Abstract
Biological membranes are heterogeneous assemblies of lipids, proteins, and cholesterol that are organized as asymmetric bimolecular leaflets of lipids with embedded proteins. Modulated by the concentration of cholesterol lipids and proteins may segregate into two or more liquid phases with different physical properties that can coexist in the same membrane. In this review, we summarize recent advances on how this situation can be recreated in a supported bilayer format and how this system has been used to demonstrate the induction of ordered lipid domains in lipid compositions that are typical for the inner leaflet by lipid compositions that are typical for the outer leaflet of mammalian plasma membranes. Proteins are shown to differentially target such induced inner leaflet domains.
Keywords: Lipid domains, lipid asymmetry, lipid raft, cholesterol, phase coupling in lipid bilayers, supported planar bilayer, fluorescence microscopy
1. Introduction
It has been long recognized that the classical Singer-Nicholson fluid-mosaic model [1], while remaining valid as a basic concept, provides a drastically oversimplified picture of the true nature of biological membranes. Real membranes are actually more mosaic than fluid as was recently pointed out by Engelman [2]. They are heterogeneous patchworks of groups of lipids, integral membrane proteins, and cholesterol that all cover substantial fractions of the entire membrane area. A contemporary model of a biological membrane is depicted in Figure 1. Phosopholipids are organized in a bilayer with the polar headgroups exposed to the surrounding aqueous environments and the hydrocarbon tails forming a hydrophobic core in the center of the membrane.
Figure 1.
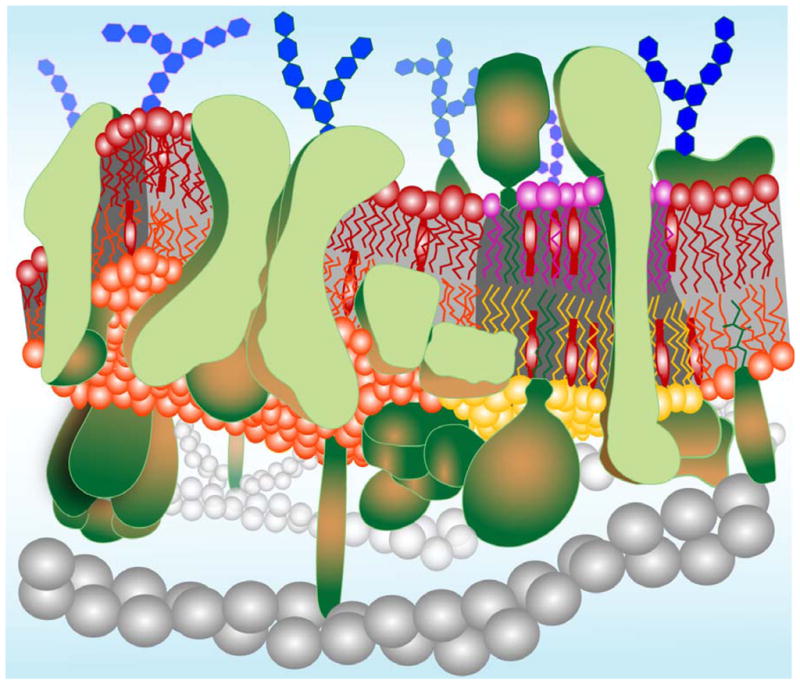
A contemporary model of a biological membrane. Lipids are organized in a dynamic bimolecular film with asymmetry across the membrane and a lateral organization in cholesterol-rich and cholesterol-poor domains. The outer leaflet (purple and red) is enriched in sphingomyelin and the inner leaflet (orange and yellow) is enriched in phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol. Both leaflets contain similar amounts of phosphatidylcholine and cholesterol (lollipop-shaped red structures). Cholesterol-rich lipid domains (“rafts”) are shown in register in both leaflets as determined experimentally (see text). Transmembrane, monotopic (partially inserted), and lipid-anchored proteins (green) occupy much of the total available membrane area and are also clustered into functional complexes in many cases. Glycosylations on proteins and lipids of the outer leaflet are shown as blue hexagon-shaped structures. Gray beaded helical structures symbolize elements of the cytoskeleton, which interact with proteins on the cytoplasmic leaflet of the membrane.
Many biological membranes are asymmetric with respect to lipid distributions across the bilayer. For example, the plasma membranes of eukaryotic cells are rich in sphingomyelin (SM) and phosphatidylcholine (PC) in the outer exoplasmic leaflet and rich in PC, phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidylinositols (PIs) in the inner cytoplasmic leaflet (Figure 2). This asymmetry is maintained by a set of lipid translocases (“flippases”), which are ATP-dependent integral membrane ABC transporters [3]. Several classes of lipid-anchored membrane proteins are also asymmetrically distributed across plasma membranes of eukaryotic cells. Biosynthetic pathways are responsible for establishing the asymmetry of these classes of proteins. Glycophosphatidylinositol(GPI)-anchored membrane proteins are exclusively present on the exoplasmic leaflet, whereas prenylated proteins are only found on the cytoplasmic face of plasma membranes. Similarly, carbohydrates are biosynthetically attached to membrane proteins and lipids in the lumen of the Golgi. When membrane vesicles are transported from the Golgi to fuse with the plasma membrane, the luminal face of Golgi membranes becomes the extracellular face of plasma membranes. Membrane proteins, whether they contain a single or multiple transmembrane (TM) domains are also always uni-directionally incorporated during their biogenesis in the endoplasmic reticulum (ER) and therefore end up in a specific orientation in any ER-derived membranes including plasma membranes.
Figure 2.
