Abstract
The dairy starter bacterium Lactococcus lactis is able to synthesize folate and accumulates >90% of the produced folate intracellularly, predominantly in the polyglutamyl form. Approximately 10% of the produced folate is released into the environment. Overexpression of folC in L. lactis led to an increase in the length of the polyglutamyl tail from the predominant 4, 5, and 6 glutamate residues in wild-type cells to a maximum of 12 glutamate residues in the folate synthetase overproducer and resulted in a complete retention of folate in the cells. Overexpression of folKE, encoding the bifunctional protein 2-amino-4-hydroxy-6-hydroxymethyldihydropteridine pyrophosphokinase and GTP-cyclohydrolase I, resulted in reduction of the average polyglutamyl tail length, leading to enhanced excretion of folate. By simultaneous overexpression of folKE and folC, encoding the enzyme folate synthetase or polyglutamyl folate synthetase, the average polyglutamyl tail length was increased, again resulting in normal wild-type distribution of folate. The production of bioavailable monoglutamyl folate and almost complete release of folate from the bacterium was achieved by expressing the gene for γ-glutamyl hydrolase from human or rat origin. These engineering studies clearly establish the role of the polyglutamyl tail length in intracellular retention of the folate produced. Also, the potential application of engineered food microbes producing folates with different tail lengths is discussed.
Folate is a B vitamin and an essential nutrient in the human diet. Folate deficiency is correlated with numerous physiological disorders, such as neural-tube defects (24) and early spontaneous abortion (9). Moreover, altered folate homeostasis as a consequence of poor nutrition and/or genetic variability is associated with higher risks of cardiovascular diseases (3, 16), several types of cancer (6, 15, 20), and mental disorders among the elderly and decreased cognitive performance (5, 13). A large proportion of natural folates are derived from the consumption of fermented dairy products and other fermented foods. The current recommended daily intake of folates for adults is 400 μg (600 μg for pregnant woman).
“Folate” is a general term for a large number of folic acid derivatives that differ in their states of oxidation, one-carbon substitution of the pteridine ring, and the number of glutamate residues. The in vivo function of reduced folate is that of a cofactor that donates one-carbon units in a variety of reactions involved in the de novo biosynthesis of purines and thymidylate and for the methylation of homocysteine to methionine. In most biological systems, folate is present in a conjugated form containing a poly-γ-glutamyl tail. Polyglutamyl folates are better substrates for the enzymes of one-carbon metabolism than the corresponding monoglutamyl folates (27). Moreover, this charged tail may prevent the vitamin from leaking out of the cell (23, 26, 33). Therefore, the polyglutamyl tail length is assumed to be a dominant factor in the distribution of folate over the cell membrane.
In earlier work, increased extracellular folate levels in Lactococcus lactis that were achieved by metabolic engineering were described(34). This lactic acid bacterium is widely applied in the dairy industry for the manufacture of fermented dairy products, such as cheese, butter, and buttermilk. It was also shown that the intracellular accumulation of folate was changed by the overexpression of specific genes involved in folate biosynthesis (34). Here, we describe the targeted engineering of the folate polyglutamyl tail length by the controlled expression of homologous genes involved in folate biosynthesis and heterologous genes encoding glutamyl hydrolase. We describe the impact of these modulations on folate distribution between the intra- and extracellular spaces. These strategies could be applied to control the degree of accumulation of folate or its release during fermentation in order to modulate the bioaccessibility of folate in fermented foods. Finally, we discuss how L. lactis could be used as a vehicle for delivering extra activity of polyglutamate hydrolase in the intestine.
MATERIALS AND METHODS
Bacterial strains and media.
L. lactis strain NZ9000 (19) and its derivatives were grown at 30°C in M17 medium (Merck, Darmstadt, Germany) (37) supplemented with 0.5% glucose (GM17) or in chemically defined SA medium (14). When appropriate, chloramphenicol was used at 10 μg/ml. Nisin induction was performed as described previously (34).
DNA techniques, construction of plasmids, and transformations.
The plasmids used in this study are listed in Table 1. The lactococcal plasmid pNZ8048 (19) is a vector that allows nisin-controlled expression of genes; it contains the nisA promoter followed by a multiple cloning site. pNZ8048 and its derivatives and lactococcal chromosomal DNA were isolated as described previously (21, 38). PCRs were performed with Pwo DNA polymerase (Boehringer, Mannheim, Germany) in a Mastercycler PCR apparatus (Eppendorf, Hamburg, Germany) with the following regime: denaturation at 94°C for 15 s (3 min in the first cycle), annealing at 50°C for 30 s, and extension at 72°C for 1 min for a total of 30 cycles.
TABLE 1.
