Indication
Denosumab (Prolia®) [1, 2] offers a new approach in the treatment of osteoporosis. It decreases bone resorption by inhibiting osteoclast formation, function and survival. The current indication covers osteoporosis in postmenopausal women at increased risk of fracture and men receiving hormonal therapy for prostate cancer.
Mechanism
Osteoporosis is characterized by an imbalance between bone formation and bone resorption. The latter process is performed by osteoclasts and occurs at a higher rate in osteoporosis. Receptor activator of nuclear factor-κB ligand (RANKL) is an essential factor in osteoclast differentiation, activation and survival. RANKL, a member of the tumour necrosis factor (TNF) superfamily, is expressed on the osteoblast membrane and binds to receptor activator of nuclear factor-κB (RANK, the receptor) on the osteoclast membrane (Figure 1). Osteoprotegerin (OPG) is the endogenous regulator of this RANKL-mediated activation of osteoclasts. OPG is a soluble RANKL-binding protein that binds RANKL, preventing it from combining with RANK on the osteoclast membrane and thus inhibiting its action. RANK is a membrane-bound, TNF-like receptor that recognizes RANKL through direct cell-to-cell interaction with osteoblasts.
Figure 1.
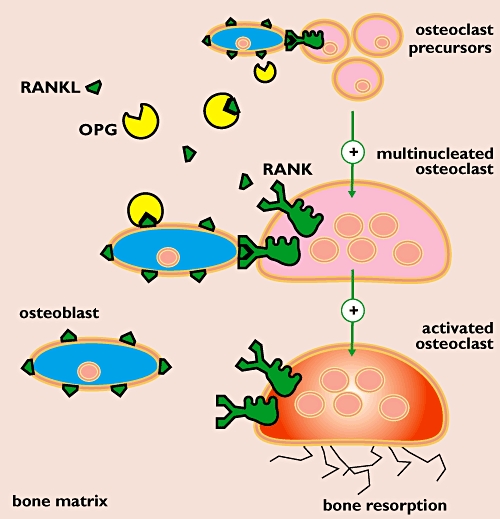
Pathophysiology of osteoporosis. Osteoporosis is characterized by a higher rate of bone resorption compared with bone formation. Bone resorption requires differentiation and activation of osteoclasts. Receptor activator of nuclear factor-κB ligand (RANKL) is an essential, stimulating factor in this regulatory process. Binding of RANKL (expressed on the osteoblast membrane) to its receptor activator of nuclear factor-κB (RANK, the receptor) in the osteoclast membrane triggers osteoclast activation and leads to bone resorption. Osteoprotegerin (OPG) is a soluble RANKL receptor and thus acts as an endogenous inhibitor of this RANKL-mediated activation process
Denosumab is a human monoclonal IgG2 antibody that binds RANKL with high affinity and specificity, preventing interaction with RANK on the osteoclast membrane (Figure 2). Thus, it mimics endogenous OPG. By attaching to and blocking RANKL, denosumab inhibits osteoclast differentiation, activation and survival. This favours bone formation over bone resorption, increasing bone mass and reducing the risk of fractures [3].
Figure 2.
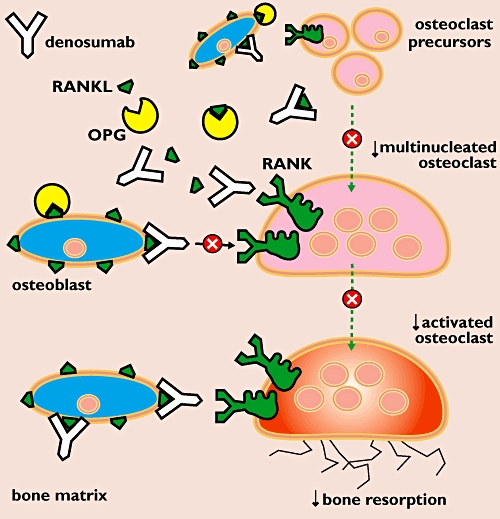
Mechanism of action of denosumab. Denosumab binds to RANKL thereby preventing interaction with RANK on the osteoclast membrane. By attaching to and blocking RANKL, denosumab inhibits osteoclast differentiation and activation. This mechanism of action decreases bone resorption which leads to improved bone density
Denosumab is administered once every 6 months as a 60-mg subcutaneous injection. During treatment, calcium and vitamin D supplementation is important.
Adverse effects
Hypocalcaemia is not a concern during denosumab therapy when patients are adequately supplemented with calcium and vitamin D. Patients with hypocalcaemia and/or chronic kidney disease may develop symptomatic hypocalcaemia upon treatment with denosumab. Therefore, hypocalcaemia should be corrected before therapy and serum calcium concentration monitored. A dental check and treatment if needed may be advised before starting treatment because of a risk of osteonecrosis of the jaw.
Because RANKL also has a function in the immune system denosumab could adversely influence infections of the urinary and upper respiratory tracts. Cataracts were observed in men receiving treatment for prostate cancer but a mechanism has not been elucidated and the findings may be coincidental. The development and progression of cataracts will be studied prospectively in planned clinical studies [4].
Literature
- 1.European Public Assessment Report. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001120/human_med_001324.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d124 (last accessed 4 April 2011)
- 2.Rizzoli R, Yasothan U, Kirkpatrick P. Fresh from the Pipeline: denosumab. Nat Rev Drug Discov. 2010;9:591–2. doi: 10.1038/nrd3244. [DOI] [PubMed] [Google Scholar]
- 3.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C, FREEDOM Trial Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 4.ClinicalTrials.gov Identifier: NCT00925600. Available at http://clinicaltrials.gov/ct2/show/NCT00925600.


