Abstract
Objective
To identify predictors of survival after resection of retroperitoneal sarcoma (RPS) and to evaluate the performance of the American Joint Committee on Cancer (AJCC) staging system for RPS.
Summary Background Data
Previous studies of survival after RPS resection are restricted to at most several institutions, yet the current AJCC staging system for RPS is based entirely on these relatively small studies.
Methods
Patients undergoing resection of primary RPS from 1988 to 2005 were identified from the Surveillance, Epidemiology, and End Results (SEER) database. Cox proportional hazards models were used to analyze survival and evaluate AJCC staging.
Results
In 1365 patient undergoing resection of primary RPS, the most prevalent histologies were liposarcoma (50%), leiomyosarcoma (26%), and malignant fibrous histiocytoma (11%). Median, 5-year, and 10-year survival after resection were 55 months, 47%, and 27%. Histological subtype (P < 0.001), histological grade (grade 3–4 vs. grade 1; HR, 2.42; P < 0.001), and tumor invasion of adjacent structures (HR, 1.37; P < 0.001) were associated with survival on multivariable analysis. However, tumor size had no prognostic value. Consequently, the AJCC T classification system demonstrated poor discriminatory ability (c = 0.50). The AJCC stage grouping system demonstrated moderate discriminatory ability (c = 0.66) but performed no better than a much simpler system that omits information on tumor size and lymph node metastasis (c = 0.67).
Conclusions
Indicators of tumor aggressiveness (histological grade and invasion of adjacent structures) as well as histological subtype predict survival after RPS resection. Tumor size, however, does not impact survival. The AJCC staging system for RPS is in need of revision.
Keywords: retroperitoneal sarcoma, soft tissue sarcoma, SEER, surgery, survival, staging
Retroperitoneal sarcoma (RPS) is a relatively uncommon tumor that accounts for approximately 15% of all soft tissue sarcomas (STS). Complete surgical resection represents the only chance at cure but may be limited by the involvement of adjacent structures, and recurrence is common.1–11 As such, several studies have sought to identify factors that predict prognosis after resection of RPS with the goal of identifying patients who may benefit from more aggressive follow-up or investigational adjuvant therapies. However, all of these studies have been limited to at most a few high-volume institutions, and none have included more than a few hundred resected patients.1–11 Consequently, their findings may not be generalizable to a broader population. For example, the impact of histologic subtype on survival reported in some studies1,4,5,7,9–11 needs to be confirmed in a broader population. Although the use of radiation therapy in RPS has been studied on a population level,12 population-based data on the survival of patients after resection of RPS have not been published previously.
The American Joint Committee on Cancer sixth edition staging system for STS, including RPS, was developed and validated for extremity STS.13–15 Its relevance for RPS is questionable. For example, the AJCC T-classification system for STS uses a tumor size threshold of 5 cm as the sole discriminating prognostic feature. However, several previous studies have failed to demonstrate any association of tumor size with survival in RPS,2,3,5,7–9,11 while 2 analyses have found that a size threshold of 10 cm is significant.4,10 The AJCC staging system also regards lymph node metastasis as conferring a prognosis similar to that conferred by distant metastasis, but previous studies of RPS included too few patients with N1 disease to address this issue. We sought to define predictors of survival after primary RPS resection and critically appraise the performance of the AJCC staging system for RPS using a large population-based cohort of patients.
METHODS
A retrospective cohort study was performed using data from the SEER Program (available at: www.seer.cancer.gov) SEER*Stat Database, released April 2008, based on the November 2007 submission. The characteristics and representativeness of this database have been discussed previously.16 All patients diagnosed with primary RPS between 1988 and 2005 were identified using International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3),17 histology codes 8800 to 8806 (sarcoma not otherwise specified, NOS), 8810 to 8815 (fibrosarcoma), 8830 (malignant fibrous histiocytoma [MFH]), 8850 to 8858 (liposarcoma), 8890 to 8896 (leiomyosarcoma), 8900 to 8921 (rhabdomyosarcoma), 8990 to 8991 (malignant mesenchymoma), 9120 (hemangiosarcoma), 9150 (hemangiopericytoma), and 9540 (malignant peripheral nerve sheath tumor, MPNST) in combination with site code C48.0 (retroperitoneum). The histology code for gastrointestinal stromal tumors was excluded. Histologic grade is coded in the SEER database using a four-tier system.18,19 When a three-tier system20,21 is reported by the pathologist, SEER coders convert low grade to grade 2, intermediate grade to grade 3, and high grade to grade 4.13,22
Kaplan-Meier estimates of survival23 and Cox proportional hazards models24 were used to evaluate the association of survival with potential prognostic variables. The multivariable model was refined using Akaike information criteria.25 To explore the potential for bias and loss of power due to missing data,16 sensitivity analyses were performed using multiple imputation to assess the impact of missing data on survival analyses.26–28 The discriminative ability of the current AJCC staging system was evaluated using the bootstrap-corrected concordance index (c-statistic),28,29 a generalization of the area under the receiver operating characteristic curve that quantifies the proportion of all patient pairs for whom the predicted and observed survival outcomes are concordant.29 A value of c = 0.5 indicates no predictive ability as compared with chance alone, while a value of 1 indicates perfect discrimination. All tests of statistical significance were 2-sided, and statistical significance was established at α = 0.05. Statistical analyses were performed using Stata/MP 10.0 for Windows (StataCorp, College Station, TX). This study was deemed exempt from review by the Johns Hopkins University School of Medicine Institutional Review Boards.
