX at 1 μM, the PSP toxins were added to the tested wells 30 min prior to the addition of VTD and VAN to simulate natural the conditions of toxic cultures. In these experiments, the positive controls consisted of samples exposed to VTD-VAN added with lidocaine hydrochloride at 1 μM. Replicates dosed with amiloride at 10 mM were used as negative controls. In a previous study, lidocaine hydrochloride was demonstrated to induce an increase in total cellular Na+, whereas amiloride reduced the cellular ion levels in cyanobacteria (Pomati et al., unpublished).
In both assays, the microtiter plates were incubated at room temperature (25°C) for 5 to 8 h (minimum time for complete cell lysis observed in the VTD-VAN samples). Determination of cell lysis was performed by light microscopic inspection every 30 to 60 min from the onset of the test, and cells were counted by using a Neubauer improved counting chamber (0.1 mm deep). Complete cyanobacterial lysis in the VTD-VAN samples was utilized as the endpoint of the assay. If no cyanolysis was observed in the inoculated wells, the presence of a channel-blocking agent, including PSP toxins, was indicated. Three to five trials were performed for each strain or treatment to test the reproducibility of the results.
Cell titer assay for metabolic activity.
Microtiter plate cytotoxicity assays on cyanobacterial cells were performed by using the CellTiter 96 nonradioactive cell proliferation assay kit (Promega Corp., Madison, Wis.). This method utilizes, as an indicator of a cell's metabolic activity, the cellular conversion of a tetrazolium salt into a formazan product that can be quantified with a spectrophotometer plate reader. Assays were performed essentially, as suggested in the standard protocol provided with the kit. C. raciborskii cells in mid-exponential growth were centrifuged (15 min at 4,000 × g) and concentrated to reach approximately an optical density at 750 nm of 1. Subsequently, 100-μl aliquots of concentrated cyanobacterial suspension were inoculated in 96-well microtiter plates and then exposed to the test agents. For comparison between the metabolic responses of C. raciborskii strains AWT205 and T3, the culture samples were tested with a combination of 4 μl of 10 mM VTD and 4 μl of 10 mM VAN, yielding a final concentration of 400 μM for each compound. Controls consisted of untreated cyanobacteria. In the evaluation of the effect of combined VTD and STX on C. raciborskii AWT205, cyanobacterial cells were exposed to 1 μM STX, incubated at room temperature for 30 min, and then added to VTD at 100 μM. Controls consisted, for each sample, of untreated cells and cyanobacteria exposed to VTD at 100 μM. The dye solution was added at different times, and the stop solution after 4 h of incubation at room temperature (25°C). Plates were allowed to rest overnight, and then the absorbance at 600 nm was determined with a Metertech Σ960 microplate reader (Metertech, Inc., Taipei, Taiwan). All experiments were performed in quadruplicate, and the data were expressed as an average percent variation of sample values versus levels in untreated control.
Luminescent bacteria test.
Inhibition of bioluminescence in cultures of V. fischeri NRRL-B-11177 was performed by using a commercially available standard luminescent bacterium LUMIStox test kit (Dr Bruno Lange GmbH & Co., Dusseldorf, Germany) and specific analytical equipment, including the LUMIStox 300 measuring station and a Lumistherm thermostat. Reactivation of freeze-dried bacteria and preparation of samples was done according to the instructions provided. Light emission of reactivated bacteria was adjusted to a relative intensity of ∼1,000 by dilution with sterile 2% NaCl. For the test, 0.5 ml of luminescent bacterial suspension was combined with 0.5 ml of the test solutions. Test solutions were prepared in sterile saline medium (2% NaCl) and adjusted to pH 7 with 10 mM phosphate buffer. When needed, STX supplemented the solutions to the desired final concentration. Bacterial suspensions and samples were maintained at 15°C, combined, and monitored with the luminometer, while allowing bacteria to adapt for 5 to 10 min. Subsequently, 100 μM VTD was added, and the light emission was measured over time. Positive and negative controls were included for each test and consisted of bacteria exposed to only VTD and unexposed samples, respectively. The effect on bioluminescence was monitored and was expressed as the percent inhibition relative to the untreated controls. Values were calculated by using, as a correction factor, the changes in intensity of the controls. This was achieved by subtracting the trace negative control reading from the test samples over the duration of the experiment.
