Abstract
Intracranial hemorrhage during blastic crisis is a rare but critically important occurrence in children with acute lymphoblastic leukemia. This form of hemorrhage has not been described since the advent of magnetic resonance imaging (MRI), leaving radiologists and clinicians unfamiliar with its unique imaging features. The authors describe 2 boys with severe intracranial pathology secondary to blastic hyperleukocytosis. Both patients were followed with serial MRI. Imaging findings in relation to the pathophysiology of white matter leukostasis are discussed and implications for treatment and prognosis are considered.
Keywords: leukemia, intracranial hemorrhage
Introduction
The association of blastic crisis, hyperleukocytosis, and intracranial hemorrhage is a rare but critically important occurrence in children with acute lymphoblastic leukemia. The pathophysiology has been well described previously,1,2 although the clinical outcome has not been well studied. We describe 2 boys with severe intracranial pathology secondary to blastic hyperleukocytosis followed by serial magnetic resonance imaging (MRI). We discuss the imaging findings related to the pathophysiology of white matter leukostasis and consider implications for treatment and prognosis.
Case Reports
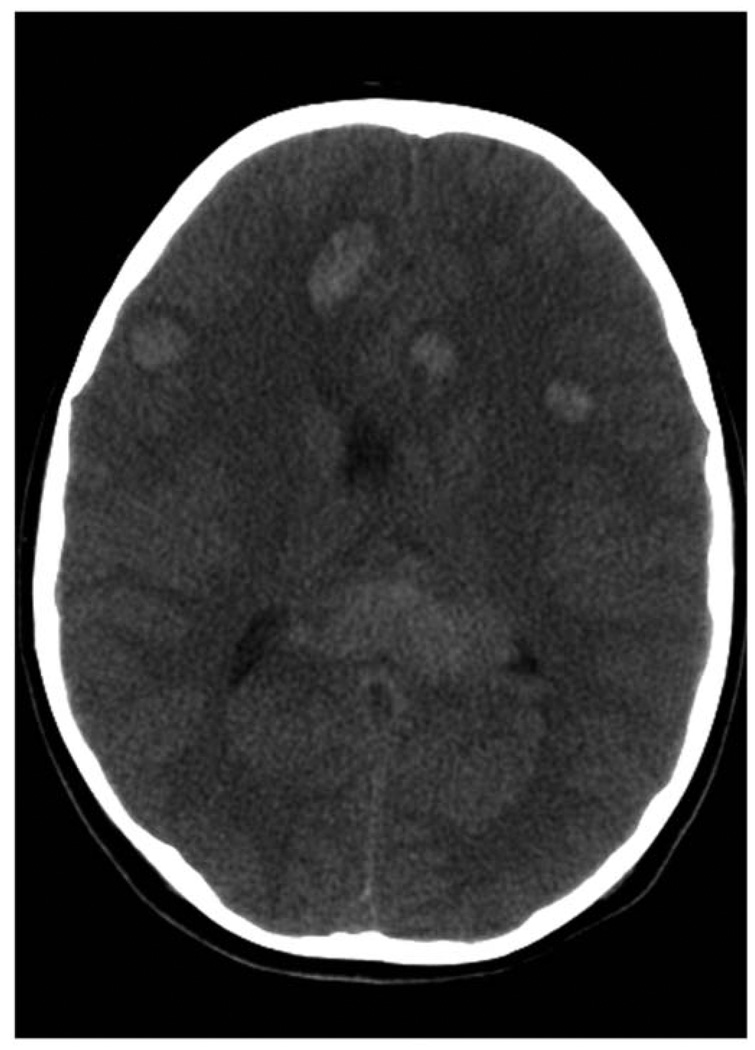
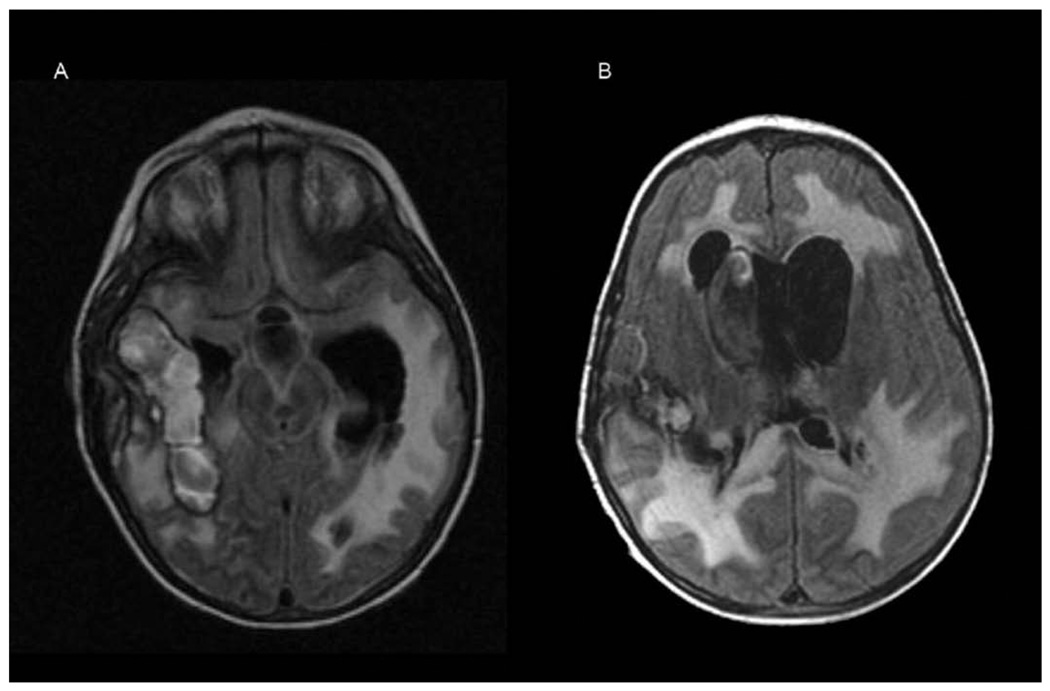
A 9-year-old boy was evaluated by his pediatrician for a 10-day history of progressive lower-extremity pain, night sweats, anorexia, and fatigue. Physical examination revealed altered mental status, diffuse petechiae, lymphadenopathy, and hepatosplenomegaly. His leukocyte count was 211 000/µL. He was referred