RESULTS
Our selection criteria identified 2500 patients with RPS. Of these, 1365 (55%) received curative-intent surgery (excluding biopsies and local ablative therapies). The characteristics of the operative cohort are described in Table 1. The median age of the operative cohort was 63 years, and 754 (55%) were female. Most patients were white (n = 1135, 83%), and the remainder were black (n = 109, 8%), Asian/Pacific Islander (n = 113, 8%), or of another or unknown race (n = 8, <1%). Most of the patients were diagnosed later in the study period (n = 724, 53% in 2000–2005). Of those patients who did not receive curative-intent surgery (n = 1135, 45%), 28% had metastatic disease. Those who did not receive curative-intent surgery also tended to be older (median age, 66 vs. 63 years; P <0.001) and were more often male (52% vs. 45%, P = 0.001) than patients in the operative cohort.
TABLE 1.
Patient and Tumor Characteristics (n = 1365)
| Variable | Number | Percent |
|---|---|---|
| Age in yr (median, range) | 63 | 0–95 |
| Female | 754 | 55 |
| Year of diagnosis | ||
| 1988–1993 | 276 | 20 |
| 1994–1999 | 365 | 27 |
| 2000–2005 | 724 | 53 |
| Histological subtype | ||
| Liposarcoma | 682 | 50 |
| Leiomyosarcoma | 358 | 26 |
| Malignant fibrous histiocytoma | 146 | 11 |
| Fibrosarcoma | 24 | 2 |
| Rhabdomyosarcoma | 21 | 2 |
| MPNST | 15 | 1 |
| Hemangiopericytoma | 13 | <1 |
| Hemangiosarcoma | 10 | <1 |
| Malignant mesenchymoma | 5 | <1 |
| Sarcoma NOS | 91 | 7 |
| Histological grade | ||
| Grade 1 | 360 | 26 |
| Grade 2 | 225 | 17 |
| Grade 3 | 213 | 16 |
| Grade 4 | 292 | 21 |
| Unknown grade | 275 | 20 |
| Tumor size | ||
| Size in cm (median, range)* | 17 | 0.5–99 |
| Size >5 cm | 1121 | 82 |
| Size ≤5 cm | 66 | 5 |
| Unknown size | 178 | 13 |
Tumors >99 cm in size are reported as 99 cm by SEER.
The most common histologic subtypes were liposarcoma (n = 682, 50%), leiomyosarcoma (n = 358, 26%), MFH (n = 146, 11%), and sarcoma NOS (n = 91, 7%). Among tumors ≤5 cm in size (n = 66), the most common histologic subtypes were again liposarcoma (n = 28, 42%) and leiomyosarcoma (n = 23, 35%). Histologic grade was grade 1 in 360 patients (26%), grade 2 in 225 (17%), grade 3 in 213 (16%), grade 4 in 292 (21%), and unknown in 275 (20%). Overall, median tumor size was 17 cm; most of patients had tumors >5 cm (n = 1121, 82%). Tumor size was unknown in 178 patients (13%) overall and in 135 patients (11%) with M0 disease.
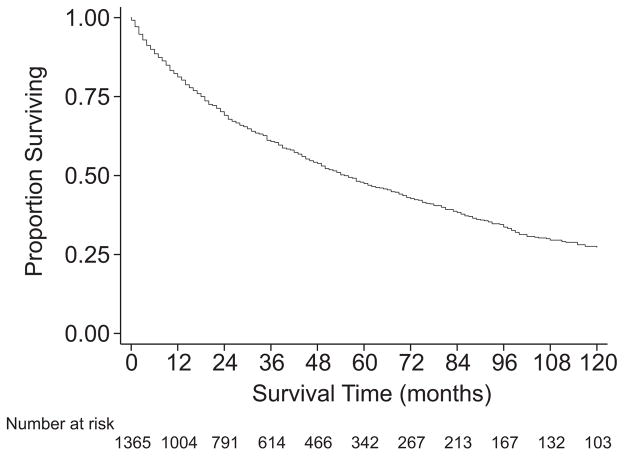
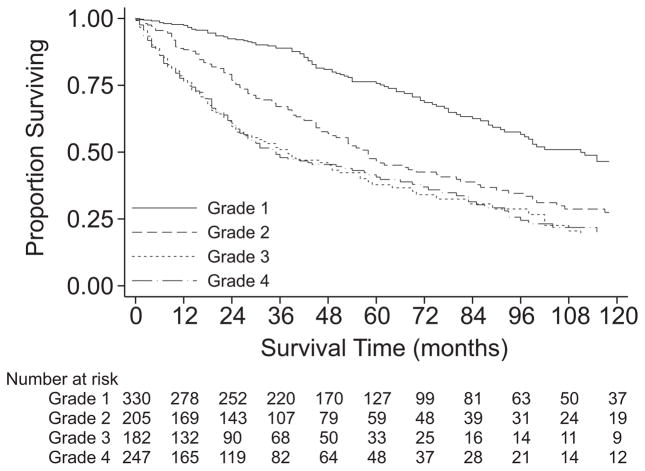
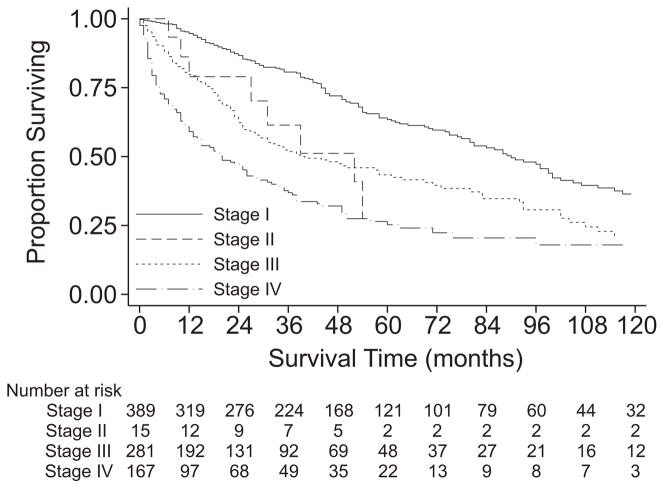
Overall survival of the cohort undergoing resection was 61% at 3 years, 47% at 5 years, and 27% at 10 years with median survival of 55 months (Table 2, PFig. 1). The survival of patients with M0 disease was significantly better than that of patients with M1 disease ( <0.001). Among those with M0 disease, histologic grade was clearly associated with survival (P <0.001, Fig. 2), with 5-year survival ranging from 76% for grade 1 tumors to 41% for grade 4 tumors. However, the survival estimates for grade 3 and grade 4 tumors were quite similar (P = 0.9). Among those with M0 disease, the survival of patients with T1 (≤5 cm) disease was similar to that of patients with T2 (>5 cm) disease (P = 0.4, Fig. 3). Descriptive survival statistics were also calculated for all patients in each of the AJCC stage groupings (Table 2, Fig. 4), including those with metastatic disease.
