Abstract
Osteosarcoma (OSA) is the most frequently occurring malignant primary bone tumour in dogs and children and arises from cells of the osteoblast lineage. Inappropriate Wnt signalling activity has been implicated in human OSA. Altered expression of β-catenin, an integral member of the Wnt signalling pathway, has been associated with numerous human cancers, including OSA. In this study, 30 of the 37 primary canine OSA tissues and 2 of the 3 metastatic OSAs were positive for β-catenin expression as determined by immunohistochemistry, whereas 2 normal bones stained negative for β-catenin. No mutations were identified in exon 3 of β-catenin in the three OSA cases in which DNA sequencing was performed. Finally, there was no relationship between β-catenin expression and overall survival time or disease-free interval. Our results indicate β-catenin is frequently expressed within the cytoplasm of neoplastic cells in canine OSA but contains no detectable mutations in exon 3, similar to human OSA.
Keywords: comparative oncology, immunohistochemistry, Wnt signalling
Introduction
β-Catenin is an intracellular protein with two important cellular functions: cell–cell adhesion and transmission of extracellular-initiated Wnt signals to the nucleus.1 The Wnt signalling pathway is involved in cellular differentiation and proliferation in a cell-type dependent fashion. In the absence of Wnt signalling, β-catenin is targeted for destruction by a complex consisting of glycogen synthase kinase-3β (GSK-3β), adenomatous polyposis coli tumour suppressor protein and axin.2,3 This multiprotein complex phosphorylates specific serine/threonine residues within the amino terminal region of β-catenin necessary for recognition and destruction via the ubiquitin–proteosome pathway. Activation of the Wnt pathway occurs through ligand-specific binding to receptors. Ligand-bound receptors activate the dishevelled protein (Dsh/Dv1), which in turn binds to proteins in the β-catenin degradation complex and prevents their binding to β-catenin. This sequence of events results in the stabilization of β-catenin within the cytoplasm. Stabilized β-catenin translocates into the nucleus, where it interacts with members of the T-cell factor/lymphoid enhancer factor (Tcf/Lef) family of DNA-binding proteins to promote the transcription of Wnt-responsive genes including cyclin D1, c-myc and numerous matrix metalloproteinases.1
Abnormalities in Wnt signalling pathway activity are associated with a variety of human cancers including neoplasia of the colon, breast, brain and liver.4 In these cancers, there is aberrant accumulation of β-catenin in the cytoplasm and/or nucleus relative to non-cancerous tissues. The accumulation of β-catenin may be attributable to alterations of regulatory proteins associated with β-catenin degradation, mutations of serine/threonine residues within β-catenin necessary for its targeted destruction or overexpression of Wnt ligands resulting in excess pathway activity.4
The Wnt signalling pathway has been identified as an essential pathway in mammalian skeletal development.5 Activation of the Wnt signalling pathway is necessary for the commitment of mesenchymal stem cells to the osteoblast lineage.6 Both excessive and inadequate Wnt pathway activities are associated with pathologic bone conditions such as osteopetrosis and osteoporosis, respectively.7 Additionally, Wnt signalling pathway alterations have been reported in human osteosarcoma (OSA) primary tissues and cell lines, though additional work is necessary to determine this pathway’s contribution to OSA development.8–11 Recently, a study using gene expression profiling of canine OSA indicated Wnt signalling pathway alterations were one of several deregulated pathways in canine OSA.12 Although this study did not identify β-catenin as being overexpressed at the RNA level, other gene changes associated with Wnt pathway activation are likely to result in stabilization of the β-catenin protein by preventing its degradation. Therefore, it is not surprising that β-catenin RNA was not found to be overexpressed given that most regulation of β-catenin expression occurs at the post-translational level via ubiquitin-mediated degradation.2,3 Currently, controversy exists regarding the importance of Wnt signalling alterations in OSA with some arguing the pathway is selected against in OSA cells.13,14
OSA in dogs and humans shares numerous similarities including sites of primary disease development, metastatic pattern and prognostic factors.15–18 The relevance of canine OSA as a model for human OSA is becoming more widely accepted because of the similarities in the clinical course, common molecular alterations, the increased incidence of OSA development in dogs relative to humans and the comparatively shorter disease course in dogs.19 Given the importance of Wnt signalling in bone biology, controversy regarding its involvement with human OSA and the utility of canine OSA as a model for human OSA we are interested in determining the status of Wnt signalling in canine OSA. Therefore, this study aimed to determine the frequency of cytoplasmic or nuclear accumulation of β-catenin in canine OSA, partially explore its mutational status and interrogate its impact on disease outcome in canine OSA.