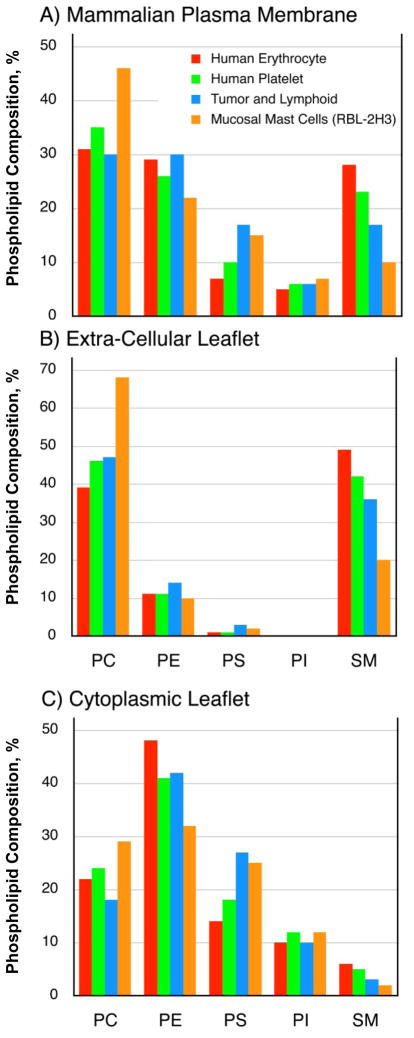
Inner and outer leaflet lipid compositions of some mammalian plasma membranes. (A) Estimated total lipid headgroup compositions of the human erythrocyte (red, [36]), human platelet (green; [37]), tumor and lymphoid cell (blue; [38]), and mucosal mast cell (orange; [39]) plasma membranes. (B) Estimated lipid headgroup compositions of the extra-cellular leaflets of mammalian plasma membranes based on the works of refs. [36, 37]. (C) Estimated lipid headgroup compositions of the cytoplasmic leaflets of mammalian plasma membranes based on the works of refs. [36, 37].
Biological membranes are also very heterogeneous with respect to the lateral organization of both lipids and proteins. It has long been recognized that membrane proteins may assemble in functional clusters in the plane of the membrane. For example, the electron transfer systems I through IV are thought by some authors to form large functional assemblies in inner mitochondrial membranes [4]. More recent attention has focused on lateral assemblies of lipids in plasma and other biomembranes. Cholesterol has been recognized as a major modulator of lateral membrane structure. Cholesterol interacts with different lipids in different ways creating transient structures [5] that can phase separate into domains with different structural and dynamical properties. These domains are sometimes also called “lipid rafts.” Since these structures have been inferred initially from rather harsh biochemical treatments of cell membranes and since they cannot be directly visualized in cells, they have remained rather controversial. Cholesterol and SM and certain proteins are left behind when other lipids and other proteins are extracted from cell membranes with the detergent Triton X-100 at low temperatures. Since the detergent resistant mixtures of SM, PC and Chol have been known to form more ordered lipid phases than other typical cell lipid mixtures without SM, these liquid-ordered phases are thought to occur also in cell membranes, but perhaps in quite small clusters so that they have so far escaped direct detection in cells. Although it is clear that cold detergent extracts do not directly resemble anything physiological in cell membranes [6], there are still interesting empirical correlations. Many membrane proteins, including most GPI-linked proteins, signaling receptors in various cells of the immune system, as well as many viral envelope proteins have been found to remain in detergent resistant membrane residues and might therefore be associated with domains of liquid-ordered lipid phases or “rafts” in intact cell membranes. When cholesterol is depleted from such cells, these putative raft-associated proteins are extracted by the same detergent treatment, which supports the notion of their association with lipid rafts in cells. An intriguing correlate of the raft hypothesis is that these structures play a central role in trans-membrane signaling [7]. Importantly, rafts have been hypothesized (but never really strictly proven) to be the organizing platforms for transducing signals from ligand-activated receptors on the extracellular side of the membrane to downstream effector proteins that reside on the intracellular side of the plasma membrane.
Lipid model membranes prepared from typical lipid mixtures have the great advantage (i) that liquid-ordered domains are much bigger than in cells so that they can be directly observed and studied by biophysical techniques, and (ii) that the lipid composition can be easily manipulated, which allows investigators to study their properties as a function of lipid composition. Two systems have been particularly useful in this regard: giant unilamellar vesicles (GUVs) and supported planar lipid bilayers (SPBs). Each of these systems has certain advantages and disadvantages and it depends on the questions, which system is more suitable to answer them. GUVs are particularly powerful to map compositional phase diagrams of coexisting liquid-ordered (lo) and liquid-disordered (ld) lipid phases [8–11], measuring lipid diffusion by fluorescence correlation spectroscopy (FCS, [12]), and to determine other thermodynamic parameters like line tension [13, 14], tie lines, and miscibility critical points [15–17], etc. The planar geometry of SPBs makes them the preferred system for determining relative area fractions of coexisting liquid phases [18, 19], measuring local lateral diffusion coefficients by single particle tracking (SPT), and setting up systems with asymmetric trans-bilayer lipid distributions [18–20]. In addition, potential light-induced artifacts are minimized because much lower light levels are typically used in epi-fluorescence microscopy of SPBs compared to confocal microscopy of GUVs.