to MD Anderson Cancer Center within 48 hours, and a repeat leukocyte count was 1 037 000/µL. A peripheral blood smear showed 96% blasts. Leukopheresis was performed. Bone marrow biopsy established the diagnosis of T-cell acute lymphoblastic leukemia. He was admitted to the intensive care unit, and his mental status rapidly deteriorated. A computed tomography (CT) scan of his brain showed multiple discreet areas of hemorrhage and associated mass effect in both frontal lobes and within the genu of the corpus callosum (Figure 1). Continued deterioration occurred during the next 24 hours, leading to unresponsiveness, posturing, and pupillary fixation. A repeat CT scan showed diffuse multifocal edema and bilateral uncal herniation. He was intubated and treated with dexamethasone and mannitol to reduce intracranial pressure with simultaneous chemotherapy induction using daunorubicin and vincristine. CT findings guided the management of intracranial hypertension and demonstrated preservation of the posterior fossa and brainstem. The option to discontinue ventilation was considered and rejected. MRI was first available 2 weeks after presentation. It showed multiple areas of fluid-attenuated inversion recovery (FLAIR) T2 hyperintensity and T1 postcontrast enhancement suggesting subacute hemorrhage and ischemic changes in the right posterior temporal region, both medial temporal lobes, the optic chiasm, and bilateral hypothalami (Figure 2A). No leptomeningeal enhancement was noted. A repeat MRI 3 weeks later demonstrated reduction in the volume of hemorrhages as well as diffuse reduction in cerebral volume with ex-vacuo ventricular dilation (Figure 2B).