Materials and methods
Sample selection
Archived tissue blocks of canine OSA and normal bone from the Pathology Service at the University of Wisconsin-Madison Veterinary Medical Teaching Hospital (UWVMTH) were identified by medical records search. Canine OSA tissue was collected at the time of diagnostic biopsy, surgical amputation or necropsy. Normal bone was collected from dogs presenting to the UWVMTH necropsy service with no history of OSA or other orthopaedic disease. The normal bone was collected from the humerus of a 13-year-old female-spayed (FS) Papillon and the femur of a 7-year-old FS Labrador Retriever. Haematoxylin–eosin (H&E) and unstained slides were made using 5-μm slices from paraffin-embedded sections of OSA tissue and normal bone. One investigator (A. M.) from the histopathology service reviewed all H&E-stained slides cases to confirm the diagnosis of either OSA or normal bone.
Medical records were reviewed for all cases with a histopathologic diagnosis of OSA. The following information was recorded from the medical records: signalment, age at diagnosis, date of diagnosis, location of primary tumour, type of treatment elected by owners (surgery, chemotherapy, radiation or combination), date of metastatic disease development (if occurred) and date of death. The overall survival time, defined as the number of days from the date of diagnosis until death, was determined for all patients. Disease-free interval (DFI) was calculated for patients in which no metastatic lesions could be detected at the time of diagnosis and had surgery to remove their primary OSA lesion. DFI was defined as the number of days between surgery and development of metastatic disease, recurrent disease or death as a result of any cause.
Detection of β-catenin by Western blot analysis
To determine whether the β-catenin antibody would cross-react with β-catenin in canine tissues (BD Transduction Laboratories; 610154, BD Biosciences, San Jose, CA, USA), Western blot analysis was performed on cell lysates from canine (D17 and Abrams) and human (Saos-2) OSA cell lines. Lysate from the Saos-2 cell line served as a positive control, as β-catenin has been detected in this cell line.9 Cells were lysed using a mammalian protein extraction reagent (Pierce, Rockford, IL, USA) and protein lysates collected. Proteins from cell lysates were separated on a 7.5% sodium dodecyl sulphate–polyacrylamide gel at 150 V for 1.5 h. Proteins were transferred onto a nitrocellulose membrane at 100 V for 1 h and blocked with tris-buffered saline (TBS) containing 5% non-fat dry milk and 1% bovine serum albumin for 1 h. The membranes were incubated overnight with a mouse anti-β-catenin antibody diluted 1:2000 in blocking solution. Primary antibody was removed by washing in TBS/0.05% Tween-20 (TBST) three times for 5 min. Membranes were exposed to a horseradish peroxidase-conjugated antimouse secondary antibody (for 1 h at room temperature, washed in TBST three times for 5 min and treated with a chemiluminescent substrate (Pierce; #34080, Thermo Scientific, Rockford, IL, USA). Blots were visualized after exposure to film and analysed using a Gel Logic 100 Imaging System (Kodak, Rochester, NY, USA).
Detection of β-catenin by immunohistochemistry
Immunohistochemistry (IHC) was performed on unstained slides of canine OSA and normal bone samples to detect β-catenin protein. Slides were deparaffinized in CitriSolv, rehydrated through an ethanol series and rinsed in type II H2O. Endogenous peroxidase activity was blocked with 0.5% hydrogen peroxide in methanol. Slides were rinsed in tap water for 5 min and heat treated in 0.1 M citrate buffer for antigen retrieval. Tissue sections on the slide were soaked in a solution of dried milk in phosphate-buffered saline (PBS) (0.5 g 100 mL−1) for 5 min. To block non-specific binding, normal horse serum from the mouse IgG Vector Vectastain Elite ABC Kit (PK-6102, Vector Laboratories, Burlingame, CA, USA) was mixed with PBS/milk solution and applied to each slide. Excess blocking solution was blotted from the slides and the mouse anti-β-catenin primary antibody, diluted 1:100 in PBS/milk solution, applied to each slide. Slides serving as negative controls were treated with the PBS/milk solution in which the primary antibody was omitted. All slides were rinsed in PBS, treated with a Vectastain Elite biotinylated antimouse antibody followed by Vectastain Elite ABC reagent, rinsed in PBS and treated with 3,3′-diaminobenzidine substrate solution (Vector Laboratories; PK-6102). Slides were rinsed in PBS and counterstained with Mayer’s haematoxylin, dehydrated through an ethanol series, soaked in CitriSolv and coverslipped.
