Abstract
The inability to quantify the risk for disorders, such as substance use disorders (SUD), hinders etiology research and development of targeted intervention. Based on the concept of common transmissible liability to SUD related to illicit drugs, a method enabling quantification of this latent trait has been developed, utilizing high-risk design and item response theory. This study examined properties of a SUD transmissible liability index (TLI) derived using this method. Sons of males with or without SUD were studied longitudinally from preadolescence to young adulthood. The properties of TLI, including its psychometric characteristics, longitudinal risk assessment and ethnic variation, were examined. A pilot twin study was conducted to analyze the composition of TLI’s phenotypic variance. The data suggest that TLI has concurrent, incremental, predictive and discriminant validity, as well as ethnic differences. The data suggest a high heritability of the index in males. The results suggest applicability of the method for genetic and other etiology-related research, and for evaluation of individual risk.
Keywords: Addiction, Phenotype, IRT, Ethnicity, Race, Transmissibility
Introduction
The individual risk for substance use disorder (SUD) can be viewed as a phenotype for a continuous latent (unobserved) complex trait termed liability. This term was introduced to human genetics by Falconer (1965) and defined as encompassing the effects of all factors influencing the probability of the disorder. Phenotypic values that surpass a certain point on the liability scale, the threshold, are likely to be ascribed a clinical diagnosis. For liability to a behavioral disorder such as SUD, without a natural clear boundary between the norm and pathology, the threshold is defined and described by consensually accepted and changing diagnostic criteria. There are as many as 466 possible DSM-III-R diagnostic combinations of symptoms for dependence only (three or more out of nine symptoms), not counting variation in the substances used (Vanyukov et al. 2003a). Each of the symptoms may have different relevance to the actual latent threshold phenotype. The categorical SUD diagnosis thus collapses the continuous trait into two heterogeneous phenotypic classes. While needed for clinical work, the diagnosis is therefore not an optimal research tool. It also cannot inform primary prevention of SUD.
Whereas drug- or drug-class-specific mechanisms of metabolism and pharmacologic action may be involved in SUD risk, studies have documented considerable commonalities between the various SUD (reviewed in Vanyukov and Tarter 2000; Vanyukov et al. 2003c). Indeed, the different substance-specific SUD diagnoses are indicators of a unidimensional latent continuous trait (Kirisci et al. 2002). Only a small proportion of variance in liability for SUD related to different types of illicit drugs is substance-specific (Kendler et al. 2003a, 2007). A large proportion of genetic variance is accounted for by genetic factors shared in common between various SUD, with twin data indicating little or no specific genetic contribution for different illicit drugs (Tsuang et al. 1998; Kendler et al. 2003a, 2007). Common liability, rather than drug/“stage”-specific factors posited by the “gateway theory” (Kandel 1975), underlies transitions in drug abuse development (Tarter et al. 2006).
Measurement of this latent trait, needed for genetic and other etiology research as well as risk assessment, is complicated. Liability can be characterized dimensionally among the affected (or at least symptomatic) individuals, insofar as indices of disorder severity (e.g., symptom counts) are related to this trait. This, however, can be done only when the population reaches the age of risk and starts using drugs, which constrains the ability to distinguish causes (or even premorbid phenotypic characteristics) and effects of substance abuse. Moreover, the resultant scale would be truncated, with many nonaffected or asymptomatic phenotypes largely collapsed into one class, limiting analyses related to the risk for SUD in power and scope.
Previous discussions have detailed the theoretical foundation (Vanyukov et al. 2003c; Vanyukov and Tarter 2000) and methodological strategy (Vanyukov et al. 2003a; Vanyukov and Tarter 2000) for quantifying liability to SUD related to illicit drugs, using high-risk/family design and item response theory (IRT) in the development of a liability index (TLI). The present study examines properties and utility of TLI, including its psychometric characteristics, longitudinal risk assessment, and ethnic variation. We hypothesized that the index, regardless of ethnicity, would be predictive of the risk for and the rate of SUD development from preadolescence to young adulthood, and that the accuracy of prediction would be greater for SUD related to illicit drugs as compared to alcohol use disorder. In addition, we conducted a pilot twin study to further validate the method and assess TLI’s utility in genetic research. Whereas the index may measure transmissible SUD liability or a proportion thereof, transmissibility [a component of phenotypic variance correlated between parents and offspring (Rice et al. 1980; Vanyukov et al. 2003b)] may be due to both genes (heritability) and environment. Considering that genetic studies of SUD based on categorical diagnoses have largely not found a significant contribution of shared environment (e.g., Kendler et al. 2007; Tsuang et al. 1998), an index derived as a measure of transmissible SUD liability was also expected to have high heritability and little, if any, contribution of shared environment.