TABLE 2.
Descriptive Survival Statistics
| Group | 3 yr | 5 yr | 10 yr | Median (mo) | P* |
|---|---|---|---|---|---|
| Overall (n = 1365) | 61% | 47% | 27% | 55 | <0.001 |
| Metastatic disease | |||||
| M0 (n = 1189) | 64% | 51% | 29% | 63 | |
| M1 (n = 132) | 31% | 19% | 17% | 15 | |
| Mx (n = 44) | 65% | 33% | 26% | 54 | |
| Histological grade (M0, n = 1189) | <0.001 | ||||
| Grade 1 (n = 330) | 89% | 76% | 47% | 111 | |
| Grade 2 (n = 205) | 67% | 47% | 27% | 58 | |
| Grade 3 (n = 182) | 51% | 38% | 18% | 38 | |
| Grade 4 (n = 247) | 48% | 41% | 20% | 35 | |
| Unknown grade (n = 225) | 53% | 42% | 22% | 42 | |
| AJCC T classification (M0, n = 1189) | 0.4 | ||||
| T1 (n = 58) | 67% | 54% | 38% | 67 | |
| T2 (n = 996) | 63% | 50% | 28% | 61 | |
| Unknown size (n = 135) | 68% | 56% | 31% | 75 | |
| AJCC stage grouping (n = 1365) | <0.001 | ||||
| Stage I (n = 389) | 81% | 63% | 36% | 90 | |
| Stage II (n = 15) | 61% | 27% | 27%† | 52 | |
| Stage III (n = 281) | 52% | 43% | 21% | 40 | |
| Stage IV (n = 167) | 37% | 25% | 18% | 20 | |
| Unknown stage (n = 513) | 59% | 46% | 27% | 52 | |
P values reflect comparisons between complete cases only, except for histological grade.
Last observed death at 54 mo, last censored observation at 165 mo.
FIGURE 1.
Kaplan-Meier survival estimates, all patients undergoing curative-intent surgery.
FIGURE 2.
Kaplan-Meier survival estimates, M0 patients, by histologic grade.
FIGURE 3.
Kaplan-Meier survival estimates, M0 patients, by AJCC T-classification.
FIGURE 4.
Kaplan-Meier survival estimates, by AJCC stage grouping.
Because the presence of metastatic disease was likely to obscure the impact of other determinants of survival, further analyses focused on patients with M0 disease who underwent resection (n = 1189). Of these patients, 135 had unknown tumor size and were therefore excluded, leaving 1054 patients for the subsequent analyses of survival (Tables 3, 4). Among these 1054 patients, lymph node metastasis was present in 30 patients (3%), absent in 768 patients (73%), and unknown in 256 patients (24%), reflecting the fact that lymph node dissection is not routinely performed during sarcoma resection. Invasion of adjacent structures by the tumor was reported by SEER as present in 434 patients (41%), absent in 618 patients (59%), and unknown in 2 patients (<1%). Radiation therapy was administered to 272 patients (26%). The timing of radiation therapy was postoperative only in 226 patients, preoperative only in 15 patients, and both pre- and postoperative in 2 patients. Intraoperative radiation therapy was administered to 28 patients. In 1 patient, the timing of radiation therapy was unknown.
TABLE 3.