An intriguing question that has plagued the hypothesis of transmembrane signaling through rafts for as long as it has been around is: how can rafts, that based on knowledge of the phase behavior of symmetric lipid systems, are only supposed to exist in the outer leaflets of plasma membranes, transmit signals across the membrane to inner leaflets with lipid compositions that by themselves are known to not form rafts? Since under favorable conditions rafts with asymmetric lipid compositions can be produced by step-wise assembly on solid supports, SPBs have been employed recently to answer this important question for the first time. In this short review, we will summarize what we have learned so far from this system about domain coupling in asymmetric supported planar bilayers. Before we get into lipid domain coupling in Sections 3 through 5, we briefly recapitulate in Section 2 the methods for setting up asymmetric supported lipid systems with coexisting lo and ld phase domains. Finally in Section 6, we demonstrate the utility of this system to determine the targeting of proteins to different domains in complex asymmetric heterogeneous membranes.
2. Preparation of Asymmetric Supported Bilayers
Supported membranes are often produced by simple vesicle adsorption to an appropriate hydrophilic surface. This method forms bilayers with symmetric lipid distributions and, therefore, is inadequate to produce asymmetric lipid bilayers. The other two methods to prepare supported bilayers are to sequentially deposit lipid monolayers by the Langmuir-Blodgett/Langmuir-Schäfer (LB/LS, [21]) or the Langmuir-Blodgett/vesicle fusion (LB/VF, [22]) methods (for recent technical descriptions of these methods, see [23]). We have carefully examined these two methods for the purpose of asymmetric supported membrane preparation and made some unexpected discoveries [24]. We found that the lipid compositions in the two leaflets were often very far from the intended compositions that one would expect from the step-wise assembly procedures (see below). A very useful method to ascertain lipid asymmetry and composition in supported membranes has been fluorescence interference contrast (FLIC) microscopy adapted to measure lipid flip-flop across supported membranes [24]. Supporting membranes on an inert highly hydrated polymer cushion on the solid substrate turned out to also be very critical to achieving our goal of maintaining lipid asymmetry in supported membranes. Therefore, we first describe FLIC microscopy and the preparation of tethered polymer supported bilayers as two important advances that were critical prerequisites for enabling the studies described in this review.
FLIC microscopy was originally developed to measure distances of cell membranes and supported membranes from solid surfaces [25, 26]. Briefly, membranes are placed on patterned silicon wafers with oxide layers of different heights. Since the oxide is transparent to visible light, but silicon is not, these substrates act as mirrors on which the membranes are deposited at different heights from the reflecting surface. Excitation and emission light is reflected and forms interference patterns on the surface that can be precisely predicted by a theoretical optical layer model if all optical parameters and the step sizes of the oxide layers are known. The single remaining unknown is the distance of the supported membrane from the oxide surface. Therefore, this distance can be determined by fitting the experimental FLIC data to the optical FLIC theory. It turns out that in a well optimized system, these distances can be determined to an amazing accuracy of ±0.5 nm with light in the 500 to 600 nm wavelength range. If this distance is already known, for example from an independent measurement, the method can be adapted to measure the distribution of fluorescent lipid analogs across the supported membrane [24]. Let us assume a bilayer is labeled with fluorescent lipids (e.g. headgroup-labeled rhodamine-PE) only in the distal leaflet from the reflecting surface. FLIC theory then would predict that the fluorophor distance is the water cleft distance between the bilayer and the substrate, dw, plus 4 nm, i.e. the thickness of a typical fluid lipid bilayer. If we now measure dw plus 2 nm, we know that the bilayer has randomized with respect to lipid asymmetry. If we measure dw plus 0 nm, all lipids have flipped from the outer to the inner surface, and if we measure dw plus 4 nm, the bilayer has remained asymmetric as intended when it was constructed. Other values between 0 and 4 nm may be interpreted in terms of fractions of flipped lipids. The reciprocal experiment, i.e. starting with all labeled lipids in the proximal leaflet is also possible.
The other important technological advance is the development of tethered polymer supported bilayers. Supported bilayer work has occasionally been criticized by claiming that the support affects the properties of the bilayer in important ways [27]. Although we agree that supported bilayers are not ideal to measure delicate phase boundaries in phase diagrams of complex ternary lipid systems (there are disagreements also about details in the use of GUV and multilamellar lipid systems for this purpose, see [11, 28]) and that whole domains are not moving in supported membranes, we believe that many of the other criticisms are unfounded. For example, phase transitions are preserved in supported membranes [21, 29] and the diffusive behavior of lipids in uniform bilayers and within individual domains in heterogeneous bilayers is not appreciably affected by the solid support [19, 29], especially if the bilayer is decoupled from the surface by an intermediate to thick polymer cushion [30]. It thus appears that the typical distinct structural and dynamical properties of lo and ld phases are preserved in well constructed supported bilayer systems.
Although other similar systems have been developed with much success in other laboratories, our preferred polymer is polyethylene glycol (PEG) of molecular weight >3000 that has been covalently attached to a fraction (typically about 3 mol %) of the lipids in the leaflet that faces the substrate. In most cases, we have also tethered these PEG units at the other end to the substrate via silane chemistry [30], but more recently, we found that this is not absolutely required (unpublished results). We have measured by FLIC microscopy that the polymer with an average of 77 glycol units (MW=3400) lifts the bilayer 4 nm from the solid surface of a quartz-like substrate, as would be expected from the Flory radius of this polymer [31]. A water-filled cleft is apparently sufficient to uncouple the bilayer from the surface. For certain applications, the 1–2 nm water-filled cleft that exists in non-polymer supported bilayers is sufficient. It should be noted, however, that these are average distances. Bilayers may undergo distance fluctuations and quartz surfaces are certainly not atomically flat. We believe that small contact points (pins) exist between the support and the bilayer, which are responsible, for example, for the observation that whole domains do not move in these systems. However, these pins may account for as little as 1 % or less of the total bilayer area and, therefore, are negligible when dynamical properties of bilayer components are measured by spectroscopic techniques.