Methods
Participants
One group of subjects consisted of participants in the Center for Education and Drug Abuse Research (CEDAR), a longitudinal family/high-risk study of etiology of substance use disorder (SUD; substance abuse or dependence). The probands in this study are adult males with or without a lifetime DSM-III-R diagnosis (DSM-IV was introduced after the study started) of SUD consequent to use of illicit drugs (SUD+and SUD−, respectively), who had a 10–12 year old biological child (index case, IC) (Tarter and Vanyukov 2001). The SUD+ probands were recruited from substance abuse treatment programs, social service agencies, newspaper and radio advertisements, public service announcements, and random digit telephone calls. Depending on one or even a few recruitment sites heightens the risk of sampling bias (Merikangas et al. 1998). The SUD− men were recruited using the same method as SUD+ probands except that none were acquired from treatment facilities. This study was reviewed and approved by the Institutional Review Board of the University of Pittsburgh, and participants provided written informed consent prior to implementing the research protocol.
The family was excluded from study if the father had a history of neurological disorders, schizophrenia or uncorrectable sensory incapacity, or the IC child had a history of neurological injury requiring hospitalization, IQ less than 70, chronic physical disability, uncorrectable sensory incapacity or psychosis. Offspring of SUD+ probands are assigned to the “high average risk” (HAR) group, while children of SUD− probands are assigned to the “low average risk” (LAR) group (N = 250 each).
Children undergo regular assessments on a large number of individual and environmental characteristics. For the purpose of this study, we used data from all available assessments of the probands’ sons, which have been conducted when these subjects are aged approximately 10–12 (1st visit), 14, 16, 19, 22, 25, 28 and 30. Their current maximal age ranged from 10 to 28 years (mean ± SD = 19.5 ± 4.32). The recruitment of families with female IC started later than those with male IC, and the female sample is thus not yet sufficient to derive a liability index and conduct longitudinal analyses. Analyses of the sample indicate no attrition bias as pertains to the TLI score, ethnicity, socioeconomic status, or education (Tarter et al. 2006; Kirisci et al. 2006, 2009). The sample is 75.6% European–American, 21.2% African–American, and 3.2% other ethnicities.
Another group were participants in a twin study of TLI, same-sex pairs recruited at the Twins Days Festival in Twinsburg, Ohio, during 2006 and 2007. This study was also reviewed and approved by the University of Pittsburgh IRB. The twins were 9–18 years of age, and had at least one parent available to participate. Parents were required to consent to their own and their children’s participation in the study, and the children’s assent was also obtained. Each family member independently completed anonymous paper-and-pencil questionnaires. A brief zygosity questionnaire developed by Nichols and Bilbro (1966), with the corresponding zygosity determination algorithm developed by Eley and colleagues for the Twins Early Development Study (TEDS) in London [personal communication (Strassberg et al. 2002; Jenkins et al. 2006)] was used for zygosity determination. The questionnaire is composed of 15 items to evaluate the similarity and differences between members of the twin pair. Forty-nine pairs (33 male and 16 female) were diagnosed as dizygotic (DZ), and 183 pairs (110 male and 73 female), as monozygotic (MZ). These individuals were also administered a questionnaire containing the TLI item set.
Diagnosis
The diagnoses in parents and children (if age 19 or older) in CEDAR were determined using an expanded version of the Structured Clinical Interview for DSM-III-R-outpatient version (SCID-OP) (Spitzer et al. 1987), or K-SADS-E for children younger than 19 (Orvaschel et al. 1982) and finalized at a consensus conference according to the best estimate procedure (Kosten and Rounsaville 1992). The expanded SCID evaluates current episode (past 6 months) and worst past episode of psychopathology (before the past 6 months). The K-SADS-E, a semistructured diagnostic interview for children and adolescents aged 6–17, covers current and lifetime psychopathology. The standard procedure is to first interview the mother about the psychiatric status of the child. During the subsequent interview of the child, the interviewer attempts to resolve any discrepancies between parent and child in case of disagreement. A summary score is obtained based on positive ratings of either informant. The diagnoses were finalized at a consensus conference according to the best estimate procedure (Kosten and Rounsaville 1992).
Development of transmissible liability index
The rationale and the measurement model for the SUD risk index (transmissible liability index, TLI) derivation procedure have been described in detail elsewhere (Vanyukov et al. 2003a, c). Briefly, it is based on the known transmissibility of liability to SUD related to illicit drugs and on the application of item response theory (IRT). SUD liability is largely non-specific (common), both phenotypically (most of the variance is shared in common for various classes of illicit drugs) and genetically (twin data support the overwhelming contribution of nonspecific genetic variation) (Kendler et al. 2003a). Inasmuch as SUD risk is transmissible (mostly due to its heritability), children’s characteristics that discriminate groups with affected and nonaffected parents are likely to be indicators of children’s transmissible SUD liability, which could be then analyzed by IRT. IRT (e.g., Embretson and Reise 2000) is a psychometric test theory that relates the performance of an examinee on a test item to a latent trait that the test is intended to measure. This relationship (e.g., in a simple case, between the trait and the probability of a correct response) is described by an item response function (IRF). While ability level is a characteristic of the examinee, performance also depends on parameters characterizing items themselves and defining the IRF. In the widely used two-parameter model, these are the location (difficulty) parameter (b, the trait value at which the probability of a correct response exceeds 0.5), and the discrimination parameter (a, proportional to the slope of the IRF at the point b on the trait scale). These parameters allow for taking into account that different items have different difficulty and different ability to discriminate between values of the trait. In contrast to the classical psychometric test theory, IRT provides testable models. A data-fitting IRT model provides estimates with features that are uniquely valuable for the trait measurement: item parameters are invariant of the sample (subpopulation) of the subjects (the trait distribution does not influence the estimates), and trait estimates are invariant of items used.
