Abstract
AIM
The aim of the study was to assess the influence of national and international warnings on the prescription rates of cough and cold medicines (CCMs) in the youngest children (<2 years) in the Netherlands and Italy.
METHODS
Analysis of outpatient electronic medical records of children <2 years in Italy and the Netherlands was carried out. Age and country specific prescription prevalence rates were calculated for the period 2005–08. Comparisons of prescription rates in 2005 (pre) and 2008 (post) warnings were done by means of a chi-square test.
RESULTS
The cohort consisted of 99 176 children <2 years of age. After international warnings, overall prescription rates for CCMs decreased slightly from 83 to 77/1000 person years (P = 0.05) in Italy and increased in the Netherlands from 74 to 92/1000 children per year. Despite the international warnings, prescription rates for nasal sympathomimetics and opium alkaloids increased in the Netherlands (P < 0.01). In Italy a significant decrease in the prescription rates of opium alkaloids and other cough suppressants (P < 0.01) was observed, and also a significant reduction in use of combinations of nasal sympathomimetics.
CONCLUSION
Despite the international safety warnings and negative benefit-risk profiles, prescription rates of cough and cold medicines remain substantial and were hardly affected by the warnings, especially in the Netherlands where no warning was issued. The hazards of use of these medicines in young children should be explicitly stipulated by the European Medicines Agency and all national agencies, in order to increase awareness amongst physicians and caretakers and reduce heterogeneity across the EU.
Keywords: cough and cold medicines, drug utilization, regulatory warnings
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Cough and cold medicines are frequently used in children to treat upper respiratory tract infections without solid proof of benefits.
Safety issues have been raised about the use of these drugs in young children.
In 2007 international warnings were issued advising against use of these drugs in young children.
WHAT THIS STUDY ADDS
Cough and cold medicines prescribing by primary care physicians has not really been influenced by international warnings in the Netherlands, where no additional national warnings were made and only partially in Italy.
A concerted action should be taken in Europe to advise strongly against the OTC use and prescription of cough and cold medicines in young children.
Introduction
Cough and cold medicines (CCMs) are frequently used to treat upper respiratory tract symptoms in children. This group of medicines includes expectorants, mucolytics, opium alkaloids (for cough), nasal sympathomimetics and anti-histamines. CCMs carry a risk of serious and potentially life-threatening adverse events, such as cardiac arrhythmias, depressed levels of consciousness and encephalopathy, and should be avoided in the very young [1].
As a result of these safety issues, in 2007 various regulatory actions were taken in many countries: in the United States, the Food and Drug Administration (FDA) issued a recommendation advising parents and caregivers not to use these drugs in children younger than 2 years of age [2]; in the United Kingdom, the Medicine and Healthcare Products Regulatory Agency (MHRA) advised against the use of many CCMs in children under the age of 6 years [3] and Health Canada required manufacturers to re-label certain over-the-counter (OTC) CCMs to indicate that these should not be used in children under the age of 6 years [4]. In Italy a warning was issued in September 2007 regarding only the use of nasal sympathomimetics in children <12 years. However in the Netherlands and many other countries, no national warning was issued. The safety concerns on the use of CCMs in young children, and the regulatory actions, also received a lot of attention from the scientific community. Sharfstein et al. published an important paper in the New England Journal of Medicine which was followed by publications in many lay press journals and media attention in most countries [1].
Most of the utilization data that have been published focus on OTC use of CCMs, as this is the major market in the USA [5–10]. Although CCMs can be bought as OTC in many countries, they are also regularly prescribed by physicians, especially when being re-imbursed. Previous studies have shown that prescription rates of, for example, antidepressants and antipsychotics drop after regulatory warnings [11–13]. However, to our knowledge, no studies have been performed that evaluate the effect of regulatory warnings on CCMs prescription rates. We aimed to investigate the extent of use and to study the effects of the warnings on the prescription rates of CCMs to children under the age of 2 years (since this is the group included in all international warnings) in the Netherlands and Italy, two countries with different types of direct national warnings (the Netherlands none, and Italy only on nasal sympathomimetics).
Methods
Data collection
Data were obtained from general practice medical record databases in two countries and were extracted and elaborated according to a common protocol. Databases comprised the Pedianet database in Italy [14] and the Integrated Primary Care Information (IPCI) database in the Netherlands [15]. These databases include complete automated medical records of primary care physicians. The patient population in each database is representative of the respective Dutch and Italian population regarding age and gender. The electronic records contain anonymous and coded information on patient demographics, symptoms and diagnoses, referrals, clinical findings, laboratory assessments, drug prescriptions and hospitalizations. Details of these databases regarding their paediatric data are described elsewhere [16].