A second unanticipated benefit of the polymer supported bilayer system is that lipids are less prone to randomization when asymmetric bilayers are prepared than in bilayers that are directly supported on oxidized silicon wafers [24]. This same study also showed that lipid asymmetry is better retained when the asymmetric bilayers are constructed by the LB/VF technique than by the LB/LS technique. Apparently, the LB/VF technique is more gentle especially when the LB layer is polymer supported. Capillary forces probably cause water to rush in too quickly in the non-polymer supported bilayers when the first monolayer is touched down onto the second monolayer in the LS step. By contrast, smaller capillary forces in polymer supported bilayers and slower hydration when flowing in vesicles in the VF step preserve lipid asymmetry much better in the polymer supported LB/VF system. We are able to retain nearly 100 % lipid asymmetry shortly after preparation in this system. However, as expected, the asymmetry decays by spontaneous natural lipid flip-flop with a 1/e decay time of ~15 hours in a uniform ld phase PC bilayer [19]. Therefore, LB/VF prepared bilayers can be used for ~2 hours after preparation for lipid asymmetry studies, which is plenty of time to record many images and perform numerous dynamic measurements.
3. Natural Lipid Systems
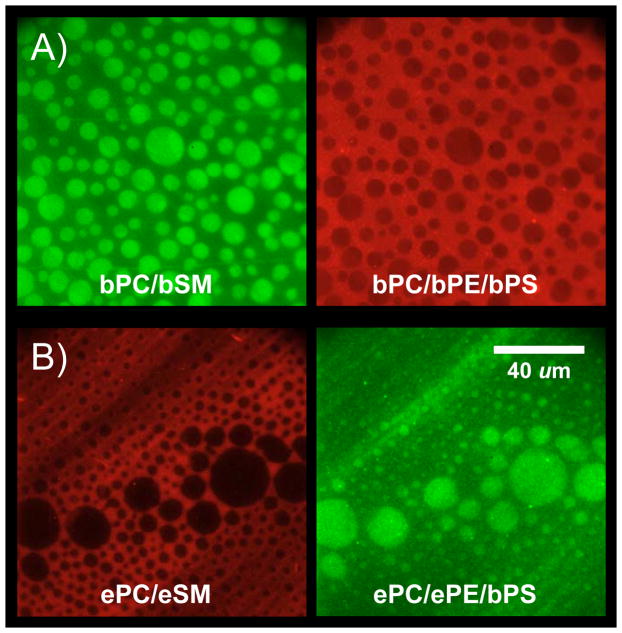
Lipid domain coupling has been examined in asymmetric bilayers made from natural extracts of porcine brain and chicken egg phospholipids (Figure 3). To mimic the exoplasmic leaflet of cell membranes (cf. Figure 2B) equimolar concentrations of the PC and SM fractions of these natural extracts were mixed with 20 mol % cholesterol to form the bottom leaflet of supported bilayers. These mixtures are known to form coexisting lo and ld phase domains. At 20 mol % cholesterol the lo domains form round islands (“rafts”) in a single continuous background of ld phase lipid [18]. To visualize the domains 0.5 mol % NBD-DPPE was included in the PC/SM/Chol leaflet in the brain lipid system (Figure 3A) and 0.02 mol % rhodamine-DPPE in the PC/SM/Chol leaflet in the egg lipid system (Figure 3B). NBD-DPPE preferentially partitions into and labels more ordered lipid domains, whereas rhodamine-DPPE labels more disordered domains [8, 18]. To mimic the cytoplasmic leaflet of cell membranes (cf. Figure 2C) equimolar concentrations of the PC, PE and PS fractions of these natural brain or egg lipid extracts were mixed with 20 mol % cholesterol to form the top leaflets of the respective bilayers. Although these mixtures cannot form phase-separated domains in symmetric bilayers by themselves [19, 32], they are clearly induced to form domains when adjacent to domains of a PC/SM/Chol leaflet in asymmetric bilayers. Rhodamine-DPPE and NBD-DPPE were included in the top leaflets formed from the brain (Figure 3A) or egg (Figure 3B) lipids, respectively. It is clear that in both systems the more ordered domains in the PC/PE/PS/Chol leaflets line up exactly with the more ordered domains in the PC/SM/Chol leaflets [33].
Figure 3.
Outer-to-inner leaflet coupling in asymmetric lipid raft domains formed from natural lipid mixtures. (A) Brain lipid system. The first leaflet of the supported bilayer (green) is composed of brainPC:brainSM:cholesterol (2:2:1) and labeled with lo-phase partitioning NBD-DPPE. The second leaflet (red) is composed of brainPC:brainPE:brainPS:cholesterol (1:1:1:0.75) and labeled with ld-phase partitioning rhodamine-DPPE. (B) Egg lipid system. The first leaflet of the supported bilayer (red) is composed of eggPC:eggSM:cholesterol (2:2:1) and labeled with ld-phase partitioning rhodamine-DPPE. The second leaflet (green) is composed of eggPC:eggPE:eggPS:cholesterol (1:1:1:0.75) and labeled with lo-phase partitioning NBD-DPPE. Adapted from ref. [33].
Single molecule tracking experiments indicate that the lateral motions of fluorescent lipid analogs are almost the same in the more ordered as in the less ordered compartments of both leaflets [19]. The lateral diffusion coefficients derived from these experiments are 0.20 and 0.23 μm2/sec in the lo and ld phases of the PC/SM/Chol leaflet and 0.18 and 0.16 μm2/sec in the lo and ld phases of the PC/PE/PS/Chol leaflets, respectively, with estimated errors of about 0.01 μm2/sec. A more detailed analysis shows the presence of two diffusive components in each of these four compartments [19]. However, the general conclusion that the lateral diffusion coefficients of lipids differ only by small amounts between the different compartments still holds with these more complicated models of diffusion.