Plasmids used in this study and their characteristics
| Plasmid | Relevant characteristics | Reference |
|---|---|---|
| pNZ8048 | Cmr; inducible expression vector carrying the nisA promoter | 19 |
| pNZ7001 | Cmr; pNZ8048 derivative containing a functional mature HGH gene under control of the nisA promoter | This study |
| pNZ7002 | Cmr; pNZ8048 derivative containing a functional mature rat γ-glutamyl hydrolase gene under control of the nisA promoter | This study |
| pNZ7010 | Cmr; pNZ8048 derivative containing a functional lactococcal folKE gene under control of the nisA promoter | 34 |
| pNZ7011 | Cmr; pNZ8048 derivative containing functional lactococcal folKE and folC genes under control of the nisA promoter | 34 |
| pNZ7016 | Cmr; pNZ8048 derivative containing a functional lactococcal folC gene under control of the nisA promoter | This study |
The gene folC encoding the biprotein folate synthetase-polyglutamyl folate synthetase, was amplified from chromosomal DNA using the primers folC-F (5′-GGTCCATGGTTTCTATTGAACAAGCATTAGAATGG-3′) and folC-R (5′-TCTCTAGACTACTTTTCTTTTTTCAAAAATTCACG-3). The forward primer, folC-F, contains an NcoI restriction site (underlined), allowing translational fusion of the folC gene to the nisA promoter region in pNZ8048. This NcoI site resulted in the introduction of an additional valine residue at position 2 of the encoded FolC protein. The reverse primer, folC-R, introduces an XbaI restriction site (underlined). The amplification product was digested with NcoI and XbaI and cloned in similarly digested pNZ8048. The resulting plasmid was designated pNZ7016 and put the folC gene under nisA promoter control, allowing nisin-controlled human γ-glutamyl hydrolase (HGH) expression in an appropriate lactococcal nisin-controlled expression host like NZ9000 (19). This strain contains the nisRK genes, required for nisin-mediated regulation, integrated in the chromosomal pepN locus. The construction of pNZ7010, developed for the overexpression of folKE, and pNZ7011, developed for the simultaneous overexpression of folKE and folC, was described before (34).
The gene hgh, encoding the mature HGH protein, was amplified from the corresponding full-length cDNA (41) cloned in vector pCR3, kindly provided by the Laboratory of Molecular Diagnostics, Wadsworth Center, Albany, N.Y., by using the sense primer HGH-f (5′-CATGCCATGGGACCCCACGGCGACACCGCCAAG-3′) and the antisense primer HGH-r (5′-GCTCTAGATCAATCAAATATGTAACATTGCTG-3′). The PCR product was digested with NcoI and XbaI (the sites are underlined) and cloned in similarly digested pNZ8048. The introduction of an NcoI site resulted in the insertion of an additional methionine at position 1 and a replacement of arginine by glycine at position 2 of the encoded HGH protein, resulting in the synthesis of MG-HGH. The resulting plasmid was designated pNZ7001 and contained the human glutamyl hydrolase gene translationally fused to the nisA gene and under nisA promoter control.
The gene rgh, encoding the mature rat γ-glutamyl hydrolase, was amplified from the corresponding full-length cDNA (42) cloned in vector pCR2, kindly provided by the Laboratory of Molecular Diagnostics, by using the sense primer RGH-f (5′-CATGCCATGGGATCCTATGAGCGCGGCTCCAAG-3′) and the antisense primer RGH-r (5′-GCTCTAGATCAGTTAAACATATAAGCTTGCTG-3′). The PCR product was (partially) digested with NcoI and XbaI (the sites are underlined) and cloned in similarly digested pNZ8048. The introduction of an NcoI site resulted in the insertion of an additional methionine at position 1, resulting in the synthesis of M-RGH. The new plasmid was designated pNZ7002 and contained the rat glutamyl hydrolase gene translationally fused to the nisA gene and under nisA promoter control.
Restriction enzymes and T4 DNA ligase were purchased from Life Technologies BV (Breda, The Netherlands) and used according to the manufacturer's protocol. All other DNA manipulations were performed using established procedures (30). L. lactis was electroporated as described before (40).
Quantification of folate.
Folate was quantified using a Lactobacillus casei microbiological assay (12), including postsampling enzymatic deconjugation, as described before (34). A 1% yeast extract medium solution (Difco, Becton DickinsonMicrobiology Systems, Sparks, Md.) containing almost exclusively polyglutamyl folates, with a previously determined total folate content, was used as a positive control for deconjugation.
Folate measurement by HPLC.
Intracellular folate levels were measured by high-performance liquid chromatography (HPLC) as described previously (35). HPLC columns, pumps, and chromatographic conditions were as described previously (35). The freshly prepared mobile phase consisted of 20% methanol and 1.5% formic acid, pH 3.0 (A), and 1.5% formic acid, pH 3.0 (B). Elution conditions were 25% A and 75% B for 30 min, followed by 75% A and 25% B from 32 to 100 min. Prior to analysis, the column was washed with 60% acetonitrile. Fluorimetric detection using a Waters 470 fluorescence detector was done at an excitation wavelength of 310 nm and an emission setting of 352 nm. The optimal signal-to-noise ratio for sensitive detection was an attenuation of 512 or 32 for detection of intra- and extracellular folate, respectively, and a gain value of 100 with a filter value of 4 s. UV detection was performed using a Shimadzu SPD-M10A photodiode array detector. Photodiode array detector data were collected between 220 and 500 nm at 2-nm optical resolution in order to discriminate fine structural details of the mono- and polyglutamyl folate spectra. Postanalysis routines were achieved using Shimadzu Class VP 5.0 software. UV absorption at 360 nm enables the discrimination of 5,10-methenyl tetrahydrofolates (25), while 5-formyl tetrahydrofolate derivatives are clearly discriminated by fluorimetric detection.
Extracellular folate levels were analyzed by HPLC after purification and concentration by solid-phase extraction using C18 columns (500 μg; 3 ml; Sopachem BV, Wageningen, The Netherlands). For this purpose, cells were grown in chemically defined SA medium (14), and 50 ml of fermentation broth was acidified with formic acid to pH 2.8 and loaded on the column, which was equilibrated with 5 ml of methanol, 5 ml of H2O, and 5 ml of 20 mM NaPO4 (pH 2.8). After passage of the sample by gravity, the column was washed with 10 ml of 20 mM NaPO4 (pH 2.8). Finally, the folates were eluted with 5 ml of 10 mM acetic acid- 25% methanol (pH 7.0), and 100 μl of the eluate was analyzed by HPLC as described above.