Characterization of β-catenin expression in canine OSA
β-Catenin expression was quantified in canine OSA and normal bone by one investigator (A. M.) using a score based on the percentage of neoplastic cells staining positive and the intensity of staining.20,21 A total of 10 fields viewed at 400× magnification were assessed for each sample. The positive-stained samples were evaluated for membranous, cytoplasmic and/or nuclear staining pattern. The percentage labelling score (%LS) for β-catenin was scored as follows: 0% = LS0,1 –<10% = LSI, 10 –30% = LS2, >30 –60% = LS3, >60% = LS4. The intensity score (IS) for staining was scored as follows: negative = IS0, mild = IS1, moderate = IS2, intense = IS3. The β-catenin score (BCS) was the product of LS and IS.
Determination of β-catenin exon 3 mutational status
To determine if mutations in exon 3 were associated with β-catenin staining, genomic DNA was isolated from paraffin-embedded OSA tissue. Paraffin-embedded tissue was removed from the slide with a razor blade, placed into a micro-centrifuge tube and treated twice with xylene to remove the paraffin wax. The tissue was washed in 100% ethanol at 37 °C for 16 min and genomic DNA isolated using a commercial kit (Qiagen DNeasy Blood & Tissue Kit; #69504, Qiagen Inc., Valencia, CA, USA). Primers to canine β-catenin exon 3 were designed to cover the intron/exon junction of exon 3 using the sequence information for canine β-catenin available from NCBI accession number NM_001137652 (Fig. SI, Supporting Information). Forward and reverse primers were generated with the following sequences:(F) 5′-GGGTAGCACAAATTCAGGTGAATGC-3′ and (R) 5′-CATTCTGAGGCTCCTTGAGAGTT-3′ (Eurofms MWG Operon, Huntsville, AZ, USA). Amplification of β-catenin exon 3 was accomplished using 50 pmol forward primer, 50 pmol reverse primer, 15 μL H2O, 8 μL template and 25 μL of master mix containing a high fidelity polymerase (Qiagen; #203443). The quantity of template DNA in each amplification reaction ranged from 10 to 35 ng. Cycling conditions were 95 °C for 15 min, 90 cycles of 95 °C for 1 min, 58 °C for 1 min and 72 °C for 1 min, followed by 72 °C for 10 min. Polymerase chain reaction products were visualized on a 1% agarose gel containing 0.1% ethidium bromide. The Qiagen Gel Extraction Kit (#28404) was used to extract the amplified β-catenin exon 3 from the gel. The extracted product was concentrated by ethanol precipitation and resuspended in tris-ethylenediaminetetraacetic acid buffer. For DNA sequencing of exon 3, the following mixture was used: 7 μL of DNA template, 5 pmol of forward primer, 3 μL of sequencing buffer, 2 μL of big dye terminator, 1 μL of dimethyl sulphoxide and 6 μL of H2O. The DNA sequencing mixture was exposed to 95 °C for 3 min, followed by 50 cycles of 96 °C for 10 s, 58 °C for 4 min and 72 °C for 7 min. Sequencing of the product was performed by the University of Wisconsin-Madison Biotechnology Center. Sequence information was viewed using FinchTV Version 1.4.0 (Geospiza, Seattle, WA, USA).
Statistical analysis
Kaplan–Meier curves were generated for overall survival times and DFI for dogs treated with surgery and adjuvant chemotherapy. Using the median β-catenin staining score as the cut-off for comparison (BCS ≤ 4.1 versus β-catenin score >4.1) we used the log rank test to determine if there was an association between β-catenin staining and DFI or overall survival time for dogs treated similarly. A P value <0.05 was considered to be significant.
Results
Canine OSA cases selected for evaluation
Thirty-seven cases of primary canine OSA arising from the appendicular skeleton and 3 cases of pulmonary metastatic OSA were used in this study. One of the three cases of pulmonary metastatic OSA had the corresponding primary tumour available for analysis. Samples of bone collected at the time of necropsy from two dogs without OSA were used to determine the presence of β-catenin in normal bone.