Not all lipid mixtures in the second leaflet couple to domains in the first leaflet. For example, using 80 % PC and 20 % cholesterol in the top layer does not couple to pre-existing exoplasmic leaflet domains in the bottom layer. The fluorescence distribution is uniform in these cases and the local diffusion coefficients are larger (0.40 μm2/sec) in these homogeneous top layers than in the previously discussed coupled cases and they are independent of the presence of lo or ld phase domains underneath [19, 33]. Adding one of the higher melting lipid components PE or PS to the PC/Chol top leaflets in these systems recouples the domains in the two leaflets of the asymmetric bilayers [33]. PEs and PSs have chain melting phase transitions (Tm) that are about 20 and 12 °C higher, respectively, than those of PCs with the same chain compositions. Although the compositional space has not been exhaustively sampled by our experiments, it is quite clear that at least one high Tm lipid, one low Tm lipid, and cholesterol need to be present for domain formation in the top leaflet and for efficient ordering of the lipids by coupling to ordered lipid domains in the bottom leaflet.
4. Simple Synthetic Lipid Systems
The natural lipid systems discussed above may be approximated with simpler lipid systems composed of only synthetic lipids. The natural PC/SM/Chol ternary system has been modeled very frequently with the synthetic DOPC/DPPC/Chol ternary system. Detailed phase diagrams have been published using symmetric bilayers with this mixture in GUVs. Coexisting lo and ld phase domains are found over quite broad compositional and temperature regions of the phase diagram of these better defined synthetic lipid mixtures. Very similar to the results described above, domains of this composition did not induce domains in adjacent leaflets that contained only the fluid lipid mixtures POPC or DOPC and cholesterol.
Another synthetic ternary lipid system that has been frequently used in this field is composed of diphytanyolPC (DiPhPC), DPPC, and cholesterol. DiPhPC is a low Tm lipid with no double bonds and therefore cannot be oxidized by peroxidation of olefinic bonds in the fatty acyl chains. Lipid oxidation has been claimed by some to be the reason for domain formation in GUVs and SPBs. The non-oxidizable lipid system DiPhPC/DPPC/Chol forms very similar albeit smaller lo phase domains in a background of ld phase bilayers compared to those found in the DOPC/DPPC/Chol system. Again, when a leaflet of DiPhPC/DPPC/Chol domains is overlayed with a leaflet of the low melting lipids DiPhPC, POPC, or DOPC with cholesterol, no domains are induced [33]. This proves that domains are not induced by light-induced or spontaneous lipid oxidation in these systems.
5. Complex Synthetic Lipid Systems
Contrary to the experiments described in Section 4, domains are induced in the second leaflet on top of lo and ld phase domains in the first leaflet if the second leaflet is composed of ternary mixtures of synthetic low melting PCs and a higher melting synthetic PS and cholesterol. This has been demonstrated with a DOPC/DOPS/Chol leaflet on top of a DOPC/DPPC/Chol leaflet and a DiPhPC/DLPS/Chol leaflet on top of a DiPhPC/DPPC/Chol leaflet [33]. Replacing the PS component with a PE component did not work because stable asymmetric bilayers could not be formed in these systems. The physical properties of the lipids in these pure PE-containing three component systems may be too different from each other and may not have enough degrees of freedom to adapt to the required properties for stable transbilayer phase coupling.
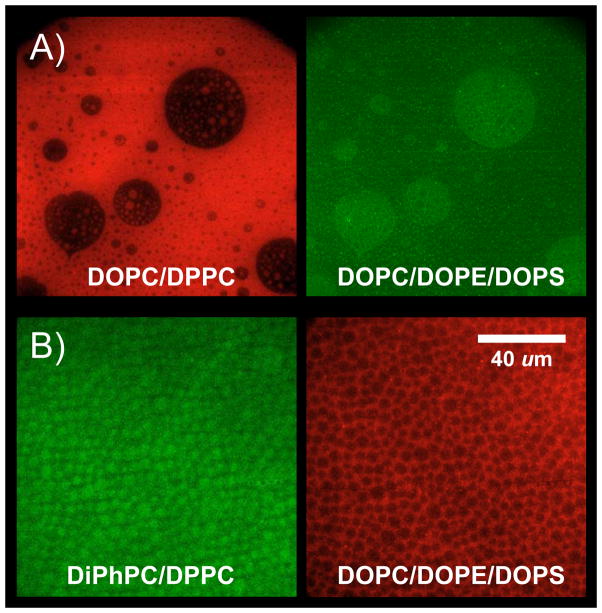
However, quaternary mixtures of synthetic representatives of the four major inner leaflet lipid classes readily formed stable asymmetric bilayers and domains were always induced in the top leaflet by lo and ld phase domains in the bottom leaflet [33]. Examples are shown in Figure 4. Domains in the bottom leaflet composed of DOPC/DPPC/Chol induced domains in the top leaflet composed of DOPC/DOPE/DOPS/Chol (Figure 4A); and domains in the bottom leaflet composed of DiPhPC/DPPC/Chol induced domains in the top leaflet composed of DOPC/DOPE/DOPS/Chol (Figure 4B). In keeping with the notion that more degrees of freedom generate bilayers with higher stability, we found that the more complex natural lipid systems described in Section 3 were generally easier to handle in asymmetric supported bilayers than lipid systems made from pure components. The systems with the fewest pure components were experimentally the most difficult to treat. While the results described here are very reproducible whenever stable bilayers can be formed, the difficulty with membranes made from few synthetic components is their overall stability and not whether they do or do not form induced domains in stable bilayers. It is very conceivable that these stability issues also affect natural membranes in cells and that this may be a previously underestimated reason for the large diversity of lipids that are always found in cell membranes [33].
