Abstract
AIM(S)
Although scopolamine is a frequently used memory impairment model, the relationships between exposure and corresponding central nervous system (CNS) effects are mostly unknown. The aim of our study was to characterize these using pharmacokinetic–pharmacodynamic (PK–PD) modelling.
METHODS
In two double-blind, placebo-controlled, four-way crossover studies, 0.5-mg scopolamine was administered i.v. to 90 healthy male subjects. PK and PD/safety measures were monitored pre-dose and up to 8.5 h after administration. PK–PD relationships were modelled using non-linear mixed-effect modelling.
RESULTS
Most PD responses following scopolamine administration in 85 subjects differed significantly from placebo. As PD measures lagged behind the plasma PK profile, PK–PD relationships were modelled using an effect compartment and arbitrarily categorized according to their equilibration half-lives (t1/2keo; hysteresis measure). t1/2keo for heart rate was 17 min, saccadic eye movements and adaptive tracking 1–1.5 h, body sway, smooth pursuit, visual analogue scales alertness and psychedelic 2.5–3.5 h, pupil size, finger tapping and visual analogue scales feeling high more than 8 h.
CONCLUSIONS
Scopolamine affected different CNS functions in a concentration-dependent manner, which based on their distinct PK–PD characteristics seemed to reflect multiple distinct functional pathways of the cholinergic system. All PD effects showed considerable albeit variable delays compared with plasma concentrations. The t1/2keo of the central effects was longer than of the peripheral effects on heart rate, which at least partly reflects the long CNS retention of scopolamine, but possibly also the triggering of independent secondary mechanisms. PK–PD analysis can optimize scopolamine administration regimens for future research and give insight into the physiology and pharmacology of human cholinergic systems.
Keywords: healthy subjects, non-linear mixed effect modelling, pharmacokinetic–pharmacodynamic relationships, pharmacology, scopolamine
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The cholinergic system is important for different central nervous system functions, including memory, learning and attention. Scopolamine, a centrally active muscarinic antagonist, has been used to model dementia and to demonstrate the pharmacological effects of cholinergic drugs, but for most effects the concentration–effect relationships are unknown.
WHAT THIS STUDY ADDS
We determined the pharmacokinetic–pharmacodynamic relationships of scopolamine using a multidimensional central nervous system test battery in a large group of healthy volunteers. The results suggested there are various functional cholinergic systems with different pharmacological characteristics, which can be used to study the effects of drugs that directly or indirectly modify cholinergic systems. The design of such studies should take the different concentration–effect relationships into account.
Introduction
Scopolamine, also known as hyoscine, is an antimuscarinic agent approved for use in the prevention of motion sickness. Scopolamine competitively antagonizes the effect of acetylcholine on the muscarinic receptors by occupying postsynaptic receptor sites [1]. Scopolamine has a high affinity for the muscarinic receptor family with little selectivity for any of the five receptor subtypes M1–M5 [2], and it has a negligible affinity for histaminergic and dopaminergic receptors [3]. The peripheral effects of scopolamine include typical anticholinergic effects like a dry mouth, skin and throat, decreased blood pressure, decreased heart rate, difficulty urinating, constipation, pupil dilatation and impaired eye focusing (mydriasis and cycloplegia). The central nervous system (CNS) effects consist of drowsiness, reduced attention and memory impairment, and a range of other CNS effects including changes in several EEG frequency bands [4–6].
At present, scopolamine is the most extensively studied and documented model for cognitive deficits [7–10]. The scopolamine model is frequently used in preclinical studies of cognitive impairment. Studies in animals showed that scopolamine induced memory impairments similar to those seen in humans [11–13]. Scopolamine has been used in humans for several different purposes, e.g. to study the role of cholinergic systems in memory processing [14]. As cholinergic systems play an important role in memory processing, scopolamine has been applied as a disease model for dementia [10] and working memory impairment in schizophrenia [15]. The challenge test has been used to show the pharmacological activity of putative cognition enhancers by reversal of scopolamine-induced cognitive and other CNS deficits in healthy volunteers, including cholinergic drugs [16, 17], oxiracetam [18] and d-cycloserine [19].
A methodological disadvantage of the model is the sedation induced by scopolamine. This is not a feature of dementia and may contribute to scopolamine-induced impairment of memory and cognition. However, in many scopolamine studies measures of sedation were unrelated to memory impairment effects [20–24]. Additionally, no cognitive impairment was produced by lorazepam at a dose which induced similar sedation as scopolamine [25]. Moreover, most stimulant drugs that were added to scopolamine in animals and humans were able to reverse the scopolamine-induced ‘fatigue’, but not the cognitive impairment [11, 26, 27], whereas cholinergic agents were [14].
Another source of debate is the dissimilarity between scopolamine effects and the features of memory failure associated with dementia in some studies [28, 29]. For example, Alzheimer's disease (AD) is also characterized by other neuropharmacological deficits that are not captured by a single cholinergic deficiency model. These differences are not a reason to invalidate completely the model per se, but they would essentially limit the application to cognitive deficits specifically associated with cholinergic dysfunction, of which loss of recent memory might be the most prominent example [11]. With these limitations and caveats, the scopolamine model seems to simulate essential elements of the deficits of AD, and can help studying the physiologic (particularly cholinergic) mechanisms involved in learning and memory [30].
A major part of the uncertainty about the value of the scopolamine challenge is related to the large variability in exposure (because of different administration routes, doses, study populations, etc.) and/or corresponding effect(s). This limitation is inherent to many pharmacological challenge tests [31], and much of the variability can be better understood by linking exposure and effect(s) in a pharmacokinetic–pharmacodynamic (PK–PD) model [32–34]. Once identified and validated, these PK–PD models can be used to predict the concentration–effect relationship of various dosing regimens and their subsequent optimization.
At present, there is only one study correlating the plasma PK of scopolamine to its corresponding PD effects. However, this study was limited to EEG as a measure of central activity [6]. Therefore, the aim of our study was to evaluate and characterize the PK–PD relationships of scopolamine for a wide range of central nervous system functions in a large group of healthy volunteers using PK–PD modelling.
Methods
Subjects
A total of 90 male subjects aged 18–55 years with a BMI of 18–28.5 kg m−2 were recruited by the Centre of Human Drug Research (Leiden, the Netherlands). After giving written informed consent, subjects were medically screened within 3 weeks prior to study participation. Exclusion criteria included the use of agents known to affect CNS performance (including smoking and drug or alcohol abuse), consuming more than five cups of caffeine-containing drinks per day and evidence of relevant clinical abnormalities. The use of medication was not allowed during the entire study period. Also, subjects were not allowed to consume caffeine-containing drinks from 48 h prior to dosing until the end of each treatment period.
The Ethics Review Board of the Leiden University Medical Centre approved the study protocols.
Study design
Data for this analysis were obtained in two separate studies evaluating the effect of investigational glycinergic compounds on cognitive dysfunction in schizophrenia [35, 36]. In these studies, scopolamine was used as a transient cognition impairment model. Both studies had equal double-blind, placebo-controlled, four-period crossover ascending dose designs. This article is limited to the study visits on which scopolamine or placebo were administered without the experimental glycinergic compounds. Study periods were separated by a washout period of at least 1 week. Scopolamine (0.5 mg) or placebo were administered as a short-term i.v. infusion over 15-min starting at t = 0 h. The sampling and measurement schedules for the scopolamine challenges were identical for both studies.
Clinical observations
Adverse events, electrocardiogram (ECG), body temperature, blood pressure and heart rate (after a supine position for minimally 5 min) were monitored throughout the study. ECGs were assessed using a Cardioperfect ECG recorder (Welch Allyn, Delft, the Netherlands). Blood pressure and heart rate were measured using an automated device (Nihon Kohden, Life Scope EC, Tokyo, Japan).
