Abstract
Contrary to a clinical aphorism that early head and neck cancer is painless, we show that patients who develop head and neck cancer experience significant pain at the time of initial diagnosis. We compared orofacial pain sensitivity in groups of patients with normal oral mucosa, oral precancer and newly diagnosed oral cancer. The UCSF Oral Cancer Pain Questionnaire was administered to these patients at their initial visit, before being prescribed analgesics for pain and before any treatment. In contrast to those with biopsy-proven normal oral mucosa and oral precancer, only oral cancer patients reported significant levels of spontaneous pain and functional restriction from pain. Moreover, oral cancer patients experienced significantly higher function-related, rather than spontaneous pain qualities. These findings suggest an important predictor for the transition from oral precancer to cancer may be the onset of orofacial pain that is exacerbated during function. Screening patients who have new-onset orofacial pain may lead to a diagnosis of early, resectable head and neck cancer and may improve quality of life and survival for head and neck cancer patients.
1. Introduction
The overall 5-year survival rate for head and neck cancer has not significantly improved in the last 40 years [17]. The most important goal is to establish an early diagnosis at the first signs and symptoms of disease [20]. Early diagnosis is the most important determining factor for improving head and neck cancer survival since overall rates as high as 80–90% for the first stages of head and neck cancer may be achieved. The clinical presentation of head and neck cancer in advanced stages often demonstrates characteristic signs of malignancy [1]. In contrast, the clinical presentation of early malignant lesions, usually in the form of an erythroleukoplakic lesion, is often indistinguishable from precancerous lesions. Although pain is often recognized as an important symptom, a clinical aphorism has been that early head and neck cancers often go unnoticed because they are asymptomatic and pain usually arises only when the cancer has reached a remarkable size [1,22].
The purpose of the present study is to compare orofacial pain sensitivity in groups of patients with normal oral mucosa, oral precancer and newly diagnosed oral cancer. Here we demonstrate that patients who develop head and neck cancer experience significant pain at the time of initial diagnosis.
2. Methods
2.1. Study population and design
A cross-sectional analysis of 44 oral cancer patients (25 men, 19 women; mean age=63±2), 20 oral precancer patients (12 men, 8 women; mean age=65±3), and 21 normal oral mucosa patients (9 men, 12 women; mean age=41±4) referred to the University of California San Francisco (UCSF) Department of Oral & Maxillofacial Surgery from 2002–2010 were studied. The normal oral mucosa patients were healthy volunteers undergoing dental procedures such as wisdom tooth extraction or dental implant placement. The research protocol is in compliance with the Committee on Human Research at the University of California San Francisco.
Inclusion criteria for patients in the study were (1) untreated, biopsy-proven normal oral mucosa, oral precancer, or oral squamous cell carcinoma (SCC) and (2) comprehension of the UCSF Oral Cancer Pain Questionnaire [7,13]. Exclusion criteria were (1) having a diagnosed psychiatric condition, (2) addiction to pain medications or recreational drugs, and (3) having taken pain medications in the previous 6 months.
2.2. Clinical examination and oral biopsy
A comprehensive history and examination was performed on all patients to rule out a history of medical conditions or disorders that may alter their pain perception. Oral biopsy specimens of all patients meeting the inclusion criteria were evaluated by the UCSF Department of Pathology and the Oral Pathology Biopsy Service for diagnostic confirmation of normal oral mucosa, precancer (proliferative verrucous leukoplakia, dysplasia, carcinoma in situ) or oral cancer (SCC). Demographic information was also collected for each patient including age, sex, racial/ethnic identity, current smoking and high-risk drinking status, oral lesion location and tumor size.
2.3. UCSF Oral Cancer Pain Questionnaire
The UCSF Oral Cancer Pain Questionnaire was administered to patients meeting the inclusion criteria at their initial visit, before being prescribed analgesics for any orofacial pain and before any treatment. This questionnaire, consisting of 8 questions on a visual analog scale of 0–100 mm, has been validated previously [7,13]. Briefly, the 8 questions differentiate spontaneous and function-related pain and determine the quality of pain. Questions 1 through 6 examined the intensity, sharpness, and aching nature of orofacial pain. Question 7 focused on the degree of sensitivity to touch. Question 8 determined the level of functional restriction as a result of orofacial pain. Patients were instructed to place a vertical line along the scale to approximate their orofacial pain level (if any).
