Abstract
AIMS
To evaluate the relative plasma and tissue availability of diclofenac after repeated topical administration of a novel diclofenac acid-based delivery system under development (DCF100C).
METHODS
This was a single-centre, open-label, three-period, crossover clinical trial of five discrete diclofenac formulations. Test preparations comprised two concentrations (1.0% and 2.5%) of DCF100C, with and without menthol and eucalyptus oil (total daily doses of 5 mg and 12.5 mg). Voltaren® Emulgel® gel (1.0%) was the commercially available comparator (total daily dose of 40 mg). Topical application was performed onto the thigh of 20 male healthy subjects for 3 days. Applying a Youden square design, each drug was evaluated in 12 subjects, with each subject receiving three test preparations. Blood sampling and in vivo microdialysis in subcutaneous adipose and skeletal muscle tissues were performed for 10 h after additional final doses on the morning of day 4.
RESULTS
All four DCF100C formulations demonstrated a three- to fivefold, dose-dependent increase in systemic diclofenac availability compared with Voltaren® Emulgel® and were approximately 30–40 times more effective at facilitating diclofenac penetration through the skin, taking different dose levels into account. Tissue concentrations were low and highly variable. The 2.5% DCF100C formulation without sensory excipients reached the highest tissue concentrations. AUC(0,10 h) was 2.71 times greater than for Voltaren® Emulgel® (90% CI 99.27, 737.46%). Mild erythema at the application site was the most frequent adverse event associated with DCF100C. There were no local symptoms after treatment with the reference formulation.
CONCLUSION
DCF100C formulations were safe and facilitated greater diclofenac penetration through the skin compared with the commercial comparator. DCF100C represents a promising alternative to oral and topical diclofenac treatments that warrants further development.
Keywords: bioavailabilty, dermal penetration, diclofenac, microdialysis, topical
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Therapy with topical non-steroidal anti-inflammatory drugs (NSAIDs) relies on the ability of the active drug to penetrate the skin in sufficiently high amounts to exert a clinical effect, which is linked to the specific galenic properties of the formulation.
WHAT THIS STUDY ADDS
This phase 1 study characterizes the transdermal penetration and plasma exposure of different dose levels with galenic differences of a novel topical diclofenac formulation under development and indicates greater diclofenac penetration through the skin when compared with a commercially available formulation.
Introduction
Diclofenac is a non-steroidal anti-inflammatory drug (NSAID) with a well-established therapeutic usage, which is considered safe and effective [1]. It is administered for the treatment of acute and chronic pain of different aetiologies and exerts its action by cyclo-oxygenase inhibition and modulation of arachidonic acid release and uptake [2]. The dose-dependent risk of serious gastrointestinal, cardiovascular or renal adverse effects of oral NSAIDs has promoted the development of different topical formulations with the aim to reduce the detrimental systemic effects and simultaneously provide symptom control comparable with the oral counterparts.
Topical NSAID use is sometimes questioned because of higher costs than generic oral NSAIDs [3]. There is, however, evidence for their efficacy and they are regarded as safe and practical alternatives to oral NSAID treatment in many instances [4]. Topical diclofenac has been available in Europe for more than 20 years to relieve pain and inflammation due to rheumatic disease or osteoarthritis in muscles, tendons and joints and was approved for osteoarthritis therapy in the USA in 2007 [5].
The crucial step during topical NSAID therapy is the ability of the free active drug to penetrate the skin in sufficiently high amounts to exert its clinical effect, which largely depends on the composition of the drug formulation. Thus, to enhance diclofenac bioavailability, a novel topical formulation of diclofenac (DCF100C) has been developed. Most topical diclofenac preparations are usually supplied as the diethylamine, sodium or potassium salt. DCF100C uniquely uses diclofenac acid in a novel optimized solvent system (DermaSys®), because of its intrinsically higher permeability than the commonly used salts [6]. In vitro studies in human skin have shown over 10-fold enhancement of skin transport of diclofenac from DCF100C compared with the reference product Voltaren® Emulgel® (internal data, Futura Medical Developments Limited). Based on these results, it was anticipated that DCF100C would locally deliver higher drug concentrations than currently marketed formulations without achieving clinically significant systemic drug concentrations.
For the in vivo evaluation of the novel DCF100C gel formulation of diclofenac, two different dose levels (1.0% and 2.5% gels) with galenic differences (with or without menthol and eucalyptus oil excipients) were selected. Transdermal penetration and plasma exposure of DCF100C as well as local in vivo tissue delivery of diclofenac were assessed and compared with Voltaren® Emulgel® gel in male healthy subjects. Voltaren® Emulgel® gel was chosen as the reference formulation, as it is the current global sales leader for topical pain relief and because it has been studied previously using microdialysis (MD) [7–12]. Compared with other experimental techniques, MD uniquely enables the measurement of free, active local drug concentrations in different target tissues. Consequently, MD has been considered a promising approach for the evaluation of bioavailability and bioequivalence of topically applied drug formulations [13, 14].