Figure 1.
Computed tomography (CT) brain demonstrating multiple discreet areas of hemorrhage in both frontal lobes and in the genu of the corpus callosum.
Figure 2.
(A) Magnetic resonance imaging (MRI) brain demonstrating multiple areas of mixed signal likely representing hemorrhage surrounded by increased T2 signal compatible with edema or ischemic changes. (B) MRI brain (fluid-attenuated inversion recovery [FLAIR] T2) demonstrating reduction in the volume of hemorrhages and diffuse cerebral volume loss manifested as dilation of the ventricular system.
His neurological status improved during the next 2 months, and he was awake and alert by discharge. Chemotherapy and craniospinal irradiation were continued on an outpatient basis. Chemotherapy was completed 3 years after diagnosis, and he is currently in remission. His long-term neurological follow-up examination, including clinical mental status testing, is normal with the exception of blindness. Visual loss is presumed to have been caused secondary to hemorrhagic injury in the posterior optic pathways.
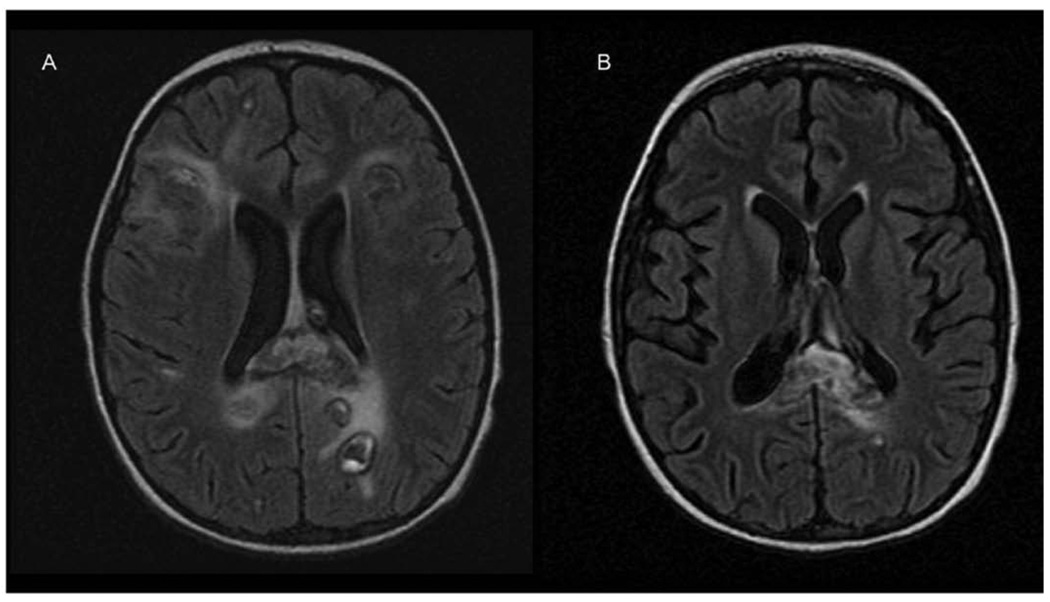
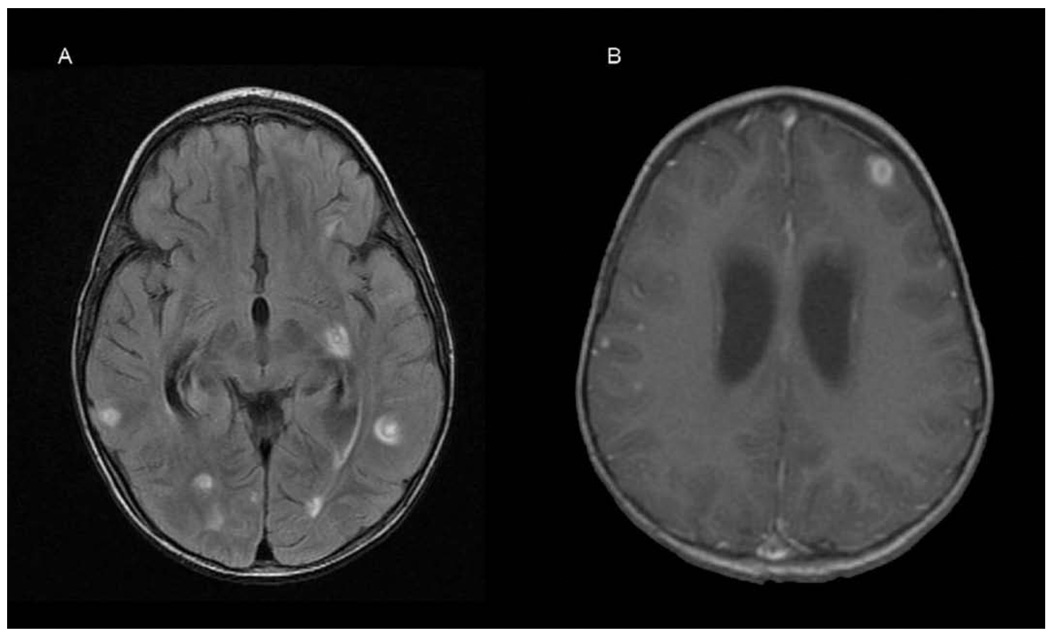
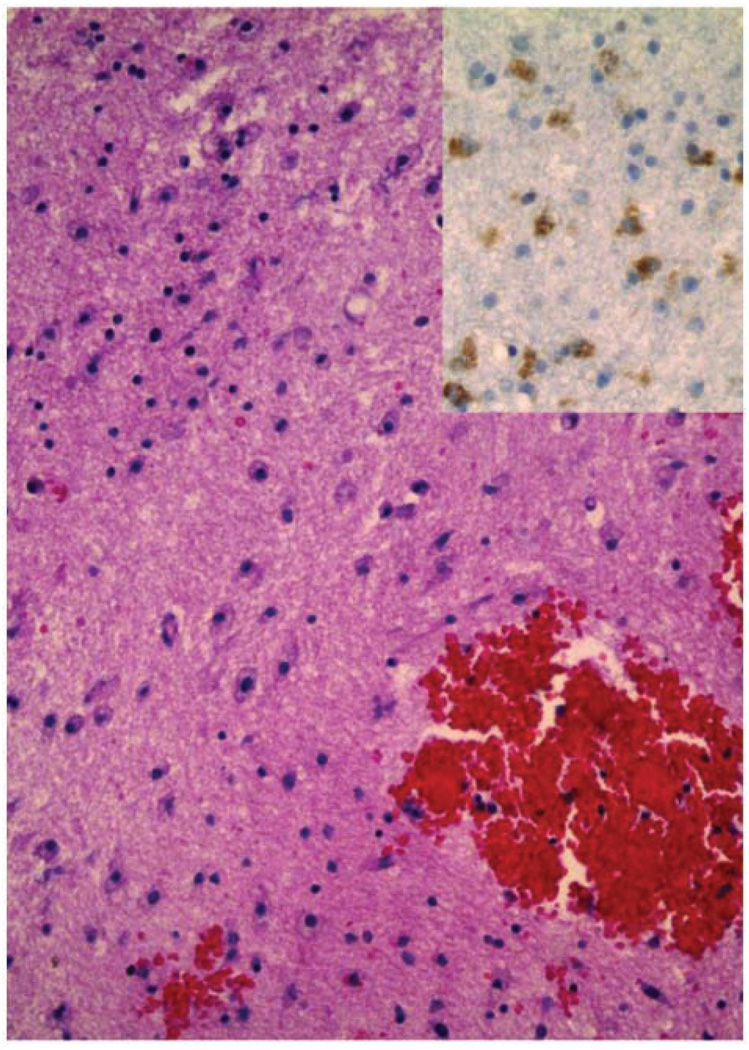
A 2-year-old boy was evaluated by his pediatrician for a 1-week history of fatigue, bleeding gums, swollen neck glands, and rash. Physical examination revealed diffuse petechiae, lymphadenopathy, and hepatosplenomegaly. His leukocyte count was 274 000/µL. He was referred to MD Anderson Cancer Center within 12 hours, where a repeat leukocyte count was 412 500/µL. Peripheral blood smear showed 95% blasts. Treatment was initiated with methylprednisolone and intravenous fluids with alkalinization. Lumbar puncture, intrathecal administration of