Folate derivatives used as standards—(6R,S)-5-formyl-5,6,7,8-tetrahydropteroyl mono-, di-, tri-, tetra-, and penta-γ-l-glutamic acid (lithium salt) and (6R,S)-5,10-methylene-5,6,7,8-tetrahydrofolic acid (magnesium salt)—were purchased from Schircks (Jona, Switzerland). Small volumes of folate stock solutions were prepared at a concentration of 1 mg/ml and stored at −20°C. Working solutions were prepared by dilution to a concentration within the range of 1 to 100 ng/ml. The tail lengths of the concentrated 5-formyl polyglutamyl folate samples were analyzed by mass spectrometry using a VG Quattro II mass spectrometer (Micromass UK Ltd., Manchester, United Kingdom). All chromatographic separations performed in this study were largely reproducible without influencing the results and conclusions.
Functional expression of γ-glutamyl hydrolase monitored in vitro.
An overnight culture of L. lactis harboring pNZ7001 or pNZ8048 was diluted (1:100) in 20 ml of GM17 supplemented with 10 μg of chloramphenicol/ml and grown to an optical density at 600 nm (OD600) of 0.5. The cells were induced with nisin (1 ng/ml) and incubated to an OD600 of 2.5, at which point cells were harvested for extraction. A cell extract was made as described above. Five hundred microliters of concentrated cell extract was added to 20 ml of 0.5-g/liter yeast extract (Difco Laboratories, Detroit, Mich.), as a source of polyglutamyl folate, dissolved in 0.1 M Na-PO4 buffer, pH 7.0-1% ascorbic acid. Incubation continued for 4 h at 37°C, and samples were taken periodically. The reaction was stopped by heating the samples for 5 min at 100°C, followed by determination of the folate concentration.
RESULTS
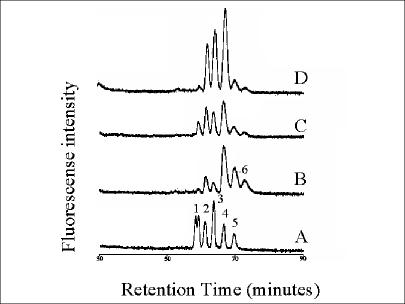
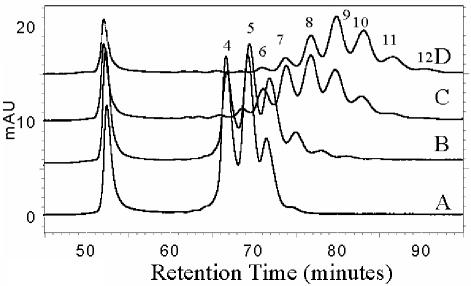
The folate distribution and the nature of the polyglutamyl folates produced were studied in the different engineered L. lactis strains. Analysis by HPLC of the intracellular folate pool in late-exponential-phase cells of L. lactis strain NZ9000 harboring pNZ8048 (empty vector) showed the presence of 5-formyl tetrahydrofolate with two, three, four, five, and six glutamate residues (Fig. 1B). The total folate production levels were found to be ∼50 ng/ml/OD600 unit, as determined by the folate microbiological assay, including deconjugase treatment of long-tailed polyglutamyl folates. This assay has nearly equal responses to mono-, di-, and triglutamyl folates, while the response to folates with longer polyglutamyl tails (>3 glutamyl residues) decreases markedly in proportion to the chain length (36). Approximately 90% of the total folate pool was accumulated inside the L. lactis cell. The intracellular folate concentration, as measured by the microbiological assay, increased after enzymatic deconjugation, confirming the presence of polyglutamyl folates. Approximately 10% of the total folate pool was excreted into the environment. Further analysis of folate distribution and polyglutamyl tail length was done in strain NZ9000 harboring pNZ7016, overexpressing folC, which encodes the bifunctional enzyme folate synthetase-polyglutamyl folate synthetase. The extracellular folate levels in this engineered strain were decreased, and the relative accumulation of folate was increased, upon induction with nisin, resulting in overexpression of FolC (results not shown). The growth rate and total folate production decreased a maximum of 20% upon gradual overexpression of folC using nisin concentrations ranging from 0 to 2 ng/ml. Moreover, upon high-level overexpression of folC, intracellular folate could no longer be measured without enzymatic deconjugation, suggesting that all folate molecules present had extended polyglutamyl tails. HPLC analysis of the intracellular folate pool of cells overexpressing folC confirmed this elongation of the folate polyglutamyl tail. It could be shown that glutamyl tail length increased stepwise with the increase of the nisin concentration used for folC induction. Under maximal induction conditions (2 ng of nisin/ml), folates with polyglutamyl tails containing up to 12 glutamyl residues could be detected, while in these cells polyglutamyl folates with <5 glutamate residues could hardly be visualized (Fig. 2). Based upon the specific UV absorption spectra of these polyglutamyl folates, characterized by a maximum at 360 nm, and the retention time of 5,10-methenyl tetrahydromonoglutamyl folate, we identified these folates as 5,10-methenyl tetrahydrofolate derivatives. Moreover, after enzymatic deconjugation and subsequent chromatographic separation, only 5,10-methenyl tetrahydromonoglutamyl folate could be detected. No extracellular folate could be detected, even when the culture supernatant was concentrated (results not shown).