Pharmacokinetics
Blood samples (4 ml) were drawn at 0.5, 0.75, 1.0, 2.5, 6.5 h post dosing of scopolamine. Samples were protected from light at all times. Scopolamine concentrations were determined by using a validated, selective and sensitive liquid LC-MS/MS method (LLOQ = 10 pg ml−1). The method consisted of a liquid–liquid extraction sample clean-up with 1-chlorobutane. After evaporation of the organic layer, the residue was reconstituted in a mixture of acetonitrile and 0.1% ammonia (24%) in water (10:90, v : v). The extract (15 µl) was injected onto an X-Bridge shield C18 column (50 × 2.1 mm, 3.5 µm; Waters Corporation,Milford, MA, USA) operated at 30°C. The mobile phase, a mixture of 0.1% ammonia (24%) in water as solvent A and acetonitrile as solvent B, was delivered at 0.4 ml min−1. Quantitation was achieved by MS/MS detection in the positive ion mode, using a PE Sciex (Foster City, CA, USA) API 4000 mass spectrometer, equipped with a Turboionspray™ interface. The inter-run precision (expressed as % CV) and accuracy (expressed as % bias) ranged from 1.9% to 5.6% and from −1.0% to 4.3% respectively (n = 18). Scopolamine bioanalysis was performed by Pharma Bio-Research Group B.V., Zuidlaren, the Netherlands.
Pharmacodynamics
A battery of quantitative PD measurements was chosen for its repeatability and sensitivity to many different CNS active drugs in healthy volunteers [35–39] and was incorporated to provide background information on general CNS performance and functional CNS domains (neurophysiological, cognitive, neuroendocrine and autonomic changes). Eleven blocks of pharmacodynamic measurements were performed: twice pre-dose (within 1 h before scopolamine administration) and approximately 0.75, 1.0, 1.5, 2.0, 2.5, 3.5, 4.5, 6.5 and 8.5 h post dose. Tests were performed in the following order (relative time difference to the approximate time points): body sway (−10 min), saccadic eye movements (−8 min), smooth pursuit measurement (−7 min), pupil : iris ratio (−5 min), pharmaco-EEG (−4 min), visual analogue scale (VAS) Bond & Lader (−3 min), VAS Bowdle (−2 min), adaptive tracking (+2 min), finger tapping (+6 min) and Stroop test (+8 min). A memory task (i.e. the Visual Verbal Learning Test) was performed once per study period. Blood for hormones (FSH, LH and prolactin) was taken regularly from 4 min up to 24 h after scopolamine administration. Average baseline values for each variable were obtained by calculation of the mean of the two baseline assessments. Pharmacodynamic tests were performed in a quiet room with ambient illumination with only one subject in the same room per session. Subjects had a standardized breakfast 1 h before scopolamine administration. All subjects were thoroughly trained and familiarized with the psychometric tests within 14 days preceding study start to minimize learning effects during the study.
Neurophysiological
For body sway, the sum of all spontaneous anterior–posterior movements over 2 min (in mm) was used for statistical analysis [37]. Saccadic and smooth pursuit eye movements were performed as described before [38]. Average values of saccadic peak velocity (SPV), latency (= reaction time) and inaccuracy were calculated for all artefact-free saccades. The average percentage of smooth pursuit for all stimulus frequencies was used as response parameter for smooth pursuit eye movements.
Pharmaco-EEG was measured as previously described [39]. Fast Fourier transform analysis was performed to obtain the sum of amplitudes in the delta- (0.5–3.5 Hz), theta (3.5–7.5 Hz), alpha- (7.5–11.5 Hz) and beta- (11.5–30 Hz) frequency ranges. The square root of the total power (in µV) was analysed.
The diameter of the iris and the pupil of both eyes was determined as described previously [35, 40].
The adaptive tracking test, as originally described by Borland & Nicholson [41], was performed as previously explained [42]. The average performance and the standard deviation of scores over a 3.5-min period were used for analysis.
The finger tapping test (adapted from the Halstead Reitan Test Battery [43]) was performed as previously described [35] and evaluated motor activation and fluency. Speed of finger tapping was measured for the index finger of the dominant hand and a session contained five performances of 10 s. The mean tapping rate and the standard deviations were used for statistical analysis.
Cognitive
The Stroop colour–word conflict test is helpful in understanding attention, perception and reading as well as the cognitive and neural mechanism of mental inhibition, interference and controlled vs. automatic processing [44] and was performed as previously described [36]. Outcome parameters are the number of correct answers and the reaction time in basic and conflict situation.
The Visual Verbal Learning Test (VVLT) addresses different components of learning behaviour, such as acquisition, consolidation, storage and retrieval. The VVLT contains three different subtests that cover immediate and delayed word recall and a delayed word recognition [44]. The immediate word recall test was administered 2 h and 8 min after the start of the scopolamine infusion. For the delayed word recall test this was 2 h and 38 min and for the delayed word recognition test 2 h and 43 min. The 30-word learning test avoids ceiling effects in healthy students, and has shown CNS effects, e.g. for benzodiazepines [32, 38, 45], cannabinoids [33] and antipsychotics [46]. For each study period, a different 30-word learning list was used to avoid learning effects over time. All versions of the 30-word learning are validated for differences in complexity. Main parameters for the immediate and delayed word recall were the average and the maximum number of correct responses. For the delayed word recognition, the number of correct items and mean response time for correct responses were analysed.
Subjective
The Bond & Lader Visual Analogue Scale was performed to measure subjective alertness, mood and calmness [38]. The Bowdle VAS evaluates psychedelic effects. These could cluster into two distinct total sum scores (see [33] for further details): internal perception (reflects inner feelings that do not correspond with reality, including mistrustful feelings) and external perception (reflects a misperception of an external stimulus or a change in the awareness of the subject's surroundings). The VAS ‘feeling high’ and ‘colour intensity’ are subscales of the VAS Bowdle.
Neuroendocrine
Blood samples (3 ml) for LH, FSH and prolactin were kept at room temperature for at least 15 and maximally 60 min. The hormones were analysed by the Central Clinical Chemistry Laboratory (Leiden University Medical Centre, Leiden, the Netherlands) using an electrochemoluminescence-immunoassay (ECLIA) for prolactin, and a fluoro-immunoassay for LH and FSH. The assays have a lower limit of quantification (LLOQ) of 0.12 (LH), 0.05 (FSH) U l−1 and 0.047 µg l−1 (prolactin). Intra-assay precision (expressed as coefficient of variation) was 3.8–5.1% (LH), 3.0–14.8% (FSH) and 1.81–1.90% (prolactin). Inter-assay precision was 5.5–6.8% (LH), 3.8–5.3% (FSH) and 2.39–2.64% (prolactin).
Statistical analysis
Pharmacodynamics
Pharmacodynamics parameters were analysed by mixed-model analyses of variance using SAS PROC MIXED (SAS Institute, Inc., Cary, NC, USA). with treatment, period, time and treatment by time as fixed effects, with subject, subject by time and subject by treatment as random effects, and with the baseline value as covariate, where baseline is defined as the average of the available values obtained prior to dosing. Body sway, EEG and neuroendocrine parameters were analysed after log-transformation and back-transformed after analysis (results may be interpreted as percentage change). The analysis resulted in least square means (LSM) estimates that indicate the change from baseline where baseline in the graph is set at 0 for t = 0 h. Treatment effects were reported as the contrasts between placebo and scopolamine, where the average of the measurements up to and including 8.5 h (22 h for neuroendocrine parameters) were calculated within the statistical model. Contrasts were reported along with 95% confidence intervals and analyses were two-sided with a significance level of 0.05.
Pharmacokinetics and PK–PD analysis
Pharmacokinetics and PK–PD modelling and simulations were performed using non-linear mixed-effect modelling as implemented in the NONMEM program [47]. Non-linear mixed-effect modelling allows the description of a population of individuals using a common structural model while allowing the individuals to vary. Estimates of location and spread between individuals are estimated for the model parameters. Using the population values, individual-specific empirical Bayes estimates can be determined that allow description of individual time profiles. Competing models can be compared using the likelihood ratio test, which compares the difference between log-likelihoods for the models (or minimum value of the objective function) to a chi-squared distribution with degrees of freedom corresponding to the difference in number of parameters between the two models. First-order conditional estimation was used throughout, with additive (on log-scale for concentrations) residual error models.