Methods
The study protocol was approved by the local research ethics committee of the Medical University of Vienna, Austria. All subjects were given a detailed description of the study and their written consent was obtained prior to enrolment in the study. The study was conducted according to the harmonized European standards of Good Clinical Practice enshrined in ICH E6 1.24.
Study design
The study was conducted as a single-centre, open-label, five-treatment, three-period crossover study to compare the relative bioavailability of diclofenac in plasma, subcutaneous adipose and skeletal muscle tissue after repeated administration using two dose strengths of topically applied DCF100C with or without menthol and eucalyptus oil and topically applied Voltaren® Emulgel® gel (1.16%) in 20 evaluable subjects. To evaluate each test preparation in a group of 12 subjects, a Youden square design was applied, with subjects randomly allocated to one of four blocks containing five treatment sequences each. Each subject was exposed to the three test articles according to the prospective randomization sequence in three study phases (separated by a wash out period) and with comparable trial activities.
Study population
Twenty male, healthy, drug-free, White volunteers were included in the study. Their mean (±SD) age was 29.8 ± 8.8 years; mean bodyweight was 77.4 ± 8.8 kg with a body mass index of 24.1 ± 2.0 kg m−2. Baseline SBP, DBP and HR were 125.6 ± 5.9 mmHg, 72.5 ± 10.6 mmHg and 73.4 ± 11.2 beats min−1, respectively.
Investigational products
The four DCF100C test products were manufactured at Specials Clinical Manufacturing (SCM Pharma Northumberland, UK) and were supplied by Futura Medical Developments Ltd (Guildford, Surrey, UK).
DCF100C1 (1.0%) and DCF100C1 (2.5%) contained menthol and eucalyptus oil and were applied onto the skin twice daily (at 08.00 h ± 1 h and 20.00 h ± 1 h) for 3 days with a total daily diclofenac dose of 5 mg (DCF100C1, 1.0%) or 12.5 mg (DCF100C1, 2.5%), respectively. DCF100C2 (1.0%) and DCF100C2 (2.5%) were similar to the DCF100C1 formulations, except that they did not contain menthol and eucalyptus oil. These were applied twice daily for 3 days (as for the DCF100C1 formulations) and provided the same total daily diclofenac doses.
The reference substance, Voltaren® Emulgel® gel 1.0%, was provided by the local hospital pharmacy and was applied onto the skin 4 times daily (at 08.00 h ± 1 h, 12.00 h ± 1 h, 16.00 h ± 1 h and 20.00 h ± 1 h) for 3 days with a total daily diclofenac dose of 40 mg.
For all treatments, a last drug application was performed on the morning of day 4. The first and the last drug applications were performed by the investigator at the clinical trial centre. The interim applications were performed by the subjects at home.
Experimental design
Two weeks prior to the first drug application, a screening visit was performed, which included a physical examination, determination of body mass index (BMI), collection of blood and urine for routine laboratory and drug-screening tests, evaluation of vital signs and an electrocardiogram (ECG). Prior to the first study day, eligible subjects were allocated to the next available treatment schedule according to a prospective randomization sequence.
On the morning of the first study day of each study period, baseline blood sampling was performed. Thereafter, the area of topical drug application at the anterior right or left thigh was marked, shaved, and the first topical drug application was performed onto the marked area (10 × 10 cm = 100 cm2). Volunteers were given diary cards and were instructed how to apply the appropriate daily doses at home, to record the exact times of test preparation application and to accurately and fully complete the diary cards. For the rest of the first day and during the next 2 days, subjects administered the allocated medications at home. In the evening of the third study day, subjects were confined at the clinical trial centre. The last drug application was performed in the morning of day 4 and was followed by blood and tissue diclofenac concentration measurements for up to 10 h.
For the measurement of diclofenac in subcutaneous adipose and skeletal muscle tissue, commercially available microdialysis probes (cut-off 20 000 kD, CMA, Solna, Sweden) were inserted. After probe equilibration and steady-state sampling, the final administration of the allocated medication was performed. Thereafter, microdialysates and venous blood samples were collected every hour for 10 h post dosing. All samples were stored at approximately −80°C prior to analysis. Microdialysis probes were calibrated in vivo at the end of the study day as described previously [11]. Local tolerability was assessed at 1 h and 10 h after the last administration of the test preparation. The second and third study periods were performed as described above for period 1, with washout periods of at least 2 weeks between study periods. A final clinic visit was performed 1 week following the last study period.