The number and breeds of dogs from which tumour tissue was collected included 12 Labrador Retrievers, 3 Golden Retrievers, 3 Saint Bernards, 2 Boxers, 2 Great Danes and an Akita, Australian Shepherd, Belgian Tervuran, Doberman, English Springer Spaniel, Great Pyrenees, Greyhound, Leonberger, Siberian Husky, Rottweiler and a West Highland White Terrier. Primary OSA tissue was from 20 male neutered (MN), 14 FS, 2 male intact and 1 female intact dogs with a median age of 8 years (range: 2–14 years). The number of samples and locations of the primary tumours are 12 distal radius, 9 proximal humerus, 5 distal femur, 4 distal tibia, 2 proximal tibia, 1 calcaneous, 1 carpus, 1 femoral head, 1 scapula and 1 proximal radius. The pulmonary metastatic OSA tissue was from two Labrador Retrievers (1 MN/1 FS) and one MN Giant Schnauzer, with a median age of 9 years (range: 8–11 years). Twenty-five dogs were treated with amputation and chemotherapy, 4 dogs underwent amputation only, 1 dog was treated with palliative radiation therapy only and the treatment for 7 dogs was unknown. Information on survival time was available for 34 of the 37 cases. The median survival time for all dogs analysed was 224 days. The DFI could be determined for 22 of the 25 dogs treated with surgery and adjuvant chemotherapy, with the median DFI being 157 days.
Validation of anti-β-catenin antibody in dogs
To determine if the anti-β-catenin antibody would react with canine β-catenin we performed Western blot analysis using one human OSA cell line (Saos-2) known to express β-catenin and two canine OSA cell lines (D-17 and Abrams), in which the β-catenin expression status is unknown. Using the anti-β-catenin antibody for Western blot analysis a protein of appropriate size (approximately 92 kDa in humans and 88 kDa in canines) was identified in all three OSA cell lines (Fig. 1).
Figure 1.
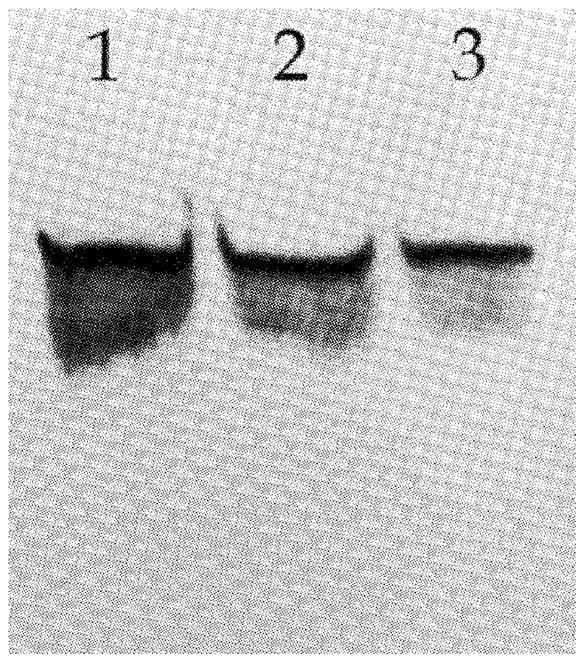
Western blot analysis of β-catenin in human (Saos-2 = lane 1) and canine (D17 = lane 2; Abrams = lane 3) OSA cell lines displaying appropriately sized bands (~90 kDa)
Characterization of β-catenin expression by IHC in canine OSA
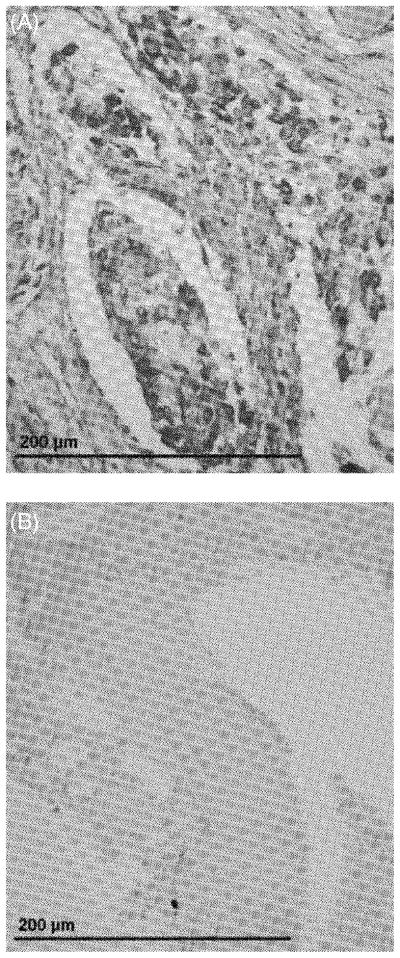
When the anti-β-catenin antibody was used for IHC, β-catenin was detected in 30 of the 37 (81%) cases of canine primary OSA (Fig. 2A and Table 1). Of the 30 samples with positive staining, all 30 (100%) had cytoplasmic staining, 9 had membranous (30%) and 3 (10%) had nuclear staining (Table 1). Reactive osteoblasts in a small amount of reactive bone adjacent to neoplastic tissue in one OSA sample displayed positive cytoplasmic staining for β-catenin. There was no positive staining for β-catenin in osteoclasts in any of the seven OSA samples in which this cell type was observed in adjacent non-neoplastic bone. Two of the three metastatic OSA samples stained positive for β-catenin, both of which displayed cytoplasmic staining. In contrast, osteoblasts and osteoclasts lining the osseous trabeculae in normal bone did not have detectable staining with the anti-β-catenin antibody (Fig. 2B). The median BCS in primary OSA tissue was 4.1 (Table 1).