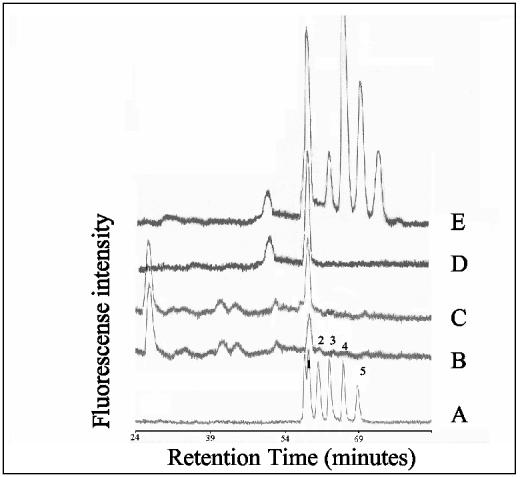
FIG. 1.
Folate chromatograms of 5-formyl tetrahydrofolate standards (A) and cell extracts of L. lactis NZ9000 harboring pNZ8048 (control strain with empty vector) (B), pNZ7010 (overexpressing folKE) (C), or pNZ7011 (overexpressing folKE and folC) (D) monitored by fluorescent detection. Cells were induced with 2 ng of nisin/ml as described in Materials and Methods. The numbers correspond to the polyglutamyl tail lengths of 5-formyl tetrahydrofolate derivatives. For 5-formyl tetrahydromonoglutamyl folate, both the S and Rdiastereoisomeres can be distinguished.
FIG. 2.
Folate chromatograms of cell extracts of L. lactis NZ9000, harboring pNZ7016 with increased overexpression of folC, induced with 0 (A), 0.01 (B), 0.1 (C), and 1 (D) ng of nisin/ml and monitored at 360 nm. The numbers correspond to thepolyglutamyl tail lengths of 5,10-methenyl tetrahydrofolate derivatives. mAU, milliabsorbance units.
In earlier work, an almost 10-fold increase in extracellular and total folate production was reported upon the overexpression of folKE, encoding the biprotein 2-amino-4-hydroxy-6-hydroxymethyldihydropteridine pyrophosphokinase and GTP-cyclohydrolase I (34). We have now shown through chromatographic analysis of the intracellular folate pool that this is the result of a decrease in the levels of 5-formyl tetrahydrofolate with four, five, and six glutamate residues and an increase in the levels of 5-formyl tetrahydrofolate with one, two, and three glutamate residues in comparison to the control strain (Fig. 1). Similar analysis of the purified and concentrated extracellular folate pool produced by cells overexpressing folKE showed the presence of predominantly 5-formyl monoglutamyl folate and, to a lesser extent, 5-formyl diglutamyl folate (results not shown). In the present work, we have now also shown that upon the combined overexpression of folC and folKE in strain NZ9000 harboring pNZ7011, the average polyglutamyl tail length is increased in relation to the FolKE-overproducing strain but is still smaller than that of the wild-type. Chromatographic separation of the intracellular folate pool revealed decreased levels of 5-formyl tetrahydrofolate with five and six glutamate residues and increased levels of 5-formyl tetrahydrofolate with two, three, and four glutamate residues compared to the wild type (Fig. 1). The higher intracellular folate concentration in cells overexpressing folKE and folC compared to the FolKE-overproducing cells reported earlier, using the microbiological assay (12), were confirmed by the chromatograms representing the intracellular accumulated folates (Fig. 1).
All folate chromatograms obtained were also analyzed by UV detection to evaluate the effects of metabolic engineering on 5,10-methenyl tetrahydrofolate derivatives in addition to 5-formyl tetrahydrofolate derivatives. These analyses showed that the polyglutamyl tail length of this folate molecule responded similarly to the engineering approaches employed for the 5-formyl tetrahydrofolates. Quantitative analysis by microbiological assay of fractions collected from the chromatographic separation of the fermentation broth revealed that the 5-formyl monoglutamyl folate and 5,10-methenyl monoglutamyl folate detected represented >90% of the total extracellular folate pool that was injected on the column (results not shown).
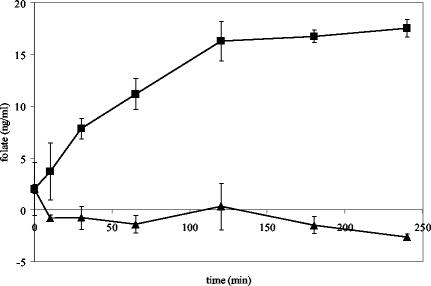
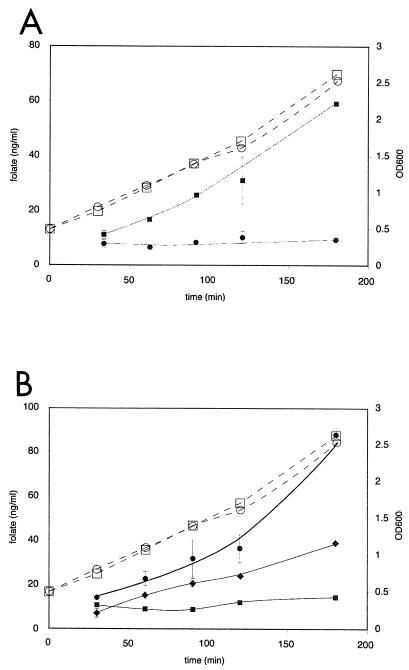
The level of polyglutamylation of folate in L. lactis was further controlled by the cloning and expression of γ-glutamyl hydrolase from human or rat origin in L. lactis (NZ9000 harboring pNZ7001 or pNZ7002, respectively). The production of HGH could be visualized on a Western blot using polyclonal anti-γ-glutamyl hydrolase antibody (results not shown). The HGH expression and activity in L. lactis was tested on yeast extract containing mostly heptaglutamyl folate (1). Figure 3 shows the progressive deconjugation of polyglutamyl folate in yeast extract by a cell extract of L. lactis NZ9000 harboring pNZ7001 induced with 1 ng of nisin/ml. The optimal enzyme activity was found at pH 7.0. In the cell extract of the control strain NZ9000 harboring pNZ8048, no deconjugase activity was found after the addition of nisin. Moreover, in a separate in vitro experiment, the strains expressing γ-glutamyl hydrolase were analyzed for the capacity to deconjugate polyglutamyl folates from different sources with different polyglutamyl tail lengths. An extract of cells expressing γ-glutamyl hydrolase from rat or human origin was mixed with a cell extract of wild-type L. lactis or strain NZ9000 harboring pNZ7016, which produces