Statistical analyzes.
All graphical and statistical analyses were performed by using PC Origin 5.0 software (Microcal Software, Inc., Northampton, Mass.).
RESULTS
Effect of Na+ stress, VTD, and STX on the total cellular Na+ and K+ content.
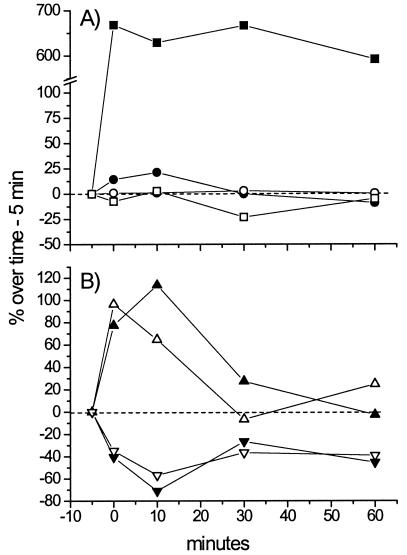
In C. raciborskii AWT205, the stress induced by 10 mM NaCl increased the total cellular Na+ levels compared to the untreated controls (Fig. 1A). Na+ uptake by the cells was shown to be very rapid, and the total cyanobacterial sodium content remained stable over the 60-min course of the experiment. The total K+ content of cells was only slightly affected by 10 mM NaCl, indicating that the homeostasis of K+ is of marginal consequence in the overall cyanobacterial Na+ stress response. On the other hand, both Na+ and K+ cellular levels in C. raciborskii AWT205 were altered due to the effects of STX at 1 μM and VTD at 100 μM (Fig. 1B). The addition of VTD dramatically stimulated cyanobacterial Na+ and K+ accumulation, whereas STX markedly inhibited the cellular uptake and thus the intracellular levels of both ions. These results suggest that Na+ flux is not the only cellular response elicited by these two compounds in cyanobacteria.
FIG. 1.
(A) Time course of total cellular Na+ and K+ levels in C. raciborskii AWT205 cultures exposed to 10 mM NaCl (Na+ ▪ and K+ □) compared to untreated control samples (Na+ • and K+ ○). (B) Effects of STX at 1 μM (Na+ ▾ and K+ ▿) and VTD at 100 μM (Na+ ▴ and K+ ▵) on total cellular Na+ and K+ concentrations in C. raciborskii AWT205. All values are the mean of four experimental replicates and are expressed as the percent variation over time.
Lysis test with toxic and nontoxic cyanobacteria.
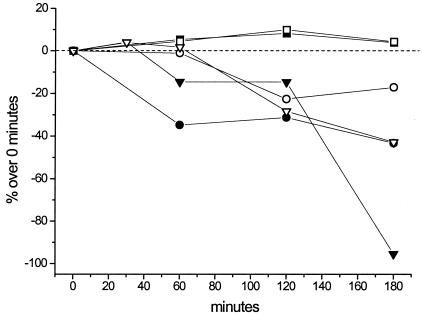
The effects of VTD and VAN each at 400 μM and in combination were monitored by microscopically counting the cells of the two strains of C. raciborskii: AWT205 and T3. Cell densities were recorded over a 180-min time period (Fig. 2). VAN had no significant effect on both strains, whereas culture samples of AWT205 were manifestly more sensitive to VTD and the combination of both VTD and VAN compared to the STX-producing strain T3. For AWT205, the first indications of cyanobacterial lysis occurred after 60 min of exposure to VTD or VTD-VAN, whereas cell lysis was evident for both T3 and AWT205 at 120 min after the onset of the experiment. The combination of VTD and VAN was more effective than VTD alone in both strains as indicated by the decrease in the number of intact cells (Fig. 2). In AWT205 cultures, 180-min exposure to VTD-VAN resulted in almost complete lysis of cells. By microscopic examination, cyanobacterial lysis occurred after evident enlargement of the cells, which subsequently burst. This indicated that lysis could have been caused by a dramatic increase in the intracellular osmotic pressure due to excessive ion uptake.