When analysing the data, it could be shown that there was no significant placebo response (flat dose–response curve) for most of the parameters. Therefore, it was decided to include a separate placebo model in the analysis for those parameters where a placebo response was observed (tracking performance and smooth pursuit performance), while this was not conducted for those PD measures where a flat dose–response curve was observed for placebo.
Results
Clinical observations
Eighty-five of the included 90 subjects completed the study. Four subjects withdrew for reasons unrelated to the study. One subject withdrew after what was considered to be a second moderate vasodepressive episode following dosing with scopolamine. The scopolamine treatment was well tolerated by all other subjects. Ninety-eight per cent of the subjects reported at least one adverse event. The most commonly reported mild adverse events following dosing with scopolamine were anticholinergic symptoms (97%), nausea (8%), dizziness (7%) and palpitations (6%). The anticholinergic symptoms consisted of variable combinations of drowsiness, dizziness, dry mouth, concentration problems and blurred vision.
The mean supine systolic and diastolic blood pressure were lower than with placebo 2 to 6.5 h after dosing with scopolamine. A similar effect was seen on pulse rate. There was a decrease in mean heart rate (approximately 23%, from 66.8 to 51.2 beats min−1) and consequently a transient increase in mean uncorrected QT interval (approximately 9%, from 381 to 415 ms) without QTc changes. There were no other clear consistent changes in vital signs, ECG or laboratory safety parameters.
Pharmacodynamics
Neurophysiological
Except for three EEG parameters (beta Fz-Cz, theta Fz-Cz and Pz-Oz), the changes in the other neurophysiological measures were highly statistically significant after scopolamine compared with placebo treatment (Table 1, Figure 1). While alpha and beta power increased, delta power decreased. Furthermore, scopolamine decreased SPV (by 22.7° s−1), adaptive tracking performance (by 9.6%), finger tapping rate [by 3.4 taps (10 s)−1] and smooth pursuit (by 6.1%). Body sway and average pupil : iris ratio were both increased by 57.4% and 0.057 respectively.
Table 1.
Pharmacodynamic differences in least square means relative to placebo for all measurements
| Least square means | ||||||
|---|---|---|---|---|---|---|
| Variable | Placebo | Scopolamine | Difference | 95% CI | P-value | |
| Neurophysiological | ||||||
| Body sway (mm) | 268 | 422 | 57.4% | 48.3 | 67.1 | <0.0001 |
| Saccadic peak velocity (o s−1) | 471.0 | 448.3 | −22.7 | −28.5 | −16.8 | <0.0001 |
| Saccadic latency (s) | 0.200 | 0.217 | 0.017 | 0.013 | 0.022 | <0.0001 |
| Saccadic inaccuracy (%) | 5.73 | 8.14 | 2.41 | 2.14 | 2.68 | <0.0001 |
| Smooth pursuit eye movements (%) | 48.37 | 42.32 | −6.05 | −8.10 | −3.99 | <0.0001 |
| EEG alpha Fz-Cz (µV) | 2.889 | 2.064 | −28.6% | −32.8 | −24.1 | <0.0001 |
| EEG alpha Pz-Oz (µV) | 5.472 | 3.133 | −42.7% | −46.9 | −38.3 | <0.0001 |
| EEG beta Fz-Cz (µV) | 1.998 | 1.982 | −0.8% | −4.3 | 2.8 | 0.6492 |
| EEG beta Pz-Oz (µV) | 2.508 | 2.010 | −19.8% | −23.1 | −16.4 | <0.0001 |
| EEG delta Fz-Cz (µV) | 1.801 | 2.064 | 14.6% | 9.6 | 19.9 | <0.0001 |
| EEG delta Pz-Oz (µV) | 1.779 | 1.983 | 11.5% | 6.4 | 16.8 | <0.0001 |
| EEG theta Fz-Cz (µV) | 2.112 | 2.134 | 1.1% | −3.3 | 5.6 | 0.6332 |
| EEG theta Pz-Oz (µV) | 2.293 | 2.310 | 0.7% | −4.8 | 6.6 | 0.8011 |
| Average pupil : iris ratio | 0.532 | 0.590 | 0.057 | 0.042 | 0.072 | <0.0001 |
| Adaptive tracking performance (%) | 21.70 | 12.07 | −9.62 | −10.29 | −8.95 | <0.0001 |
| Finger tapping rate [taps (10 s)−1] | 65.4 | 62.0 | −3.4 | −4.4 | −2.4 | <0.0001 |
| Cognitive | ||||||
| Stroop test RT conflict situation (ms) | 657.0 | 767.4 | 110.5 | 82.2 | 138.8 | <0.0001 |
| Stroop test # correct conflict situation | 19.5 | 18.9 | −0.6 | −0.9 | −0.3 | <0.0001 |
| Immediate word recall # correct third trial | 16.0 | 10.2 | −5.8 | −6.7 | −5.0 | <0.0001 |
| Delayed word recall # correct | 12.4 | 7.3 | −5.1 | −6.0 | −4.3 | <0.0001 |
| Delayed word recognition average RT correct answers (ms) | 903.1 | 951.4 | 48.4 | 17.4 | 79.3 | 0.0025 |
| Delayed word recognition # correct | 24.9 | 22.6 | −2.3 | −3.3 | −1.4 | <0.0001 |
| Subjective | ||||||
| VAS alertness (mm) | 54.7 | 45.7 | −9.0 | −10.9 | −7.2 | <0.0001 |
| VAS mood (mm) | 57.2 | 56.6 | −0.6 | −1.9 | 0.6 | 0.3243 |
| VAS calmness (mm) | 57.5 | 58.7 | 1.2 | −0.5 | 2.9 | 0.1567 |
| VAS internal perception (log mm) | 0.35 | 0.43 | 0.08 | 0.04 | 0.11 | <0.0001 |
| VAS external perception (log mm) | 0.34 | 0.50 | 0.15 | 0.11 | 0.19 | <0.0001 |
| VAS feeling high (log mm) | 0.36 | 0.68 | 0.32 | 0.24 | 0.40 | <0.0001 |
| VAS colour perception (log mm) | 0.35 | 0.42 | 0.08 | 0.04 | 0.11 | <0.0001 |
| Neuroendocrine | ||||||
| FSH (U l−1) | 3.70 | 3.90 | 5.6% | 3.7 | 7.6 | <0.0001 |
| LH (U l−1) | 4.39 | 5.28 | 20.2% | 13.6 | 27.1 | <0.0001 |
| Prolactin (U l−1) | 7.91 | 9.12 | 15.3% | 10.5 | 20.3 | <0.0001 |
# = number. RT, reaction time; VAS, visual analogue scale.
Figure 1.