polyglutamyl folates. After incubation at 37°C for 3 h, chromatographic separation of the mixed cell extracts showed the conversion of polyglutamyl folate into monoglutamyl folate (results not shown). The functional expression of the strains expressing γ-glutamyl hydrolase was also analyzed in vivo. The fermentation broth and the intracellular folate pool of growing cells expressing HGH showed an increase in the extracellular folate concentration from ∼10 to 60 ng/ml, while the extracellular folate concentration of the control strain remained at a constant level of ∼10 ng/ml (Fig. 4). Detailed chromatographic analysis of 5-formyl polyglutamyl folate standards (Fig. 5A) and of the fermentation broth from cells producing MG-HGH showed an increase in 5-formyl monoglutamyl folate compared to cells not producing MG-HGH (Fig. 5B and C). The intracellular folate pool in cells producing MG-HGH changed from polyglutamyl folates with four, five, and six glutamyl residues to monoglutamyl folate that was partially excreted by the cells (Fig. 5D and E). It can be concluded that by the expression of the gene encoding MG-HGH in L. lactis, polyglutamyl folate is deconjugated, retention of folate is decreased, and the monoglutamyl folates formed are excreted into the environment. The efflux of folate does not lead to an altered growth rate or to increased folate production, so the remaining intracellular folate levels are sufficient for normal growth of the bacteria. The expression of the gene coding for rat γ-glutamyl hydrolase (34) in L. lactis (NZ9000 harboring pNZ7002) gave results similar to those described for MG-HGH, except for the increased rate of deconjugation (data not shown).
FIG. 3.
Functional expression of the gene coding for MG-HGH in a cell extract of L. lactis pNZ9000 harboring pNZ7001 (▪) or pNZ8048 (negative control) (▴) monitored in vitro using yeast extract as a source of polyglutamyl folate at pH 7.0. Folate concentrations were measured by a microbiological assay and corrected for the production of folate by the L. lactis strain. The error bars indicate the standard deviation of the microbiological assay (measurements were done in duplicate).
FIG. 4.
(A) Growth curves (dashed lines) and functional expression of the MG-HGH gene by monitoring the extracellular concentrations of folate (solid lines) in growing cells of L. lactis NZ9000 harboring pNZ7001 (▪) or pNZ8048 (negative control) (•). (B) Growth curves (dashed lines) and functional expression of the MG-HGH gene by monitoring the intracellular concentrations of folate (solid lines) in growing cells of L. lactis NZ9000 harboring pNZ7001 (▪) or pNZ8048 (♦). Higher intracellular folate levels were detected in the control strain after the deconjugation of polyglutamyl folate (•). The cells were induced with nisin at an OD600 of 0.5. Folate concentrations were measured by a microbiological assay. The error bars indicate the standard deviation of the microbiological assay (measurements were done in duplicate). Open symbols indicate cell mass.
FIG. 5.
Chromatography of 5-formyl tetrahydrofolate standards (A), folates extracted at the end of the exponential phase from fermentation broth of L. lactis harboring pNZ7001 induced with nisin (C) or not induced with nisin (B), and intracellular folates present in cell extracts of L. lactis harboring pNZ7001 induced with nisin (D) or not induced with nisin (E). Fluorescence detection: λex, 310 nm; λem, 352 nm. The numbers correspond to the polyglutamyl tail lengths of 5-formyl tetrahydrofolate derivatives. For 5-formyl tetrahydromonoglutamyl folate, the S and R diastereoisomeres can be distinguished.
Table 2 shows the correlation between the polyglutamyl tail length, as determined by HPLC, and the intra- and extracellular distribution of folate, as determined by the microbiological assay.
TABLE 2.
Average polyglutamyl tail length and ratio between intracellular and extracellular folate concentrations in engineered L. lactis strainsa
| Strain | Average polyglutamyl tail lengthb | Ratio of intracelluar folate and extracellular folatec |
|---|---|---|
| NZ9000/pNZ7010 | 3.0 | 0.64 |
| NZ9000/pNZ7011 | 3.3 | 1.5 |
| NZ9000/pNZ8048 | 4.1 | 9 |
| NZ9000/pNZ7016 | >5 | >>d |
| NZ9000/pNZ7001 | 1 | 0.15 |
| NZ9000/pNZ7002 | 1 | 0.15 |
Determined 10 h after induction with nisin.
Determined by calculating the area under the curve in the chromatogram (standard deviation, between 0 and 20%; experiments performed in duplicate). Number of glutamate residues.
Determined by microbiological assay (standard deviation, between 0 and 15%; measurements done in duplicate).
Depends on detection limit of microbiological assay (∼1 ng/ml).