Unadjusted Cox Proportional Hazards Analyses (n= 1054)
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Age (per decade) | 1.31 | 1.23–1.39 | <0.001 |
| Female | 0.73 | 0.62–0.87 | <0.001 |
| Year of diagnosis (per yr) | 0.99 | 0.97–1.01 | 0.4 |
| Histological subtype | <0.001 | ||
| Liposarcoma | Ref. | — | |
| Leiomyosarcoma | 1.59 | 1.30–1.96 | |
| Malignant fibrous histiocytoma | 2.12 | 1.64–2.74 | |
| Fibrosarcoma | 1.31 | 0.65–2.66 | |
| Rhabdomyosarcoma | 1.93 | 0.86–4.34 | |
| MPNST | 2.57 | 1.14–5.79 | |
| Hemangiopericytoma | 0.30 | 0.08–1.22 | |
| Hemangiosarcoma | 7.06 | 3.13–16.0 | |
| Malignant mesenchymoma | 0.81 | 0.20–3.26 | |
| Sarcoma NOS | 2.27 | 1.62–3.18 | |
| Histological grade | <0.001 | ||
| Grade 1 | Ref. | — | |
| Grade 2 | 2.01 | 1.51–2.69 | |
| Grade 3 | 3.04 | 2.27–4.07 | |
| Grade 4 | 3.08 | 2.34–4.05 | |
| Unknown grade | 2.84 | 2.14–3.76 | |
| Tumor size | |||
| Size >5 vs. ≤5 cm (T2 vs. T1) | 1.16 | 0.80–1.69 | 0.4 |
| Size >10 vs. ≤10 cm | 1.12 | 0.90–1.38 | 0.3 |
| Size >20 vs. ≤20 cm | 0.84 | 0.70–1.01 | 0.06 |
| Continuous variable (per cm) | 1.00 | 0.99–1.01 | 0.8 |
| Log-transformed continuous variable | 1.01 | 0.89–1.14 | 0.9 |
| Lymph node metastasis (N1 vs. N0) | 1.25 | 0.77–2.03 | 0.4 |
| Invasion of adjacent structures | 1.51 | 1.27–1.79 | <0.001 |
| Radiation therapy | 0.95 | 0.78–1.15 | 0.6 |
HR indicates hazard ratio; CI, Confidence interval; Ref, referent.
TABLE 4.
Multivariable Cox Proportional Hazards Analyses (n= 1054)
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Age (per decade) | 1.33 | 1.25–1.42 | <0.001 |
| Female | 0.70 | 0.59–0.84 | <0.001 |
| Histological subtype | <0.001 | ||
| Liposarcoma | Ref. | — | |
| Leiomyosarcoma | 1.49 | 1.19–1.86 | |
| Malignant fibrous histiocytoma | 1.47 | 1.12–1.93 | |
| Fibrosarcoma | 1.19 | 0.58–2.43 | |
| Rhabdomyosarcoma | 3.54 | 1.52–8.23 | |
| MPNST | 2.57 | 1.13–5.87 | |
| Hemangiopericytoma | 0.25 | 0.06–1.00 | |
| Hemangiosarcoma | 3.08 | 1.34–7.07 | |
| Malignant mesenchymoma | 0.75 | 0.18–3.06 | |
| Sarcoma NOS | 2.03 | 1.43–2.87 | |
| Histological grade | <0.001 | ||
| Grade 1 | Ref. | — | |
| Grade 2 | 1.60 | 1.17–2.19 | |
| Grade 3–4 | 2.42 | 1.84–3.17 | |
| Unknown grade | 2.25 | 1.65–3.05 | |
| Invasion of adjacent structures | 1.37 | 1.15–1.63 | <0.001 |
HR indicates hazard ratio; CI, Confidence interval; Ref, referent.
Unadjusted Cox proportional hazards analyses were performed to identify potential predictors of survival (Table 3). Increasing patient age (hazard ratio [HR], 1.31 per decade; 95% CI, 1.23–1.39; P <0.001) was associated with worse survival, while female gender (HR, 0.73; 95% CI, 0.61–0.86; P <0.001) was associated with improved survival. The histologic subtype of RPS also had a significant effect on survival (P <0.001). Leiomyosarcoma, MFH, MPNST, hemangiosarcoma, and sarcoma NOS were associated with significantly worse prognoses than liposarcoma. For other histologic subtypes, survival was not significantly different from that for liposarcoma.
As expected, histologic grade also had a significant impact on prognosis (Table 3). Of note, grade 3 tumors (HR, 3.04; 95% CI, 2.27–4.07) and grade 4 tumors (HR, 3.08; 95% CI, 2.34–4.05) were both associated with worse prognoses as compared with grade 1 tumors (P <0.001 for overall comparison), but these 2 high-grade groups themselves were associated with similar prognoses (P = 0.9). For this reason, in further analyses the grade 3 and grade 4 groups were combined into a single high-grade group, consistent with AJCC staging.13 Grades 1 and 2 were analyzed separately but were both considered “low-grade” for purposes of AJCC staging.13 Finally, the survival of patients with missing histologic grade (n = 276) was consistent with what would be expected for a group containing a mixture of the 4 tumor grades.
Tumor size, specifically tumor size >5 cm versus ≤5 cm, is considered a significant prognostic factor in the AJCC staging system for RPS and constitutes the entire basis for the AJCC T-classification system. For this reason, the impact of tumor size on survival was explored in particular depth (Table 3). In unadjusted analyses, tumor size >5 cm versus ≤5 cm (ie, T2 versus T1) had no significant impact on survival (HR, 1.16; 95% CI, 0.80–1.69; P = 0.4; Fig. 3). Tumor size cutpoints of 10 cm and 20 cm yielded similar results (Table 3). Because the use of cutpoints to dichotomize continuous variables can result in a loss of statistical power, we also analyzed tumor size as an untransformed continuous variable (HR, 1.00; 95% CI, 0.99–1.0; P = 0.8) and as a log-transformed continuous variable (HR, 1.01; 95% CI, 0.89–1.14; P = 0.9). In none of these analyses did tumor size predict prognosis. Finally, we theorized that while tumor size might not predict prognosis overall, it might have a differential impact dependent on tumor invasion of adjacent structures. Again, there was no statistically significant impact of tumor size, whether in those without adjacent structure invasion (HR, 1.60; 95 CI, 0.91–2.79; P = 0.1), those with it (HR, 0.81; 95% CI, 0.48–1.34; P = 0.4), or in a stratified analysis including both groups (HR, 1.16; 95% CI, 0.79–1.68; P = 0.4).
