Abstract
AIMS
To establish the possible involvement of alprazolam (ALP) and/or opiates in the mechanism underlying the ACTH/cortisol response to physical exercise.
METHODS
Tests were carried out under basal conditions (exercise control test), exercise plus ALP (50 µg at time −90 min), naloxone (10 mg at time 0) or ALP plus naloxone. Plasma ACTH and serum cortisol concentrations were evaluated in blood samples taken before, during and after the bicycle ergometer tests.
RESULTS
ACTH and cortisol concentrations rose significantly after physical exercise. Maximum peak at time 15 min (P≤ 0.01 vs. baseline) for ACTH and at time 30 min (P≤ 0.01 vs. baseline) for cortisol. In the presence of naloxone, the ACTH and cortisol responses were significantly increased (maximum peak at time 20 min, P≤ 0.02 vs. control test for ACTH, and at time 30 min (P≤ 0.01 vs. baseline) for cortisol) whereas they were abolished by ALP. When ALP and naloxone were given together, the inhibitory effect of ALP was partial.
CONCLUSIONS
These data demonstrate an inhibitory effect of ALP in the regulation of the ACTH/cortisol response to physical exercise in man and suggest that GABAergic receptor activating benzodiazepines and opioids interact in the neuroendocrine secretion of ACTH/cortisol.
Keywords: ACTH, alprazolam, cortisol, naloxone, physical exercise
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Alprazolam (ALP), a benzodiazepine activating GABAergic receptors, is involved in ACTH secretion.
WHAT THIS STUDY ADDS
This study demonstrates a partial opioid influence in the inhibitory effect of ALP on the release of ACTH/cortisol during physical exercise.
Introduction
Alprazolam (ALP), a GABAergic receptor activating benzodiazepine, has been found capable of inhibiting ACTH and cortisol release in various animal species [1]. In man, ALP modulates the ACTH/cortisol response to various stimuli such as metabolic changes, mental stress, metyrapone, naloxone and hypoglycaemia [2–6]. Physical exercise is a classical stimulus for ACTH/cortisol release in humans. Therefore, in the present study, we wondered whether stimulation of GABA/benzodiazepine (BDZ) neurotransmission could inhibit exercise-induced ACTH/cortisol release.
Several studies have shown a modulation of ACTH secretion by opioids [2, 7, 8]. Furthermore, ALP-opioid interaction have been reported at various levels in the central nervous system [7–12].
In the light of these observations we wondered whether the inhibition exerted by ALP on the response of ACTH during physical exercise is mediated by endogenous opioids.
In order to clarify this issue, the ACTH response to physical exercise was studied in normal men with or without the administration of ALP either in the presence or absence of the opioid receptor antagonist naloxone.
Methods
Thirteen healthy male subjects (mean body mass index 22.5 kg m–2, aged 24–29 years) were studied. All subjects were informed of the purpose of the study and gave their informed consent. The study was carried out in accordance with the Helsinki II declaration and received ethical approval by the University Ethical Commission.
Men performed four exercise tests: under basal conditions (control test) and in presence of ALP (50 µg at time −90 min), naloxone (10 mg as an i.v. bolus injection at time 0, just before exercise) or the combination of ALP and naloxone. Tests were performed in random order at weekly intervals.
All men were in good health, without clinical or laboratory evidence of hepatic, renal, heart or other organic disease. None was a smoker or was addicted to excessive alcohol drinking (<30 ml ethanol week–1). None had taken any drug for at least 1 month before the study or was under drug therapy at the time of the tests. All men were used to taking regular physical exercise, but they were not trained athletes. All tests were carried out after a 10 h overnight fast and rest.
Experimental protocol
Exercise control test
At 08.30 h on the test day, an intravenous cannula was placed into an antecubital vein and was kept patent with a slow saline (NaCl 0.9%) infusion. The cannula was used for naloxone or saline injection and for blood sampling.
Basal blood samples were collected at time −90 min, just after the intravenous cannula insertion and 90 min later (time 0), when subjects started the exercise test. Further samples were taken 5, 10, 15, 20, 30, 40, 50 and 60 min later. The subjects exercised for a period on an electrically braked cycle ergometer. An initial load of 50 W was increased by 50 W every 3 min until subjective exhaustion.