FIG. 2.
Effects of VTD and VAN at 400 μM and their combination on cell numbers in samples of C. raciborskii AWT205 and T3. All values represent the average of five experimental replicates and are expressed as the percent variation over time. Symbols for C. raciborskii AWT205: ▪, VAN; •, VTD; and ▾, VAN-VTD. Symbols for C. raciborskii T3: □, VAN; ○, VTD; and ▿, VAN-VTD.
PSP toxin-producing and nonneurotoxic strains of the cyanobacterial species C. raciborskii and A. circinalis were assayed for resistance to VTD-VAN by the method described above, and the results are summarized in Table 1. All of the non-PSP-toxin-producing strains tested showed complete cell lysis after 3 to 8 h, with no filaments recorded, whereas the neurotoxic strains were characterized by intact filaments and a lack of complete cell lysis even after overnight exposure to these agents.
TABLE 1.
Cyanobacterial strains used in this study and their relative resistance to treatment with VTD (400 μM) plus VAN (400) μM
| Strain | PSP toxin production | Source or reference | Lysisa | Time (h) |
|---|---|---|---|---|
| C. raciborskii AWT205 | No | 16 | + | 3-5 |
| C. raciborskii T3 | Yes | 20 | − | 3-5 |
| A. circinalis 344B | Yes | P. Bakerb | − | 3-5 |
| A. circinalis 134C | Yes | 4 | − | 3-5 |
| A. circinalis 150A | Yes | 4 | − | 3-5 |
| A. circinalis 131C | Yes | 4 | − | 3-5 |
| A. circinalis 271C | No | P. Baker | + | 3-5 |
| A. circinalis 306A | No | P. Baker | + | 3-5 |
| A. circinalis 332H | No | P. Baker | + | 5-8 |
+, Complete cell lysis and filaments absent; −, partial cell damage with intact filaments present.
P. Baker, unpublished data.
Two non-PSP toxins producing cyanobacterial strains, C. raciborskii AWT205 and A. circinalis 271C, were also chosen to evaluate the effect of STX and neoSTX at 1 μM on the stress induced by exposure to VTD-VAN. The results, summarized in Table 2, demonstrated that the production of PSP toxins prevented complete cell lysis caused by VTD-VAN in culture samples, as seen for the positive controls and in the cyanobacteria added with lidocaine hydrochloride at 1 μM. In cyanobacteria exposed to lidocaine, cell lysis was observed at the same time as the untreated controls. On the other hand, the effects of amiloride at 1 mM, STX at 1 μM, and neoSTX at 1 μM were comparable with regard to the level of inhibition of cyanobacterial lysis.
TABLE 2.
Results of the application of channel-blocking agents to two non-PSP-toxin-producing cyanobacterium strains over a 5-h exposure time to VTD (400 μM) plus VAN (400 μM)
| Strain | Cell lysisa
|
||||
|---|---|---|---|---|---|
| Control | Lidocaine (1 μM) | Amiloride (1 mM) | STX (1 μM) | neoSTX (1 μM) | |
| C. raciborskii AWT205 | + | + | − | − | − |
| A. circinalis 271C | + | + | − | − | − |
Control, samples with no channel blocker added. +, Complete cell lysis and filaments absent; −, partial cell damage with intact filaments present.
Effect of VTD and STX on cyanobacterial metabolic activity.