Study population
The study period was from 1 January 2005 to 31 December 2008. The study population in each country consisted of all children aged 0–2 years (up to 24 months). Each patient was followed from start of the study period or date of registration with the primary care practice (whichever was latest), until the patient left the practice or end of the study period (whichever was earliest).
Classification of prescriptions
Cough and cold medicines of interest comprised the following medicines: expectorants (ATC R05CA: althea root, thyme, combinations containing promethazine, oxomemazine, terpenes, guaiacol and eucalyptus), mucolytics (ATC R05CB: ambroxol, bromhexine, acetylcysteine, carbocisteine, sobrerol, erdosteine, dornase alpha), opium alkaloids and derivatives (ATC R05DA: codeine, dextromethorphan, dihydrocodeine, noscapine and opium combinations containing pentetrazol, pseudoephedrine, triprolidine or codeine), other cough suppressants (ATC R05DB: pentoxyverine, levodropropizine, clobutinol, butarimate, dropropizine, cloperastine and combinations containing pentoxyverine, terpenes or thyme), nasal sympathomimetics (ATC R01AA: xylometazoline, oxymetazoline, phenylephrine, naphazoline) and combinations of nasal sympathomimetics (ATC R01AB). These drug classes were extracted from the databases using the World Health Organization's Anatomical Therapeutic Chemical (ATC) classification system [17].
Statistical analysis
Annual prescription rates [users per 1000 person years (PY)] of CCMs were estimated by counting the number of children being prescribed each drug or drug class divided by the number of person years attributed, categorized by calendar year, age and country. Prescription rates can be interpreted as the number of children per 1000 children who get a specific drug (class) prescribed in 1 year. Because we used annual prescription rates, a child who uses one drug (class) multiple times in 1 year will be counted only once for that year. To inspect seasonal variability monthly rates were calculated by dividing the number of users per month by the person months attributed.
To study the effect of the international warnings in 2007 on prescription rates, we compared prescription rates between 2005 and 2008 for each individual drug and drug class by a chi-square test. P values below 0.05 were considered to be significant.
Results
Patient characteristics
Our population of 99 176 children under the age of 2 years generated 110 169 person years of follow-up (Table 1). The overall distribution of children per country was fairly equal, 52% of the patients were from Italy and 48% from the Netherlands.
Table 1.
Number of children in the study population and persontime by gender and calendar year
| Italy | The Netherlands | |||||
|---|---|---|---|---|---|---|
| Patient characteristics | Children† | PY | GP practices* | Children† | PY | GP practices* |
| Females | 24 532 (48%) | 33 862 | 23 406 (49%) | 19 741 | ||
| Males | 26 890 (52%) | 37 002 | 24 348 (51%) | 19 564 | ||
| 2005 | 19 070 | 16 338 | 158 | 11 331 | 8 422 | 134 |
| 2006 | 19 424 | 18 780 | 176 | 12 189 | 8 340 | 136 |
| 2007 | 18 611 | 18 234 | 160 | 13 128 | 10 845 | 138 |
| 2008 | 17 596 | 17 512 | 170 | 13 219 | 11 698 | 138 |
| Total | 51 422 | 70 864 | 47 754 | 39 305 | ||
The number of contributing general practitioner's practices to the study population.
The number of children in various years does not add up to the total since one child can contribute in more than 1 year during the study period. PY, person years.
Prescription rates of cough and cold medicines
In the Netherlands CCMs prescription rates increased significantly between 2005 and 2008 from 74 to 92/1000 PY (P < 0.01), but decreased slightly from 83 to 77/1000 PY (P = 0.05) in Italy (Figure 1). In both countries a strong seasonal prescription pattern could be observed, with peaks during winter time (Figure 2). The seasonal patterns of CCMs prescription rates were similar before and after the international warnings in Italy and the Netherlands.
Figure 1.