The presence of lymph node metastasis, when known, had no significant effect on prognosis (HR, 1.24; 95% CI, 0.76–2.03; P = 0.4). Tumor invasion of adjacent structures did have a significant association with survival (HR, 1.51; 95% CI, 1.27–1.80; P <0.001). However, information on completeness of resection (such as margin status) is not available in the SEER database. Receipt of radiation therapy had no significant impact on prognosis (HR, 0.95; 95% CI, 0.78–1.15; P = 0.6).
In multivariable analyses (Table 4), patient age (HR, 1.33 per decade; 95% CI, 1.25–1.42; P <0.001) and female gender (HR, 0.70; 95% CI, 0.59–0.84; P <0.001) remained significant predictors of survival. The histologic subtype of RPS also remained significantly associated with survival (P <0.001). Hemangiosarcoma (HR, 3.08; 95% CI, 1.34–7.07) conferred worse prognoses than liposarcoma. Leiomyosarcoma, MFH, MPNST, and sarcoma NOS were also associated with significantly worse prognoses than liposarcoma. As in unadjusted analyses, histologic grade also predicted survival (P <0.001), with grade 2 (HR, 1.60; 95% CI, 1.17–2.19) and grade 3–4 tumors (HR, 2.42; 95% CI, 1.84–3.17) conferring a worse prognosis than grade 1 tumors. Finally, tumor invasion of adjacent structures had a detrimental effect on survival (HR, 1.37; 95% CI, 1.15–1.63; P <0.001) similar to that observed in unadjusted analyses. Importantly, tumor size was not a significant predictor of survival when included in the multivariable model (whether as a continuous variable, a log-transformed continuous variable, or a categorical variable with a cutpoint of 5 cm, 10 cm, or 20 cm). Tumor size was therefore excluded from the final model.
Because of the significant amount of missing data on tumor size and the prominence of tumor size in the AJCC staging system, we conducted further analyses to assess the potential impact of these missing data. Using the cohort of 1189 patients with resected M0 RPS, including the 135 patients with missing tumor size, the regression analyses of survival were repeated using multiple imputations to account for missing data on tumor size, lymph node metastasis, and histologic grade. None of these analyses revealed any statistically significant effect of tumor size or lymph node metastasis in either unadjusted or multivariable analyses. The effect of tumor grade in multiple imputation analyses was nearly identical to that observed in complete-case analyses.
A final set of analyses focused on the discriminative ability of the AJCC staging system for RPS (Table 5). The first analysis focused only on patients with M0 disease and known tumor size (n = 1054) to specifically focus on the T-classification system. In this analysis, T-classification had no statistically significant association with survival (HR, 1.16; 95% CI, 0.80–1.69; P = 0.4; Fig. 3). The c-statistic for this analysis was 0.50, indicating that for patients with M0 disease the discriminative ability of the AJCC T-classification system was no better than that obtained by chance alone. The second analysis focused on all patients for whom sufficient data were available to assign a stage grouping (n = 854, Fig. 4). Patients with stage II disease did not have a statistically significant difference in survival as compared with those with stage I disease (HR, 1.68; 95% CI, 0.82–3.42). Stage III disease did confer a worse prognosis than stage I disease (HR, 2.02; 95% CI, 1.60–2.54), and the magnitude of the effect estimate was similar to that for stage II disease, suggesting that the incorporation of histologic grade into the staging system was responsible for the observed augmentation of discriminative ability. Again, this was consistent with the results of the previous survival analyses.
TABLE 5.
Cox Proportional Hazards Analyses of AJCC Staging System
| Variable | HR | 95% CI | P | c-Statistic |
|---|---|---|---|---|
| T-Classification (M0, known size, n = 1054) | ||||
| T2 (n = 996) vs. T1 (n = 58) | 1.16 | 0.80–1.69 | 0.4 | 0.50 |
| Stage grouping (n = 854) | <0.001 | 0.66 | ||
| Stage I (n = 389): low- grade, any T, N0M0 | Ref. | — | ||
| Stage II (n = 15): high- grade, T1, N0M0 | 1.68 | 0.82–3.42 | ||
| Stage III (n = 281): high-grade, T2, N0M0 | 2.02 | 1.60–2.54 | ||
| Stage IV (n = 167): N1 and/or M1 | 3.28 | 2.56–4.19 | ||
HR indicates hazard ratio; CI, Confidence interval; Ref, referent.
Resected patients with stage IV disease had markedly worse prognoses than those with stage I disease (HR, 3.30; 95% CI, 2.58–4.22). However, the AJCC staging system groups N1M0 disease and M1 disease together. Because this was inconsistent with our analyses that demonstrated no prognostic relevance of lymph node metastasis, the validity of this grouping was further explored. Indeed, stage IV patients with M1 disease (n = 134) had significantly worse prognoses than those with N1M0 disease (n = 35) (HR, 1.87; 95% CI, 1.15–3.04, P = 0.01), suggesting that further stratification of these patients may be necessary.
