Abstract
AIMS
We aimed to investigate the effects of tyrosine kinase inhibitors (TKIs) on paracetamol (acetaminophen) glucuronidation.
METHODS
The inhibition of nine small molecule TKIs on paracetamol glucuronidation was investigated in human liver microsomes (HLMs) and recombinant human UDP-glucuronosyltransferases (UGTs).
RESULTS
Sorafenib, dasatinib and imatinib exhibited mixed inhibition against paracetamol glucuronidation in pooled HLMs, and potent inhibition in UGT1A9 and UGT2B15. Dasatinib and imatinib also inhibited UGT1A1-mediated paracetamol glucuronidation. Axitinib, erlotinib, gefitinib, lapatinib, nilotinib and vandetanib exhibited weak inhibition of paracetamol glucuronidation activity in HLMs.
CONCLUSIONS
The inhibition of paracetamol glucuronidation by TKIs might be of particular concern when they are co-administered.
Keywords: acetaminophen, inhibition, paracetamol, toxicity, tyrosine kinase inhibitors, UDP-glucuronosyltransferase
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Clinical cases reported that fatal acute liver failure occurred when paracetamol (acetaminophen) was co-administrated with some tyrosine kinase inhibitors (TKIs). The direct inhibition of UDP-glucuronosyltransferase activities has been identified as a mechanism of potentiation of paracetamol hepatotoxicity. However, the effects of TKIs on paracetamol glucuronidation are not known.
WHAT THIS STUDY ADDS
The TKIs, sorafenib, dasatinib and imatinib exhibited potent mixed inhibition against paracetamol glucuronidation in pooled human liver microsomes, implying a possible increase in paracetamol hepatotoxicity when they are co-administrated with paracetamol.
Introduction
Paracetamol (acetaminophen) accounts for the majority of drug-induced hepatotoxicity in the USA. Over 55% of paracetamol is eliminated by glucuronidation, which competes with a pathway involving cytochrome P450-catalysed bioactivation to a hepatotoxic reactive intermediate [1]. UDP-glucuronosyltransferase (UGT) 1A1, UGT1A6, UGT1A9 and UGT2B15 are the major UGTs catalysing paracetamol glucuronide formation in vitro[2, 3]. The direct inhibition of UGT enzymes has been identified recently as a mechanism of potentiation of paracetamol toxicity [3, 4].
Some small molecule tyrosine kinase inhibitors (TKIs) have been found to have effective antitumour activity, and dozens are in clinical trials [5]. However, fatal acute liver failure has been reported with the co-administration of paracetamol and some TKIs, such as imatinib and sunitinib [6, 7]. As several TKIs, including erlotinib, gefitinib and sorafenib, have been identified as inhibitors of UGTs [8, 9], these findings prompted us to consider whether other TKIs could increase paracetamol-mediated hepatotoxicity via the inhibition of UGTs.
In this study, we investigated the effects of nine TKIs on paracetamol glucuronidation activity in human liver microsomes (HLMs) and recombinant UGTs.
Methods
Chemicals and reagents
Erlotinib was purchased from Biaffin GmbH & Co KG (Kassel, Germany). Gefitinib, sorafenib, lapatinib, dasatinib, vandetanib, nilotinib, imatinib mesylate and axitinib were purchased from Toronto Research Chemicals, Inc. (North York, Canada). Paracetamol, paracetamol glucuronide, 3-acetamidophenol, alamethicin, phenobarbital and uridine 5’-diphosphoglucuronic acid (UDPGA) were purchased from Sigma-Aldrich (St Louis, MO, USA). Pooled HLMs and recombinant human UGTs were purchased from BD Gentest Corp. (Woburn, MA, USA). All other reagents were of HPLC grade or of the highest grade commercially available.
Kinetic study of paracetamol glucuronidation in HLMs
Incubations were conducted using conditions reported previously [10] with a slight modification. In brief, paracetamol (0.2–40 mm) was pre-incubated with pooled HLMs (0.5 mg ml−1 protein) in a final volume of 200 µl of 50 mm Tris-HCl buffer (pH 7.4) containing 10 mm MgCl2, and 50 µg mg−1 protein alamethicin. The reaction was started by adding UDPGA (final concentration 5 mm). The reaction mixtures were incubated for 60 min at 37°C. The metabolites were analysed by HPLC (Hitachi High-Technologies America, Schaumburg, IL, USA) with UV detection at 254 nm. Intra- and inter-day variation in paracetamol glucuronidation was less than 10%. All experiments were performed in duplicate.