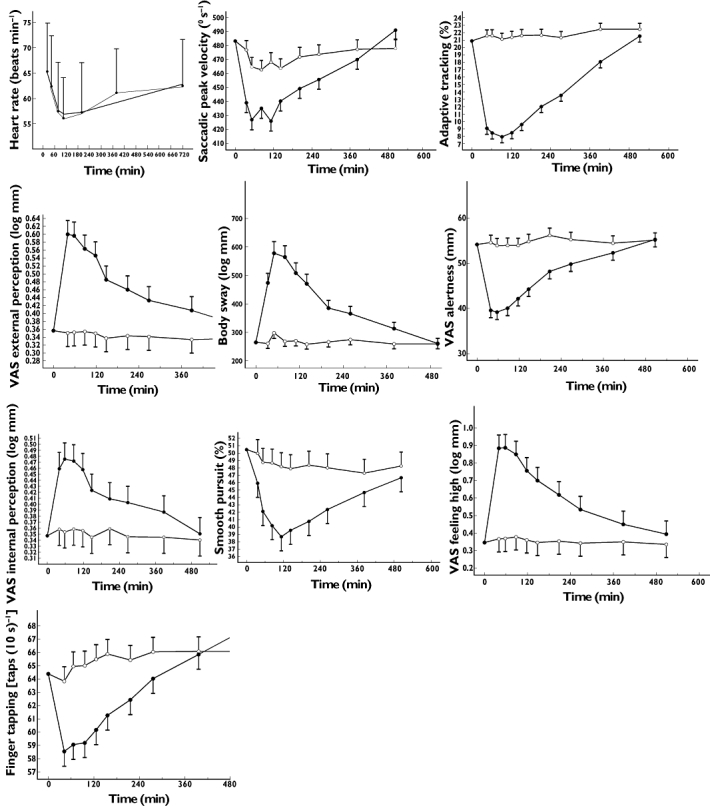
Time–effect profiles for all pharmacodynamic measurements which resulted in a successful pharmacokinetic–pharmacodynamic analysis. ○ placebo, • scopolamine 0.5 mg i.v. VAS, visual analogue scale
Cognitive
Of the cognitive function tests, the reaction time of the Stroop colour–word conflict test increased after scopolamine with 110.5 ms compared with placebo, while the number of correct responses of this test decreased by 0.6 (Table 1). Memory performance deteriorated after scopolamine, as compared with placebo the number of correct responses decreased by 5.8 items for the third immediate recall trial, and by 5.1 items during delayed recall. The average reaction time of the delayed word recognition test (for correct responses) was 48.4 ms longer after scopolamine compared with placebo. The number of correct responses for this same test decreased by 2.3.
Subjective measurements
Except for the VAS mood and VAS calmness, all subjective measures were statistically significantly affected by scopolamine (Table 1, Figure 1). The Bond & Lader VAS alertness scale decreased 9.0 mm compared with placebo. For the Bowdle VAS, an increase in VAS internal perception, external perception, feeling high and colour perception was seen.
Neuroendocrine measurements
All hormones were increased after scopolamine compared with placebo: FSH by 5.6%, LH by 20.2% and prolactin by 15.3% (Table 1).
Pharmacokinetics
Scopolamine pharmacokinetics were described using a two-compartment model. Individual empirical Bayes estimates were determined that were used to describe the concentration profiles in subsequent PK–PD analyses (see Figure 2 for average concentration–time profile with the average NONMEM predicted profile superimposed).
Figure 2.
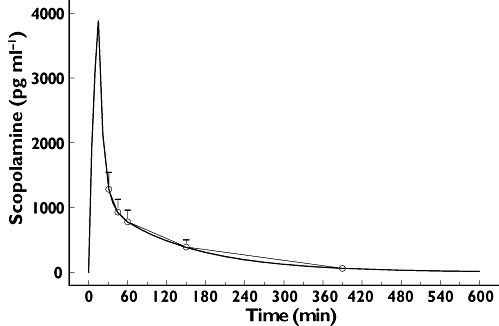
Average observed (with SD error bars) and predicted (two-compartment model) scopolamine plasma concentrations
After 30 min scopolamine showed a mean maximum concentration of 1287 pg ml−1. Plasma concentrations declined rapidly with a mean terminal half-life of 1.5 (range 1.2–2.49) h (Table 2). The AUC(0,∞) was 2752 (range 1737–4604) pg ml−1 h.
Table 2.
Population pharmacokinetic parameters of scopolamine
| Mean* | SEM | IIVar | |
|---|---|---|---|
| Clearance (l min−1) | 2.53 | 0.0791 | 15.4% |
| Central volume (l) | 66.3 | 8.37 | 36.6% |
| Intercompartmental clearance (l min−1) | 4.78 | 0.309 | 8.9% |
| Steady-state Vd (l) | 250 | 12.6 | 7.2% |
| Residual error (SD/mean) | 0.102 | 0.00736 |
Mean: population average. SEM, standard error population mean; IIVar, inter-individual variability.
Pharmacokinetic–pharmacodynamic relationships
When plotting scopolamine plasma concentrations against corresponding effects, PD responses clearly lagged behind the plasma PK profile and showed a much more gradual increase and decline (see Figure 3A for SPV and 4A for VAS alertness). In order to overcome this temporal disconnect, data were modelled using an effect compartment, and the relationship between effect compartment concentration and response was assumed to be linear (see Figures 3B and 4B). Exploration of the mean predicted and observed time profile for these models did not indicate sufficient model misspecification to warrant more complex models. With this model average graphs of predicted and observed time–effect curve were made (Figures 3C and 4C). In Table 3 the PK–PD parameters intercept, slope, equilibration half-life (t1/2keo) and residual error are presented. t1/2keo is a measure of hysteresis and is the time in which the effect compartment concentration reaches half the concentration of the central compartment. In this study, we were not able to measure a maximal effect (Emax) for any of these outcome parameters. These parameters were categorized according to their equilibration half-lives in four arbitrarily divided groups, i.e. shorter than 0.5 h, 1–1.5 h, 2.5–3.5 h and longer than 8 h. No simple PK–PD model could be fitted adequately for blood pressure, hormones (LH, FSH, prolactin), EEG, pupil : iris ratio, Stroop test, VVLT and VAS scales for calmness, mood and colour (reasons for this are given in the discussion).
Figure 3.
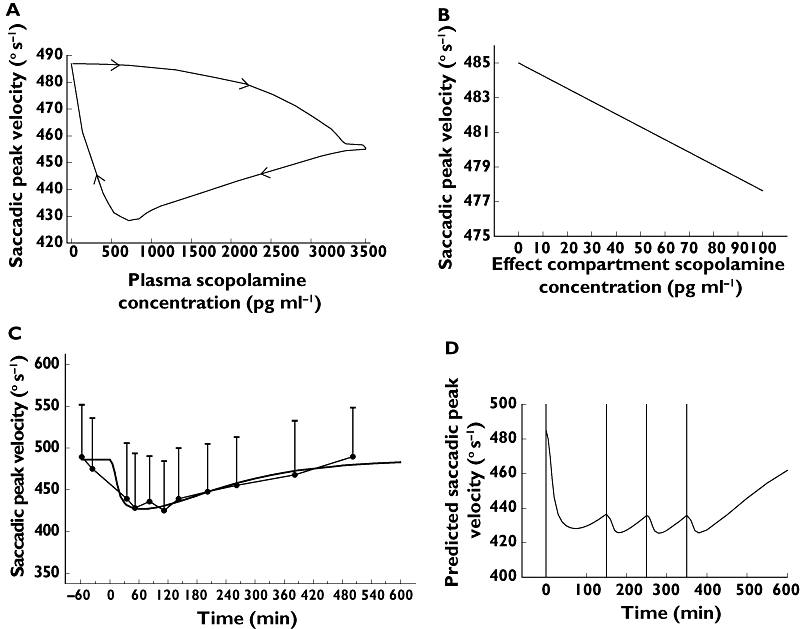
Saccadic peak velocity: (A) clockwise hysteresis loop formed by the plasma concentration–effect curve; (B) effect compartment concentration–effect curve; (C) predicted (dark line) and observed (closed circles) time–effect curve with 95% CI error bars; (D) mean predicted dosing regimen (0.45-mg i.v. infusion 15 min at t = 0 and 0.15-mg i.v. infusion over 15 min at t = 150, t = 250 and t = 350 min) to attain a saccadic peak velocity reduction of 50° s−1
Figure 4.