Despite these limitations—namely, the inappropriate use of tumor size and lymph node metastasis as prognostic variables—the AJCC staging system demonstrated moderate discriminative ability (c = 0.66). However, it should be emphasized that the prognostic value of the AJCC staging system was essentially due to its consideration of histologic grade and the presence of metastatic disease. To illustrate this point, we constructed a staging system that is identical to the AJCC system except that it omits tumor size and lymph node metastasis from consideration (stage I: low-grade, M0; stage II: high-grade, M0; stage III: M1). The c-statistic for this system was 0.67, indicating that information on tumor size and lymph node metastasis adds no discriminative ability to the AJCC staging system.
DISCUSSION
Previous studies of survival after resection of RPS have analyzed data from selected institutions and have included at most several hundred patients each.1–11 Although these studies have advanced our understanding of RPS, they may be susceptible to institutional biases, and there remains a need for generalizable information on this uncommon malignancy. The present population-based study of survival after resection of primary RPS has the advantages of large size and generalizability beyond a few institutions. Using data on 1365 patients with resected RPS, we identified predictors of survival and evaluated the performance of the AJCC staging system. Overall survival at 5 years was 47%, which is consistent with the range of 43% to 65% reported in other recently published series.1,2,4–8,10,11 Our work presents generalizable information on outcomes after surgical resection of RPS and convincingly addresses a lingering controversy regarding the prognostic significance of tumor size in this disease.
Our analyses identified histologic grade and invasion of adjacent structures, both indicators of tumor aggressiveness, as predictors of survival after RPS resection. These findings are consistent with those of previous studies that have demonstrated the association of histologic grade with survival.2,4–11 Our results confirm the appropriateness of retaining information on histologic grade in the AJCC staging system. Previous analyses have also identified completeness of resection as another important prognostic factor.1– 4,6,7,11,30 Although the SEER database does not report margin status, patients who had tumor invasion of adjacent structures may have been more likely to have had residual microscopic or gross disease after RPS resection, as has been suggested by other authors.5,30 The negative prognostic impact of tumor invasion of adjacent structures and the high incidence of margin-positive resection in patients with such tumor extension have recently been emphasized in a large multicenter French study.11
In the present study, histologic subtype was also an important predictor of prognosis. In the SEER database, as in previous institutional series,1,4,5,7,9,11 liposarcoma was the most prevalent histologic subtype among resected patients and was associated with a favorable prognosis. However, most previous studies have grouped other histologic subgroups into a nonlipomatous group, potentially obscuring important differences in prognosis. Perez et al found that leiomyosarcoma and MFH were associated with poor prognoses as compared with liposarcoma, but their analysis included both truncal and retroperitoneal tumors.9 Gronchi et al separately analyzed leiomyosarcoma, liposarcoma, fibrosarcoma, and MPNST, and notably found poor survival for liposarcoma versus leiomyosarcoma.10 Finally, Bonvalot et al separately analyzed well-differentiated liposarcoma, other liposarcoma, leiomyosarcoma, and MFH, and found that well-differentiated liposarcoma had the best prognosis.11 However, both of these studies grouped less common histologies together. In the present study, leiomyosarcoma, MFH, MPNST, and sarcoma NOS were all found to adversely affect prognosis. Two rare tumors—rhabdomyosarcoma and hemangiosarcoma—were associated with dramatically worse prognoses as compared with liposarcoma. Taken together with previously published data, our results suggest that it may be useful for future editions of the AJCC staging system to identify specific histologic subtypes of RPS as being associated with better or worse prognoses, as appropriate.
The 5-cm threshold used in the AJCC staging system is of limited value for RPS because of the small number of patients who actually have such small RPS tumors, both in the present study and others.2,4,5,7 However, at least 2 studies have suggested that the tumor size threshold should be revised upward to 10 cm.4,10 In this large study, we rigorously evaluated the potential prognostic role of tumor size, using several functional forms of the variable and several different cutpoints for dichotomization. Tumor size was not associated with survival in any of these analyses, strongly suggesting that no tumor size threshold should be used in predicting prognosis in RPS. In light of these findings, the AJCC T-classification system performed predictably poorly, yielding no more information than a coin toss regarding prognosis. Several studies have similarly found no prognostic role for tumor size in RPS.2,3,5,7–9 The only other aspect of the T-classification system for STS is the categorization of a tumor as superficial or deep, which has no relevance for RPS (which are all deep). As such, the current AJCC T-classification system for RPS has no prognostic relevance and is in need of revision.
The prognostic value of the AJCC staging system was essentially due to its consideration of histologic grade and the presence of metastatic disease. A simpler system that omits information on tumor size and lymph node metastasis performed just as well, reinforcing the notion that information on tumor size and lymph node metastasis is not useful in the staging of RPS. Rather, information on histologic grade and distant metastasis is responsible for the moderate discriminative ability of the AJCC staging system. A recently proposed classification system for resected RPS emphasizes these elements while also adding information on completeness of resection.7 This system categorizes histologic grade as either low or high, although our analyses suggest that the use of 3 categories may be warranted. Completeness of resection in this system is determined solely by gross examination and without regard to the microscopic resection margin. While this approach may not be ideal, it recognizes both the high rate of microscopic margin positivity in RPS4,31 and the inherent limitations of microscopic margin examination in typically large RPS resection specimens. Our study does not shed light on the validity of this approach, as SEER does not report data on the completeness of resection.