Figure 2.
IHC of a representative case of (A) canine OSA and (B) normal bone stained for the presence of β-catenin.
Table 1.
Immunohistochemical staining patterns of β-catenin in canine OSA
| Primary OSA (n = 37)
| |||
|---|---|---|---|
| %LSa | ISb | BCSc | Staining pattern |
| LS0 (7) | IS0 (7) | BCS0 (7) | Total number of positive cases = 30/37 |
| LS1 (0) | IS1 (17) | BCS2 (3) | Membranous = 9/30 |
| LS2 (4) | IS2 (13) | BCS3 (4) | Cytoplasmic = 30/30 |
| LS3 (6) | IS3 (0) | BCS4 (9) | Nuclear = 3/30 |
| LS4 (20) | BCS6 (2) | ||
| BCS8 (12) | |||
|
| |||
|
Metastatic OSA (n = 3)
| |||
| %LSa | ISb | BCSc | Staining pattern |
|
| |||
| LS0 (1) | IS0 (1) | BCS0 (1) | Total number of positive cases = 2/3 |
| LS2 (1) | IS1 (2) | BCS2 (1) | Membranous = 0/2 |
| LS4 (1) | BCS4 (1) | Cytoplasmic = 2/2 Nuclear = 0/2 |
|
Numbers in parentheses following LS#, IS# and BCS# indicate number of dogs in that category.
Labelling scores: LS0 = 0%, LS1 = <10%, LS2 = 10–30%, LS3 = >30–60%, LS4 = >60%.
intensity scores: IS0 = negative, IS1 = mild, IS2 = moderate, IS3 = intense.
BCS: LS × IS.
Sequence analysis of β-catenin exon 3
DNA sequencing was performed to determine if β-catenin expression in canine OSA was associated with mutations in exon 3. β-Catenin exon 3 was successfully amplified and sequenced from three OSA tissue samples representative of the different staining patterns (non-staining OSA, membranous staining, cytoplasmic staining and nuclear staining). Exon 3 of β-catenin was selected for mutational evaluation as it contains the consensus coding sequence for GSK-3β phosphorylation, necessary for subsequent ubiquitination and proteosomal degradation.1–3 No mutations were identified in exon 3 of β-catenin in any of the samples (non-staining, membranous, cytoplasmic and nuclear).
Assessment of β-catenin staining characteristics and disease outcomes
Finally, we compared overall survival times and DFIs of dogs based on their BCS (LS × IS), using the median value as a cut-off between groups. We found no difference in overall survival times when patients were compared according to the β-catenin staining product score (PS ≤ 4.1 versus PS > 4.1) (Fig. 3). Similarly, when the DFI for dogs treated with surgery and adjuvant chemotherapy having a PS ≤ 4.1 was compared with similarly treated dogs with a PS > 4.1 there was no difference (196 days versus 161 days, respectively; P = 0.2) (Fig. 4).
Figure 3.
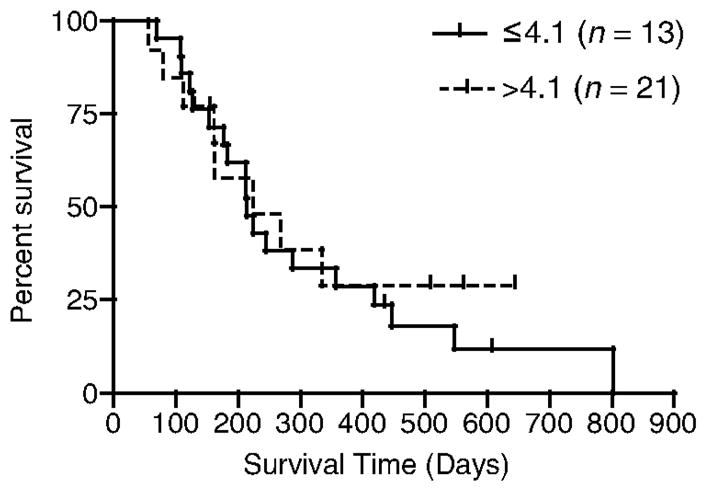
Overall survival of dogs with OSA based on BCS.