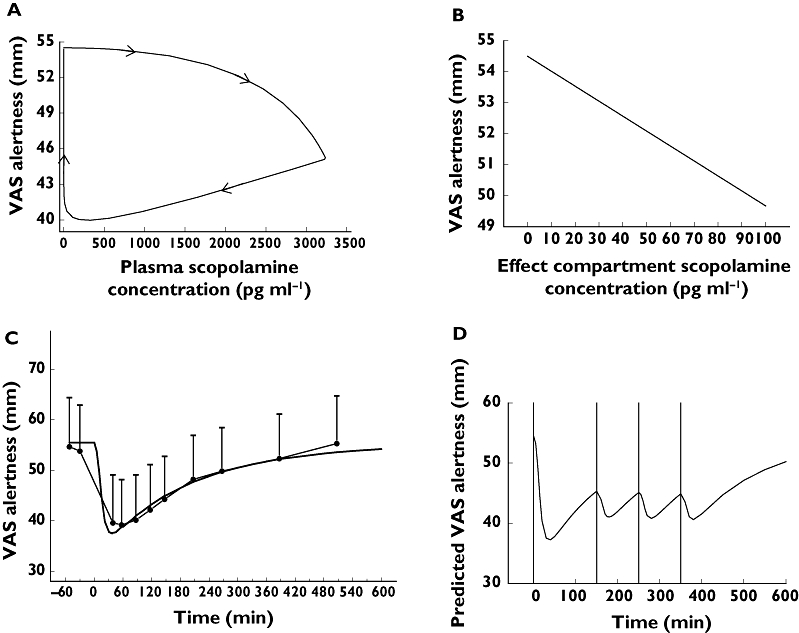
For visual analogue scale (VAS) alertness (A) clockwise hysteresis loop formed by the plasma concentration–effect curve; (B) effect compartment concentration–effect curve; (C) predicted (dark line) and observed (closed circles) time–effect curve with 95% CI error bars; (D) mean predicted dose regimen (0.45-mg i.v. infusion over 15 min at t = 0 and 0.15-mg i.v. infusion over 15 min at t = 150, t = 250 and t = 350 min) to attain a VAS alertness reduction of 10 mm
Table 3.
Population parameters of pharmacokinetic–pharmacodynamic analysis sorted by equilibration half-life; depicted as population mean (SEM – IICV*)
| Outcome parameter mean (SEM – IICV) | Intercept | Slope† | Equilibration t1/2 (min) | Residual error |
|---|---|---|---|---|
| <0.5 h | ||||
| Heart rate (beats min−1) | 55.2 (0.96–1.72) | −0.00675‡ (−0.000976–−14.46) | 16.8 (2.73–16.25) | 0.103 (NA*) |
| 1–1.5 h | ||||
| Saccadic peak velocity (o s−1)§ | 485 (NA¶–55.05) | −0.0737 (NA¶–45.3%) | 65.1 (NA¶–0.0%) | 28.4 (NA*) |
| Adaptive tracking (%) | 0.0479 (0.457–4.336) | −0.0217 (0.000931–22.6%) | 86.6 (4.14–28.4%) | 2.98 (0.155) |
| 2.5–3.5 h | ||||
| VAS external perception (log mm)§ | 0.34 (0.00931–28.6%) | 0.000633 (0.000109–107%) | 161 (25.5–86.5%) | 0.0935 (0.0062) |
| Body sway (log mm)§ | 2.4 (0.0219–8.2%) | 0.00147 (0.000105–32.4%) | 181 (12–30.6%) | 0.102 (0.0053) |
| VAS alertness (mm)§ | 53.6 (NA¶–14.8%) | −0.0622 (NA¶–62.9%) | 199 (NA¶–53.8%) | 4.05 (NA*) |
| VAS internal perception (log mm)§ | 0.336 (NA¶–28.1%) | 0.000331 (NA¶–130%) | 200 (NA¶–1.6%) | 0.083 (NA*) |
| Smooth pursuit (%)§ | 3.1 (0.951–12.25) | −0.0264 (0.0029–44.4%) | 221 (31.5–97.8%) | 6.28 (0.238) |
| >8 h | ||||
| VAS feeling high (log mm)§ | 0.328 (NA¶–21.0%) | 0.00313 (NA¶–93.9%) | 483 (NA¶–207%) | 0.216 (NA*) |
| Finger tapping [taps (10 s)−1]§,** | 63.4 (0.895–12.6%) | −0.0797 (0.0571–25.3%) | 649 (472–62.9%) | 3.09 (0.217) |
For the residual error in most cases, only the SEM is depicted.
Unit of the slope = unit of the outcome parameter (pg ml−1).
Effect is parameterized: a decrease from baseline is depicted as a positive value.
Placebo results not subtracted.
NA = not available because of aborted covariance step.
Finger tapping has a time slope of 19.3 (9.187) number per day. SEM, standard error of the mean; IICV, inter-individual variability coefficient of variation.
Discussion
In this study the PK, PD and PK–PD relationships following the i.v. administration of 0.5-mg scopolamine were assessed using an extensive CNS test battery, frequent measurements and a sizeable study population. The scopolamine challenge proved to be an accurate, reproducible and safe model for cognitive impairment.
With regard to autonomic changes, the paradoxical reduction in heart rate of 15.6 beats min−1 on average was larger than is typically observed with scopolamine doses of 0.4–0.6 mg, but probably still because of the blockade of M1-receptors on postganglionic parasympathetic neurons, as suggested previously [45].
Scopolamine affected most of the measured PD parameters of this study. Most effects were reasonably consistent with the effects described in the literature [5, 18, 23, 26, 27, 46, 48] and disappeared within 8 h. Some of the more noticable PD results will be briefly discussed below.
The very long duration of pupil dilation, in line with the prolonged cycloplegic effects of anticholinergic ocular mydriatics [49], could possibly be explained by an element of retention of the drug in the anterior chamber of the eye and/or a high sensitivity of the iris to cholinergic inhibition. This could not be examined any further, because the effect lasted much longer than the observation period of this study, which refuted PK–PD analyses.
All tested components of memory showed a significant impairment compared with placebo. In our study, this was particularly because of a reduction of the capacity to immediately actively reproduce memorized words, from 53% (16/30) after placebo to 34% (10.2/30) with scopolamine. This difference was comparable when these words were reproduced 30 min later (41% vs. 24%). Memory storage (assessed using the delayed word recognition test) was also affected, but to a smaller extent: 75% (22.6/30) of the presented words were still recognized 3 h after scopolamine treatment, compared with 83% (24.9/30) with placebo.
Performance of the Stroop test was also significantly impaired, similar to the results of Mintzer & Griffiths [50]. A central mechanism seems the most logical cause for these changes in Stroop performance as the effects were larger in the conflict than in the basic situation, where the colours were the same. Reduced attention could have contributed, as Mintzer & Griffiths [50] also found a strong difference between conflict and basic situation of the Stroop test after benzodiazepine administration. The observed changes in VAS colour intensity could have been influenced by pupil dilation or cholinergic retinal impairment [51].
The increases in VAS of internal perception, external perception and feeling high confirms that scopolamine has some psychomimetic properties [2]. When comparing the increase in VAS feeling high with that of tetrahydrocannabinol (THC, the psychoactive component of cannabis), the effects of THC (2, 4, 6 mg intrapulmonary [39]) were twice as large as those of scopolamine. The effects of zolpidem (10 mg orally) on VAS feeling high were close to the values observed with scopolamine [52].
In the PK–PD analysis of our study, all the effects showed hysteresis and Emax could not be estimated for any of the effects using a single dose of 0.5 mg. This delay was modelled by assuming a direct relationship between the concentrations in the effect compartment (estimated from the pharmacodynamic effect) and those in the central compartment. Variations in t1/2keo can be caused by strong retention of the drug within a certain compartment (like for lipophilic compounds in the CNS) or subsequent pharmacological and physiological processes that can lead to a delay or prolongation of a pharmacodynamic effect [34]. It should be kept in mind that the descriptive approach of our model is a limitation of the analysis. As we are evaluating a large variety of effects that certainly cannot be characterized by a single mechanism-based model, we decided to keep it reasonably simple and consistent for all outcome parameters. We arbitrarily divided the equilibration half-lives in four groups, namely shorter than 0.5 h, 1–1.5 h, 2.5–3.5 h and longer than 8 h. Although these differences seem large enough to be related to meaningful functional distinctions, a physiological explanation is not immediately apparent and may in fact differ among pharmacodynamic effects even within the same group.