DISCUSSION
All living cells contain folate, mostly in the polyglutamyl form. Many folate-dependent enzymes have a higher affinity for polyglutamyl folates than for the corresponding monoglutamyl folates (27). The enzyme responsible for polyglutamyl folate synthesis and the corresponding chain length elongation is polyglutamyl folate synthetase (EC 6.3.2.17), encoded by folC. So far, all organisms for which the entire genome sequence has been determined appear to have a homologue of folC. Nevertheless, different species are known to contain a large variety of actual folate polyglutamyl chain lengths, ranging from 1 to 10 glutamate residues (32). It is generally assumed that polyglutamyl folates determine the retention of folate molecules inside the cells. However, in bacteria, this assumption is based on observations that the polyglutamyl tail lengths of folates found externally in the growth medium are shorter than the tail lengths found intracellularly (4). Here, by engineering this property in a directed manner, we provide experimental evidence for a correlation between the polyglutamyl tail length and intracellular accumulation or extracellular release of folate in bacterial cells. The overexpression of folC increases the polyglutamylation of folate enormously, and polyglutamyl folates with up to 12 glutamyl residues could be detected. As a consequence, retention of folate in the cells was increased and release of folate into the environment was below detection limits. The elongation of the intracellular polyglutamyl tail affected the growth rate and total folate production slightly, which could be caused by a reduced affinity of the folate-dependent enzymes for folates with longer polyglutamyl tails. Shortening of the polyglutamyl tail length is possible by the overexpression of folKE, which resulted in conversion of intracellular polyglutamyl folates with predominantly four, five, and six glutamate residues in the wild type to folates with predominantly one, two, and three glutamyl folates. Concomitantly, increased levels of 5-formyl monoglutamyl folate could be measured in the culture supernatants of these folKE-overexpressing strains. Simultaneous overexpression of folC and folKE can partially counteract the folKE overexpression effect, generating a partial recovery of the polyglutamyl tail length and spatial folate distribution toward the wild-type situation. We conclude that the capacity of folate synthetase and polyglutamyl folate synthetase to add glutamate residues to the folate precursor dihydropteroate and subsequent mono- and polyglutamyl folates is limited when the flux through the folate biosynthesis pathway is increased, for instance, by overproduction of the first enzyme of the pathway, GTP cyclohydrolase I. As a consequence, production of short-tailed polyglutamyl folates is favored over the synthesis of long-tailed polyglutamyl folates. Further reduction of the polyglutamyl tail length is possible by the expression of heterologous γ-glutamyl hydrolase in L. lactis, which results in the deconjugation of polyglutamyl folate and subsequent decreased retention of folate. The HPLC data show that in L. lactis only monoglutamyl folates can be transported over the cell membrane. In general, our results provide the first direct evidence that polyglutamyl folates are responsible for retention of this vitamin in the bacterial cell.
The chromatographic separation of L. lactis cell extracts and subsequent analysis of folate species by UV absorption or fluorescence have enabled the detection and quantification of several forms of folate with different polyglutamyl tail lengths. Although the maximum polyglutamyl tail length of the 5-formyl tetrahydrofolate standards used does not exceed five glutamyl residues, the appearance of 5-formyl monoglutamyl folate after enzymatic deconjugation of the polyglutamyl folates justifies the conclusion that the polyglutamyl folates produced are also derivatives of 5-formyl tetrahydrofolate. A similar conclusion can be made for the detection of 5,10-methenyl tetrahydrofolate derivatives with their unique and characteristic absorption maximum at 360 nm (25). Other folate derivatives were not detected in our experiments.
In humans, most folate is consumed in the polyglutamyl form; however, folate is absorbed by tissue cells in the monoglutamyl form. Polyglutamyl folates are available for absorption and metabolic utilization only after enzymatic deconjugation in the small intestine by a mammalian deconjugase enzyme (11, 29). In animal and human trials (7; A. Melse-Boonstra, C. E. West, M. B. Katan, F. J. Kok, and P. Verhoef, submitted for publication), it has been reported that the bioavailability of monoglutamyl folate is higher than that of polyglutamyl folate. The controlled production of monoglutamyl folates could have an impact on the bioavailability of folate, i.e., folate that can be directly absorbed in the gastrointestinal tract, for two reasons. (i) The total monoglutamyl folate levels will increase, and consequently, the need for intestinal hydrolase activity to deconjugate polyglutamyl folate will be relieved, which may be advantageous under conditions where hydrolase activity is reduced by certain food components present in the diet (2, 28, 31, 39) or by genetic polymorphism (8). (ii) The intracellular deconjugation of endogenously produced folate to monoglutamyl folate will result in increased excretion of folate into the environment, resulting in improved bioaccessibility. Especially when microorganisms tend to survive the passage through the gastrointestinal tract, release of folate by these cells increases the effective folate consumption. By contrast, the production of polyglutamyl folates could also have advantages. Bacterial strains used as probiotics, which are generally consumed in large amounts in relatively small absolute volumes, may deliver higher folate concentrations when the produced polyglutamyl folates are accumulated intracellularly during growth. However, a prerequisite of efficient folate delivery by such probiotic strains is limited survival of the cells during intestinal passage. Human trials have indicated that the majority of L. lactis cells actually lyse during passage through the gastrointestinal tract (17).
Another benefit of the expression of γ-glutamyl hydrolase was shown in the in vitro experiments, which clearly indicated that the γ-glutamyl hydrolase expressed in L. lactis can also be active on polyglutamyl folates from other sources or with different polyglutamyl tail lengths. Consequently, under conditions where intestinal deconjugase activity is limited, conversion of polyglutamyl folates to monoglutamyl folates will be enhanced within the gastrointestinal tract by extra delivery of active γ-glutamyl hydrolase.