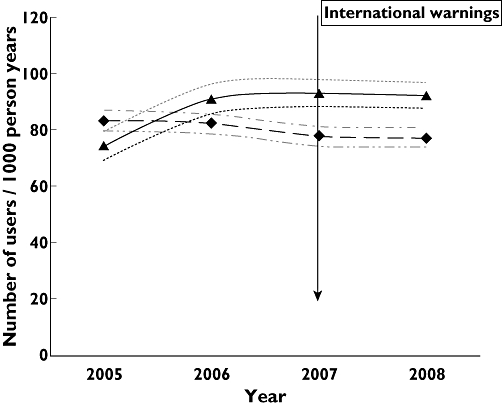
Prescription rates of cough and cold medicines by database and year with 95% confidence intervals. IPCI Dutch database, Pedianet Italian database. IPCI ( ); IPCI 95% CI upper limit (
); IPCI 95% CI upper limit ( ); IPCI 95% CI lower limit (
); IPCI 95% CI lower limit ( ); Pedianet (
); Pedianet ( ); Pedianet 95% CI upper limit (
); Pedianet 95% CI upper limit ( ); Pedianet 95% CI lower limit (
); Pedianet 95% CI lower limit ( )
)
Figure 2.
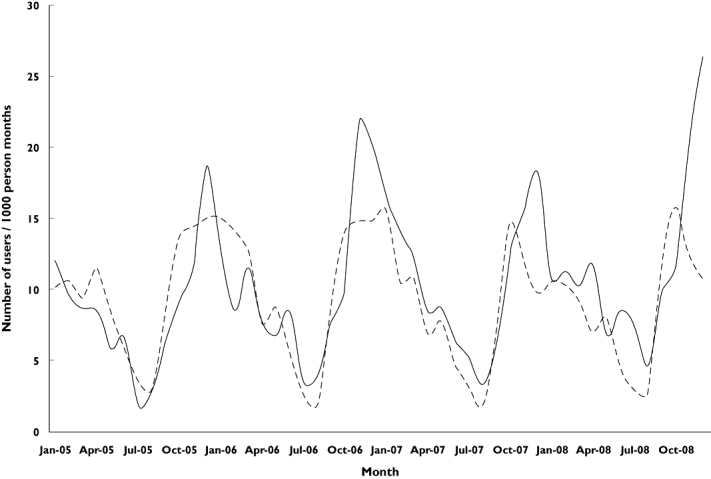
Prescription rates of cough and cold medicines by database and calendar month. IPCI Dutch database, Pedianet Italian database. IPCI ( ); Pedianet (
); Pedianet ( )
)
When looking at classes of CCMs, different patterns were observed, both in terms of preferred medicines between countries and changes in annual prescription rates. Expectorants were mostly prescribed in the Netherlands, and rates decreased significantly from 10.8 to 4.3/1000 PY (P < 0.01) (Table 2), mostly because of a decrease in prescription of both thyme syrup (P < 0.01) and combination products (P < 0.01) (Table 3). In Italy the only expectorants prescribed were combination products, and prescription rates for these increased slightly.
Table 2.
Prescription rates of cough and cold medicines per 1000 person years by year and country
| CCMs | Cough relievers | Cough suppressants | Nasal decongestants | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Expectorants | Mucolytics | Opium alkaloids and derivatives | Other | Sympathomimetics | Sympathomimetic combinations | ||||||||
| Year | NL | IT | NL | IT | NL | IT | NL | IT | NL | IT | NL | IT | NL | IT |
| 2005 | 74 | 83 | 10.8 | 4.1 | 6.6 | 54 | 4.2 | 7.4 | 7.7 | 21 | 51 | 0.9 | – | 7.3 |
| 2006 | 91 | 82 | 9.4 | 5.6 | 7.1 | 56 | 6.4 | 5.4 | 7.2 | 22 | 67 | 1.1 | – | 4.6 |
| 2007 | 93 | 78 | 6.8 | 5.2 | 8.6 | 58 | 10.9 | 3.6 | 9.7 | 17 | 70 | 0.3 | – | 1.4 |
| 2008 | 92 | 77 | 4.3 | 5.0 | 5.2 | 58 | 13.4 | 3.6 | 6.2 | 16 | 73 | 0.1 | – | 0.3 |
| Overall | 88* | 79 | 7.1* | 5.0 | 6.8 | 57 | 9.3* | 5.0* | 7.7 | 19* | 66* | 0.6* | – | 3.3* |
Significant change between 2005 and 2008 (P < 0.05). IT, Italy; NL, the Netherlands. –, not available in NL.
Table 3.