Heart rate showed a short t1/2keo of 17 min. The fact that an effect compartment with a significant equilibration half-life was needed suggests that both central and peripheral effects are involved. In the case of purely peripheral effects, we would have expected a more direct link of the plasma PK to the corresponding PD effect. However, the physiological situation is more complex. An apparently long equilibration half-life can also ensue from a high affinity of the drug for cardiac tissue leading to prolongation of the (peripheral) effects of scopolamine on the heart. Moreover, heart rate is determined by complex peripheral cardiovascular regulatory systems, and changes in one part of the system (like muscarinic blockade) will usually cause compensatory changes in the components. Similar t1/2keo values are found for cannabis-induced tachycardia [34].
Adaptive tracking and SPV were both characterized by longer equilibration half-lives of about 1 h (see Table 3). SPV is one of the most sensitive parameters for alertness [53–55]. Adaptive tracking performance is a visuomotor task that is influenced by attention and vigilance. These measurements usually show fairly direct concentration–effect relationships with other CNS active drugs [56–59] indicating that an underlying prolonged physiological mechanism is unlikely to be the cause for an extended effect duration. It is more likely that an effect prolongation is mainly because of retention of scopolamine in a certain part of the central nervous system, and not solely to induction of independent physiological processes. This was confirmed by a PET study performed by Frey et al. [60], who showed that 11C-scopolamine was retained in different brain regions for many hours. The compound was most rapidly removed from the cerebellum, where receptor binding still had a half-life of several hours. Functionally, this region is most relevant for body sway, which also had an effect half-life of approximately 2.5 h. The tracer showed even longer retention times in other brain regions (pons, thalamus, occipital cortex and caudate nucleus), which cannot be easily related to functional parameters in our study. It is possible therefore that some very long-lasting effects, like on some of the VAS scales and finger tapping (see Table 3), are still related to the active presence of scopolamine in relevant brain regions. Obviously, it cannot be excluded that functional adaptations play a role in the final effect duration and the length of its equilibration half-life. Although all effects seem closely related to the activity (i.e. inhibition) of cholinergic systems in the brain, the features of these relationships vary considerably. This indicates that different mechanisms could be involved in the effects of scopolamine, and that they are not caused by a single underlying anticholinergic effect like for instance ‘CNS depression’. More complex functional mechanisms may have refuted an adequate PK–PD analysis, which was not possible for all end points. This was the case for blood pressure, possibly because of homeostatic mechanisms interfering between the direct relation between scopolamine concentration and blood pressure. Once LH and FSH are activated (by scopolamine), these hormones have their own complicated and poorly described system dynamics. For EEG parameters, the complex profiles during placebo treatment (which probably not only reflect diurnal rhythms but also unspecified variability) made PK–PD modelling too complicated. The excessive duration of the effects on the pupil : iris ratio and the absence of measurements in this late phase refuted an adequate description of the PK–PD relationships. For many cognitive processes (e.g. Stroop and memory tasks), it is hard, if not impossible, to include enough measurements in a study to have reliable PK–PD, as repeated assessments are complicated by learning effects, different versions and long test durations. PK–PD relationships could also not be described for three other VAS scales (calmness, mood and colour), mainly because the effects were too small and/or inconsistent.
The results of this study can be used for the design of new studies of the cholinergic system. One example would be when stable CNS effects (e.g. a certain level of objective alertness) of scopolamine are required for a functional imaging study of longer duration. The dosing regimen predicted to result in reasonably stable reductions of SPV (an objective measure of sedation) of 50° s−1 and of VAS alertness of 10 mm for approximately 7 h (Figures 3D and 4D), would result in a 0.45-mg continuous infusion for 15 min and 0.15-mg infusions for 15 min at t = 150, t = 250 and t = 350 min.
The models can also be used for individual dose optimization in patient studies when large inter-individual differences are anticipated, and to study the integrity of the cholinergic system in different individuals, populations or disorders (i.e. disease progression or effects of treatment on the cholinergic system in dementia). However, as this study was carried out in healthy young men, it is not yet known whether the results are also applicable to older (or demented) men.
In conclusion, the results of our PK–PD analyses show that scopolamine causes a range of different effects, which are regulated by systems that are functionally and pharmacologically at least partly distinct. The rather extensive data set obtained in this study allowed for accurate analyses of the effects of scopolamine and their concentration–effect relationships across a wide range of different CNS domains, and provides a useful set of normal reference values for the design optimization of future studies.
Acknowledgments
The authors would like to thank Ria Kroon, Esther Davidse, Ibrahim Ince and Dennis Calkhoven for their help with this study and Jasper Janssen for designing the Qpupil program.
Competing Interests
The study was executed at the Centre for Human Drug Research in Leiden, the Netherlands, with financial support from Johnson and Johnson, Pharmaceutical Research and Development. PdB and MT are employees of Janssen Pharmaceutical Companies at Johnson and Johnson. PdB has shares in the company.
REFERENCES
- 1.Deutsch JA. The cholinergic synapse and the site of memory. Science. 1971;174:788–94. doi: 10.1126/science.174.4011.788. [DOI] [PubMed] [Google Scholar]
- 2.Renner UD, Oertel R, Kirch W. Pharmacokinetics and pharmacodynamics in clinical use of scopolamine. Ther Drug Monit. 2005;27:655–65. doi: 10.1097/01.ftd.0000168293.48226.57. [DOI] [PubMed] [Google Scholar]
- 3.Peroutka SJ, Snyder SH. Antiemetics: neurotransmitter receptor binding predicts therapeutic actions. Lancet. 1982;1:658–9. doi: 10.1016/s0140-6736(82)92206-1. [DOI] [PubMed] [Google Scholar]
- 4.Ebert U, Kirch W. Scopolamine model of dementia: electroencephalogram findings and cognitive performance. Eur J Clin Invest. 1998;28:944–9. doi: 10.1046/j.1365-2362.1998.00393.x. [DOI] [PubMed] [Google Scholar]
- 5.Ebert U, Siepmann M, Oertel R, Wesnes KA, Kirch W. Pharmacokinetics and pharmacodynamics of scopolamine after subcutaneous administration. J Clin Pharmacol. 1998;38:720–6. doi: 10.1002/j.1552-4604.1998.tb04812.x. [DOI] [PubMed] [Google Scholar]