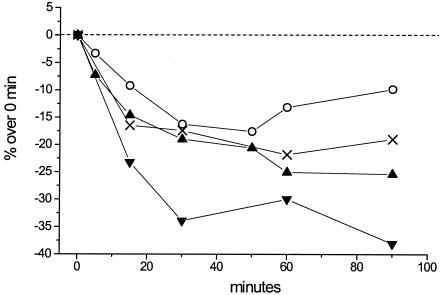
In order to investigate whether the increased Na+ uptake in cyanobacterial cells was coupled to a consequent measurable decrease in general metabolic activity, cultures of C. raciborskii AWT205 were treated with 400 μM VTD-400 μM VAN, 100 μM VTD, or 100 μM VTD-1 μM STX and then monitored by using a CellTiter 96 cell proliferation assay. As a control, cultures of the STX-producing C. raciborskii T3 were also exposed to 400 μM VTD-400 μM VAN, and the metabolic activity was measured over time. The treatments with VTD and VTD-VAN both resulted in a reduced metabolic activity of C. raciborskii strains over a 90-min exposure; however, the toxic strain T3 was affected less than AWT205 (Fig. 3). Treatment with STX at 1 μM alone did not result in any adverse effect on cyanobacterial metabolism (data not shown). The addition of 1 μM STX to cultures of AWT205, followed by 100 μM VTD, induced a less dramatic decrease in metabolic activity compared to exposure to 100 μM VTD alone (Fig. 3).
FIG. 3.
Time course of metabolic activity in culture samples of C. raciborskii AWT205 treated with 100 μM VTD (▴), 400 μM VTD-400 μM VAN (▾), and 100 μM VTD-1 μM STX (○). Control culture samples of C. raciborskii T3 were also tested with 400 μM VTD-400 μM VAN (×). All values are the average of 4 experimental replicates and are expressed as the percent variation over time.
Effect on bioluminescence by V. fischeri.
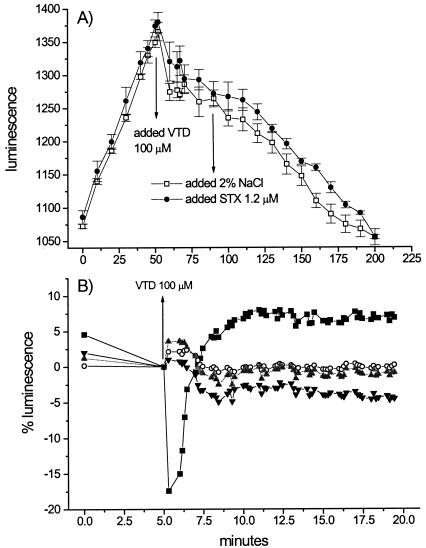
In the standard toxicity test LUMIStox, no significant effect of varied concentrations of STX (from 100 nM to 1 μM) on the luminescence of V. fischeri was observed. On the other hand, treatment with VTD at 100 μM reduced bacterial light emission over time, as shown in Fig. 4A. The addition of STX (1 μM) to the Vibrio cultures after exposure to 100 mM VTD resulted in a slight recovery of bioluminescence (maximum of 4.4% after 70 min) compared to samples with no STX added (Fig. 4A). Exposure of the bioluminescent bacteria to STX prior to the addition of 100 μM VTD, however, led to different responses over time for the test samples compared to the controls with no STX added. Figure 4B shows the time course of the percent light emission for the various treatments, with values corrected by subtracting the trace levels of unexposed control samples to eliminate threshold variations in bioluminescence. In the first 2 min after the addition of 100 μM VTD, samples not exposed to STX drastically decreased their light emission compared to bacterial solutions treated with the channel-blocking toxin. STX at 600 nM was the most effective treatment for preventing the VTD effect (21% compared to VTD controls), followed by STX at 300 nM (19.5%) and at 1.2 μM (18.3%). By 5 min after the addition of VTD, samples with no STX added reached a level of bioluminescence that were, on average, 6% higher than the untreated controls. Bacterial solutions with STX at 300 and 600 nM showed no significant variation over control levels. Samples with STX added to 1.2 μM did not completely recover control levels of light emission, attaining an average of −3.8% of the luminescence in unexposed bacteria.