Pre- and post-warning prescription rates per 1000 person years of individual cough and cold medicines
| The Netherlands | Italy | |||||||
|---|---|---|---|---|---|---|---|---|
| Class of CCM | 2005 | 2008 | P value | 2005 | 2008 | P value | ||
| Expectorants | Combinations* | 4.3 | 1.7 | <0.01 | Combinations‡ | 4.1 | 5.0 | 0.24 |
| Thyme syrup | 14.8 | 0.8 | <0.01 | |||||
| Althea root | 0 | 0.08 | 0.40 | |||||
| Mucolytics | Bromhexine | 4.4 | 3.2 | 0.16 | Ambroxol | 17.6 | 19.8 | 0.13 |
| Acetylcysteine | 2.0 | 2.0 | 0.93 | Sobrerol | 16.0 | 20.2 | <0.01 | |
| Carbocisteine | 0.2 | 0 | 0.47 | Carbocisteine | 13.4 | 14.0 | 0.65 | |
| Dornase alpha | 0 | .08 | 0.40 | Bromhexine | 9.7 | 8.2 | 0.14 | |
| Opium alkaloids and derivatives | Noscapine | 3.7 | 14.1 | <0.01 | Dextromethorphan | 3.8 | 1.8 | <0.01 |
| Codeine | 0.8 | 0.4 | 0.88 | Codeine | 2.3 | 0.9 | <0.01 | |
| Combinations§ | 0.7 | 0.2 | 0.01 | |||||
| Other cough suppressants | Pentoxyverine | 7.4 | 6.2 | 0.30 | Levodropropizine | 14.8 | 10.8 | <0.01 |
| Combinations† | 0.6 | 0 | 0.28 | Cloperastine | 5.6 | 5.0 | 0.40 | |
| Clobutinol | 0.2 | 0 | 0.42 | |||||
| Nasal sympathomimetics | Xylometazoline | 51 | 73 | <0.01 | Phenylephrine | 0.8 | 0.1 | <0.01 |
| Oxymetazoline | 0.1 | 0.4 | 0.21 | Naphazoline | 0.1 | 0 | 0.48 | |
| Combinations of nasal sympathomimetics | – | Ephedrine based | 4.6 | 0.2 | <0.01 | |||
| Tuaminoheptane based | 2.9 | 0.1 | <0.01 | |||||
Combinations containing promethazine, oxomemazine, guaiacol, ipecacuanha.
Combinations containing pentoxyverine, terpenes, thyme.
Combinations containing terpenes, eucalyptus, guaiacol, thyme.
Combinations containing pentetrazol, dihydrocodeine, codeine, dextromethorphan, pseudoephedrine, triprolidine.
–, not available in the Netherlands.
Mucolytics were 10 times more often prescribed in Italy than in the Netherlands. In both countries, overall rates did not change before and after the warning, but small changes occurred in prescription of individual products. In particular prescription rates of sobrerol increased in Italy and dornase alpha in the Netherlands (Tables 2 and 3).
Opium alkaloids and derivatives for cough suppression were most frequently prescribed in the Netherlands and prescription rates even tripled between 2005 and 2008 from 4.2 to 13.4/1000 PY (P < 0.01). This increase was due to a substantial rise in prescription rates of noscapine. In contrast, in Italy prescription rates of opium alkaloids dropped by half from 7.4 in 2005 to 3.6/1000 PY in 2008 (P < 0.01). This decrease was seen for all products in this class (Tables 2 and 3). Prescription rates for other (non-opioid) cough suppressants were highest in Italy, and this was mostly accounted for by levodropropizine. Rates decreased by one third after the warning (P < 0.01, Tables 2 and 3). In the Netherlands, use of other cough suppressants did not change significantly.
Single product nasal sympathomimetics (mostly xylometazoline) were primarily prescribed in the Netherlands, and prescription rates increased significantly from 51 in 2005 to 73/1000 PY in 2008, mostly due to use of xylometazoline (P < 0.01) (Table 2). In Italy, xylometazoline was hardly prescribed even before the warning, and prescription rates decreased to almost 0 in 2008. On the other hand, combinations of nasal sympathomimetics were only prescribed in Italy, and a strong effect of the warning was seen on their prescription rates (from 7.3 in 2005 to 0.3/1000 PY in 2008, P < 0.01). Medicines prescribed in this class were combinations with ephedrine or tuaminoheptane.