Figure 4.
Outer-to-inner leaflet coupling in asymmetric lipid raft domains formed from synthetic lipid mixtures. (A) Unsaturated PC system. The first leaflet of the supported bilayer (red) is composed of DOPC:DPPC:cholesterol (2:2:1) and labeled with ld-phase partitioning rhodamine-DPPE. The second leaflet (green) is composed of DOPC:DOPE:DOPS:cholesterol (1:1:1:0.75) and labeled with lo-phase partitioning NBD-DPPE. (B) Saturated PC system. The first leaflet of the supported bilayer (green) is composed of DiPhPC:DPPC:cholesterol (2:2:1) and labeled with lo-phase partitioning NBD-DPPE. The second leaflet (red) is composed of DOPC:DOPE:DOPS:cholesterol (1:1:1:0.75) and labeled with ld-phase partitioning rhodamine-DPPE. Adapted from ref. [33].
6. Binding and Partitioning of Proteins in Heterogeneous Asymmetric Membranes
Liquid-ordered domains or “rafts” have often been implicated as mediators of signal transduction in various signaling pathways across cell membranes. Key to this hypothesis is that certain but not other proteins would partition to rafts and thus assemble with proteins with similar properties on these membrane platforms. Now that we have established that asymmetric bilayers with close to natural lipid compositions and distributions can be assembled in supported bilayers, it is of high interest to use these systems to measure the distribution of proteins between different membrane compartments. Fluorescence microscopy of supported membranes, either by direct observation of the final result or snapshots of the binding or by continuous monitoring of binding reactions by total internal reflection fluorescence microscopy (TIRFM), is exquisitely adequate for quantitative measurements of protein partioning between rafts, non-rafts, and aqueous solution.
As a preliminary illustration of these capabilities, we show in Figure 5 the targeting of C2A domains of synaptotagmin to induced inner leaflet lipid domains in an asymmetric lipid bilayer system. Three fluorescent dyes emitting at different wavelengths are used to image (A) the bottom lipid leaflet composed of bPC/bSM/Chol, (B) the top lipid leaflet composed of bPC/bPE/bPS/Chol, and (C) bound C2A domains on top of the bilayer, all in the same region of the supported membrane. C2 domains, which are common in many signaling proteins including protein kinase C, cytosolic phospholipase A2, and the synaptic vesicle protein synaptotagmin, bind via Ca2+ to acidic lipids in negatively charged bilayers [34, 35]. Significant binding of the C2A domains shown in Figure 5 is only observed in the presence of Ca2+, but not in the presence of EGTA. Binding is thus thought to occur to the PS component in the top leaflet. Since more protein binds to the ld than to the lo domains, we assume that PS also preferentially partitions into the more fluid lipid domains in this system. However, the selectivity is not dramatic; quite a large fraction of the protein also binds to lo phase domains, i.e., induced inner leaflet lipid rafts. Although this example demonstrates quite nicely the usefulness of the system for measuring protein partitioning to raft and non-raft components in asymmetric supported membranes, more experiments are needed to quantify this partitioning as well as the presumed underlying partitioning of specific acidic lipids to which these proteins bind.
Figure 5.
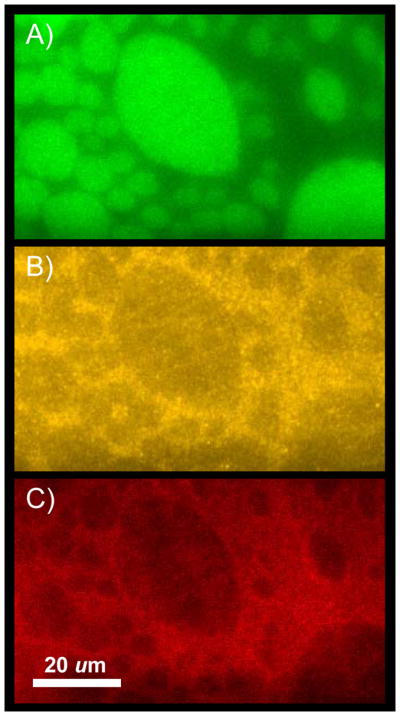
Targeting of synaptotagmin C2A domains to induced inner leaflet lipid rafts in an asymmetric lipid system. The first leaflet of the supported bilayer (green) is composed of brainPC:brainSM:cholesterol (1:1:1) and labeled with lo-phase partitioning NBD-DPPE. The second leaflet (yellow) is composed of brainPC:brainPE:brainPS:cholesterol (1:1:1:1.33) and labeled with ld-phase partitioning rhodamine-DPPE. (C) 0.2 μM Alexa-647-labeled synaptotagmin I C2A (red) bound to asymmetric bilayer in the presence of 1mM Ca2+.