FIG. 4.
(A) Time course of bioluminescence in V. fischeri. VTD (100 μM) was added 52 min after the onset of the experiment. Subsequently, half of the experimental replicates were supplemented with 1 μM STX (•), whereas the other half were supplemented with physiological saline solution (□). (B) Effects of 100 μM VTD on light emission by samples of V. fischeri exposed to STX at 0 (▪), 300 (○), 600 (shaded triangles), and 1,200 (▾) nM, expressed as the percent variation over time and corrected for the control levels. All values are the average of four experimental replicates.
DISCUSSION
During the present study, we observed opposing effects of STX and VTD on cyanobacterial Na+ and K+ ion fluxes, as measured by flame photometry analysis. The effects detected were rapid but not Na+ specific, as predicted by the interaction of these compounds with eukaryotic cells. Our results suggested either that STX and VTD have less specific effects on prokaryotic cells than those reported in the literature for eukaryotic sodium fluxes or that the target of these two agents on cyanobacterial cells is a binding protein involved in both Na+ and K+ homeostasis. The latter hypothesis is consistent with several reports in the literature demonstrating the presence, in cyanobacterial cells, of channel proteins that are permeable to both sodium and potassium ions (19, 22, 23).
Based on these observations, PSP toxin-producing and nonneurotoxic cyanobacterial strains were assayed to investigate whether, in vivo, the production of STX would have prevented the excessive ion uptake and subsequent cell lysis induced by VTD stress. As noted from microscopic observations, non-PSP-toxin-producing cyanobacteria under VTD-VAN stress were swollen, probably due to an increase in the internal osmotic pressure, and subsequently collapsed within 5 to 8 h. Similar findings were reported in previous studies with animal cells subjected to the activity of the sodium channel activator and the ion pump inhibitor ouabain (29). In contrast, PSP toxin-producing cultures exhibited lower rates of cell lysis. This differential sensitivity to VTD and VAN exposure is proposed to be due to the presence of channel-blocking compounds in the cultures, which interfere with the action of the channel-activating agents. To verify this hypothesis and to exclude the possibility of a variable intrinsic sensitivity of the toxic strains to the chemicals used, the two nonneurotoxic cyanobacteria C. raciborskii AWT205 and A. circinalis 271C were exposed to VTD-VAN in the presence of STX and neoSTX at 1 μM. This experiment clearly revealed the acquired resistance to lysis of nonneurotoxic strains after the addition of PSP toxins to the cultures. Therefore, in vivo, a direct antagonism of STX and VTD was demonstrated in a prokaryotic microorganism, a result similar to what has been noted in the eukaryotic cell-based assays for channel-blocking toxins (21).
Consistent with our hypothesis that the main effect of VTD on cyanobacterial cells was due to the increased uptake of Na+ and K+ ions, we investigated whether such stress was correlated with a decrease in the metabolic activity of the cyanobacteria. Sodium, above a certain critical concentration, represents a threat to normal cellular functions. This ion, if in excess compared to normal physiological levels, can disrupt several crucial biological functions, such as photosynthetic and electron transport activities in cyanobacterial cells (2). The cell titer toxicity assays, applied in the present study with the addition of VTD-VAN, demonstrated that antagonistic effects of STX and VTD can also be detected via the monitoring of bacterial metabolic activity.