Inhibition of paracetamol glucuronidation activity
Paracetamol was incubated in the presence of TKIs (0–100 µm) in 0.5 mg ml−1 pooled HLMs or 0.5 mg ml−1 recombinant UGTs. The concentrations of substrate, paracetamol, were 12, 5, 4, 9 and 20 mm in HLMs, UGT1A1, UGT1A6, UGT1A9 and UGT2B15, respectively, close to its Km values [3]. Phenobarbital, a known inhibitor of paracetamol glucuronidation [4], was used as positive control. According to the manual of UGT supersomes (provided by the manufacturer), DMSO can be used at final concentration of up to 2% with little impact on activity. All tested TKIs were dissolved in DMSO (final concentration was 1% v : v).
Kinetics analysis
Kinetic constants were obtained by fitting of the Michaelis-Menten equation (Equation 1) [11].
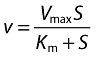 |
(1) |
The type of inhibition was determined from the fitting of enzyme inhibition models to data. The inhibition constant (Ki) values were calculated with non-linear regression according to the equation for mixed inhibition (Equation 2) [11].
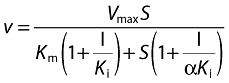 |
(2) |
IC50 values (concentration of inhibitor that reduces enzyme activity by 50% between the fitted top and bottom of the curve) were determined according to the four parameter logistic model (Equation 3).
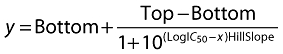 |
(3) |
where y is the per cent activity and x is the inhibitor concentration.
Calculation of AUCi : AUC ratio
The magnitudes of inhibitory interactions of sorafenib, dasatinib and imatinib were estimated as the ratio of the area under the plasma concentration–time curve in the presence and absence of the inhibitor (AUCi : AUC). This ratio was predicted as described in the supporting information file (Appendix S1).
Results
Kinetic studies in pooled HLMs
The values (mean ± SE) of apparent Km and Vmax of paracetamol in HLMs were 12 ± 0 mm and 3065 ± 24 pmol min−1 mg−1 protein, respectively (Figure 1A).
Figure 1.
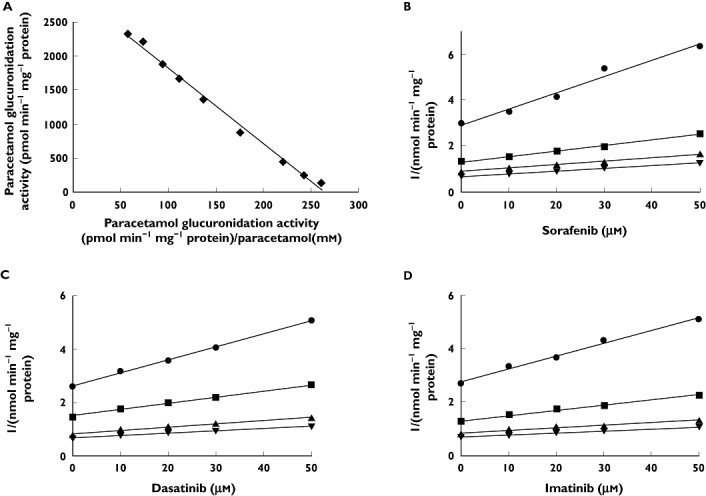
Representative Eadie–Hofstee plot for paracetamol glucuronidation (A) and Dixon plots of the effects of sorafenib (B), dasatinib (C) and imatinib (D) on paracetamol glucuronidation in pooled human liver microsomes. Reactions were performed as described in Methods. All data points shown represent the mean of duplicate measurements. 2 mm paracetamol ( ); 5 mm paracetamol (
); 5 mm paracetamol ( ); 10 mm paracetamol (
); 10 mm paracetamol ( ); 15 mm paracetamol (
); 15 mm paracetamol ( )
)
Inhibition of paracetamol glucuronidation activity by TKIs
The effects of TKIs on paracetamol glucuronidation activity in HLMs and recombinant UGTs are listed in Table 1. Kinetic experiments showed that sorafenib, dasatinib and imatinib exerted mixed inhibition against the formation of paracetamol glucuronide in HLMs with Ki (mean ± SE) of 52 ± 7, 48 ± 7 and 49 ± 7 µm, and with αKi of 63, 157 and 306 µm, respectively. The data are graphically represented by Dixon plots in Figures 1B, C and D.