The cycling period ended when muscle fatigue and pain forced the subjects to stop exercise. In the same individuals exercise lasted for the same time in all tests. Two subjects with a low maximal capacity to perform exercise, because of early symptoms of muscle fatigue and pain (as established in a preliminary test carried out at least 1 week before the study) pedalled for 3 min against no workload at the beginning of the test, so that the exercise lasted about the same time (15 min) in all individuals.
During exercise, the subjects breathed through a low resistance one-way valve connected to a PK Morgan measurement system (Avinton Corp, Seattle, WA, USA), which had been appropriately calibrated. The following non-endocrine physiological parameters (NEPP) were measured: ventilation, frequency of breathing, tidal volume, oxygen consumption (VO2), carbon dioxide production (VCO2) and respiratory exchange ratio (R). Determinations of heart rate and blood pressure were carried out by an experienced cardiologist. Heart rate was measured by auscultation over the precordium; blood pressure was evaluated with a sphygmomanometer. Measurements were performed just before the beginning of exercise (at rest) and at the end of exercise (at exhaustion).
Exercise plus ALP test
This test was performed as the previously described exercise control test, except for the oral administration of 50 µg ALP at time −90 min. In the control test and in the naloxone test, a placebo was given instead ALP at time −90 min.
Exercise plus naloxone test
This test was performed as the previously described exercise control test, except for the injection of an i.v. bolus of 10 mg naloxone at time 0. In the control and ALP tests normal saline was given instead of naloxone.
Exercise plus ALP plus naloxone test
This test was performed as the previously described exercise control test, except for the administration of ALP and naloxone as described in each individual test. The amount of alprazolam and naloxone administered to our subjects has been successfully used in previous studies which demonstrated the influence of alprazolam and naloxone on hormonal secretion during physical exercise [2, 13].
Assay
Plasma ACTH, serum cortisol and blood glucose concentrations, osmolality, haematocrit and serum sodium concentrations were evaluated in all samples. Blood for ACTH and cortisol measurement was chilled to 4°C immediately after withdrawal, plasma was separated and stored at −20°C until assayed. ACTH and cortisol concentrations were measured with a specific immunometric assay, using a commercial kit (Siemens Medical Diagnostics, Los Angeles, CA, USA). All samples were assayed in duplicate in the same assay. Intra-assay and inter-assay coefficients of variation were 8.3% and 14% for ACTH and 4.0% and 7.0% for cortisol, respectively. The sensitivity of this assay was 1.1 pmol l−1 for ACTH, and 16.5 nmol l−1 for cortisol. Serum sodium concentrations were evaluated by flame photometry.
Statistical analysis was performed with the paired t-test and analysis of variance followed by a specific mean comparison test, as appropriate. Results are reported as mean ± SE.
Results
None of the subjects experienced untoward side effects after ALP and/or naloxone administration. No significant differences in maximum work load and work time between the four tests was observed in each individual subject and in the whole group of 13 subjects (data not shown).
Physical exercise significantly modified osmolality, haematocrit, serum sodium concentrations and all the examined NEPP (Table 1). Exercise-induced changes were not altered by stimulation of ALP, naloxone or the combination (Table 1).
Table 1.
Basal and peak values (mean ± SE) of non-endocrine physiological parameters and biochemical variables during physical exercise following the administration of normal saline (control test), alprazolam (ALP), naloxone or ALP plus naloxone in 13 normal men