Discussion
This paper provides information on the effects of national and international warnings on rates of prescriptions of cough and cold medicines in a country with and a country without additional national warnings. The formal regulatory review of OTC drugs, including CCMs, started in 1972 in the United States [18]. At that time, most OTC CCMs were generally recognized as safe and effective. Until recently CCMs received little attention regarding their safety and efficacy in children. In 2007, this changed dramatically by a citizen petition submitted to the FDA, raising significant concerns about the safety and efficacy of CCMs in children under the age of 6 years. This petition resulted in a public health advisory by the FDA, recommending that CCMs should not be used in children under 2 years of age because of the risk of serious and life-threatening effects [2]. The FDA warning was followed by other countries issuing warnings and several literature publications.
Despite the international warnings and important publications in high impact journals and lay press advising against the use of CCMs in young children, overall prescription rates for CCMs increased in the Netherlands, where no national warning was issued. Use of opium alkaloids and nasal sympathomimetics increased significantly, although these drugs are contra-indicated in young children [19]. In Italy, where a specific warning was issued by the Agenzia Italiana del Farmaco only against the use of nasal sympathomimetics, a significant reduction in use of nasal sympathomimetics was seen and also a decrease in use of cough suppressants (opioids and non-opioids).
Although the warnings did not show a considerable impact on the overall prescription rates in the Netherlands, there were shifts in the use of individual medicines. Rates for expectorant combinations decreased (comprising of promethazine and oxomemazine), whereas the prescription rates for noscapine, an opium alkaloid, increased substantially. Substantial evidence of adverse events related to the use of promethazine and oxomemazine in children exists [1, 20]. There are no studies evaluating the safety of noscapine in children, and no safety issues have been raised so far on the use of these drugs in young children. The Dutch GP society recommends noscapine for the symptomatic treatment of cough in young children [21]. This change from expectorant combinations to noscapine may be seen as a favourable trend although safety of noscapine in these young children should be further investigated. The observed increase in prescription rates of nasal sympathomimetics in the Netherlands is of high concern, since these drugs may cause serious adverse events such as lethargy, tachycardia, convulsions and even coma [22–26]. Furthermore, a recent Cochrane review concluded that insufficient data are available on the safety and efficacy of many nasal decongestants in children. Therefore, these drugs are not recommended for use in children <12 years of age [27].
In Italy, safety warnings were issued in September 2007 advising against the use of nasal sympathomimetics in children <12 years. Prescription rates of these drugs reduced significantly after the warning.
Our data show that CCMs are still prescribed to very young children, and prescription rates were hardly influenced by the international safety warnings, especially in the Netherlands where no national regulatory action was taken. In Italy we could observe positive effects from the safety warnings in some drug classes. Overall however, more action should be taken since use of these drugs in the very young may raise safety issues, and there is no evidence of efficacy [20, 28–30]. The few studies that evaluated the efficacy of CCMs in children with cough and cold found no significant benefit in symptomatic relief when compared with placebo [31–35]. Besides a lack of efficacy, CCMs are more prone to dosing errors. Variations in liquid CCMs dosing with spoons can be as much as 20%, leading to a higher risk of adverse events [36]. A recent study investigating therapeutic errors in children found that 23% of the dosing errors were related to use of CCMs [37]. Another study exploring paediatric fatalities associated with OTC CCMs use concluded that 85% of the fatalities were associated with an overdose of CCMs, mainly in children under the age of 2 years [38].
Not only are the country specific differences of the international warnings on the prescription rates of CCMs of interest but also the differences in the types of CCMs that are prescribed. In Italy sympathomimetics are hardly used but very commonly prescribed in the Netherlands, whereas in the Netherlands use of mucolytics is very low, but frequently prescribed in Italy. This is probably due to country specific guidelines on the symptomatic relief of cough and cold.
Many of the CCMs, which are still prescribed as seen in this study, are contraindicated in children younger than 6 years in the United Kingdom and Canada and younger than 2 years in the US [2, 3, 39]. This makes prescription by primary care physicians to young children even more alarming. To minimize the use of CCMs in children, these drugs should no longer be available OTC. Carer and physician education about the self-limiting nature of coughs and colds, the lack of efficacy and the risks associated with these drugs is needed [1, 40].
As for all observational research, our data have limitations. First of all, since we used primary care prescription data, we did not capture OTC use of these drugs and had no data on actual intake. However, our aim was to analyze prescription rates of CCMs by primary care physicians as these health professionals are approached directly by regulators/inspectorates. Furthermore, we could not compare our results with pre- and post-warning prescription rates in countries where more extensive warnings have been given, since no data have been published on this subject.