Table 1.
The effects of TKIs on paracetamol glucuronidation activity in pooled human liver microsomes (HLMs), recombinant UDP-glucuronosyltransferase (UGT) 1A1, UGT1A6, UGT1A9 and UGT2B15
| IC50 (µm) | |||||
|---|---|---|---|---|---|
| pooled HLMs | UGT1A1 | UGT1A6 | UGT1A9 | UGT2B15 | |
| Sorafenib | 27 ± 3 | >100 | >100 | 2 ± 0 | 13 ± 1 |
| Dasatinib | 49 ± 3 | 2 ± 1 | >100 | 1 ± 0 | 45 ± 2 |
| Imatinib | 69 ± 6 | 29 ± 4 | >100 | 47 ± 3 | 31 ± 3 |
| Axitinib | >100 | NA | NA | NA | NA |
| Erlotinib | >100 | NA | NA | NA | NA |
| Gefitinib | >100 | NA | NA | NA | NA |
| Lapatinib | >100 | NA | NA | NA | NA |
| Nilotinib | >100 | NA | NA | NA | NA |
| Vandetanib | >100 | NA | NA | NA | NA |
| Phenobarbital | 1745 ± 199 | NA | NA | NA | NA |
IC50 is expressed as mean ± SE. NA, not applicable.
Quantitative prediction of AUCi : AUC ratio
A 400-mg twice-daily dose of sorafenib will result in a 51% increase in the AUC of co-administered paracetamol. A 200-mg daily dose of imatinib will result in a 22% increase. However, for a 120-mg twice-daily dose of dasatinib, the AUC ratio is 1.06.
Discussion
Previous studies have shown that the enhanced susceptibility of UGT-deficient rats to paracetamol toxicity is because of decreased glucuronidation resulting in enhanced bioactivation [12]. Our findings imply a possible increase of paracetamol hepatotoxicity when these three TKIs are co-administrated with paracetamol.
Thus, it is important to know the possible effects in vivo of these TKIs. Our data suggest that at clinically relevant doses, imatinib and sorafenib could cause a considerable increase in the AUC of co-administered paracetamol via UGT inhibition. In addition, as in vitro data tend to underestimate inhibition of glucuronidation in vivo[13], it is likely that the inhibition of imatinib and sorafenib, but not dasatinib, against paracetamol glucuronidation observed in vitro might occur in vivo. These findings might explain the hepatotoxicity observed in the reported case of co-administration of paracetamol and imatinib [6].
UDP-glucuronosyltransferase 1A9 has been identified as the predominant isoform responsible for paracetamol glucuronidation over a clinically relevant paracetamol concentration range [2]. Therefore, the inhibition of UGT1A9-mediated paracetamol glucuronidation by these TKIs might be of particular concern.
However, various factors are responsible for paracetamol-mediated hepatotoxicity. The extrapolations of in vitro data must be made with great caution. Conversion of paracetamol to its hepatotoxic metabolite does not seem to be increased in patients induced with phenobarbital or phenytoin, the inhibitors of UGTs and also some metabolizing enzyme inducers [14]. Therefore, further systemic studies are needed to clarify the in vivo effects of these TKIs on paracetamol-mediated hepatotoxicity.
Acknowledgments
This work was supported by Pharmacogenetics of Anticancer Agents Research Group, National Institutes of Health/National Institute of General Medical Sciences Grant U01GM61393.