7. Conclusions and Future Directions
Signal transduction through lipid rafts has been an attractive hypothesis for the stimulation cells of the immune system and other humoral and cell-mediated triggers of cell proliferation, migration, and attachment to substrata. One of the early caveats about this hypothesis was that lipid rafts were very difficult, if not impossible to visualize in cell membranes and that the evidence for their existence was very circumstantial by being based mostly on questionable detergent extraction procedures. However, since these early studies, technologies of cellular imaging have dramatically improved so that ever smaller clusters of proteins can be seen. Also, more detailed localized lipid fluidity assays have been developed that can be applied to cell membranes in situ. At the same time, biophysicists have made great strides towards visualizing and characterizing the structures and thermodynamics of liquid-liquid lipid phase separations in large liposomes. These different lines of research are converging in support of the raft hypothesis even if the ultimate and most direct proof of directly seeing such structures in cell membranes is still elusive. The work summarized in this review adds another piece towards building a bridge between the biophysical model systems and cell biological observations and hopefully towards completing this long-held puzzle. The asymmetric bilayer preparations specifically address and remove the second caveat that was held against the raft hypothesis, namely that the lipids that are typically found in the inner leaflets of plasma membranes do not form rafts or liquid-ordered domains on their own. The supported bilayer work described in ref. [18, 19] and in expanded form in ref. [33] showed for the first time that inner leaflet ordered domains can be induced by outer leaflet ordered domains in a reconstituted system as had been postulated by the raft hypothesis a decade earlier [7].
It should be recognized that asymmetric bilayers have since also been produced in free-standing “black lipid” membranes suspended in a small hole of a teflon septum [27]. Although in this case outer leaflet mixtures of different compositions were used in both leaflets, there is no principal reason why outer and inner leaflet mixtures could not also be combined with this method in the future. The black lipid membranes have the advantage that there is no influence of an underlying solid support (which is minimal for SPBs on a polymer cushion) and the SPB method has the advantage that there is no solvent required for the preparation of the membranes (which may influence lipid asymmetry in free standing membranes). Therefore, converging results from both methods should be viewed as reinforcing each other.
Both asymmetric lipid systems are now open for further experimentation. One of the obvious and most important future directions is to determine which proteins can and which proteins cannot assemble on different asymmetric membrane platforms. The partitioning and functioning of specific proteins in these membranes almost certainly will also depend on the particular lipid compositions. What we see in these experiments is the partitioning of fluorescent lipid tracers and that is always a relative and not an absolute quantity. Changing lipid compositions in either leaflet does change the physical properties of the darker and brighter domains and they may have quite different relative and average lipid orders over the range of lipid compositions that is available to cells. Therefore, not all “rafts” may be equal. It is very likely that there are many different types of lipid domains with different physical properties that can be fine-tuned in model systems and that may co-exist in cell membranes. By changing, for example, the cholesterol concentration in the plasma membrane, a cell may quite dramatically alter not only the sizes and numbers of lipid rafts, but also their quality and connectivity and thereby affect protein partitioning, binding, and enzymatic activity. Even if rafts in cells turn out to be very small, perhaps down to a few tens or hundreds of molecules, this seems to be a powerful mechanism of signal transduction and membrane-mediated regulation that asks to be further explored, for example with a biophysical system as described in this review.
Acknowledgments
This work was supported by NIH grant P01 GM072694. We thank Dr. Jonathan Crane for the preparation of Figure 2.
References
- 1.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 2.Engelman DM. Membranes are more mosaic than fluid. Nature. 2005;438:578–580. doi: 10.1038/nature04394. [DOI] [PubMed] [Google Scholar]
- 3.Daleke DL. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res. 2003;44:233–242. doi: 10.1194/jlr.R200019-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson-Miller S, Hochman J, Schindler M. Aggregation and diffusion in the mitochondrial electron-transfer chain: role in electron flow and energy transfer. Biochem Soc Trans. 1986;14:822–824. doi: 10.1042/bst0140822. [DOI] [PubMed] [Google Scholar]
- 5.McConnell HM, Vrljic M. Liquid-liquid immiscibility in membranes. Annu Rev Biophys Biomol Struct. 2003;32:469–492. doi: 10.1146/annurev.biophys.32.110601.141704. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenberg D, Goni FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci. 2005;30:430–436. doi: 10.1016/j.tibs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich C, Bagatolli LA, Volovyk ZN, Thompson NL, Levi M, Jacobson K, Gratton E. Lipid rafts reconstituted in model membranes. Biophys J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korlach J, Schwille P, Webb WW, Feigenson GW. Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy. Proc Natl Acad Sci U S A. 1999;96:8461–8466. doi: 10.1073/pnas.96.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veatch SL, Keller SL. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys J. 2003;85:3074–3083. doi: 10.1016/S0006-3495(03)74726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao J, Wu J, Heberle FA, Mills TT, Klawitter P, Huang G, Costanza G, Feigenson GW. Phase studies of model biomembranes: complex behavior of DSPC/DOPC/cholesterol. Biochim Biophys Acta. 2007;1768:2764–2776. doi: 10.1016/j.bbamem.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahya N, Scherfeld D, Bacia K, Poolman B, Schwille P. Probing lipid mobility of raft-exhibiting model membranes by fluorescence correlation spectroscopy. J Biol Chem. 2003;278:28109–28115. doi: 10.1074/jbc.M302969200. [DOI] [PubMed] [Google Scholar]