2.4. Data analysis
Data are reported as mean ± SE. RM ANOVA, RM ANOVA-on-ranks, Wilcoxon signed rank, Fisher exact and Pearson correlation tests were used as appropriate (P<0.05 considered to reflect statistical significance). Current smoking was defined as any cigarette use within the past 30 days. High-risk drinking was defined for men as more than 2 drinks/day, more than 14 drinks/week and/or more than 4 drinks/occasion; for women and patients >65 years of age, as more than 1 drink/day, more than 7 drinks/week and/or more than 3 drinks/occasion (if >65 years, more than 1 drink/occasion) as per the Substance Abuse and Mental Health Services Administration (SAMHSA) guidelines.
3. Results
A total of 44 oral cancer patients (25 men, 19 women; mean age=63±2, range 32–90), 20 oral precancer patients (12 men, 8 women; mean age=65±3, range 35–82), and 21 normal oral mucosa patients (9 men, 12 women; mean age=41±4, range 17–72) were studied. Of the 44 oral cancer patients, racial/ethnic identity was comprised of 82% Caucasian, 9% Asian, 2% Middle Eastern, and 7% Hispanic; 31% were current smokers and 13% were high-risk drinkers. Of the oral precancer patients, 65% Caucasian, 20% Asian, 5% Middle Eastern, and 10% Hispanic; 15% were current smokers and 10% were high-risk drinkers. Finally, of the normal oral mucosa patients, 62% Caucasian, 33% Asian, and 5% Middle Eastern; 19% were current smokers and 5% were high-risk drinkers. There was no significant difference in the incidence of smoking or drinking status between the oral cancer, oral precancer and normal oral mucosa patient groups (P>0.05, Fisher exact test).
3.1. Clinical examination and oral biopsy
The oral precancer lesions were clinically indistinguishable from the oral cancer lesions- both demonstrating an erythroleukoplakic appearance (Figure 1). Upon review of the oral biopsy specimens, 44 patients had oral cancer (44 SCC), 20 patients had oral precancer (3 mild dysplasia, 1 moderate dysplasia, 14 severe dysplasia/carcinoma in situ, 2 proliferative verrucous leukoplakia) and 21 patients had normal oral mucosa. Both oral precancers and oral cancers demonstrated dysplastic epithelium with varying degrees of cellular and nuclear pleomorphism. However, the characteristic histopathological feature distinguishing oral precancer from cancer was the invasion of dysplastic epithelial cells into the underlying tissues (Figure 2).
Figure 1. Oral precancer and oral cancer are clinically indistinguishable.
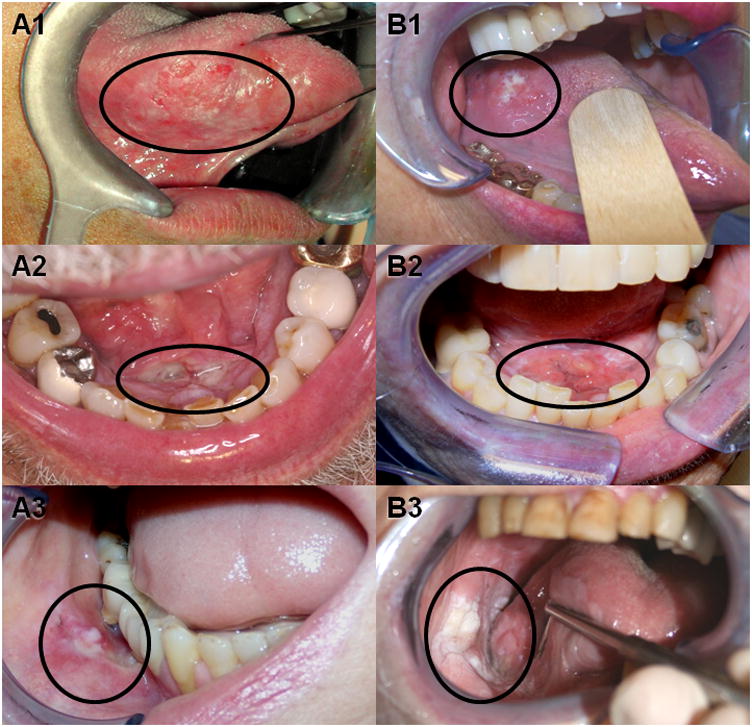
Precancerous lesions such as carcinoma in situ affecting the lateral tongue (A1), floor of mouth (A2), and buccal mucosa (A3) are not distinguishable from their respective squamous cell carcinoma counterparts (B1, B2, and B3) on physical examination alone. Black circles indicate approximate extent of oral lesion.
Figure 2. Oral precancer and oral cancer may be distinguished by histopathological signs of invasion.