Sample analysis
Total diclofenac concentrations in plasma and free, non-protein bound diclofenac concentrations in microdialysates were determined by Pharmakin GmbH, Germany, using validated LC-MS/MS methods with electrospray ionization in the positive mode [ESI (+)] and multiple reaction monitoring (MRM) with a limit of quantification (LLQ) of 150 pg ml−1. Analyses were performed in compliance with GLP regulations {OECD, [C (97) 186 Final]} using fully validated methods.
Data analysis
Pharmacokinetic parameters of diclofenac were determined from total drug concentrations in plasma and free, non-protein bound drug concentrations in microdialysates and with reference to pharmacokinetic modelling software. For pharmacokinetic calculations, the program package WinNonlin® version 4.1 (WinNonlin V4.1, Pharsight Corporation, California, USA, 2003) was employed. The following pharmacokinetic parameters were calculated for each study preparation:
Plasma AUC(0,10 h) (pg ml−1 h) = area under the plasma concentration−time curve, calculated by the linear trapezoidal rule based on total plasma concentrations following drug administration up to 10 h.
Tissue AUC(0,10 h) (pg ml−1 h) = area under the microdialysate concentration−time curve, calculated by the linear trapezoidal rule based on free MD concentrations (for each probe) following drug administration up to 10 h.
Cmax (pg ml−1) = maximum concentration (in plasma or microdialysate)
tmax (h) = time after dosage to reach Cmax (in plasma or microdialysate)
Cmin (pg ml−1) = minimum concentration (in plasma), evaluated for additional exploratory purposes
Additionally, AUC(0,3 h) was calculated to provide further information on early local delivery post dose. Diclofenac half-life (t1/2) was not calculated for plasma, because steady-state drug concentrations were only measured for a 10-h period and the 10-h concentrations were still relatively high. Half-life calculations for microdialysates were not possible because of fluctuating concentrations throughout the observation period. Given in vivo recovery values > 85% [i.e. mean recovery for skeletal muscle microdialysis probes was 88.0 ± 5.2% (SD), mean recovery for subcutaneous tissue was 85.4 ± 5.7%], tissue diclofenac concentrations were not corrected for individual or mean recoveries.
Statistical analysis
Statistical analysis was performed as a valid case analysis with all primary target variables available for measurement using WinNonlin. AUC(0,10 h) and Cmax were primary target variables, whereas tmax and AUC(tissue) : AUC(plasma) were considered as secondary pharmacokinetic target variables. In order to achieve a better approximation to a normal distribution, AUC(0,10 h) and Cmax data (including Cmin for plasma) were logarithmically transformed before analysis and tested parametrically (anova). A linear mixed effects model appropriate for a Youden square design was fitted to the data, with components of treatment, period, sequence and subject within sequence. From the results of anova, 90% confidence intervals of two one-sided t-tests were calculated for test : reference parameter ratios of geometric means by retransformation of the shortest confidence interval for the difference of the ln-transformed values. The parameters tmax and AUC(tissue) : AUC(plasma) were tabulated including calculation of descriptive statistics. Comparative bioavailability was evaluated by 90% confidence intervals for test : reference ratios (point estimators) of geometric means for AUC(0,10 h) and Cmax. Statistical analysis was performed using WinNonlin V4.1.
Post-hoc pharmacokinetic analysis
Voltaren® Emulgel® was dosed 4 times a day and DCF100 formulations twice a day for 3 days. Multiple-dose plasma concentrations were measured after the 13th and 7th dose, respectively, on day 4. Using a simple pharmacokinetic correction, single-dose pharmacokinetics could be predicted from the multiple-dose variable-regimen dosing used in the study. The pharmacokinetic model assumed a simple single compartment with a single first-order elimination process.
Safety and tolerability
Safety and tolerability assessments were based on recording of adverse events and the assessment of local drug tolerability at the application site by visual inspection, at home (performed by the subjects) and at 1 and 10 h after last drug application at the clinical trial centre (performed by the investigator). Assessment of local tolerability included the documentation and scoring of symptoms, i.e. erythema, itching and burning.
Results
For all four DCF100 preparations, a three- to fivefold, dose-dependent increase in systemic availability of diclofenac compared with Voltaren® Emulgel® was observed during the 10-h post final dose after 3 days multiple drug application (Table 1, Figure 1). Therefore, all four formulations were approximately 30–40 times more effective at facilitating diclofenac penetration through the skin, taking different dose levels into account. This increased systemic availability was sustained throughout the observation period, such that total diclofenac plasma concentrations were still up to threefold higher for DCF100C formulations compared with the reference substance at 10-h post dose. By contrast, the systemic concentrations in subjects dosed with the reference substance gradually declined after the last drug application. AUC(0,10 h) point estimators showed an increase in diclofenac plasma concentrations by 2.84-fold for DCF100C1 (1.0%), 2.82-fold for DCF100C2 (1.0%), 4.69-fold for DCF100C1 (2.5%) and 4.80-fold for DCF100C2 (2.5%), as compared with Voltaren® Emulgel®, respectively (Table 2).