Since measurements of metabolic activity, compared to cell lysis, represent a more precise, easy-to-quantify, and standardized means of investigation, we applied the principle of inducing ion uptake by using VTD in the bioluminescent bacterium V. fischeri. Such stress, resulting in decreased primary metabolism, can be measured in this microorganism as variations in light emission by a commercially standardized method. In suspensions of V. fischeri, VTD exposure reduced bioluminescence. Light emission also followed a similar pattern after the post-VTD addition of STX (Fig. 4A). This effect could be explained by the occurrence of nonreversible cell damage caused by either the initial VTD stress or a critical increase in cytoplasmic Na+ levels (2). Alternatively, these data could indicate a difference in the affinity of VTD and STX for a putative binding site on the bacterial cells. STX may have less specificity than VTD for the receptor molecule in V. fischeri, or the binding protein(s) on the bacterial cell membranes could have a completely different structure compared to the defined targets of these two agents, i.e., the eukaryotic voltage-gated Na+ channels.
On the other hand, exposure to STX prior to the addition of VTD to bioluminescent bacteria resulted in the observed difference in the time course of cellular metabolic activity. As expected for changes in membrane ion fluxes, the effect displayed by V. fischeri was rapid and dose dependent. A 1.2 μM concentration of STX was shown to have a minor level of toxicity to bioluminescent bacteria. This effect could be a result of the prolonged inhibition of basal bacterial Na+ and K+ activity by such high concentrations of the channel-blocking toxin. In general, however, during the course of the present study, STX alone did not have any particular effect on bacterial (i.e., Vibrio sp. or cyanobacteria) growth or metabolism. Accordingly, exposure to STX could not be used directly in a bacterial bioassay. These data were consistent with reports that show low to no toxicity of STX on other microorganisms (14, 30). In contrast, no investigations of the effects of STX on prokaryotic ion fluxes have been reported. Bacterial ion channels are single-domain proteins (for reviews, see references 3 and 7) that have been reported to be insensitive to both STX and TTX and confirmed by recent findings regarding the voltage-gated prokaryotic Na+ channel in the halophilic bacterium Bacillus halodurans (27).Further comprehensive investigations of the effect of STX on microorganisms may lead to important evolutionary findings regarding an ancestral ion channel sensitive to neurotoxins.
The application of the bioluminescent bacterium method described here for toxicity assays showed a nanomolar order of magnitude detection range for STX and a limit of <300 nM. Preliminary data also confirmed (29) that varying the concentrations of VTD or VAN or the number of cells in the assay can affect the sensitivity of the test. In the present study, the parameters were selected to provide the best results in a short time scale, as required for a rapid bioassay. We predict that additional development and standardization of this test would afford a novel and accurate method for the detection and quantification of PSP toxins. All three gram-negative bacteria tested with VTD showed a lower affinity to STX compared to their potency against eukaryotic cells. However, the use of microorganisms represents an easy, economic, and ethical alternative to animal tests for screening environmental, clinical, and industrial samples for neurotoxins, including PSP toxins, tetrodotoxin, ciguatoxins, and brevetoxins.
In conclusion, the present study presents the first evidence of the effect of the Na+ channel blocker STX, as well as the sodium channel activator VTD, on the Na+ and K+ ion fluxes and metabolism of bacterial cells. Previously, these two agents were thought to act almost exclusively on eukaryotic membrane channels. In addition, we demonstrated the applicability of VTD and STX antagonism in prokaryotic cells for the development of a novel PSP toxin bioassay.
Acknowledgments
We thank G. Manarolla for helpful experimental assistance and L. Llwellyn for advice.
F.P. is the recipient of research scholarships from the University of New South Wales and the School of Biotechnology and Biomolecular Sciences, together with an A. Lee Travel Scholarship kindly granted by the School of Microbiology and Immunology.
REFERENCES
- 1.Alam, M., M. Ikawa, J. J. Sasner, Jr., and P. J. Sawyer. 1973. Purification of Aphanizomenon flos-aquae toxin and its chemical and physiological properties. Toxicon 11:65-72. [DOI] [PubMed] [Google Scholar]
- 2.Allakhverdiev, S. I., A. Sakamoto, Y. Nishiyama, M. Inaba, and N. Murata. 2000. Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol. 123:1047-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, P. A., and R. M. Greenberg. 2001. Phylogeny of ion channels: clues to structure and function. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 129:17-28. [DOI] [PubMed] [Google Scholar]
- 4.Baker, P. D., A. R. Humpage, and D. A. Steffensen. 1993. Cyanobacterial blooms in the Murray-Darling basin: their taxonomy and toxicity. Report 8/93. Australian Centre for Water Quality Research, Adelaide, Australia.