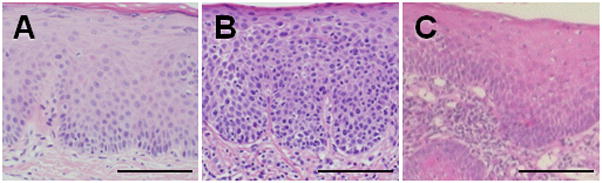
(A) Normal oral mucosa showing stratified squamous epithelium with normal cytoarchitecture. (B) Precancer (carcinoma in situ) with dysplastic changes extending the entire thickness of the epithelium. The main histopathological sign distinguishing oral precancer (B) from oral squamous cell carcinoma (C) is invasion of dysplastic epithelial cells through the basement membrane into the underlying tissues. Black scale bar = 100μm.
3.2. Orofacial pain
Oral cancer patients reported significantly greater spontaneous and function-related pain in comparison to both normal oral (P<0.001) and oral precancer patients (P<0.001, RM ANOVA-on-ranks, Dunn’s; Figure 3). The majority of the oral cancer patients presented with moderate to severe pain (84%, >30 mm) at the time of initial diagnosis. In contrast, oral precancer pain levels were not significantly different from patients with normal oral mucosa (P>0.05, RM ANOVA-on-ranks).
Figure 3. Orofacial pain distinguishes oral precancer from cancer at the time of diagnosis.
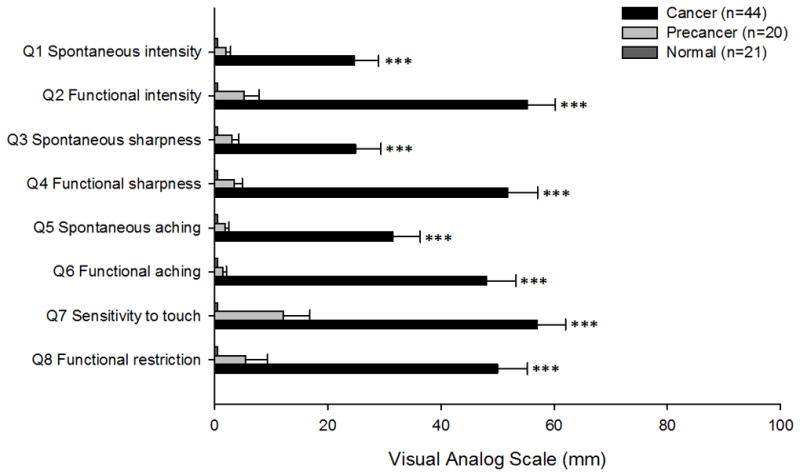
Mean visual analogue scale pain scores with standard error for each of the eight questions were compared between normal oral mucosa, oral precancer and oral cancer patients. Oral cancer patients experienced marked spontaneous and function-related pain in comparison to the relatively pain-free normal oral (***P<0.001) and oral precancer patients (***P<0.001, RM ANOVA-on-ranks, Dunn’s).
Oral cancer patients also experienced a significantly higher function-related, rather than spontaneous, pain intensity (P<0.001, questions 1 and 2), sharpness (P<0.001, questions 3 and 4), and aching (P<0.05, questions 5 and 6) (Wilcoxon signed rank test). Oral cancer patients had tumors of varying sizes (27% T1, 50% T2, 8% T3 and 15% T4). Consistent with our previous findings in head and neck cancer patients [6], there was no correlation between tumor size and reported pain levels or functional restriction (P>0.05, Pearson correlation test).
4. Discussion
Head and neck cancer patients reported significant spontaneous and function-related pain at the time of initial diagnosis in the present study. In contrast to those patients with oral precancers that were clinically indistinguishable from oral cancer, only oral cancers resulted in significant levels of orofacial pain. In addition, the character of cancer pain was markedly more function-related rather than spontaneous in nature. These findings suggest an important predictor for the progression of oral precancer to cancer may be the onset of orofacial pain that is exacerbated during function.
The present study provides some insight into the involved peripheral nociceptive mechanisms in head and neck cancer pain. Peripheral sensitization in cancer patients has been proposed to occur through cancer invasion and/or compression of surrounding tissues [2,3,6,27]. Since the distinguishing histopathological characteristic between non-painful oral precancer and painful oral cancer is tissue invasion, the present findings suggest a role for malignant epithelial cell invasion into underlying tissues in head and neck cancer pain. However, the lack of correlation between oral cancer size and patient pain levels in the present study suggests that head and neck cancer pain is likely independent of any possible cancer compression or mass-related effect.