Table 1.
Mean (±SD) pharmacokinetic parameters of diclofenac in plasma (total concentrations) and microdialysates from skeletal muscle (MD i.m.) and subcutaneous adipose tissue (MD s.c.) (free, non-protein bound concentrations)
| DCF100C1 (1%) | DCF100C2 (1%) | DCF100C1 (2.5%) | DCF100C2 (2.5%) | Voltaren® Emulgel® | |
|---|---|---|---|---|---|
| Plasma | |||||
| AUC(0,10 h) (pg ml−1 h) | 23 771.6 ± 11 308.1 | 19 733.7 ± 8 202.7 | 42 103.6 ± 19 474.4 | 35 704.7 ± 15 872.0 | 7306.2 ± 4790.7 |
| Cmax (pg ml−1) | 4 210.2 ± 2 110.6 | 3484.7 ± 1 612.8 | 8 222.6 ± 4 750.7 | 6 578.5 ± 3 877.9 | 1102.9 ± 852.9 |
| tmax (h) | 5.67 ± 2.19 | 6.42 ± 1.93 | 4.50 ± 2.32 | 4.50 ± 2.15 | 1.25 ± 1.96 |
| MD i.m. | |||||
| AUC(0,10 h) (pg ml−1 h) | 1 089.2 ± 1 443.6 | 2 089.5 ± 1 045.1 | 1 477.7 ± 1 732.4 | 12 031.0 ± 34 572.3 | 2004.5 ± 3257.4 |
| Cmax (pg ml−1) | 368.5 ± 449.9 | 1 071.2 ± 832.7 | 746.2 ± 673.3 | 6 277.4 ± 18 339.8 | 448.2 ± 435.3 |
| tmax (h) | 4.13 ± 2.90* | 4.92 ± 3.58 | 4.36 ± 3.17† | 4.64 ± 3.80† | 4.00 ± 3.43‡ |
| MD s.c. | |||||
| AUC(0,10 h) (pg ml−1 h) | 1 408.6 ± 1 234.8 | 2 429.8 ± 2 507.2 | 1 957.6 ± 2 138.8 | 3 709.4 ± 3 794.0 | 2925.5 ± 3501.2 |
| Cmax (pg ml−1) | 545.3 ± 366.7 | 1065.9 ± 1261.4 | 811.1 ± 970.0 | 1235.2 ± 1456.6 | 611.4 ± 498.9 |
| tmax (h) | 3.10 ± 2.77‡ | 5.64 ± 3.72† | 2.55 ± 1.63† | 2.00 ± 1.61† | 3.55 ± 3.64† |
n = 8
n = 11
n = 10. n = 12; unless otherwise specified. DCF100C1 (1%) and DCF100C1 (2.5%) contained menthol and eucalyptus oil as sensory signals.
Figure 1.
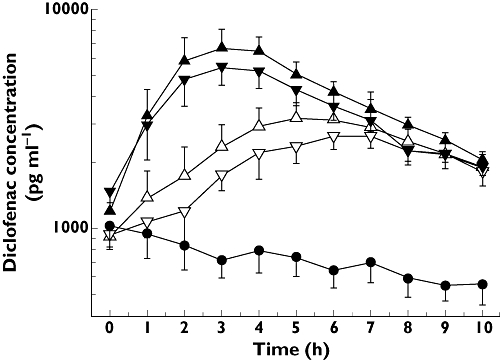
Mean time vs. total plasma concentration profiles of diclofenac after a 3-day multiple-dose regimen and a last dose on the morning of day 4 of topically applied DCF100C1 (1%) (▵) DCF100C1 (2.5%) (▵), DCF100C2 (1%) (▿), DCF100C2 (2.5%) (▿) and Voltaren® Emulgel® gel (•) in 12 healthy male subjects per formulation. Results are presented as means ± SE. DCF100C1 formulations contained menthol and eucalyptus oil as sensory signals
Table 2.