| Exercise | Exercise + | Exercise + | Exercise + | |||||
|---|---|---|---|---|---|---|---|---|
| ALP | Naloxone | ALP+ | Naloxone | |||||
| Test Variable | Basal | Peak | Basal | Peak | Basal | Peak | Basal | Peak |
| Heart rate (beats min−1) | 72±4 | 166±9* | 73±4 | 170±10* | 73±4 | 168±7* | 72±3 | 168±7 |
| Systolic BP (mmHg) | 114±7 | 158±8* | 111±8 | 157±9* | 111±8 | 156±9* | 113±9 | 153±11* |
| Diastolic BP (mmHg) | 73±6 | 51±5* | 74±7 | 53±5* | 70±7 | 54±6* | 72±7 | 52±6* |
| Respiratory rate (min−1) | 12.8±0.9 | 31.5±3* | 12.7±0.7 | 31.6±±3* | 12.9±0.8 | 32.2±3.4* | 12.9±0.9 | 31.6±3* |
| Tidal volume (l) | 0.8±0.3 | 2.5±0.3* | 0.8±0.2 | 2.4±0.3* | 0.7±0.3 | 2.3±0.3* | 0.9±0.2 | 2.3±0.4* |
| Ventilation (l min−1) | 10.8±0.6 | 76±5 | 10.4±0.6 | 70±3* | 10.7±0.5* | 75±3 | 10.5±0.7 | 72±4* |
| VO2 (ml min−1) | 342±13 | 2133±149* | 345±12 | 2117±148* | 345±14 | 2092±145* | 340±14 | 2081±157* |
| VCO2 (ml min−1) | 291±18 | 2270±145* | 295±12 | 2280±127* | 294±18 | 2287±130* | 295±16 | 2280±146* |
| R | 0.85 | 1.07* | 0.85 | 1.09* | 0.85 | 1.09* | 0.86 | 1.09* |
| Serum sodium (mEq l−1) | 138.1±0.9 | 140.8±0.9* | 139.5±0.7 | 141.8±0.7** | 139.7±0.8 | 142.6±1.0** | 140.7±0.6 | 141.6±0.8** |
| Plasma osmolality (mOsmol kg−1) | 284.6±0.9 | 287.5±0.8* | 284.9±0.8 | 290.0±0.8* | 284.7±0.8 | 290.2±0.9* | 284.2±0.9 | 289.8±0.8* |
| Haematocrit (%) | 44.0±0.7 | 49.1±1.0* | 44.5±0.8 | 48.7±0.9* | 43.8±0.7 | 48.7±0.9* | 44.0±0.8 | 48.7±0.7* |
P<0.01 vs. basal value
P<0.05 vs. basal value. R, respiratory exchange ratio.
Physical exercise induced a significant increase (P < 0.01) in heart rate, haematocrit, sodium, osmolality, blood pressure, ventilation, VO2, and VCO2 (Table 1). Both basal and exercise-induced values were unaltered by drug interventions (Table 1).
Plasma ACTH concentrations rose significantly during physical exercise (P≤ 0.01 at time 20 min and P≤ 0.02 at time 10 min vs. baseline in all subjects) (Figure 1). Pre-treatment with naloxone increased the ACTH response to exercise (at time 30 min P < 0.01, at time 20 min P < 0.02 and at time 10 min P < 0.05 vs. exercise control test), whereas ALP completely abolished the ACTH rise during exercise (at time 20 min P < 0.05 and at time 10 min P < 0.01 vs. exercise control test) (NS vs. baseline at any time point).
Figure 1.
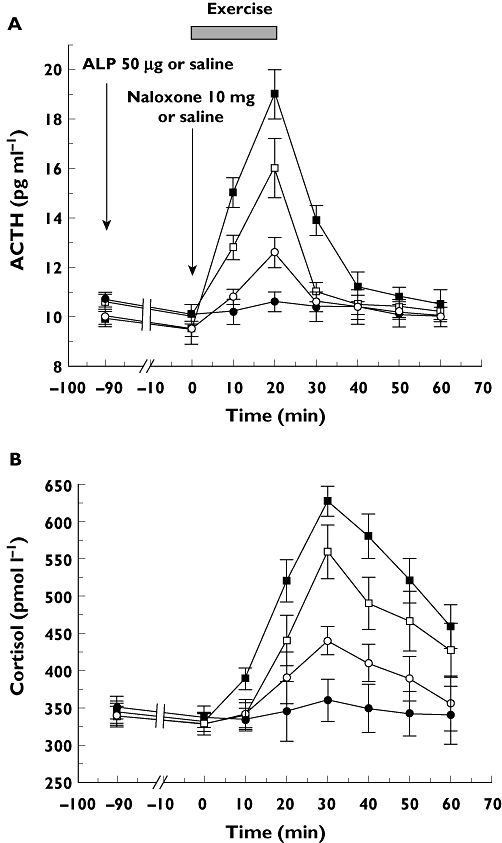
A) ACTH and B) cortisol response to exercise (from time 0 for about 15 min) and exercise plus alprazolam (ALP) (50 µg given orally at time −90 min), naloxone (10 mg as an i.v. bolus at time 0) or ALP plus naloxone in 13 healthy normal male subjects. Each point represents the mean ± SE of the observations. Exercise ( ); Exercise + ALP (
); Exercise + ALP ( ); Exercise + Naloxone (
); Exercise + Naloxone ( ); Exercise + Naloxone + ALP (
); Exercise + Naloxone + ALP ( )
)
When the effect of ALP on the ACTH response to physical exercise was studied in the presence of naloxone, the inhibitory effect of ALP was partial (at time 10 and 20 min P < 0.05 vs. exercise control test and at time 20 min P < 0.05 vs. exercise plus ALP test). Serum cortisol concentrations significantly increased during physical exercise with a maximum peak at time 30 min (P < 0.01 vs. baseline). Pretratment with naloxone increased the cortisol response to exercise (at time 10 min, 20 min, 30 and 40 min P < 0.05 vs. exercise cortisol test), whereas ALP inhibited the cortisol increase during exercise (at time 30 min and 40 min P < 0.01, at time 50 min P < 0.02 and at time 10 min P < 0.05 vs. exercise test, NS vs. baseline at any time point). When the effect of ALP on cortisol response to physical exercise was studied in the presence of naloxone, the inhibitory effect of ALP was partial (at time 10 min, 20 min, 30 min, 40 min and 50 min P < 0.05 vs. exercise control test and at time 30 min and 40 min P < 0.05 vs. exercise plus ALP test).