- 6.Ebert U, Grossmann M, Oertel R, Gramatte T, Kirch W. Pharmacokinetic-pharmacodynamic modeling of the electroencephalogram effects of scopolamine in healthy volunteers. J Clin Pharmacol. 2001;41:51–60. doi: 10.1177/00912700122009836. [DOI] [PubMed] [Google Scholar]
- 7.Vitiello B, Martin A, Hill J, Mack C, Molchan S, Martinez R, Murphy DL, Sunderland T. Cognitive and behavioral effects of cholinergic, dopaminergic, and serotonergic blockade in humans. Neuropsychopharmacology. 1997;16:15–24. doi: 10.1016/S0893-133X(96)00134-0. [DOI] [PubMed] [Google Scholar]
- 8.Hall ST, Puech A, Schaffler K, Wesnes K, Gamzu ER. Early clinical testing of cognition enhancers: prediction of efficacy. Pharmacopsychiatry. 1990;23(Suppl. 2):57–8. doi: 10.1055/s-2007-1014534. [DOI] [PubMed] [Google Scholar]
- 9.Riedel WJ, Jolles J. Cognition enhancers in age-related cognitive decline. Drugs Aging. 1996;8:245–74. doi: 10.2165/00002512-199608040-00003. [DOI] [PubMed] [Google Scholar]
- 10.Broks P, Preston GC, Traub M, Poppleton P, Ward C, Stahl SM. Modelling dementia: effects of scopolamine on memory and attention. Neuropsychologia. 1988;26:685–700. doi: 10.1016/0028-3932(88)90004-8. [DOI] [PubMed] [Google Scholar]
- 11.Bartus RT. Evidence for a direct cholinergic involvement in the scopolamine-induced amnesia in monkeys: effects of concurrent administration of physostigmine and methylphenidate with scopolamine. Pharmacol Biochem Behav. 1978;9:833–6. doi: 10.1016/0091-3057(78)90364-7. [DOI] [PubMed] [Google Scholar]
- 12.Araujo JA, Studzinski CM, Milgram NW. Further evidence for the cholinergic hypothesis of aging and dementia from the canine model of aging. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:411–22. doi: 10.1016/j.pnpbp.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Araujo JA, Chan AD, Winka LL, Seymour PA, Milgram NW. Dose-specific effects of scopolamine on canine cognition: impairment of visuospatial memory, but not visuospatial discrimination. Psychopharmacology (Berl) 2004;175:92–8. doi: 10.1007/s00213-004-1777-y. [DOI] [PubMed] [Google Scholar]
- 14.Ghoneim MM, Mewaldt SP. Studies on human memory: the interactions of diazepam, scopolamine, and physostigmine. Psychopharmacology (Berl) 1977;52:1–6. doi: 10.1007/BF00426592. [DOI] [PubMed] [Google Scholar]
- 15.Green A, Ellis KA, Ellis J, Bartholomeusz CF, Ilic S, Croft RJ, Phan KL, Nathan PJ. Muscarinic and nicotinic receptor modulation of object and spatial n-back working memory in humans. Pharmacol Biochem Behav. 2005;81:575–84. doi: 10.1016/j.pbb.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Siegfried KR. Pharmacodynamic and early clinical studies with velnacrine. Acta Neurol Scand Suppl. 1993;149:26–8. doi: 10.1111/j.1600-0404.1993.tb04250.x. [DOI] [PubMed] [Google Scholar]
- 17.Prohovnik I, Arnold SE, Smith G, Lucas LR. Physostigmine reversal of scopolamine-induced hypofrontality. J Cereb Blood Flow Metab. 1997;17:220–8. doi: 10.1097/00004647-199702000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Preda L, Alberoni M, Bressi S, Cattaneo C, Parini J, Canal N, Franceschi M. Effects of acute doses of oxiracetam in the scopolamine model of human amnesia. Psychopharmacology (Berl) 1993;110:421–6. doi: 10.1007/BF02244648. [DOI] [PubMed] [Google Scholar]
- 19.Jones RW, Wesnes KA, Kirby J. Effects of NMDA modulation in scopolamine dementia. Ann N Y Acad Sci. 1991;640:241–4. doi: 10.1111/j.1749-6632.1991.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 20.Caine ED, Weingartner H, Ludlow CL, Cudahy EA, Wehry S. Qualitative analysis of scopolamine-induced amnesia. Psychopharmacology (Berl) 1981;74:74–80. doi: 10.1007/BF00431761. [DOI] [PubMed] [Google Scholar]
- 21.Curran HV, Schifano F, Lader M. Models of memory dysfunction? A comparison of the effects of scopolamine and lorazepam on memory, psychomotor performance and mood. Psychopharmacology (Berl) 1991;103:83–90. doi: 10.1007/BF02244079. [DOI] [PubMed] [Google Scholar]
- 22.Bartus RT, Dean RL, III, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 23.Drachman DA, Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974;30:113–21. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- 24.Kopelman MD, Corn TH. Cholinergic ‘blockade’ as a model for cholinergic depletion. A comparison of the memory deficits with those of Alzheimer-type dementia and the alcoholic Korsakoff syndrome. Brain. 1988;111(Pt 5):1079–110. doi: 10.1093/brain/111.5.1079. [DOI] [PubMed] [Google Scholar]
- 25.Sunderland T, Weingartner H, Cohen RM, Tariot PN, Newhouse PA, Thompson KE, Lawlor BA, Mueller EA. Low-dose oral lorazepam administration in Alzheimer subjects and age-matched controls. Psychopharmacology (Berl) 1989;99:129–33. doi: 10.1007/BF00634466. [DOI] [PubMed] [Google Scholar]
- 26.Drachman DA. Memory and cognitive function in man: does the cholinergic system have a specific role? Neurology. 1977;27:783–90. doi: 10.1212/wnl.27.8.783. [DOI] [PubMed] [Google Scholar]
- 27.Martinez R, Molchan SE, Lawlor BA, Thompson K, Martinson H, Latham G, Weingartner H, Sunderland T. Minimal effects of dextroamphetamine on scopolamine-induced cognitive impairments in humans. Biol Psychiatry. 1997;41:50–7. doi: 10.1016/0006-3223(95)00674-5. [DOI] [PubMed] [Google Scholar]
- 28.Beatty WW, Butters N, Janowsky DS. Patterns of memory failure after scopolamine treatment: implications for cholinergic hypotheses of dementia. Behav Neural Biol. 1986;45:196–211. doi: 10.1016/s0163-1047(86)90772-7. [DOI] [PubMed] [Google Scholar]
- 29.Christensen H, Maltby N, Jorm AF, Creasey H, Broe GA. Cholinergic ‘blockade’ as a model of the cognitive deficits in Alzheimer's disease. Brain. 1992;115:1681–99. doi: 10.1093/brain/115.6.1681. [DOI] [PubMed] [Google Scholar]
- 30.Molchan SE, Martinez RA, Hill JL, Weingartner HJ, Thompson K, Vitiello B, Sunderland T. Increased cognitive sensitivity to scopolamine with age and a perspective on the scopolamine model. Brain Res Brain Res Rev. 1992;17:215–26. doi: 10.1016/0165-0173(92)90017-g. [DOI] [PubMed] [Google Scholar]
- 31.Gijsman HJ, Cohen AF, van Gerven JM. The application of the principles of clinical drug development to pharmacological challenge tests of the serotonergic system. J Psychopharmacol. 2004;18:7–13. doi: 10.1177/0269881104040205. [DOI] [PubMed] [Google Scholar]
- 32.Meibohm B, Derendorf H. Basic concepts of pharmacokinetic/pharmacodynamic (PK/PD) modelling. Int J Clin Pharmacol Ther. 1997;35:401–13. [PubMed] [Google Scholar]
- 33.Cawello W. Connection of pharmacokinetics and pharmacodynamics – how does it work? Int J Clin Pharmacol Ther. 1997;35:414–7. [PubMed] [Google Scholar]
- 34.Strougo A, Zuurman L, Roy C, Pinquier JL, van Gerven JM, Cohen AF, Schoemaker RC. Modelling of the concentration–effect relationship of THC on central nervous system parameters and heart rate – insight into its mechanisms of action and a tool for clinical research and development of cannabinoids. J Psychopharmacol. 2008;22:717–26. doi: 10.1177/0269881108089870. [DOI] [PubMed] [Google Scholar]
- 35.Liem-Moolenaar M, Zoethout RWM, de Boer P, Schmidt M, de Kam ML, Cohen AF, Franson KL, van Gerven JMA. The effects of a glycine reuptake inhibitor R231857 on the central nervous system and on scopolamine-induced impairments in cognitive and psychomotor function in healthy subjects. J Psychopharmacol. 2009;24:1681–7. doi: 10.1177/0269881109105573. [DOI] [PubMed] [Google Scholar]