Figure 4.
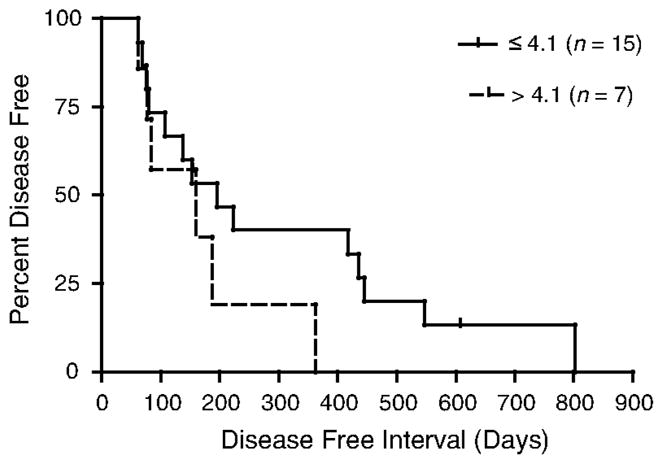
DFI for dogs with OSA treated with surgery and adjuvant chemotherapy based on BCS.
Discussion
OSA is the most common malignant primary bone tumour of dogs and humans. The disease in dogs shares numerous similarities with the human form, including sites of predilection, high metastatic potential and molecular alterations.15–18 Unfortunately, modifications to the current standard of care for canine OSA, typically a combination of surgery and chemotherapy, have resulted in relatively minimal improvements in outcome. Further improvements in patient outcome will likely result from an improved understanding of the role specific genetic and molecular alterations play in OSA development and progression. The Wnt signalling pathway has been identified as a key component of normal skeletal development and disease.5–7 Alterations within this signalling pathway have been described in human and canine OSA.8,9,12 Currently, debate exists as to whether or not alterations in this pathway contribute to human OSA development and progression. These conflicting reports indicate additional research is necessary to clarify the role of the Wnt signalling pathway in OSA development and progression. Given the numerous other similarities between human and canine OSA, we were interested in determining the status of β-catenin expression in canine OSA.
Using a combination of Western blot, IHC and DNA sequencing, we characterized the expression of β-catenin in 37 cases of primary canine OSA and 3 cases of pulmonary metastases from canine OSA. β-Catenin was detected by IHC in the majority of canine OSA samples and its intracellular location was most frequently cytoplasmic. These results are similar to human OSA, in which β-catenin was detected by IHC in approximately 70% of samples.8 In a separate study, 90% of OSA samples had membranous and/or cytoplasmic β-catenin expression detectable by IHC, with the remaining 10% of samples displaying nuclear staining.13 The importance of β-catenin’s subcellular location (i.e. membranous versus cytoplasmic versus nuclear) remains a matter of debate; however, it is the nuclear component that seems to be most relevant to carcinogenesis.22 Within the nucleus, β-catenin binds to Tcf and Lef transcription factors to upregulate expression of target genes such as c-myc, cyclin D1, survivin and matrix metalloproteinases.23–27 Previous work has found some of these target genes to be associated with OSA.28–29
To determine if the expression of β-catenin in canine OSA was associated with stabilizing mutations we performed DNA sequencing on a subset of the tumours and found no mutations. We chose to evaluate exon 3 for mutations because this sequence contains serine and threonine residues necessary for phosphorylation by GSK-3β After GSK-3β phosphorylation, β-catenin undergoes ubiquitination and is targeted for proteosomal degradation.1–3 Mutations within exon 3 often prevent the degradation of β-catenin, resulting in prolonged expression of β-catenin and subsequent upregulation of its target genes.4 No mutations in β-catenin exon 3 were detected in three OSA samples, but since this sample size was small, the possibility of exon 3 mutations occurring in other cases of canine OSA cannot be excluded. Other mechanisms by which β-catenin may be stabilized include the repression of Wnt pathway antagonists, overexpression of Wnt ligands or defects in the degradation machinery necessary for β-catenin destruction.9,11,30 A recent study by Kansara et al.11 found Wnt inhibitory factor 1 (Wif1), a Wnt pathway antagonist, was epigenetically silenced in human OSA cell lines and associated with increased β-catenin expression. Furthermore, the targeted disruption of Wif1 in mice accelerated osteosarcomagenesis.11