- 5.Carmichael, W. W., W. R. Evans, Q. Q. Yin, P. Bell, and E. Moczydlowsky. 1997. Evidence of paralytic shellfish poisons in the freshwater cyanobacterium Lyngbya wollei (Farlow ex Gomont) comb. nov. Appl. Environ. Microbiol. 63:3104-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catterall, W. A. 1980. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu. Rev. Pharmacol. Toxicol. 20:15-43. [DOI] [PubMed] [Google Scholar]
- 7.Catteral, W. A. 2001. A one-domain voltage gated sodium channel in bacteria. Science 294:2306-2308. [DOI] [PubMed] [Google Scholar]
- 8.Catteral, W. A., and M. Nirenberg. 1973. Sodium uptake associated with activation of action potential ionophores of cultured neuroblastoma and muscle cells. Proc. Natl. Acad. Sci. USA 70:3759-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eddy, M. L., and P. E. Jablonski. 2000. Purification and characterization of a membrane-associated ATPase from Natronococcus occultus, a haloalkaliphilic archaeon. FEMS Microbiol. Lett. 189:211-214. [DOI] [PubMed] [Google Scholar]
- 10.Gallacher, S., and E. A. Smith. 1999. Bacteria and paralytic shellfish toxins. Protist 150:245-255. [DOI] [PubMed] [Google Scholar]
- 11.Glimmer, H. 2000. Primary sodium plasma membrane ATPases in salt-tolerant algae: facts and fictions. J. Exp. Botany 51:1171-1178. [PubMed] [Google Scholar]
- 12.Gorham, P. R., J. McLachlan, U. T. Hammer, and W. K. Kim. 1964. Isolation and culture of toxic strains of Anabaena flos-aquae (Lyngb.) de Breb. Verh. Int. Verein. Theor. Angew. Limnol. 15:796-804. [Google Scholar]
- 13.Hafer, J., A. Siebers, and E. P. Bakker. 1989. The high-affinity K+-translocating ATPase complex from Bacillus acidocaldarius consists of three subunits. Mol. Microbiol. 3:487-495. [DOI] [PubMed] [Google Scholar]
- 14.Harada, K., F. Kondo, and L. Lawton. 1999. Laboratory analysis of cyanotoxins, p. 369-389. In I. Chorus and J. Bartram (ed.), Toxic cyanobacteria in water. World Health Organization, London, United Kingdom.
- 15.Harada, T., Y. Oshima, and T. Yasumoto. 1982. Structure of two paralytic shellfish toxins, gonyautoxins V and VI, isolated from a tropical dinoflagellate, Pyrodinium bahamense var. compressa. Agric. Biol. Chem. 46:1861-1864. [Google Scholar]
- 16.Hawkins, P. R., N. R. Chandrasena, G. J. Jones, A. R. Humpage, and I. R. Falconer. 1997. Isolation and toxicity of Cylindrospermopsis raciborskii from an ornamental lake. Toxicon 1997 35:341-346. [DOI] [PubMed] [Google Scholar]
- 17.Humpage, A. R., J. Rositano, A. H. Bretag, R. Brown, P. D. Baker, B. C. Nicholson, and D. A. Steffensen. 1994. Paralytic shellfish poisons from Australian cyanobacterial blooms. Aust. J. Mar. Freshwater Res. 45:761-771. [Google Scholar]
- 18.Kaas, H., and P. Henriksen. 2000. Saxitoxins (PSP toxins) in Danish lakes. Water Res. 34:2089-2097. [Google Scholar]
- 19.Kaneko, T., S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakamura, N. Miyajima, M. Hirosawa, M. Sugiura, S. Sasamoto, T. Kimura, T. Hosouchi, A. Matsuno, A. Musaraki, N. Nakazaki, K. Nauro, S. Okumura, S. Shimpo, C. Takeuchi, T. Wada, A. Watanabe, M. Yamada, M. Yasuda, and S. Tabata. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. PCC6803: II. Sequence determination of the entire genome and assignment of potential protein coding regions. DNA Res. 3(Suppl. 3):741-747. [DOI] [PubMed] [Google Scholar]