- 36.Liem-Moolenaar M, Zoethout RWM, de Boer P, Schmidt M, de Kam ML, Cohen AF, Franson KL, van Gerven JMA. The effects of the glycine reuptake inhibitor R213129 on the central nervous system and on scopolamine-induced impairments in psychomotor and cognitive function in healthy subjects. J Psychopharmacol. 2010;24:1671–9. doi: 10.1177/0269881109106942. [DOI] [PubMed] [Google Scholar]
- 37.de Haas SL, de Visser SJ, van der Post JP, de Smet M, Schoemaker RC, Rijnbeek B, Cohen AF, Vega JM, Agrawal NG, Goel TV, Simpson RC, Pearson LK, Li S, Hesney M, Murphy MG, van Gerven JM. Pharmacodynamic and pharmacokinetic effects of TPA023, a GABA(A) alpha(2,3) subtype-selective agonist, compared to lorazepam and placebo in healthy volunteers. J Psychopharmacol. 2007;21:374–83. doi: 10.1177/0269881106072343. [DOI] [PubMed] [Google Scholar]
- 38.de Haas SL, de Visser SJ, van der Post JP, Schoemaker RC, van Dyck K, Murphy MG, de Smet M, Vessey LK, Ramakrishnan R, Xue L, Cohen AF, van Gerven JM. Pharmacodynamic and pharmacokinetic effects of MK-0343, a GABA(A) alpha2,3 subtype selective agonist, compared to lorazepam and placebo in healthy male volunteers. J Psychopharmacol. 2008;22:24–32. doi: 10.1177/0269881107082108. [DOI] [PubMed] [Google Scholar]
- 39.Zuurman L, Roy C, Schoemaker RC, Hazekamp A, Den Hartigh J, Bender JC, Verpoorte R, Pinquier JL, Cohen AF, van Gerven JM. Effect of intrapulmonary tetrahydrocannabinol administration in humans. J Psychopharmacol. 2008;22:707–16. doi: 10.1177/0269881108089581. [DOI] [PubMed] [Google Scholar]
- 40.Twa MD, Bailey MD, Hayes J, Bullimore M. Estimation of pupil size by digital photography. J Cataract Refract Surg. 2004;30:381–9. doi: 10.1016/S0886-3350(03)00619-9. [DOI] [PubMed] [Google Scholar]
- 41.Borland RG, Nicholson AN. Visual motor co-ordination and dynamic visual acuity. Br J Clin Pharmacol. 1984;18(Suppl. 1):69S–72S. doi: 10.1111/j.1365-2125.1984.tb02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liem-Moolenaar M, Gray F, de Visser S, Franson K, Schoemaker R, Schmitt J, Cohen A, van Gerven J. Psychomotor and cognitive effects of a single oral dose of talnetant (SB223412) in healthy volunteers compared with placebo or haloperidol. J Psychopharmacol. 2010;24:73–82. doi: 10.1177/0269881108094524. [DOI] [PubMed] [Google Scholar]
- 43.Andrew JM. Delinquents and the tapping test. J Clin Psychol. 1977;33:786–91. doi: 10.1002/1097-4679(197707)33:3<786::aid-jclp2270330340>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 44.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–62. [Google Scholar]
- 45.Wellstein A, Pitschner HF. Complex dose-response curves of atropine in man explained by different functions of M1- and M2-cholinoceptors. Naunyn Schmiedebergs Arch Pharmacol. 1988;338:19–27. doi: 10.1007/BF00168807. [DOI] [PubMed] [Google Scholar]
- 46.Duka T, Ott H, Rohloff A, Voet B. The effects of a benzodiazepine receptor antagonist beta-carboline ZK-93426 on scopolamine-induced impairment on attention, memory and psychomotor skills. Psychopharmacology (Berl) 1996;123:361–73. doi: 10.1007/BF02246647. [DOI] [PubMed] [Google Scholar]
- 47.Beal SL, Sheiner LB, Boeckmann AJ, editors. Ellicott City, MD: Icon Development Solutions; 1989. NONMEM Users Guides. –2006. [Google Scholar]
- 48.Riedel W, Hogervorst E, Leboux R, Verhey F, van Praag H, Jolles J. Caffeine attenuates scopolamine-induced memory impairment in humans. Psychopharmacology (Berl) 1995;122:158–68. doi: 10.1007/BF02246090. [DOI] [PubMed] [Google Scholar]
- 49.Morrison JD, Reilly J. The effects of 0.025% hyoscine hydrobromide eye drops on visual function in man. Ophthalmic Physiol Opt. 1989;9:41–5. doi: 10.1111/j.1475-1313.1989.tb00803.x. [DOI] [PubMed] [Google Scholar]
- 50.Mintzer MZ, Griffiths RR. Lorazepam and scopolamine: a single-dose comparison of effects on human memory and attentional processes. Exp Clin Psychopharmacol. 2003;11:56–72. doi: 10.1037//1064-1297.11.1.56. [DOI] [PubMed] [Google Scholar]
- 51.Hutchins JB, Hollyfield JG. Acetylcholine receptors in the human retina. Invest Ophthalmol Vis Sci. 1985;26:1550–7. [PubMed] [Google Scholar]
- 52.de Haas SL, Schoemaker RC, van Gerven JM, Hoever P, Cohen AF, Dingemanse J. Pharmacokinetics, pharmacodynamics and the pharmacokinetic/pharmacodynamic relationship of zolpidem in healthy subjects. J Psychopharmacol. 2010;24:1619–29. doi: 10.1177/0269881109106898. [DOI] [PubMed] [Google Scholar]
- 53.van Steveninck AL, Schoemaker HC, Pieters MS, Kroon R, Breimer DD, Cohen AF. A comparison of the sensitivities of adaptive tracking, eye movement analysis and visual analog lines to the effects of incremental doses of temazepam in healthy volunteers. Clin Pharmacol Ther. 1991;50:172–80. doi: 10.1038/clpt.1991.122. [DOI] [PubMed] [Google Scholar]
- 54.van Steveninck AL. Methods of assessment of central nervous system effects of drugs in man. 1993. (Thesis). University of Leiden, Leiden.
- 55.van Steveninck AL, van Berckel BN, Schoemaker RC, Breimer DD, van Gerven JM, Cohen AF. The sensitivity of pharmacodynamic tests for the central nervous system effects of drugs on the effects of sleep deprivation. J Psychopharmacol. 1999;13:10–7. doi: 10.1177/026988119901300102. [DOI] [PubMed] [Google Scholar]
- 56.van der Post JP, de Visser SJ, Schoemaker RC, Cohen AF, van Gerven JM. Pharmacokinetic/pharmacodynamic assessment of tolerance to central nervous system effects of a 3 mg sustained release tablet of rilmenidine in hypertensive patients. J Psychopharmacol. 2004;18:221–7. doi: 10.1177/0269881104042626. [DOI] [PubMed] [Google Scholar]
- 57.Dingemanse J, van Gerven JM, Schoemaker RC, Roncari G, Oberye JJ, van Oostenbruggen MF, Massarella J, Segala P, Zell M, Cohen AF. Integrated pharmacokinetics and pharmacodynamics of Ro 48-6791, a new benzodiazepine, in comparison with midazolam during first administration to healthy male subjects. Br J Clin Pharmacol. 1997;44:477–86. doi: 10.1046/j.1365-2125.1997.t01-1-00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Gerven JM, Roncari G, Schoemaker RC, Massarella J, Keesmaat P, Kooyman H, Heizmann P, Zell M, Cohen AF, Dingemanse J. Integrated pharmacokinetics and pharmacodynamics of Ro 48-8684, a new benzodiazepine, in comparison with midazolam during first administration to healthy male subjects. Br J Clin Pharmacol. 1997;44:487–93. doi: 10.1046/j.1365-2125.1997.t01-1-00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Steveninck AL, Wallnofer AE, Schoemaker RC, Pieters MS, Danhof M, van Gerven JM, Cohen AF. A study of the effects of long-term use on individual sensitivity to temazepam and lorazepam in a clinical population. Br J Clin Pharmacol. 1997;44:267–75. doi: 10.1046/j.1365-2125.1997.t01-1-00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frey KA, Koeppe RA, Mulholland GK, Jewett D, Hichwa R, Ehrenkaufer RL, Carey JE, Wieland DM, Kuhl DE, Agranoff BW. In vivo muscarinic cholinergic receptor imaging in human brain with [11C]scopolamine and positron emission tomography. J Cereb Blood Flow Metab. 1992;12:147–54. doi: 10.1038/jcbfm.1992.18. [DOI] [PubMed] [Google Scholar]