In an ordinary diet, the daily recommended intake for folates could be achieved via the consumption of fermented dairy products (18). In some regions, foods fortified with synthetic folic acid are important sources of folate. However, the availability of these sources is not widespread. Although the difference in bioavailability of natural folate versus synthetic folic acid has been subject to debate (10), our work has provided a basis for further development of functional foods with increased levels of naturally produced, and bioavailable, folate. The use of microorganisms expressing γ-glutamyl hydrolases for the production of natural bioavailable monoglutamyl folate during food fermentation is expected to be applied only when γ-glutamyl hydrolases from (food grade) bacterial origin are used. In several Bacillus spp., carboxypeptidases that may have γ-glutamyl hydrolase activity have been described (22). However, further research is needed to determine whether bacterial carboxypeptidases that hydrolyze the γ-glutamyl tail of folate can be found.
Acknowledgments
We thank C. Olieman and J. van Riel from NIZO Food Research, Ede, The Netherlands, for assistance with HPLC analysis. We thank J. Galivan and T. J. Ryan from The Laboratory of Molecular Diagnostics, Wadsworth Center, Albany, N.Y., for providing the cDNA from human and rat γ-glutamyl hydrolases.
This work was partially financed by the European Commission (QLK1-CT-2000-01376).
REFERENCES
- 1.Bassett, R., D. G. Weir, and J. M. Scott. 1976. The identification of hexa-, hepta- and octoglutamates as the polyglutamyl forms of folate found throughout the growth cycle of yeast. J. Gen. Microbiol. 93:169-172. [DOI] [PubMed] [Google Scholar]
- 2.Bhandari, S. D., and J. F. Gregory. 1990. Inhibition by selected food components of human and porcine intestinal pteroylpolyglutamate hydrolase activity. Am. J. Clin. Nutr. 51:87-94. [DOI] [PubMed] [Google Scholar]
- 3.Boushey, C. J., S. A. Beresford, G. S. Omenn, and A. G. Motulsky. 1995. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA 274:1049-1057. [DOI] [PubMed] [Google Scholar]
- 4.Buehring, K. U., T. Tamura, and E. L. Stokstad. 1974. Folate coenzymes of Lactobacillus casei and Streptococcus faecalis. J. Biol. Chem. 249:1081-1089. [PubMed] [Google Scholar]
- 5.Calvaresi, E., and J. Bryan. 2001. B vitamins, cognition, and aging: a review. J. Gerontol. B 56:327-339. [DOI] [PubMed] [Google Scholar]
- 6.Choi, S. W., and J. B. Mason. 2002. Folate status: effects on pathways of colorectal carcinogenesis. J. Nutr. 132:2413-2418. [DOI] [PubMed] [Google Scholar]
- 7.Clifford, A. J., M. K. Heid, J. M. Peerson, and N. D. Bills. 1991. Bioavailability of food folates and evaluation of food matrix effects with a rat bioassay. J. Nutr. 121:445-453. [DOI] [PubMed] [Google Scholar]
- 8.Devlin, A. M., E. H. Ling, J. M. Peerson, S. Fernando, R. Clarke, A. D. Smith, and C. H. Halsted. 2000. Glutamate carboxypeptidase II: a polymorphism associated with lower levels of serum folate and hyperhomocysteinemia. Hum. Mol. Genet. 22:2837-2844. [DOI] [PubMed] [Google Scholar]
- 9.George, L., J. L. Mills, A. L. Johansson, A. Nordmark, B. Olander, F. Granath, and S. Cnattingius. 2002. Plasma folate levels and risk of spontaneous abortion. JAMA 288:1867-1873. [DOI] [PubMed] [Google Scholar]
- 10.Gregory, J. F. 1995. Folate: nutritional and clinical perspectives, p. 195-235. In L. Bailey (ed.), Folate in health and disease. Marcel Dekker, New York, N.Y.
- 11.Halsted, C. H. 1989. The intestinal absorption of dietary folates in health and disease. J. Am. Coll. Nutr. 8:650-658. [DOI] [PubMed] [Google Scholar]
- 12.Horne, D. W., and D. Patterson. 1988. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin. Chem. 34:2357-2359. [PubMed] [Google Scholar]
- 13.Hultberg, B., A. Isaksson, K. Nilsson, and L. Gustafson. 2001. Markers for the functional availability of cobalamin/folate and their association with neuropsychiatric symptoms in the elderly. Int. J. Geriatr. Psychiatry 16:873-878. [DOI] [PubMed] [Google Scholar]
- 14.Jensen, P. R., and K. Hammer. 1996. Minimal requirements for exponential growth of Lactococcus lactis. Appl. Environ. Microbiol. 59:4363-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, Y. I. 1999. Folate and cancer prevention: a new medical application of folate beyond hyperhomocysteinemia and neural tube defects. Nutr. Rev. 57:314-321. [DOI] [PubMed] [Google Scholar]
- 16.Klerk, M., P. Verhoef, R. Clarke, R., H. J. Blom, F. J. Kok, and E. G. Schouten. 2002. MTHFR 677C→T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA 288:2023-2031. [DOI] [PubMed] [Google Scholar]
- 17.Klijn, N., A. H. Weerkamp, and W. M. de Vos. 1995. Genetic marking of Lactococcus lactis shows its survival in the human gastrointestinal tract. Appl. Environ. Microbiol. 61:2771-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konings, E. J., H. H. Roomans, E. Dorant, R. A. Goldbohm, W. H. Saris, and P. A. van den Brandt. 2001. Folate intake of the Dutch population according to newly established liquid chromatography data for foods. Am. J. Clin. Nutr. 73:765-776. [DOI] [PubMed] [Google Scholar]