These minor criticisms aside, the system proposed by van Dalen et al7 may serve as an appropriate template for a revised AJCC staging system for RPS. A nomogram-based approach would likely provide superior calibration and discrimination than a staging system, albeit at the expense of simplicity and ease of use. Such a nomogram has been proposed by the Memorial Sloan Kettering Cancer Center group for STS generally,32 and a subsequent liposarcoma-specific revision demonstrated improved performance in patients with liposarcoma.33 Further revisions will likely allow more accurate predictions of prognosis based on both site and histologic subtype but will likely require more data. The Memorial Sloan Kettering Cancer Center nomogram includes patient age, histologic subtype, and histologic grade as prognostic variables,32 an approach that is consistent with our analysis. It also includes tumor size, but this may be a result of the high proportion of extremity sarcomas (57%) in the cohort used to derive the nomogram.32 Our analysis suggests that a similar effort directed specifically at RPS should not include tumor size.
The data presented in this manuscript, together with previously published series, emphasize the need for a distinct staging system for RPS. There is an urgent need for clinical trials to evaluate experimental therapies for RPS, especially with regard to the role of radiation therapy.34 However, appropriate design of such trials relies on the use of an accurate staging system to identify and stratify potential trial participants. The prognostic features in RPS are distinct from those in extremity STS, particularly with regard to the role of tumor size and lymph node metastasis. The options for surgical and radiation therapy are also distinct.35 The development of distinct STS staging systems would allow each system to be tailored to achieve improved prognostic accuracy for either extremity tumors or RPS.36,37 Because these staging systems are to be applied on a population level, it is important that their development and validation also be conducted in population-based data to avoid idiosyncrasies in tumor characteristics or outcomes related to individual institutions. Our population-based analysis suggests that tumor invasion of adjacent structures and histologic subtype should be considered for addition to the AJCC T-classification system. Unfortunately, the SEER database only reports information that is already included in the AJCC staging system (with a few exceptions). The more detailed pathologic data available from institutional studies will be useful in allowing the identification of prognostic features that may be incorporated in future editions of the staging system for RPS. For example, tumor multifocality has recently been implicated as a potential prognostic factor in RPS.38
Several limitations should be considered when interpreting our results. First, as a result of missing data on histologic grade and tumor size, some patients could not be assigned an AJCC stage grouping. However, our descriptive survival statistics suggested that those with missing data were not systematically different from those without, such that the missing data were unlikely to have introduced significant bias. Multiple imputation analyses were consistent with complete-case analyses, further reducing the likelihood of significant bias due to missing data. Second, because SEER does not report data on completeness of resection, we could not control for this factor. However, data on tumor invasion of adjacent structures may have served as an approximate surrogate for completeness of resection. Third, the histologic grading systems used were not standardized and could have led to variability in assignment of histologic grade. However, the important distinction for purposes of the AJCC staging system—high- versus low-grade—was likely to be less affected. Finally, the histologic classification of sarcoma has evolved over time and may not have been entirely consistent across institutions, especially those with less expertise in treating RPS. For example, some gastrointestinal stromal tumors might have been misclassified as leiomyosarcomas before the late 1990s. Dedifferentiated liposarcoma might have been misclassified as MFH in some cases. However, such misclassification would tend to obscure differences rather than exaggerate them.
In conclusion, the population-based outcomes for resected RPS presented in this manuscript provide a valuable complement to previously published reports from smaller institutional series. We emphasize that tumor size and lymph node metastasis do not predict survival in resected RPS and that the current AJCC staging system for STS is limited in its applicability to RPS. Significant revisions to this staging system are needed.
Acknowledgments
Supported by the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research, grant 1KL2RR025006–01 (to T.M.P.).
Footnotes
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Presented at the Society of Surgical Oncology 62nd Annual Cancer Symposium, March 6, 2009, Phoenix, AZ.
References
- 1.Catton CN, O’Sullivan B, Kotwall C, et al. Outcome and prognosis in retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 1994;29:1005–1010. doi: 10.1016/0360-3016(94)90395-6. [DOI] [PubMed] [Google Scholar]
- 2.Singer S, Corson JM, Demetri GD, et al. Prognostic factors predictive of survival for truncal and retroperitoneal soft-tissue sarcoma. Ann Surg. 1995;221:185–195. doi: 10.1097/00000658-199502000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heslin MJ, Lewis JJ, Nadler E, et al. Prognostic factors associated with long-term survival for retroperitoneal sarcoma: implications for management. J Clin Oncol. 1997;15:2832–2839. doi: 10.1200/JCO.1997.15.8.2832. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JJ, Leung D, Woodruff JM, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–365. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoeckle E, Coindre JM, Bonvalot S, et al. Prognostic factors in retroperitoneal sarcoma: a multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer. 2001;92:359–368. doi: 10.1002/1097-0142(20010715)92:2<359::aid-cncr1331>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Ferrario T, Karakousis CP. Retroperitoneal sarcomas: grade and survival. Arch Surg. 2003;138:248–251. doi: 10.1001/archsurg.138.3.248. [DOI] [PubMed] [Google Scholar]