cytarabine, and bone marrow biopsy were performed within 24 hours of presentation. Bone marrow pathology demonstrated pre-B cell acute lymphoblastic leukemia with 99% blasts. Induction chemotherapy was initiated on hospital day 2 using dexamethasone, daunorubicin, vincristine, and percutaneous endoscopic gastrostomy tube-asparaginase. The patient tolerated his first week of chemotherapy without complication. On the eighth day of therapy he developed clinical signs of acute renal failure confirmed by an elevated serum creatinine (1.5 mg/dL). Renal failure resolved within 3 days after administration of antihypertensive agents and diuretics, and fluid restriction. Chemotherapy was continued with dexamethasone, daunorubicin, vincristine, and a single dose of intrathecal methotrexate. On hospital day 20 he became agitated and developed focal posturing movements of his left side. A CT scan of his brain demonstrated multiple hypodensities in both cerebral hemispheres. An MRI of his brain 2 days later demonstrated multifocal areas of FLAIR T2 hyperintensity in the white matter (Figure 3A) that correlated with hypodensities seen on the CT scan. There was no mass effect or diffusion restriction visible on diffusion-weighted sequences. Two days later, clinical deterioration prompted a repeat MRI that demonstrated marked ventriculomegaly with progression of the multifocal lesions, now demonstrating T1 contrast ring enhancement and surrounding edema (Figure 3B). A ventriculoperitoneal shunt was placed and a right temporal lobe lesion was biopsied. The biopsy demonstrated normal-appearing white matter interspersed with petechial hemorrhages and macrophagic cellular infiltration (Figure 4), confirming that the hemorrhages