Comparative bioavailability
| Plasma PK parameter | DCF100C1 (1%) vs. Voltaren®Emulgel® | DCF100C2 (1%) vs. Voltaren®Emulgel® | DCF100C1 (2.5%) vs. Voltaren®Emulgel® | DCF100C2 (2.5%) vs. Voltaren®Emulgel® | |
|---|---|---|---|---|---|
| AUC(0,10 h) | PE | 284.02% | 282.38% | 468.62% | 480.19% |
| 90% CI | 201.76, 399.82% | 199.34, 400.00% | 331.01, 663.43% | 337.17, 683.88% | |
| Cmax | PE | 334.91% | 349.51% | 595.97% | 571.14% |
| 90% CI | 215.12, 521.40% | 222.69, 548.54% | 380.01, 934.65% | 361.38, 902.65% | |
| MD i.m. PK parameter | |||||
|---|---|---|---|---|---|
| AUC(0,10 h) | PE | 128.83% | 212.55% | 131.74% | 270.57% |
| 90% CI | 42.24, 392.95% | 81.58, 553.77% | 47.82, 362.96% | 99.27, 737.46% | |
| Cmax | PE | 113.65% | 190.97% | 156.11% | 258.43% |
| 90% CI | 43.32, 298.14% | 83.42, 437.15% | 64.98, 375.04% | 108.57, 615.12% | |
| MD s.c. PK parameter | |||||
|---|---|---|---|---|---|
| AUC(0,10 h) | PE | 60.67% | 59.79% | 53.56% | 95.29% |
| 90% CI | 23.13, 159.12% | 23.84, 149.98% | 21.40, 134.03% | 36.85, 246.38% | |
| Cmax | PE | 72.84% | 74.70% | 70.39% | 97.84% |
| 90% CI | 34.93, 151.90% | 37.06, 150.57% | 34.98, 141.62% | 47.43, 201.84% | |
Results are given as 90% confidence intervals for: test reference ratios (point estimators) of geometric means for AUC(0,10 h) and Cmax. Total plasma and free non-protein bound tissue concentrations are given. PE, point estimator (ratio of geometric means).
In the post hoc analysis, the output of the pharmacokinetic process gave plasma concentrations that are typical of single-dose pharmacokinetics. The dose absorbed was between 4.10 and 7.76 times (Cmax) and between 3.72 and 6.86 times [AUC(0,10 h)] for the 1% and 2.5% doses, respectively, compared with the reference Voltaren® Emulgel®.
Multiple-dosing over 3 days was performed to reach steady state. Cmin values on day 4 were 1292.2 and 1002.7 pg ml−1 for DCF100C2 (2.5%) and DCF100C1 (2.5%), respectively. DCF100C1 (1.0%), DCF100C2 (1.0%) and the reference preparation showed Cmin values of 729.9, 727.0 and 597.1 pg ml−1, respectively. As trough values in plasma or tissue were not measured during the 3-day application period, a true steady state could not be reliably demonstrated. Control of steady-state conditions at the site of action would have necessitated repeated insertion of microdialysis probes to obtain Cmin values during the application period.
Free diclofenac concentrations in subcutaneous and skeletal muscle tissue were highly variable and below the LLQ in many instances, despite substantial total diclofenac concentrations in the corresponding plasma samples (Table 1). In skeletal muscle, DCF100C2 (2.5%) demonstrated the highest bioavailability of all study preparations (Table 2). The AUC(0,10 h) was 2.71 times greater than after Voltaren® Emulgel® administration. Similarly, the other DCF100 formulations showed higher tissue diclofenac concentrations compared with the reference formulation [i.e. 1.29-, 1.32- and 2.13-fold higher AUC values for DCF100C1 (1.0%), DCF100C1 (2.5%) and DCF100C2 (1.0%), respectively]. In subcutaneous adipose tissue, AUC(0,10 h) values for all four DCF100 formulations were similar to or lower than Voltaren® Emulgel®. Because of a high variability, these differences were not statistically different.
To compare tissue availability of free diclofenac concentrations with systemically available total diclofenac concentrations, AUC(tissue) : AUC(plasma) ratios were calculated (Table 3). For the test formulations, AUC(tissue) : AUC(plasma) ratios ranged from 0.04 to 0.16, with the exception of test preparation DCF100C2 (2.5%), with an AUC(tissue i.m.) : AUC(plasma) of 0.85. This result, however, may be biased because of several extremely high drug concentrations in the microdialysates. The reference formulation, on the other hand, showed ratios of 0.62 for AUC(tissue i.m.) : AUC(plasma) and of 0.73 for AUC(tissue s.c.) : AUC(plasma).
Table 3.
Free tissue : total plasma availability ratios
| Treatment | AUCtissue i.m.: AUCplasma | AUCtissue s.c. : AUCplasma |
|---|---|---|
| Test A: DCF100C1 (1%) | 0.14 ± 0.24 (range 0.01–0.72; n = 8) | 0.10 ± 0.13 (range 0.01–0.47; n = 10) |
| Test B: DCF100C2 (1%) | 0.12 ± 0.05 (range 0.03–0.21; n = 12) | 0.13 ± 0.12 (range 0.02–0.37; n = 11) |
| Test C: DCF100C1 (2.5%) | 0.04 ± 0.04 (range 0.01–0.12; n = 11) | 0.05 ± 0.04 (range 0.01–0.12; n = 11) |
| Test D: DCF100C2 (2.5%) | 0.85 ± 2.62 (range 0–8.76; n = 11) | 0.16 ± 0.22 (range 0.01–0.79; n = 11) |
| Test E: Voltaren® Emulgel® | 0.62 ± 1.11 (range 0.02–3.36; n = 10) | 0.73 ± 1.33 (range 0.06–4.66; n = 11) |
Results are given as means ± SD (range).