- 7.van Dalen T, Hennipman A, van Coevorden F, et al. Evaluation of a clinically applicable post-surgical classification system for primary retroperitoneal soft-tissue sarcoma. Ann Surg Oncol. 2004;11:483–490. doi: 10.1245/ASO.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Gronchi A, Casali PG, Fiore M, et al. Retroperitoneal soft tissue sarcomas: patterns of recurrence in 167 patients treated at a single institution. Cancer. 2004;100:2448–2455. doi: 10.1002/cncr.20269. [DOI] [PubMed] [Google Scholar]
- 9.Perez EA, Gutierrez JC, Moffat FL, Jr, et al. Retroperitoneal and truncal sarcomas: prognosis depends upon type not location. Ann Surg Oncol. 2007;14:1114–1122. doi: 10.1245/s10434-006-9255-x. [DOI] [PubMed] [Google Scholar]
- 10.Gronchi A, Lo Vullo S, Fiore M, et al. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol. 2009;27:24–30. doi: 10.1200/JCO.2008.17.8871. [DOI] [PubMed] [Google Scholar]
- 11.Bonvalot S, Rivoire M, Castaing M, et al. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27:31–37. doi: 10.1200/JCO.2008.18.0802. [DOI] [PubMed] [Google Scholar]
- 12.Porter GA, Baxter NN, Pisters PW. Retroperitoneal sarcoma: a population-based analysis of epidemiology, surgery, and radiotherapy. Cancer. 2006;106:1610–1616. doi: 10.1002/cncr.21761. [DOI] [PubMed] [Google Scholar]
- 13.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6. New York: Springer-Verlag; 2002. [Google Scholar]
- 14.Ramanathan RC, A’Hern R, Fisher C, et al. Modified staging system for extremity soft tissue sarcomas. Ann Surg Oncol. 1999;6:57–69. doi: 10.1007/s10434-999-0057-9. [DOI] [PubMed] [Google Scholar]
- 15.Brennan MF. Staging of soft tissue sarcomas. Ann Surg Oncol. 1999;6:8–9. doi: 10.1007/s10434-999-0008-5. [DOI] [PubMed] [Google Scholar]
- 16.Nathan H, Pawlik TM. Limitations of claims and registry data in surgical oncology research. Ann Surg Oncol. 2008;15:415–423. doi: 10.1245/s10434-007-9658-3. [DOI] [PubMed] [Google Scholar]
- 17.Fritz AG. International Classification of Diseases for Oncology: ICD-O. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 18.Broders AC, Hargrave R, Meyerding HW. Pathological features of soft tissue fibrosarcoma with special reference to the grading of its malignancy. Surg Gynecol Obstet. 1939;69:267–280. [Google Scholar]
- 19.Angervall L, Kindblom LG, Rydholm A, et al. The diagnosis and prognosis of soft tissue tumors. Semin Diagn Pathol. 1986;3:240–258. [PubMed] [Google Scholar]
- 20.Costa J, Wesley RA, Glatstein E, et al. The grading of soft tissue sarcomas. Results of a clinicohistopathologic correlation in a series of 163 cases. Cancer. 1984;53:530–541. doi: 10.1002/1097-0142(19840201)53:3<530::aid-cncr2820530327>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 21.Trojani M, Contesso G, Coindre JM, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33:37–42. doi: 10.1002/ijc.2910330108. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute. [Accessed March 27, 2009];SEER Program Coding and Staging Manual. 2007 Available at: http://seer.cancer.gov/tools/codingmanuals/index.html.
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Cox DR. Regression models and life-tables. J Royal Stat Soc B. 1972;34:187–220. [Google Scholar]
- 25.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 26.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 27.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: Wiley-Interscience; 2004. [Google Scholar]
- 28.Clark TG, Altman DG. Developing a prognostic model in the presence of missing data: an ovarian cancer case study. J Clin Epidemiol. 2003;56:28–37. doi: 10.1016/s0895-4356(02)00539-5. [DOI] [PubMed] [Google Scholar]
- 29.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Anaya DA, Lev DC, Pollock RE. The role of surgical margin status in retroperitoneal sarcoma. J Surg Oncol. 2008;98:607–610. doi: 10.1002/jso.21031. [DOI] [PubMed] [Google Scholar]
- 31.Mendenhall WM, Zlotecki RA, Hochwald SN, et al. Retroperitoneal soft tissue sarcoma. Cancer. 2005;104:669–675. doi: 10.1002/cncr.21264. [DOI] [PubMed] [Google Scholar]
- 32.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20:791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 33.Dalal KM, Kattan MW, Antonescu CR, et al. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244:381–391. doi: 10.1097/01.sla.0000234795.98607.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kane JM., III At the crossroads for retroperitoneal sarcomas: the future of clinical trials for this “orphan disease”. Ann Surg Oncol. 2006;13:442–443. doi: 10.1245/ASO.2006.09.909. [DOI] [PubMed] [Google Scholar]
- 35.Kaushal A, Citrin D. The role of radiation therapy in the management of sarcomas. Surg Clin North Am. 2008;88:629–646. viii. doi: 10.1016/j.suc.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahat G, Tuvin D, Wei C, et al. New perspectives for staging and prognosis in soft tissue sarcoma. Ann Surg Oncol. 2008;15:2739–2748. doi: 10.1245/s10434-008-9970-6. [DOI] [PubMed] [Google Scholar]
- 37.Brennan MF. Staging of soft tissue sarcoma: what is new? Ann Surg Oncol. 2008;15:2643. doi: 10.1245/s10434-008-0093-x. [DOI] [PubMed] [Google Scholar]
- 38.Anaya DA, Lahat G, Liu J, et al. Multifocality in retroperitoneal sarcoma: a prognostic factor critical to surgical decision-making. Ann Surg. 2009;249:137–142. doi: 10.1097/SLA.0b013e3181928f2f. [DOI] [PubMed] [Google Scholar]