predated the biopsy. A repeat MRI showed new hemorrhagic changes in the right hemisphere, with progressive diffuse white matter FLAIR T2 abnormalities involving much of the supratentorial brain, bilateral cerebellar hemispheres, and brainstem (Figure 5A). A subsequent MRI continued to demonstrate white matter changes with enlarging ventricles. The patient underwent 4 shunt revisions without significant change in ventricular size. Intraventricular pressure measurements were consistently normal. The most recent MRI shows a slight reduction in brainstem FLAIR T2 hyperintensity, evolution of the temporal lobe hemorrhage, and no other significant changes (Figure 5B). The patient continues to show clinical improvement in motor and language skills despite persistent abnormal MRIs.
Figure 3.
(A) Magnetic resonance imaging (MRI) brain demonstrating multifocal areas of increased fluid-attenuated inversion recovery [FLAIR] T2 signal in the cortex and subcortical white matter. (B) MRI brain demonstrating T1 contrast ring enhancement with surrounding edema corresponding to the previously seen lesions.
Figure 4.
Brain biopsy, hematoxylin and eosin (100×) and CD68 staining (inset, 200×), demonstrating microvascular proliferation, infiltration of macrophages, and astrocytosis in the subcortical white matter. Also seen are multiple blood clots containing scattered lymphocytes.
Figure 5.
(A) Magnetic resonance imaging (MRI) brain demonstrating evolutionary changes of hemorrhages, progressive diffuse white matter fluid-attenuated inversion recovery (FLAIR) T2 abnormalities, and enlarging ventricles. (B) MRI brain demonstrating evolving ventriculomegaly and diffuse fluid-attenuated inversion recovery T2 white matter abnormalities.