- 19.Kuipers, O. P., P. G. de Ruyter, M. Kleerebezem, and W. M. de Vos. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J. Biotechnol. 64:15-21. [Google Scholar]
- 20.La Vecchia, C., E. Negri, C. Pelucchi, and S. Franceschi. 2002. Dietary folate and colorectal cancer. Int. J. Cancer 102:545-547. [DOI] [PubMed] [Google Scholar]
- 21.Leenhouts, K. J., J. Kok, and G. Venema. 1989. Campbell-like integration of heterologous plasmid DNA into the chromosome of Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 55:394-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leshchinskaya, I. B., E. V. Shakirov, E. L. Itskovitch, N. P. Balaban, A. M. Mardanova, M. R. Sharipova, M. B. Viryasov, G. N. Rudenskaya, and V. M. Stepanov. 1997. Glutamyl endopeptidase of Bacillus intermedius, strain 3-19. FEBS Lett. 404:241-244. [DOI] [PubMed] [Google Scholar]
- 23.Liu, Y., K. Raghunathan, C. Hill, Y. He, M. A. Bunni, J. Barredo, and D. G. Priest. 1998. Effects of antisense-based folypoly-gamma-glutamate synthetase down-regulation on reduced folates and cellular proliferation in CCRF-CEM cells. Biochem. Pharmacol. 55:2031-2037. [DOI] [PubMed] [Google Scholar]
- 24.Lucock, M. 2000. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol. Genet. Metab. 71:121-138. [DOI] [PubMed] [Google Scholar]
- 25.Lucock, M. D., M. Green, M. Priestnall, I. Daskalakis, M. I. Levene, and R. T. Hartley. 1995. Optimisation of chromatographic conditions for the determination of folates in foods and biological tissues for nutritional and clinical work. Food Chem. 55:329-338. [Google Scholar]
- 26.McGuire, J. J., and J. R. Bertino. 1981. Enzymatic synthesis and function of folylpolyglutamates. Mol. Cell. Biochem. 38:19-48. [DOI] [PubMed] [Google Scholar]
- 27.McGuire, J. J., and J. K. Coward. 1984. Pteroylpolyglutamates: biosynthesis, degradation, and function, p. 135-190. In R. L. Blakely and S. J. Bencovic (ed.), Folates and pterins. John Wiley & Sons, New York, N.Y.
- 28.Reisenauer, A. M., C. A. Buffington, J. A. Villanueva, and C. H. Halsted. 1989. Folate absorption in alcoholic pigs: in vivo intestinal perfusion studies. Am. J. Clin. Nutr. 50:1429-1435. [DOI] [PubMed] [Google Scholar]
- 29.Reisenauer, A. M., C. L. Krumdieck, and C. H. Halsted. 1977. Folate conjugase: two separate activities in human jejunum. Science 198:196-197. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 31.Seyoum, E., and J. Selhub. 1998. Properties of food folates determined by stability and susceptibility to intestinal pteroylpolyglutamate hydrolase action. J. Nutr. 128:1956-1960. [DOI] [PubMed] [Google Scholar]
- 32.Shane, B. 1986. Identification of folylpoly(gamma-glutamate) chain length by cleavage to and separation of p-aminobenzoylpoly(gamma-glutamates). Methods Enzymol. 122:323-330. [DOI] [PubMed] [Google Scholar]
- 33.Shane, B., and E. L. Stokstad. 1975. Transport and metabolism of folates by bacteria. J. Biol. Chem. 250:2243-2253. [PubMed] [Google Scholar]
- 34.Sybesma, W., M. Starrenburg, M. Kleerebezem, I. Mierau, W. M. de Vos, and J. Hugenholtz. 2003. Increased production of folate by metabolic engineering of Lactococcus lactis. Appl. Environ. Microbiol. 69:3069-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sybesma, W., M. Starrenburg, L. Tijsseling, M. H. N. Hoefnagel, and J. Hugenholtz. 2003. Effects of cultivation conditions on folate production by lactic acid bacteria. Appl. Environ. Microbiol. 69:4542-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamura, T., Y. S. Shin, M. A. Williams, and E. L. R. Stokstad. 1972. Lactobacillus casei response to pteroylpolyglutamates. Anal. Biochem. 49:517-521. [DOI] [PubMed] [Google Scholar]
- 37.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vos, P., M. van Asseldonk, F. van Jeveren, R. Siezen, G. Simons, and W. M. de Vos. 1989. A maturation protein essential for the production of active forms of Lactococcus lactis SK11 serine proteinase located in or secreted from the cell envelope. J. Bacteriol. 171:2795-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei, M. M., and J. F. Gregory. 1998. Organic acids in selected foods inhibit intestinal brush border pteroylpolyglutamate hydrolase in vitro: potential mechanism affecting the bioavailability of dietary polyglutamyl folate. J. Agric. Food Chem. 46:211-219. [DOI] [PubMed] [Google Scholar]
- 40.Wells, J. M., P. W. Wilson, and R. W. F. Le Page. 1993. Improved cloning vectors and transformation procedure for Lactococcus lactis. J. Appl. Bacteriol. 74:629-636. [DOI] [PubMed] [Google Scholar]
- 41.Yao, R., Z. Nimec, T. J. Ryan, and J. Galivan. 1996. Identification, cloning, and sequencing of a cDNA coding for rat gamma-gamma glutamyl hydrolase. J. Biol. Chem. 271:8525-8528. [DOI] [PubMed] [Google Scholar]
- 42.Yao, R., E. Schneider, T. J. Ryan, and J. Galivan. 1996. Human gamma glutamyl hydrolase: cloning and characterization of the enzyme expressed in vitro. Proc. Natl. Acad. Sci. USA 93:10134-10138. [DOI] [PMC free article] [PubMed] [Google Scholar]