Head and neck cancer pain is more likely to be a result of the sensitization and/or activation of primary nociceptive afferents by mediators liberated by the cancer and associated cells [9,10,15]. The intense spontaneous sharp and aching pain reported by oral cancer patients suggests the sensitization and/or activation of both Aδ and C fibers in head and neck cancer pain [4,21]. Since the intensity, sharpness and aching nature of cancer pain is exacerbated by mechanical function, our results further suggest that peripheral mechanisms involving mechanical allodynia and hyperalgesia contribute to head and neck cancer pain.
Cancer and associated cells in the cancer microenvironment may release a variety of pain mediators including ATP, bradykinin, cytokines, chemokines, nerve growth factor, prostaglandins and several vascular factors including endothelin 1 and vascular endothelial growth factor to either excite or sensitize nociceptive primary afferents [8,10,12,18,19,24,28,29,30]. Arachidonic acid metabolites, such as prostaglandins, are produced by various cancers, including head and neck cancer [12,24], and are well known to sensitize nociceptive primary afferents [5,25,26]. However, the poor efficacy of medications such as nonsteroidal anti-inflammatory drugs (NSAIDs) and cyclo-oxygenase-2 (COX-2) inhibitors in alleviating cancer pain [16,23] suggests that other peripheral mediators contribute to the severity of cancer pain. Indeed in a recent study involving rat models of orofacial cancer, Harano and colleagues have demonstrated that orofacial cancer pain is not significantly mediated by cancer-induced peripheral inflammation [11].
Using a novel mouse cancer model, we have recently shown that mediators released by human head and neck cancer cells alone are sufficient to produce similar mechanical hypersensitivity symptoms in animals that occur in humans secondary to cancer pain [14]. In particular, trypsin and related serine proteases released by human head and neck cancer cells generate mechanical allodynia via a protease-activated receptor 2 (PAR2)-dependent mechanism. Mast cells within the cancer microenvironment also potentiate and prolong this serine protease-induced cancer pain behavior. Thus the continual release of various pain mediators, including serine proteases, from cancer and associated cells may produce ongoing activation of primary nociceptive afferents and contribute to spontaneous pain and persistent mechanical allodynia in head and neck cancer patients.
5. Conclusion
Earlier recognition of symptoms of head and neck cancer may improve early detection of the cancer itself. Of the constellation of symptoms, recent onset of significant orofacial pain, particularly if exacerbated during function, may be the best clue and should be included in the clinical assessment of patients to identify a high-risk group to apply screening strategies for early cancer detection. Further investigations into the correlations between pain parameters and the specific biology of head and neck cancer may improve quality of life and survival for head and neck cancer patients.
Acknowledgments
We thank R. Chigurupati and C.T. Viet for data collection assistance. This work was supported by a grant from the NIH/NIDCR R21 DE018561.
Footnotes
6. Disclosure
The authors declare no conflicts of interest for this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bagan J, Sarrion G, Jimenez Y. Oral cancer: Clinical features. Oral Oncol. 2010 Apr 16; doi: 10.1016/j.oraloncology.2010.03.009. In press. [DOI] [PubMed] [Google Scholar]
- 2.Banning A, Sjogren P, Henriksen H. Pain causes in 200 patients referred to a multidisciplinary cancer pain clinic. Pain. 1991;45:45–48. doi: 10.1016/0304-3959(91)90163-R. [DOI] [PubMed] [Google Scholar]
- 3.Brant JM. Cancer-related neuropathic pain. Nurse Pract Forum. 1998;9:154–162. [PubMed] [Google Scholar]
- 4.Campbell JN, LaMotte RH. Latency to detection of first pain. Brain Res. 1983;266:203–208. doi: 10.1016/0006-8993(83)90650-9. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Tanner K, Levine JD. Mechanical sensitization of cutaneous C-fiber nociceptors by prostaglandin E2 in the rat. Neurosci Lett. 1999;267:105–108. doi: 10.1016/s0304-3940(99)00345-6. [DOI] [PubMed] [Google Scholar]
- 6.Clohisy DR, Ramnaraine ML. Osteoclasts are required for bone tumors to grow and destroy bone. J Orthop Res. 1998;16:660–666. doi: 10.1002/jor.1100160606. [DOI] [PubMed] [Google Scholar]