Discussion
In agreement with previous reports [9], the data presented here show that ALP given alone inhibits the ACTH/cortisol increase induced by physical exercise.
Blood haematocrit and sodium concentrations were not modified by ALP administration, suggesting that ALP exerts a specific action on ACTH/cortisol release which is independent of barometric or volumetric variations. ALP inhibitory action may be supposed to be mediated by central benzodiazepine receptors, which are part of the benzodiazepine receptor-aminobutyric acid A receptor chloride channel complex. Now we find that also the inhibiting effects of ALP on the exercise-induced ACTH rise decreases in the presence of naloxone. In addition, since naloxone enhanced the exercise-induced ACTH/cortisol increase when given alone, an inhibitory action of endogenous opioids on ACTH secretion during exercise may be hypothesized. In view of these observations, we suppose that the inhibitory action of ALP on exercise-stimulated ACTH secretion could be mediated by the release of endogenous opioids. Our experimental conditions cannot clarify the site of action of the opiate/GABA/BZD interactions in the inhibition of exercise-stimulated ACTH secretion. The effect of ALP might be exerted at the pituitary level, where opioids are known to reduce ACTH secretion or in the hypothalamus where endogenous opioids are thought to inhibit corticotrophin releasing hormone (CRH) release [7, 14, 15].
However, another explanation for our findings is that ALP and naloxone acted by two entirely separate pathways, one blocked by the GABA/BDZ receptors and the other by endogenous opioids and unmasked by the administration of naloxone. Our present experimental conditions cannot establish which of these possibilities is the correct one and these hypotheses require further studies to be substantiated.
The data reported here extended the broad spectrum of either a facilitatory or inhibitory role of opioids exerted in the control of the ACTH/cortisol responses to different stimuli.
In conclusion, the present study shows that the ACTH/cortisol response to physical exercise is abolished by ALP, pointing towards a relevant role of GABAergic pathway and in particular of benzodiazepine-GABA receptors in the ACTH secretion during physical exercise. Furthermore, the data show that the inhibitory action of ALP during physical activity is mediated by a naloxone-sensitive opioid pathway.