Finally, we failed to detect any association between β-catenin expression and survival time for dogs with OSA. In addition, there was no association between β-catenin expression and DFI for dogs treated with both surgery and adjuvant chemotherapy. Reports on the association of Wnt signalling pathway alterations and disease progression or outcome in humans with OSA are mixed. Haydon et al.8 detected no correlation between β-catenin expression and overall survival time in 30 patients. Conversely, the presence of LRP5, a receptor for the Wnt signalling pathway, correlated with tumour metastasis in a study involving 44 OSA patients.9 A potential reason for seeing no association with survival or disease progression and β-catenin expression in canine or human OSA could be the relative frequency with which β-catenin is expressed in OSA and the small number of patients that are available for study. Since most OSA samples are positive for β-catenin, an extremely large sample size would be required to detect any significant difference in disease progression or outcome. Conversely, the high frequency of β-catenin expression in OSA suggests this pathway is important to OSA development in general and may be of therapeutic value in the future.
In conclusion, the cytoplasmic/nuclear expression of β-catenin is a frequent event in canine OSA, though its expression is not associated with exon 3 mutations. This suggests that other members of the Wnt signalling pathway may be contributory. These findings are similar to those reported in human OSA. Similarly, we did not detect any association between survival time and β-catenin expression. Given the similarities in OSA between the two species and the increased incidence of OSA in dogs, the dog may represent a relevant model for human OSA regarding this pathway. Our findings support the model potential of spontaneously developing canine OSA with respect to further elucidating the contribution of Wnt signalling abnormalities in OSA.
References
- 1.Polakis P. Wnt signaling and cancer. Genes and Development. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 2.Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 3.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 4.Hajra KM, Fearon ER. Cadherin and catenin alterations in human cancer. Genes Chromosomes Cancer. 2002;34:255–268. doi: 10.1002/gcc.10083. [DOI] [PubMed] [Google Scholar]
- 5.Day TF, Guo X, Garret-Beal L, Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Developmental Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Developmental Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Glass DA, Karsenty G. In vivo analysis of Wnt signaling in bone. Endocrinology. 2007;148:2630–2634. doi: 10.1210/en.2006-1372. [DOI] [PubMed] [Google Scholar]
- 8.Haydon RC, Deyrup A, Ishikawa A, Heck R, Jiang W, Zhou L, Feng T, King D, Cheng H, Breyer B, Peabody T, Simon MA, Montag AG, He TC. Cytoplasmic and/or nuclear accumulation of the β-catenin protein is a frequent event in human osteosarcoma. International Journal of Cancer. 2002;102:338–342. doi: 10.1002/ijc.10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoang BH, Kubo T, Healey JH, Sowers R, Mazza B, Yang R, Huvos AG, Meyers PA, Gorlick R. Expression of LDL receptor-related protein 5 (LRP5) as a novel marker for disease progression in high-grade osteosarcoma. International Journal of Cancer. 2004;109:106–111. doi: 10.1002/ijc.11677. [DOI] [PubMed] [Google Scholar]
- 10.Guo Y, Rubin EM, Xie J, Zi X, Hoang BH. Dominant negative LRP5 decreases tumorigenicity and metastasis of osteosarcoma in an animal model. Clinical Orthopedics and Related Research. 2008;466:2039–2045. doi: 10.1007/s11999-008-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kansara M, Tsang M, Kodjabachian L, Sims NA, Trivett MK, Ehrich M, Dobrovic A, Slavin J, Choong PF, Simmons PJ, Dawid IB, Thomas DM. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. Journal of Clinical Investigation. 2009;119:837–851. doi: 10.1172/JCI37175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selvarajah GT, Kirpensteijn J, van Wolferen ME, Rao NA, Fieten H, Mol JA. Gene expression profiling of canine osteosarcoma reveals genes associated with short and long survival times. Molecular Cancer. 2009;8:72–89. doi: 10.1186/1476-4598-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai Y, Mohseny AB, Karperien M, Hogendoorn PC, Zhou G, Cleton-Jansen AM. Inactive Wnt/β-catenin pathway in conventional high-grade osteosarcoma. Journal of Pathology. 2010;220:24–33. doi: 10.1002/path.2628. [DOI] [PubMed] [Google Scholar]