A major strength of our study is the fact that we captured a large number of children in two different databases across Europe, resulting in a high generalizability of the study population and our study findings.
To conclude, our results show that despite international warnings, a negative safety profile and lack of efficacy data, CCMs are still prescribed to young children in primary care in Italy and the Netherlands, and in the latter country rates even increased. Also, CCMs were prescribed that contain specific active ingredients proven to be most harmful in young children. Two lessons can be learned from this study. First of all, primary care physicians should be more aware of the harm and lack of efficacy of these drugs and should not prescribe them to young children. Secondly, there is heterogeneity in warnings across countries in the EU, which creates inequality. In order to increase awareness among physicians and caretakers and to allow for consistent warnings, the hazards of use of these medicines have to be explicitly stipulated by the European Medicines Agency. A concerted action is needed in Europe to advise against the use and prescription of cough and cold medicines to young children.
Acknowledgments
The study was funded by the European Community's 6th Framework Programme. Project number LSHB-CT-2005-005216: TEDDY: Task force in Europe for Drug Development for the Young.
We thank all of the physicians contributing data to the PEDIANET, and IPCI databases.
Conflicts of Interest
None of the authors has a conflict of interest related to the topic of this paper. The principal investigator had full access to all of the data in the study and takes responsibility for their integrity and the accuracy of the data analysis. KV has been involved as project leader in analyses contracted by various pharmaceutical companies and received unconditional research grants from Pfizer, Yamanouchi and Boehringer-Ingelheim; none of which are related to the subject of this study. GP is director of a company who has received grants for conduct of research from Merck and Pfizer. MS has received travel reimbursement from AstraZeneca and as head of a research unit receives unconditional research grants from Pfizer, Lilley and Altana. She has been a consultant for Pfizer and Novartis.
REFERENCES
- 1.Sharfstein JM, North M, Serwint JR. Over the counter but no longer under the radar – pediatric cough and cold medications. N Engl J Med. 2007;357:2321–4. doi: 10.1056/NEJMp0707400. [DOI] [PubMed] [Google Scholar]
- 2.FDA. FDA Recommends that Over-the-Counter (OTC) Cough and Cold Products not be used for Infants and Children under 2 Years of Age. 2008. Available at: http://www.fda.gov/Drugs/DrugSafety/PublicHealthAdvisories/ucm051137.html (last accessed 3 October 2009)
- 3.MHRA. Press release: better medicines for children's coughs and colds. 2009. Available at: http://www.mhra.gov.uk/NewsCentre/Pressreleases/CON038902 (last accessed 3 October 2009)
- 4.Canada H. Health Canada Releases Decision on the Labelling of Cough and Cold Products for Children. 2008. Available at: http://www.hc-sc.gc.ca/ahc-asc/media/advisories-avis/_2008/2008_184-eng.php (last accessed 3 October 2009)
- 5.NPR/Kaiser Family Foundation/Harvard School of Public Health. Children's OTC cold medicines: the public, and parents, weigh in 2007. Available at: http://www.kff.org/kaiserpolls/upload/7725.pdf (last accessed 5 October 2009)
- 6.Gadomski AM, Rubin JD. Cough and cold medicine use in young children: a survey of Maryland pediatricians. Md Med J. 1993;42:647–50. [PubMed] [Google Scholar]
- 7.Headley J, Northstone K. Medication administered to children from 0 to 7.5 years in the Avon Longitudinal Study of Parents and Children (ALSPAC) Eur J Clin Pharmacol. 2007;63:189–95. doi: 10.1007/s00228-006-0231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kogan MD, Pappas G, Yu SM, Kotelchuck M. Over-the-counter medication use among US preschool-age children. JAMA. 1994;272:1025–30. [PubMed] [Google Scholar]