Discussion
Pathophysiology of Hyperleukocytosis
The association of blastic crisis, hyperleukocytosis, and intracranial hemorrhage is a rare but critically important occurrence in 2% to 3% of children with acute lymphoblastic leukemia. The pathophysiology was well described by Fritz, Freireich, and others.1,2 They studied a group of patients with leukocyte counts greater than 300 000 (hyperleukocytosis) and >95% blasts (blastic crisis) who died during the acute phase of their disease. A subset of these patients demonstrated a distinctive pattern of intracranial hemorrhage in the absence of coagulopathy. Postmortem examination in this subset demonstrated the hemorrhagic lesions to be limited to the white matter of the brain, centered at intravascular microscopic leukemic nodules. Another subset, without intracranial hemorrhage, was found on postmortem examination to have areas of deep brain white matter invested with intravascular microscopic leukemic nodules and blood vessels exhibiting stasis of leukemic cells (leukostasis). Patients without hyperleukocytosis or those without blastic crisis did not demonstrate leukostasis, intravascular leukemic nodules, or intracranial hemorrhage. This study demonstrated that leukemic cells accumulate in the capillaries of the white matter in patients with blastic hyperleukocytosis. This leads to leukostasis, leukemic nodule formation, capillary wall disruption, and ultimately petechial hemorrhage. Fritz and Freireich concluded that coalescence of many such microhemorrhages results in the larger hemorrhages seen grossly.
Pathology was unavailable on our first patient. The biopsy of our second patient was consistent with Fritz and Freireich’s descriptions of blastic hyperleukocytosis. The pathology demonstrated normal-appearing white matter interspersed with petechial hemorrhages. Leukemic nodules were not found in the brain specimen, nor were leukemic cells detected in any other tissue samples obtained at the time of biopsy. We propose that contemporary chemotherapeutic regimens eradicated all detectable leukemic cells.
MRI of Blastic Hyperleukocytosis
The brain MRI features of blastic hyperleukocytosis have not been described. Serial images in these cases initially raised debate about interpretation of the MRI findings. In retrospect, sequential images correlate with the progression of blastic hyperleukocytosis as described by Fritz and Freireich. Our first patient’s initial MRI was performed after significant neurological compromise and demonstrated multiple areas of acute hemorrhage consistent with the known pathophysiology. Our second patient’s scans were performed early in his course and initially demonstrated multifocal, nonhemorrhagic areas of FLAIR T2 hyperintensity without contrast enhancement. Subsequent imaging showed progression with new gadolinium enhancement of these lesions and development of diffusion restriction in the adjacent tissues. A repeat MRI demonstrated new areas of hemorrhagic changes similar to those seen in our first patient at his presentation. In both patients, serial imaging demonstrated features of hemorrhagic lesions and edema followed by rapidly progressive cerebral atrophy and ventricular enlargement without signs of elevated intracranial pressure.
Blastic Hyperleukocytosis and Prognosis
Our first patient presented with blastic hyperleukocytosis and altered mental status leading abruptly to a declaration of critical status and transfer to the intensive care unit. CT imaging demonstrated impending herniation suggesting grave prognosis. Full supportive care and aggressive treatment of his leukemia led to a surprising neurological recovery. His only deficit is cortical blindness related specifically to the hemorrhage in the genu of his corpus callosum and adjacent optic pathways, as predicted by the MRI.
Our second patient developed neurological symptoms during induction therapy, which progressed less acutely, possibly attenuated by the chemotherapy. Initial MRI findings were debated. Differential diagnoses included cytotoxic edema secondary to ischemia, vasogenic edema secondary to leukemic infiltration, leukoencephalopathy secondary to chemotherapy, or diffuse effects of central nervous system infection. The clinical course, laboratory data, and biopsy failed to confirm a diagnosis. Failure of induction, worsening neurological status, and progression of MRI features suggested a grave prognosis. Full supportive care and aggressive treatment of his leukemia led to a surprising neurological recovery. He continues to make developmental progress with no focal deficits.