To investigate drug elimination from target tissues during the first 3 h after the last drug application, AUC(0,3 h) values were additionally calculated (Table 4).
Table 4.
AUC(0,3 h) values (pg ml−1 h) (± SD) of all study formulations, calculated for plasma (total concentrations), microdialysates from skeletal muscle (MD i.m.) and microdialysates from subcutaneous adipose tissue (MD s.c.) (free, non-protein bound concentrations)
| Treatment | Plasma AUC(0,3 h) | MD i.m. AUC(0,3 h) | MD s.c. AUC(0,3 h) |
|---|---|---|---|
| DCF100C1 (1%) | 4 755.4 ± 4 809.0 | 455.4 ± 468.6 | 648.4 ± 588.7 |
| DCF100C2 (1%) | 3 623.1 ± 2 278.0 | 939.2 ± 1030.0 | 1020.9 ± 1191.9 |
| DCF100C1 (2.5%) | 13 040.4 ± 10 869.8 | 572.3 ± 854.7 | 886.4 ± 816.0 |
| DCF100C2 (2.5%) | 11 245.0 ± 8 264.9 | 929.7 ± 1216.2 | 1732.5 ± 1678.5 |
| Voltaren® Emulgel® | 2 656.4 ± 1 981.4 | 832.2 ± 1205.9 | 1122.6 ± 1218.6 |
n = 12.
Safety
The majority of adverse events were mild, non-serious and resolved spontaneously without intervention.
Erythema at the application site was the most frequent adverse event associated with the study medication. The incidence of erythema increased after the application of DCF100C2 (2.5%) > DCF100C2 (1%) > DCF100C1 (2.5%) > DCF100C1 (1%) (Table 5).
Table 5.
Incidence of erythema following exposure to test articles
| Formulation | Incidence of erythema (% dosing events) |
|---|---|
| DCF100C2 (2.5%) | 5% |
| DCF100C2 (1%) | 10% |
| DCF100C1 (2.5%) | 25% |
| DCF100C1 (1%) | 20% |
Burning was observed following application of DCF100C1 formulations, but not after DCF100C2 application. No symptoms at the application site were experienced after treatment with the reference formulation (Voltaren® Emulgel® gel).
Discussion
Topical administration of Voltaren® Emulgel® resulted in total plasma diclofenac concentrations of approximately 1 ng ml−1 at steady state. This concentration is consistent with results reported previously [12]. The finding that plasma concentrations gradually declined after the last Voltaren® Emulgel®application, however, is in contrast to a previous study that showed that plasma concentrations were increasing after the last dose of an 8-day multiple dose study indicating prolonged absorption from the application site [15]. As these increases occurred between 12 and 24 h post dose, the shorter sampling period of 10 h in the present study could be the reason for the observed differences. By contrast, topical administration of the new DCF100C formulations, with total daily doses being approximately three- to fivefold lower than those of the reference formulation, resulted in increases of up to 500% in the bioavailability of plasma diclofenac concentrations compared with Voltaren® Emulgel®, which suggests higher local tissue concentrations of DCF100C and a subsequently higher elimination from the potential target sites. This increase was dose-dependent and was slightly higher for the formulations which had eucalyptus and menthol added (Figure 1), which are known penetration enhancers [16]. Addition of sensory signals, however, did not substantially increase systemic availability as compared with the corresponding DCF100C formulations without sensory signals. There was a higher incidence of erythema at the application site, probably caused by increased local blood flow and potentially increasing dermal diclofenac clearance.
Calculation of AUC(0,3 h) was performed to evaluate additionally systemic drug absorption immediately after local test preparation application and indicated an increased elimination of the active principle from the target tissues by factors 4.2 and 4.9 for DCF100C1 (2.5%) and DCF100C2 (2.5%), and smaller increases for the other test preparations. As clinical efficacy was not evaluated in this healthy volunteer phase I study, it cannot be ascertained whether these increases also translate into differences in the time of onset of action.
Plasma concentrations after application of DCF100C formulations were consistently higher than after application of Voltaren® Emulgel®. However, they were substantially lower than those reported after oral diclofenac administration. A 3-day oral treatment regimen with 50 mg diclofenac 3 times daily resulted in maximum plasma concentrations of approximately 1000 ng ml−1[11]. In the present study, the highest systemic diclofenac plasma concentrations of 8.2 ng ml−1, i.e. those after DCF100C1 (2.5%), were approximately 150-fold lower than after oral diclofenac treatment, which indicates a wide safety margin with regard to systemic adverse drug effects.