- 9.Vernacchio L, Kelly JP, Kaufman DW, Mitchell AA. Pseudoephedrine use among US children, 1999–2006: results from the Slone survey. Pediatrics. 2008;122:1299–304. doi: 10.1542/peds.2008-0284. [DOI] [PubMed] [Google Scholar]
- 10.Vernacchio L, Kelly JP, Kaufman DW, Mitchell AA. Cough and cold medication use by US children, 1999–2006: results from the Slone survey. Pediatrics. 2008;122:e323–9. doi: 10.1542/peds.2008-0498. [DOI] [PubMed] [Google Scholar]
- 11.Katz LY, Kozyrskyj AL, Prior HJ, Enns MW, Cox BJ, Sareen J. Effect of regulatory warnings on antidepressant prescription rates, use of health services and outcomes among children, adolescents and young adults. CMAJ. 2008;178:1005–11. doi: 10.1503/cmaj.071265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurian BT, Ray WA, Arbogast PG, Fuchs DC, Dudley JA, Cooper WO. Effect of regulatory warnings on antidepressant prescribing for children and adolescents. Arch Pediatr Adolesc Med. 2007;161:690–6. doi: 10.1001/archpedi.161.7.690. [DOI] [PubMed] [Google Scholar]
- 13.Valiyeva E, Herrmann N, Rochon PA, Gill SS, Anderson GM. Effect of regulatory warnings on antipsychotic prescription rates among elderly patients with dementia: a population-based time-series analysis. CMAJ. 2008;179:438–46. doi: 10.1503/cmaj.071540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menniti-Ippolito G, Raschetti R, Da Cas R, Giaquinto C, Cantarutti L. Active monitoring of adverse drug reactions in children. Italian Paediatric Pharmacosurveillance Multicenter Group. Lancet. 2000;355:1613–4. doi: 10.1016/s0140-6736(00)02219-4. [DOI] [PubMed] [Google Scholar]
- 15.Vlug AE, van der Lei J, Mosseveld BM, van Wijk MA, van der Linden PD, Sturkenboom MC, van Bemmel JH. Postmarketing surveillance based on electronic patient records: the IPCI project. Methods Inf Med. 1999;38:339–44. [PubMed] [Google Scholar]
- 16.Sturkenboom MC, Verhamme KM, Nicolosi A, Murray ML, Neubert A, Caudri D, Picelli G, Sen EF, Giaquinto C, Cantarutti L, Baiardi P, Felisi MG, Ceci A, Wong IC. Drug use in children: cohort study in three European countries. BMJ. 2008;337:a2245. doi: 10.1136/bmj.a2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verhamme KM, Sturkenboom MC, Stricker BH, Bosch R. Drug-induced urinary retention: incidence, management and prevention. Drug Saf. 2008;31:373–88. doi: 10.2165/00002018-200831050-00002. [DOI] [PubMed] [Google Scholar]
- 18.Vassilev ZP, Kabadi S, Villa R. Safety and efficacy of over- the-counter cough and cold medicines for use in children. Expert Opin Drug Saf. 2010;9:233–42. doi: 10.1517/14740330903496410. [DOI] [PubMed] [Google Scholar]
- 19.Kuehn BM. Citing serious risks, FDA recommends no cold and cough medicines for infants. JAMA. 2008;299:887–8. doi: 10.1001/jama.299.8.887. [DOI] [PubMed] [Google Scholar]
- 20.Rimsza ME, Newberry S. Unexpected infant deaths associated with use of cough and cold medications. Pediatrics. 2008;122:e318–22. doi: 10.1542/peds.2007-3813. [DOI] [PubMed] [Google Scholar]
- 21.van Noord C, Sturkenboom MC, Straus SM, Hofman A, Witteman JC, Stricker BH. Population-based studies of antithyroid drugs and sudden cardiac death. Br J Clin Pharmacol. 2009;68:447–54. doi: 10.1111/j.1365-2125.2009.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn C, Gauthier M, Gaudreault P. Coma in a neonate following single intranasal dose of xylometazoline. Eur J Pediatr. 1993;152:541. doi: 10.1007/BF01955075. [DOI] [PubMed] [Google Scholar]
- 23.Higgins GL, III, Campbell B, Wallace K, Talbot S. Pediatric poisoning from over-the-counter imidazoline-containing products. Ann Emerg Med. 1991;20:655–8. doi: 10.1016/s0196-0644(05)82388-1. [DOI] [PubMed] [Google Scholar]