- 14.Cleton-Jansen AM, Anninga JK, Briaire-de Bruijn IH, Romeo S, Oosting J, Egeler RM, Gelderblom H, Taminiau AH, Hogendoorn PC. Profiling of high-grade central osteosarcoma and its putative progenitor cells identifies tumourigenic pathways. British Journal of Cancer. 2009;101:1909–1918. doi: 10.1038/sj.bjc.6605405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dernell WS, Ehrhart NP, Straw RC, Vail DM. Tumors of the skeletal system. In: Withrow SJ, Vail DM, editors. Withrow & MacEwen’s Small Animal Clinical Oncology. 4. St. Louis, MO: Saunders, Elsevier; 2007. pp. 540–582. [Google Scholar]
- 16.Thomas R, Wang HJ, Tsai PC, Langford CF, Fosmire SP, Jubala CM, Getzy DM, Cutter GR, Modiano JF, Breen M. Influence of genetic background on tumor karyotypes: evidence for breed–asscoiated aberrations in canine appendicular osteosarcoma. Chromosome Research. 2009;17:365–377. doi: 10.1007/s10577-009-9028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Maria R, Miretti S, Iussich S, Olivero M, Morello E, Bertotti A, Christensen JG, Levine RA, Buracco P, Di Renzo MF. Met oncogene activation qualifies spontaneous canine osteosarcoma as a suitable pre-clinical model of human osteosarcoma. Journal of Pathology. 2009;218:399–408. doi: 10.1002/path.2549. [DOI] [PubMed] [Google Scholar]
- 18.Paoloni M, Davis S, Lana S, Withrow S, Sangiorgi L, Picci P, Hewitt S, Triche T, Meltzer P, Khanna C. Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC Genomics. 2009;10:625–637. doi: 10.1186/1471-2164-10-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nature Reviews Cancer. 2008;8:147–156. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 20.Beam SL, Rassnick KM, Moore AS, McDonough SP. An immunohistochemical study of cyclooxygenase-2 expression in various feline neoplasms. Veterinary Pathology. 2003;40:496–500. doi: 10.1354/vp.40-5-496. [DOI] [PubMed] [Google Scholar]
- 21.Looper JS, Malarkey DE, Ruslander D, Proulx D, Thrall DE. Epidermal growth factor receptor expression in feline oral squamous cell carcinoma. Veterinary and Comparative Oncology. 2006;4:33–40. doi: 10.1111/j.1476-5810.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 22.Hoang BH, Kubo T, Healey JH, Yang R, Nathan SS, Kolb EA, Mazza B, Meyers PA, Gorlick R. Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-β-catenin pathway. Cancer Research. 2004;64:2734–2739. doi: 10.1158/0008-5472.can-03-1952. [DOI] [PubMed] [Google Scholar]
- 23.Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, Matrisian LM. The metalloproteinase matrilysin is a target of β-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–2891. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- 24.Tetsu O, McCormick F. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 25.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 26.Kim PJ, Plescia J, Clevers H, Fearon ER, Altieri DC. Survivin and molecular pathogenesis of colorectal cancer. Lancet. 2003;362:205–209. doi: 10.1016/S0140-6736(03)13910-4. [DOI] [PubMed] [Google Scholar]
- 27.Marchenko ND, Marchenko GN, Weinreb RN, Lindsey JD, Kyshtoobayeva A, Crawford HC, Strongin AY. β-Catenin regulates the gene of MMP-26, a novel metalloproteinase expressed both in carcinomas and normal epithelial cells. International Journal of Biochemistry and Cell Biology. 2004;36:942–956. doi: 10.1016/j.biocel.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Fife RS, Rougraff BT, Proctor C, Sledge GW. Inhibition of proliferation and induction of apoptosis by doxycycline in cultured human osteosarcoma cells. Journal of Laboratory and Clinical Medicine. 1997;130:530–534. doi: 10.1016/s0022-2143(97)90130-x. [DOI] [PubMed] [Google Scholar]
- 29.Cakir Y, Hahn KA. Direct action by doxycycline against osteosarcoma cell proliferation and collagenase (MMP-1) activity in vitro. In Vivo. 1999;13:327–331. [PubMed] [Google Scholar]
- 30.Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531–7537. doi: 10.1038/sj.onc.1210059. [DOI] [PubMed] [Google Scholar]