It has to be mentioned that the 10-h sampling period after the last drug application on study day 4 did not cover the entire dosing interval for DCF100C. Still, plasma diclofenac concentrations after the 3-day, twice daily dosing regimen of DCF100C pointed at an increased local drug elimination as compared with a the 4 times daily dosing for Voltaren® Emulgel®. It has to be considered, however, that in Europe and the rest of the world, the registered dosing regimen for Voltaren® Emulgel® (diclofenac diethylamine gel 1.16%) is, according to country and indication, two to four applications daily, based on efficacy study results. It is only for the USA that the dosing regimen for mild osteoarthritis and diclofenac sodium gel 1.0% is currently four applications daily.
In a recent multiple-dose crossover study comparing the systemic bioavailability of diclofenac after local and oral administration, detectable plasma diclofenac concentrations were found after a 2-week washout period in 23% to 68% of patients [12]. The authors, however, considered these concentrations minimal and unlikely to bias pharmacokinetic parameters [12]. In the present study, predose blood sampling did not show any carry-over effect at the beginning of the second study period. Before the third study period, three out of 18 plasma samples (16%) contained quantifiable diclofenac concentrations. As all three subjects received the study formulations with the highest diclofenac concentrations thereafter, a bias for the subsequent data analysis can largely be excluded.
AUC(tissue) : AUC(plasma) ratios indicated that all five formulations studied in the present study were able to deliver the active principle to the target tissue (Table 3). Local diclofenac concentrations in subcutaneous adipose and skeletal muscle tissue were low and variable. However, based on previous experience with diclofenac using clinical microdialysis in different experimental settings [7–11], this finding was not unexpected. Although these studies were not directly comparable, a general finding was a highly variable transdermal penetration and the inability to quantify local diclofenac concentrations in many instances [8–10]. Reasons for the observed variability might be inter-individual differences between the studied healthy volunteers, which cannot be standardized, i.e. biological variations of the human skin barrier function, differences in skin metabolism, the individual ability of dermal clearance and local blood flow, hydration status, dose control, temperature and difficult to standardize perturbation measures like shaving of the application site. Low tissue concentrations, on the other hand, might result from rapid diclofenac clearance by the local dermal circulation after skin penetration. In the present study, this is reflected by lower subcutaneous adipose tissue concentrations for the formulations with eucalyptus and menthol compared with the formulations without sensory signals.
The plasma protein binding of diclofenac has been reported to be higher than 99% [17], indicating that only a small unbound fraction is available for cyclo-oxygenase inhibition. Considering the unbound diclofenac fraction in the present study, plasma concentrations were substantially lower than corresponding unbound diclofenac concentrations in tissue layers underneath the application site. Thus, substantial direct topical penetration and not redistribution from the systemic circulation can be assumed. This effect was more pronounced for DCF100C than for Voltaren® Emulgel®.
No direct pharmacodynamic measurements were performed in the present study. However, IC50 values for diclofenac (50% inhibitory concentration for cyclo-oxygenase-2 inhibition) have been reported to be in a range of 1–500 ng ml−1[18], with COX-2 : COX-1 ratios ranging from 0.7 to 2.2 [19, 20]. In the present study, both DCF100C formulations without sensory signals attained Cmax values of approximately 1 ng ml−1, whereas Cmax values from the other formulations did not exceed this threshold. This indicates that topical application of DCF100C might lead to tissue concentrations high enough to exert a therapeutic effect.
In conclusion, DCF100C formulations applied topically may deliver more efficient diclofenac penetration compared with other topical diclofenac products. DCF100C may also represent an alternative to oral diclofenac treatment, providing efficient drug penetration to the local tissues, without the high systemic diclofenac concentrations seen with the oral forms.
Competing Interests
DD is an employee of Futura Medical plc who own the patent for the test articles. The study was conducted as part of the clinical development program. This work was supported by Futura Medical, UK. DD has shares and share options in Futura that are less than 1% of the total shares. There are no other competing interests to declare.