Competing Interests
Mark J. Ratain is a co-inventor on a pending use patent for sorafenib, has consulted on behalf of Genentech, Hoffman-LaRoche, Mylan, Novartis, Onyx, Pfizer and Teva and has received funding from Bristol-Myers Squibb for a clinical trial.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Appendix S1 Methods of AUCi/AUC ratio calculation.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- 1.Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980;10(Suppl. 2):291S–98S. doi: 10.1111/j.1365-2125.1980.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Court MH, Duan SX, von Moltke LL, Greenblatt DJ, Patten CJ, Miners JO, Mackenzie PI. Interindividual variability in acetaminophen glucuronidation by human liver microsomes: identification of relevant acetaminophen UDP-glucuronosyltransferase isoforms. J Pharmacol Exp Ther. 2001;299:998–1006. [PubMed] [Google Scholar]
- 3.Mutlib AE, Goosen TC, Bauman JN, Williams JA, Kulkarni S, Kostrubsky S. Kinetics of acetaminophen glucuronidation by UDP-glucuronosyltransferases 1A1, 1A6, 1A9 and 2B15. Potential implications in acetaminophen-induced hepatotoxicity. Chem Res Toxicol. 2006;19:701–9. doi: 10.1021/tx050317i. [DOI] [PubMed] [Google Scholar]
- 4.Kostrubsky SE, Sinclair JF, Strom SC, Wood S, Urda E, Stolz DB, Wen YH, Kulkarni S, Mutlib A. Phenobarbital and phenytoin increased acetaminophen hepatotoxicity due to inhibition of UDP-glucuronosyltransferases in cultured human hepatocytes. Toxicol Sci. 2005;87:146–55. doi: 10.1093/toxsci/kfi211. [DOI] [PubMed] [Google Scholar]
- 5.Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005;315:971–9. doi: 10.1124/jpet.105.084145. [DOI] [PubMed] [Google Scholar]
- 6.Ridruejo E, Cacchione R, Villamil AG, Marciano S, Gadano AC, Mando OG. Imatinib-induced fatal acute liver failure. World J Gastroenterol. 2007;13:6608–111. doi: 10.3748/wjg.v13.i48.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weise AM, Liu CY, Shields AF. Fatal liver failure in a patient on acetaminophen treated with sunitinib malate and levothyroxine. Ann Pharmacother. 2009;43:761–6. doi: 10.1345/aph.1L528. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Ramirez J, House L, Ratain MJ. Comparison of the drug-drug interactions potential of erlotinib and gefitinib via inhibition of UDP-glucuronosyltransferases. Drug Metab Dispos. 2010;38:32–9. doi: 10.1124/dmd.109.029660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mross K, Steinbild S, Baas F, Gmehling D, Radtke M, Voliotis D, Brendel E, Christensen O, Unger C. Results from an in vitro and a clinical/pharmacological phase I study with the combination irinotecan and sorafenib. Eur J Cancer. 2007;43:55–63. doi: 10.1016/j.ejca.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Alkharfy KM, Frye RF. High-performance liquid chromatographic assay for acetaminophen glucuronide in human liver microsomes. J Chromatogr B Biomed Sci Appl. 2001;753:303–8. doi: 10.1016/s0378-4347(00)00566-1. [DOI] [PubMed] [Google Scholar]
- 11.Copeland RA. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis. New York: Wiley-VCH; 2000. [Google Scholar]
- 12.de Morais SM, Wells PG. Enhanced acetaminophen toxicity in rats with bilirubin glucuronyl transferase deficiency. Hepatology. 1989;10:163–7. doi: 10.1002/hep.1840100207. [DOI] [PubMed] [Google Scholar]
- 13.Uchaipichat V, Mackenzie PI, Elliot DJ, Miners JO. Selectivity of substrate (trifluoperazine) and inhibitor (amitriptyline, androsterone, canrenoic acid, hecogenin, phenylbutazone, quinidine, quinine, and sulfinpyrazone) ‘probes’ for human UDP-glucuronosyltransferases. Drug Metab Dispos. 2006;34:449–56. doi: 10.1124/dmd.105.007369. [DOI] [PubMed] [Google Scholar]
- 14.Miners JO, Attwood J, Birkett DJ. Determinants of acetaminophen metabolism: effect of inducers and inhibitors of drug metabolism on acetaminophen's metabolic pathways. Clin Pharmacol Ther. 1984;35:480–6. doi: 10.1038/clpt.1984.64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