- 24.Mahieu LM, Rooman RP, Goossens E. Imidazoline intoxication in children. Eur J Pediatr. 1993;152:944–6. doi: 10.1007/BF01957538. [DOI] [PubMed] [Google Scholar]
- 25.Soderman P, Sahlberg D, Wiholm BE. CNS reactions to nose drops in small children. Lancet. 1984;1:573. doi: 10.1016/s0140-6736(84)90978-4. [DOI] [PubMed] [Google Scholar]
- 26.van Velzen AG, van Riel AJ, Hunault C, van Riemsdijk TE, de Vries I, Meulenbelt J. A case series of xylometazoline overdose in children. Clin Toxicol (Phila) 2007;45:290–4. doi: 10.1080/15563650601033326. [DOI] [PubMed] [Google Scholar]
- 27.Taverner D, Latte GJ. WITHDRAWN: Nasal decongestants for the common cold (Online) Cochrane Database Syst Rev. 2009;(2) doi: 10.1002/14651858.CD001953.pub4. CD001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC) Infant deaths associated with cough and cold medications – two states, 2005. MMWR Morb Mortal Wkly Rep. 2007;56:1–4. [PubMed] [Google Scholar]
- 29.Wingert WE, Mundy LA, Collins GL, Chmara ES. Possible role of pseudoephedrine and other over-the-counter cold medications in the deaths of very young children. J Forensic Sci. 2007;52:487–90. doi: 10.1111/j.1556-4029.2007.00391.x. [DOI] [PubMed] [Google Scholar]
- 30.Marinetti L, Lehman L, Casto B, Harshbarger K, Kubiczek P, Davis J. Over-the-counter cold medications-postmortem findings in infants and the relationship to cause of death. J Anal Toxicol. 2005;29:738–43. doi: 10.1093/jat/29.7.738. [DOI] [PubMed] [Google Scholar]
- 31.Smith SM, Schroeder K, Fahey T. Over-the-counter medications for acute cough in children and adults in ambulatory settings (Online) Cochrane Database Syst Rev. 2008;(1) doi: 10.1002/14651858.CD001831.pub3. CD001831. [DOI] [PubMed] [Google Scholar]
- 32.Smith MB, Feldman W. Over-the-counter cold medications. A critical review of clinical trials between 1950 and 1991. JAMA. 1993;269:2258–63. doi: 10.1001/jama.269.17.2258. [DOI] [PubMed] [Google Scholar]
- 33.Taylor JA, Novack AH, Almquist JR, Rogers JE. Efficacy of cough suppressants in children. J Pediatr. 1993;122(5 Pt 1):799–802. doi: 10.1016/s0022-3476(06)80031-4. [DOI] [PubMed] [Google Scholar]
- 34.Paul IM, Yoder KE, Crowell KR, Shaffer ML, McMillan HS, Carlson LC, Dilworth DA, Berlin CM., Jr. Effect of dextromethorphan, diphenhydramine, and placebo on nocturnal cough and sleep quality for coughing children and their parents. Pediatrics. 2004;114:e85–90. doi: 10.1542/peds.114.1.e85. [DOI] [PubMed] [Google Scholar]
- 35.Paul IM, Beiler J, McMonagle A, Shaffer ML, Duda L, Berlin CM., Jr. Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med. 2007;161:1140–6. doi: 10.1001/archpedi.161.12.1140. [DOI] [PubMed] [Google Scholar]
- 36.Wansink B, van Ittersum K. Spoons systematically bias dosing of liquid medicine. Ann Intern Med. 2010;152:66–7. doi: 10.7326/0003-4819-152-1-201001050-00024. [DOI] [PubMed] [Google Scholar]
- 37.Taylor DM, Robinson J, MacLeod D, MacBean CE, Braitberg G. Therapeutic errors involving adults in the community setting: nature, causes and outcomes. Aust N Z J Public Health. 2009;33:388–94. doi: 10.1111/j.1753-6405.2009.00413.x. [DOI] [PubMed] [Google Scholar]
- 38.Dart RC, Paul IM, Bond GR, Winston DC, Manoguerra AS, Palmer RB, Kauffman RE, Banner W, Green JL, Rumack BH. Pediatric fatalities associated with over the counter (nonprescription) cough and cold medications. Ann Emerg Med. 2009;53:411–7. doi: 10.1016/j.annemergmed.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Canada H. Health Canada Advisory: cough and cold products for children. 2009. Available at: http://chd.region.waterloo.on.ca/web/health.nsf/vwSiteMap/E7742A6975D6895E852570E50055EEFF/$file/PHYSUP_JAN09.pdf?openelement (last accessed 7 October 2009)
- 40.Use of codeine- and dextromethorphan-containing cough remedies in children. American Academy of Pediatrics. Committee on Drugs. Pediatrics. 1997;99:918–20. doi: 10.1542/peds.99.6.918. [DOI] [PubMed] [Google Scholar]