- 13.Esposito C, Tian A, Melamed S, Johnson C, Tee SY, Baumgart T. Flicker spectroscopy of thermal lipid bilayer domain boundary fluctuations. Biophys J. 2007;93:3169–3181. doi: 10.1529/biophysj.107.111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Saez AJ, Chiantia S, Schwille P. Effect of line tension on the lateral organization of lipid membranes. J Biol Chem. 2007;282:33537–33544. doi: 10.1074/jbc.M706162200. [DOI] [PubMed] [Google Scholar]
- 15.Honerkamp-Smith AR, Cicuta P, Collins MD, Veatch SL, den Nijs M, Schick M, Keller SL. Line tensions, correlation lengths, and critical exponents in lipid membranes near critical points. Biophys J. 2008;95:236–246. doi: 10.1529/biophysj.107.128421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veatch SL, Gawrisch K, Keller SL. Closed-loop miscibility gap and quantitative tie-lines in ternary membranes containing diphytanoyl PC. Biophys J. 2006;90:4428–4436. doi: 10.1529/biophysj.105.080283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veatch SL, Keller SL. Miscibility phase diagrams of giant vesicles containing sphingomyelin. Phys Rev Lett. 2005;94:148101. doi: 10.1103/PhysRevLett.94.148101. [DOI] [PubMed] [Google Scholar]
- 18.Crane JM, Tamm LK. Role of cholesterol in the formation and nature of lipid rafts in planar and spherical model membranes. Biophys J. 2004;86:2965–2979. doi: 10.1016/S0006-3495(04)74347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiessling V, Crane JM, Tamm LK. Transbilayer effects of raft-like lipid domains in asymmetric planar bilayers measured by single molecule tracking. Biophys J. 2006;91:3313–3326. doi: 10.1529/biophysj.106.091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garg S, Ruhe J, Ludtke K, Jordan R, Naumann CA. Domain registration in raft-mimicking lipid mixtures studied using polymer-tethered lipid bilayers. Biophys J. 2007;92:1263–1270. doi: 10.1529/biophysj.106.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamm LK, McConnell HM. Supported phospholipid bilayers. Biophys J. 1985;47:105–113. doi: 10.1016/S0006-3495(85)83882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalb E, Frey S, Tamm LK. Formation of supported planar bilayers by fusion of vesicles to supported phospholipid monolayers. Biochim Biophys Acta. 1992;1103:307–316. doi: 10.1016/0005-2736(92)90101-q. [DOI] [PubMed] [Google Scholar]
- 23.Crane JM, Tamm LK. Fluorescence microscopy to study domains in supported lipid bilayers. Methods Mol Biol. 2007;400:481–488. doi: 10.1007/978-1-59745-519-0_32. [DOI] [PubMed] [Google Scholar]
- 24.Crane JM, Kiessling V, Tamm LK. Measuring lipid asymmetry in planar supported bilayers by fluorescence interference contrast microscopy. Langmuir. 2005;21:1377–1388. doi: 10.1021/la047654w. [DOI] [PubMed] [Google Scholar]
- 25.Braun D, Fromherz P. Fluorescence interference-contrast microscopy of cell adhesion on oxidized silicon. Appl Phys A. 1997;65:341–348. [Google Scholar]
- 26.Lambacher A, Fromherz P. Fluorescence interference-contrast microscopy on oxidized silicon using a monomolecular dye layer. Appl Phys A. 1996;63:207–216. [Google Scholar]
- 27.Collins MD, Keller SL. Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers. Proc Natl Acad Sci U S A. 2008;105:124–128. doi: 10.1073/pnas.0702970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao J, Wu J, Shao H, Kong F, Jain N, Hunt G, Feigenson G. Phase studies of model biomembranes: macroscopic coexistence of Lalpha+Lbeta, with light-induced coexistence of Lalpha+Lo Phases. Biochim Biophys Acta. 2007;1768:2777–2786. doi: 10.1016/j.bbamem.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamm LK. Lateral diffusion and fluorescence microscope studies on a monoclonal antibody specifically bound to supported phospholipid bilayers. Biochemistry. 1988;27:1450–1457. doi: 10.1021/bi00405a009. [DOI] [PubMed] [Google Scholar]
- 30.Wagner ML, Tamm LK. Tethered polymer-supported planar lipid bilayers for reconstitution of integral membrane proteins: silane-polyethyleneglycol-lipid as a cushion and covalent linker. Biophys J. 2000;79:1400–1414. doi: 10.1016/S0006-3495(00)76392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiessling V, Tamm LK. Measuring distances in supported bilayers by fluorescence interference-contrast microscopy: polymer supports and SNARE proteins. Biophys J. 2003;84:408–418. doi: 10.1016/S0006-3495(03)74861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang TY, Silvius JR. Cholesterol does not induce segregation of liquid-ordered domains in bilayers modeling the inner leaflet of the plasma membrane. Biophys J. 2001;81:2762–2773. doi: 10.1016/S0006-3495(01)75919-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan C, Kiessling V, Tamm LK. Coupling of cholesterol-rich lipid phases in asymmetric bilayers. Biochemistry. 2008;47:2190–2198. doi: 10.1021/bi7021552. [DOI] [PubMed] [Google Scholar]
- 34.Cho W, Stahelin RV. Membrane binding and subcellular targeting of C2 domains. Biochim Biophys Acta. 2006;1761:838–849. doi: 10.1016/j.bbalip.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 36.Quinn PJ. Plasma membrane phospholipid asymmetry. Subcell Biochem. 2002;36:39–60. doi: 10.1007/0-306-47931-1_3. [DOI] [PubMed] [Google Scholar]
- 37.Devaux PF. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991;30:1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- 38.Mescher MF, Apgar JR. The plasma membrane ‘skeleton’ of tumor and lymphoid cells: a role in cell lysis? Adv Exp Med Biol. 1985;184:387–400. doi: 10.1007/978-1-4684-8326-0_26. [DOI] [PubMed] [Google Scholar]
- 39.Friddriksson EK, Shipkova PA, Sheets ED, Holowka D, Baird B, McLafferty FW. Quantitative analysis of phospholipds in functionally important membrane domains from RBL-2H3 mast cells using tandem high-resolution mass spectroscopy. Biochemistry. 1999;38:8056–8063. doi: 10.1021/bi9828324. [DOI] [PubMed] [Google Scholar]