- 7.Connelly ST, Schmidt BL. Evaluation of pain in patients with oral squamous cell carcinoma. J Pain. 2004;5:505–510. doi: 10.1016/j.jpain.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 8.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 9.Diener KM. Bisphosphonates for controlling pain from metastatic bone disease. Am J Health Syst Pharm. 1996;53:1917–1927. doi: 10.1093/ajhp/53.16.1917. [DOI] [PubMed] [Google Scholar]
- 10.Fujita T, Matsui M, Takaku K, Uetake H, Ichikawa W, Taketo MM, Sugihara K. Size-and invasion-dependent increase in cyclooxygenase 2 levels in human colorectal carcinomas. Cancer Res. 1998;58:4823–4826. [PubMed] [Google Scholar]
- 11.Harano N, Ono K, Hidaka K, Kai A, Nakanishi O, Inenaga K. Differences between orofacial inflammation and cancer pain. J Dent Res. 2010;89:615–620. doi: 10.1177/0022034510363095. [DOI] [PubMed] [Google Scholar]
- 12.Jung TT, Berlinger NT, Juhn SK. Prostaglandins in squamous cell carcinoma of the head and neck: A preliminary study. Laryngoscope. 1985;95:307–312. doi: 10.1288/00005537-198503000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Kolokythas A, Connelly ST, Schmidt BL. Validation of the University of California San Francisco oral cancer pain questionnaire. J Pain. 2007;8:950–953. doi: 10.1016/j.jpain.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam DK, Schmidt BL. Serine proteases and protease-activated receptor 2-dependent allodynia: a novel cancer pain pathway. Pain. 2010;149:263–272. doi: 10.1016/j.pain.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantyh PW, Clohisy DR, Koltzenburg M, Hunt SP. Molecular mechanisms of cancer pain. Nat Rev Cancer. 2002;2:201–209. doi: 10.1038/nrc747. [DOI] [PubMed] [Google Scholar]
- 16.Mercadante S, Portenoy RK. Opioid poorly-responsive cancer pain. Part 3. Clinical strategies to improve opioid responsiveness. J Pain Symptom Manage. 2001;21:338–354. doi: 10.1016/s0885-3924(01)00250-0. [DOI] [PubMed] [Google Scholar]
- 17.McCann MF, Macpherson LM, Gibson J. The role of the general dental practitioner in detection and prevention of oral cancer: a review of the literature. Dent Update. 2000;27:404–408. doi: 10.12968/denu.2000.27.8.404. [DOI] [PubMed] [Google Scholar]
- 18.Opree A, Kress M. Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1 beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: Effects on heat-evoked calcitonin gene-related peptide release from rat skin. J Neurosci. 2000;20:6289–6293. doi: 10.1523/JNEUROSCI.20-16-06289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pacharinsak C, Beitz A. Animal models of cancer pain. Comp Med. 2008;58:220–233. [PMC free article] [PubMed] [Google Scholar]
- 20.Peacock ZS, Pogrel MA, Schmidt BL. Exploring the reasons for delay in treatment of oral cancer. J Am Dent Assoc. 2008;139:1346–1352. doi: 10.14219/jada.archive.2008.0046. [DOI] [PubMed] [Google Scholar]
- 21.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 22.Scully C, Bagan J. Oral squamous cell carcinoma overview. Oral Oncol. 2009;45:301–308. doi: 10.1016/j.oraloncology.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Sorge J. The lesson from cancer pain. Eur J Pain. 2000;4:3–7. [PubMed] [Google Scholar]
- 24.Sumitani K, Kamijo R, Toyoshima T, Nakanishi Y, Takizawa K, Hatori M, Nagumo M. Specific inhibition of cyclooxygenase-2 results in inhibition of proliferation of oral cancer cell lines via suppression of prostaglandin E2 production. J Oral Pathol Med. 2001;30:41–47. doi: 10.1034/j.1600-0714.2001.300107.x. [DOI] [PubMed] [Google Scholar]
- 25.Taiwo YO, Goetzl EJ, Levine JD. Hyperalgesia onset latency suggests a hierarchy of action. Brain Res. 1987;423:333–337. doi: 10.1016/0006-8993(87)90858-4. [DOI] [PubMed] [Google Scholar]
- 26.Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neurosci. 1989;32:577–580. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- 27.Twycross RG, Fairfield S. Pain in far-advanced cancer. Pain. 1982;14:303–310. doi: 10.1016/0304-3959(82)90137-3. [DOI] [PubMed] [Google Scholar]
- 28.Watkins LR, Goehler LE, Relton J, Brewer MT, Maier SF. Mechanisms of tumor necrosis factor-alpha (TNF-alpha) hyperalgesia. Brain Res. 1995;692:244–250. doi: 10.1016/0006-8993(95)00715-3. [DOI] [PubMed] [Google Scholar]
- 29.Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimaki A. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998;58:4997–5001. [PubMed] [Google Scholar]
- 30.Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: The contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]