Blastic Hyperleukocytosis and Clinical Management
Aggressive management of our 2 cases resulted in survival and recovery, providing an opportunity to correlate their MRI with clinical courses. The cases are quite similar in pathophysiology, differing only in the time of onset of clinical neurological signs. Serial MRI demonstrated progressive white matter pathology that we speculate is related to white matter leukostasis with microscopic hemorrhagic transformation.
The severity of white matter changes in our patients and the need for rapid intervention raises the question of whether MRI of the brain should be part of the initial staging for ALL patients presenting with blastic hyperleukocytosis. Knowing the presence of leukostasis prior to administration of chemotherapy could be useful in anticipation of intracranial hemorrhage, prompting early intensive care for cranial radiation, leukopheresis, or plasmaphoresis.3–6 Patients could also be protected from the risk of craniotomy for biopsy or ventriculoperitoneal shunt placement if the presence of intracranial lesions is known early in the course of the disease and if there is no ancillary evidence to suggest central nervous system infection. Our second patient had both a brain biopsy and a ventriculoperitoneal shunt placed. Shunt placement and revision did not improve his condition. In retrospect, it is likely that the ventricular dilatation in this condition is related to rapid white matter changes and cerebral atrophy and not related to increased intracranial pressure.
Dramatic MRI abnormalities brought forth questions about the need for emergent intrathecal chemotherapy. Both patients had multiple cerebral spinal fluid studies that were negative for malignancy and did not meet the definition of central nervous system leukemia. Biopsy in our second case confirmed absence of leukemic cells in the brain parenchyma. We conclude that the pathophysiology of their condition was intravascular, without leukemic cells transgressing the blood–brain barrier. In this situation, systemic chemotherapy appears to be the treatment of choice.
Conclusions
MRI defines focal pathology for prognostic and management decisions in blastic hyperleukocytosis. This rare condition has not been described since the advent of MRI, and radiologists and clinicians are unlikely to be familiar with its unique imaging features. Our patients demonstrate that clinical recovery is possible with the current chemotherapeutic regimens and supportive care despite the severity of intracranial pathology. Recognition of this rare condition is crucial to avoid unnecessary procedures and provide appropriate treatment and prognostic information.
Footnotes
Work was performed at The University of Texas Health Science Center, Houston, and at MD Anderson Cancer Center. There was no financial support related to this publication, and this information has not been previously presented.
References
- 1.Freireich EJ, Thomas LB, Frei E, et al. A distinctive type of intracerebral hemorrhage associated with “blastic crisis” in patients with leukemia. Cancer. 1960;13:146–154. doi: 10.1002/1097-0142(196001/02)13:1<146::aid-cncr2820130126>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Fritz RD, Forkner CE, Freireich RJ, et al. The association of fatal intracranial hemorrhage and “blastic crisis” in patients with acute leukemia. N Engl J Med. 1959;261:59–64. doi: 10.1056/NEJM195907092610202. [DOI] [PubMed] [Google Scholar]
- 3.Lowe EJ, Pui CH, Hancock ML, et al. Early complications in children with acute lymphoblastic leukemia presenting with hyperleukocytosis. Pediatr Blood Cancer. 2005;45:10–15. doi: 10.1002/pbc.20178. [DOI] [PubMed] [Google Scholar]
- 4.Bunin NJ, Kunkel K, Callihan TR. Cytoreductive procedures in the early management in cases of leukemia and hyperleukocytosis in children. Med Pediatr Oncol. 1987;15:232–235. doi: 10.1002/mpo.2950150503. [DOI] [PubMed] [Google Scholar]
- 5.Dearth JC, Fountain KS, Smithson WA, et al. Extreme leukemic leukocytosis (blast crisis) in childhood. Mayo Clin Proc. 1978;53:207–211. [PubMed] [Google Scholar]
- 6.Maurer HS, Steinherz PG, Gaynon PS, et al. The effect of initial management of hyperleukocytosis on early complications and outcome of children with acute lymphoblastic leukemia. J Clin Oncol. 1988;9:1425–1432. doi: 10.1200/JCO.1988.6.9.1425. [DOI] [PubMed] [Google Scholar]