- 20.Lagos, N., H. Onodera, P. A. Zagatto, D. Andrinolo, S. M. F. Q. Azevedo, and Y. Oshima. 1999. The first evidence of paralytic shellfish toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii, isolated from Brazil. Toxicon 37:1359-1373. [DOI] [PubMed] [Google Scholar]
- 21.Manger, R. L., L. S. Leja, S. Y. Lee, J. M. Hungerford, and M. M. Wekell. 1993. Tetrazolium-based cell bioassay from neurotoxins active on voltage-sensitive sodium channels: semiautomated assay for saxitoxin, brevetoxin, and ciguatoxins. Anal. Biochem. 214:190-194. [DOI] [PubMed] [Google Scholar]
- 22.Murata, T., K. Takase, I. Yamato, K. Igarashi, and Y. Kakiuma. 1996. The ntpJ gene in the Enterococcus hirae ntp operon encodes a component of KtrII potassium transport system functionally independent of vacuolar Na+-ATPase. J. Biol. Chem. 271:10042-10047. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura, T., R. Yuda, T. Unemoto, and E. P. Bakker. 1998. KtrAB, a new type of bacterial K+-uptake system from Vibrio alginolyticus. J. Bacteriol. 180:3491-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oshima, Y., M. Hasegawa, T. Yasumoto, G. Hallegaeff, and S. Blackburn. 1987. Dinoflagellate Gimnodium catenatum as the source of paralytic shellfish toxins in Tasmanian shellfish. Toxicon 25:1105-1111. [DOI] [PubMed] [Google Scholar]
- 25.Pereira, P., H. Onodera, D. Andrinolo, S. Franca, F. Araujo, N. Lagos, and Y. Oshima. 2000. Paralytic shellfish toxins in the freshwater cyanobacterium Aphanizomenon flos-aquae, isolated from Montargil reservoir, Portugal. Toxicon 38:1689-1702. [DOI] [PubMed] [Google Scholar]
- 26.Pomati, F., S. Sacchi, C. Rossetti, S. Giovannardi, H. Onodera, Y. Oshima, and B. A. Neilan. 2000. The freshwater cyanobacterium Planktothrix sp. FP1: molecular identification and detection of paralytic shellfish poisoning toxins. J. Phycol. 36:553-562. [DOI] [PubMed] [Google Scholar]
- 27.Ren, D., B. Navarro, H. Xu, L. Yue, Q. Shi, and D. E. Clapham. 2001. A prokaryotic voltage-gated sodium channel. Science 294:2372-2375. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu, Y. 1977. Chemistry and distribution of deleterious dinoflagellate toxins, p. 261-269. In D. J. Faulkner and W. H. Fenical (ed.), Marine natural products chemistry. Plenum Press, Inc., New York, N.Y.
- 29.Shimojo, R. Y., and W. T. Iwaoka. 2000. A rapid hemolysis assay for the detection of sodium channel-specific marine toxins. Toxicology 154:1-7. [DOI] [PubMed] [Google Scholar]
- 30.Tisa, L. S., J. J. Sekelsky, and J. Adler. 2000. Effects of organic antagonists of Ca2+, Na+, and K+ on chemotaxis and motility of Escherichia coli. J. Bacteriol. 182:4856-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zingone, A., and H. O. Enevoldsen. 2000. The diversity of harmful algal blooms: a challenge for science and management. Ocean Coastal Management 43:725-748. [Google Scholar]