Acknowledgments
This work was supported by a MIUR (Ministero Italiano Università e Ricerca) grant.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Kalogeras KT, Calogero AE, Kuribayashi T, Khan I, Gallucci WT, Kling MA, Chrousa GP, Gold PW. In vitro and in vivo effects of the the triazolobenzodiazepine alprazolam on hypothalamic-pituitary-adrenal function: pharmacological and clinical implications. J Clin Endocrinol Metab. 1990;70:1462–71. doi: 10.1210/jcem-70-5-1462. [DOI] [PubMed] [Google Scholar]
- 2.Torpy DJ, Grice JE, Hockings GI, Walters MM, Crosbie GV, Jackson RV. Alprazolam blocks the naloxone-stimulated hypothalamus-pituitary-adrenal axis in man. J Clin Endocrinol Metab. 1993;76:388–91. doi: 10.1210/jcem.76.2.8381800. [DOI] [PubMed] [Google Scholar]
- 3.Giordano R, Grottoli S, Brossa PC, Pellegrino M, Destefanis S, Lanfranco F, Gianotti L, Ghigo E, Arvat E. Alprazolam (a benzodiazepine activating GABA receptor) reduces the neuroendocrine responses to insulin-induced hypoglycaemia in humans. Clin Endocrinol. 2003;59:314–20. doi: 10.1046/j.1365-2265.2003.01847.x. [DOI] [PubMed] [Google Scholar]
- 4.Arvat E, Maccagno B, Ramunni J, Di Vito L, Giordano R, Gianotti L, Broglio F, Camanni F, Ghigo E. The inhibitory effect of alprazolam, a benzodiazepine, overrides the stimulatory effect of metyrapone-induced lack of negative cortisol feedback on corticotroph secretion in humans. J Clin Endocrinol Metab. 1999;84:2611–5. doi: 10.1210/jcem.84.8.5911. [DOI] [PubMed] [Google Scholar]
- 5.Breier A, Davis O, Buchanan R, Listwak SJ, Holmes C, Pickar D, Goldstein DS. Effects of alprazolam on pituitary-adrenal and catecholaminergic responses to metabolic stress in humans. Biol Psychiatry. 1992;32:880–90. doi: 10.1016/0006-3223(92)90177-2. [DOI] [PubMed] [Google Scholar]
- 6.Roher T, von Richthofen V, Schultz C, Beyer J, Lehnert H. The stress, but not corticotropin-releasing hormone-induced activation of the pituitary-adrenal axis in man is blocked by alprazolam. Horm Metab Res. 1994;53:200–6. doi: 10.1055/s-2007-1000811. [DOI] [PubMed] [Google Scholar]
- 7.Grossman A. Brain opiates and neuroendocrine function. J Clin Endocrinol Metab. 1983;12:725–46. doi: 10.1016/s0300-595x(83)80062-0. [DOI] [PubMed] [Google Scholar]
- 8.Morley JE. Neuroendocrine effects of endogenous opioid peptides in human subjects, a review. Psychoneuroendocrinology. 1983;8:361–79. doi: 10.1016/0306-4530(83)90016-1. [DOI] [PubMed] [Google Scholar]
- 9.Deuster PA, Faraday MM, Chrousos GP, Poth MA. Effects of dehydroepiandrosterone and alprazolam on hypothalamic-pituitary response to exercise. J Clin Endocrinol Metab. 2005;90:4777–83. doi: 10.1210/jc.2004-2504. [DOI] [PubMed] [Google Scholar]
- 10.Aronin GN, DiFiglia M, Graveland GA, Schwart WJ, Wu JW. Localization of immunoreactive enkephalins in GABA synthesizing neurons of the rat neostriatum. Brain Res. 1984;300:376–80. doi: 10.1016/0006-8993(84)90850-3. [DOI] [PubMed] [Google Scholar]
- 11.Zamm DS, Zaborsky L, Alones VE, Heimer L. Evidence for the coexistence of glutamate decarboxylase and metenkephalin immunoreactivity in axon terminals of rat ventral pallidum. Brain Res. 1984;300:376–80. doi: 10.1016/0006-8993(85)90331-2. [DOI] [PubMed] [Google Scholar]
- 12.Ortel WH, Riethmuller G, Mugnaini E. Opioid peptide-like immunoreactivity localized in GABAergic neurons of rat neostriatum and central amygdaloid nucleus. Life Sci. 1987;33:73–6. doi: 10.1016/0024-3205(83)90447-2. [DOI] [PubMed] [Google Scholar]
- 13.Coiro V, Casti A, Rubino P, Manfredi G, Maffei ML, Volta E, Cataldo S, Melani A, Saccani-Jotti G, Chiodera P. Effect of naloxone on somatostatin inhibition of arginine-vasopressin response to physical exercise in normal men. J Neural Transm. 2008;115:803–7. doi: 10.1007/s00702-008-0026-7. [DOI] [PubMed] [Google Scholar]
- 14.Bicknell RJ. Endogenous opioid peptides and hypothalamic neuroendocrine neurons. J Endocrinol. 1985;101:437–46. doi: 10.1677/joe.0.1070437. [DOI] [PubMed] [Google Scholar]
- 15.Estienne MJ, Kesner JS, Barb CR, Kraeling RR, Rampacek GB. On the site of action of naloxone stimulated cortisol secretion in gilts. Life Sci. 1988;43:161–6. doi: 10.1016/0024-3205(88)90293-7. [DOI] [PubMed] [Google Scholar]