REFERENCES
- 1.European Medicines Agency. Available at http://www.emea.europa.eu/pdfs/human/press/pr/41313606.pdf (last accessed 13 July 2010)
- 2.Zacher J, Altman R, Bellamy N, Brühlmann P, Da Silva J, Huskisson E, Taylor RS. Topical diclofenac and its role in pain and inflammation: an evidence-based review. Curr Med Res Opin. 2008;24:925–50. doi: 10.1185/030079908x273066. [DOI] [PubMed] [Google Scholar]
- 3.Castelnuovo E, Cross P, Mt-Isa S, Spencer A, Underwood M. TOIB study team. Cost-effectiveness of advising the use of topical or oral ibuprofen for knee pain; the TOIB study [ISRCTN: 79353052] Rheumatology (Oxford) 2008;47:1077–81. doi: 10.1093/rheumatology/ken128. [DOI] [PubMed] [Google Scholar]
- 4.Barthel HR, Haselwood D, Longley S, 3rd, Gold MS, Altman RD. Randomized controlled trial of diclofenac sodium gel in knee osteoarthritis. Semin Arthritis Rheum. 2009;39:203–12. doi: 10.1016/j.semarthrit.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Altman RD, Dreiser RL, Fisher CL, Chase WF, Dreher DS, Zacher J. Diclofenac sodium gel in patients with primary hand osteoarthritis: a randomized, double-blind, placebo-controlled trial. J Rheumatol. 2009;36:1991–9. doi: 10.3899/jrheum.081316. [DOI] [PubMed] [Google Scholar]
- 6.Cordero JA, Camacho M, Obach R, Domenech J, Vila L. In vitro based index of topical anti-inflammatory activity to compare a series of NSAIDS. Eur J Pharm Biopharm. 2001;51:135–42. doi: 10.1016/s0939-6411(00)00149-1. [DOI] [PubMed] [Google Scholar]
- 7.Müller M, Rastelli C, Ferri P, Jansen B, Breiteneder H, Eichler HG. Transdermal penetration of diclofenac after multiple epicutaneous administration. J Rheumatol. 1998;25:1833–6. [PubMed] [Google Scholar]
- 8.Müller M, Mascher H, Kikuta C, Schäfer S, Brunner M, Dorner G, Eichler HG. Diclofenac concentrations in defined tissue layers after topical administration. Clin Pharmacol Ther. 1997;62:293–9. doi: 10.1016/S0009-9236(97)90032-1. [DOI] [PubMed] [Google Scholar]
- 9.Burian M, Tegeder I, Seegel M, Geisslinger G. Peripheral and central antihyperalgesic effects of diclofenac in a model of human inflammatory pain. Clin Pharmacol Ther. 2003;74:113–20. doi: 10.1016/S0009-9236(03)00165-6. [DOI] [PubMed] [Google Scholar]
- 10.Dehghanyar P, Mayer BX, Namiranian K, Mascher H, Müller M, Brunner M. Topical skin penetration of diclofenac after single- and multiple-dose application. Int J Clin Pharmacol Ther. 2004;42:353–9. doi: 10.5414/cpp42353. [DOI] [PubMed] [Google Scholar]
- 11.Brunner M, Dehghanyar P, Seigfried B, Martin W, Menke G, Müller M. Favourable dermal penetration of diclofenac after administration to the skin using a novel spray gel formulation. Br J Clin Pharmacol. 2005;60:573–7. doi: 10.1111/j.1365-2125.2005.02484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kienzler JL, Gold M, Nollevaux F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J Clin Pharmacol. 2010;50:50–61. doi: 10.1177/0091270009336234. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt S, Banks R, Kumar V, Rand KH, Derendorf H. Clinical microdialysis in skin and soft tissues: an update. J Clin Pharmacol. 2010;48:351–64. doi: 10.1177/0091270007312152. [DOI] [PubMed] [Google Scholar]
- 14.Holmgaard R, Nielsen JB, Benfeldt E. Microdialysis sampling for investigations of bioavailability and bioequivalence of topically administered drugs: current state and future perspectives. Skin Pharmacol Physiol. 2010;23:225–43. doi: 10.1159/000314698. [DOI] [PubMed] [Google Scholar]
- 15.Sioufi A, Pommier F, Boschet F, Godbillon J, Lavoignat D, Salliere D. Percutaneous absorption of diclofenac in healthy volunteers after single and repeated topical application of diclofenac Emulgel. Biopharm Drug Dispos. 1994;15:441–9. doi: 10.1002/bdd.2510150602. [DOI] [PubMed] [Google Scholar]
- 16.Tas C, Ozkan Y, Okyar A, Savaser A. In vitro and ex vivo permeation studies of etodolac from hydrophilic gels and effect of terpenes as enhancers. Drug Deliv. 2007;14:453–9. doi: 10.1080/10717540701603746. [DOI] [PubMed] [Google Scholar]
- 17.Verbeeck RK, Blackburn JL, Loewen GR. Clinical pharmacokinetics of non-steroidal anti-inflammatory drugs. Clin Pharmacokinet. 1983;8:297–331. doi: 10.2165/00003088-198308040-00003. [DOI] [PubMed] [Google Scholar]
- 18.Miyatake S, Ichiyama H, Kondo E, Yasuda K. Randomized clinical comparisons of diclofenac concentration in the soft tissues and blood plasma between topical and oral applications. Br J Clin Pharmacol. 2009;67:125–9. doi: 10.1111/j.1365-2125.2008.03333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci USA. 1994;90:11693–7. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pairet M, Churrchill L, Trummlitz G, Engelhardt G. Diffential inhibition of cyclooxygenase-1 (COX-1) and -2 (COX-2) by NSAIDs: consequences on anti-inflammatory activity versus gastric and renal safety. Inflammopharmacology. 1996;4:61–70. [Google Scholar]