The TLI derivation method involves using a large set of items (303 in the current study) from numerous psychological and psychiatric instruments (here, 24), originally selected in CEDAR based on their potential for measuring variables related to SUD risk and psychopathology. These items were submitted to conceptual (identification of item groups judged to indicate core psychological traits), factor and item response theory (IRT) analysis to derive theoretically based unidimensional constructs (here, 19) characterizing individual behavior/personality (e.g., antisociality, attention, mood). The HAR and LAR groups were then compared on these constructs. This comparison relates the constructs to parental SUD liability and, inasmuch as liability is transmissible, to the child’s own SUD liability (see details in Vanyukov et al. 2003a), consistent with the construct validity of TLI [its ability to capture the construct of transmissible liability; this and other standard research validities as defined, e.g., in Nunnally and Bernstein (1994)]. The constructs demonstrating significant group differences were retained for further analysis. The items that are indicators of these constructs were then submitted to factor analysis to both ensure the presence of a single dominant dimension and further reduce the item set. Exploratory factor analysis of the set thus derived estimated a ratio of 3.2 of first to second eigenvalues, consistent with the unidimensionality of the TLI scale. Confirmatory factor analysis with weighted least squares method confirmed the unidimensional factor structure of the TLI scale, a prerequisite for IRT modeling. The 45-item set thus selected (see the listing in Appendix) for sons of the probands at age 10–12 was used to assess the quality of items and estimate TLI in this study, using IRT.
Whereas this item set includes—by design—many items that have long been known to be related to SUD risk, the procedure has selected from disparate diagnostic and psychological instruments a large comprehensive initial list of potentially useful and the most relevant items, selected out many redundant ones, thus enabling substantial data reduction without a loss of information, as well as calibrated the items as indicators of the unidimensional transmissible liability trait. Because the liability distribution shifts to the right as the population matures from prepuberty, age was regressed out of the TLI in the twin sample.
Statistical analysis
Mean values were compared using the t-test and ANOVA. The relationship between the liability index (TLI) and the subsequent categorical SUD diagnosis in the probands’ sons was explored using logistic regression and receiver operating characteristic (ROC) curve analysis. Linear hierarchical regression was used to test the influence of risk group membership and ethnicity on TLI. Comparisons involving risk groups (HAR and LAR) were conducted in the full sample. In comparisons of affected and nonaffected individuals, however, there is a possibility that some individuals were not affected because they had not yet reached the age of risk due to protracted recruitment and consequently fewer assessments. To minimize this possibility, only those nonaffected subjects who had been last assessed at visit 4 (age 19) or later were selected for disorder frequency comparisons between the risk groups, for logistic regression analyses with the diagnosis as the dependent variable, and for ROC curve analyses. This selection substantially lessens the age difference between affected and nonaffected individuals in the available sample, decreasing the probability that the latter will develop a disorder later: while the former were aged 22.2 ± 3.01, 3.7 years older than nonselected non-SUD boys who were 18.6 ± 4.31, the difference drops to 0.7 years for the selected non-SUD ones, aged 21.5 ± 2.63. By this selection criterion, there were 224 nonaffected and 126 affected individuals. The relationship between the liability index and the rate of disorder development was analyzed using survival analysis (Cox proportional hazard regression) in the full sample (survival analysis properly treats observations that did not reach maximal follow-up as censored). Two-tailed P values are presented in all cases. These analyses as well as standard statistics were obtained using SPSS® for Windows® release 15.
In addition, to assess stability and accuracy of the results across variations in sampling, we applied the bootstrap procedure to determine the 95% confidence interval of the estimates for odds ratios and hazard ratios (Efron and Tibshirani 1993), using Stata® 9. Accordingly, 1,000 successive random samples were drawn with replacement from the original sample with the same sample size. The logistic regression model and proportional hazard model were thus tested using each generated sample and statistics of interest were computed. Confidence intervals obtained using the bootstrap procedure were compared with estimates obtained from the normal approximation method to show that the original sample was represented in the bootstrap samples.
Twin analysis
Within the boundaries of known assumptions (equal environment—the equality of environmental contribution to phenotypic variance in MZ and DZ twins,—random mating, nonsignificance of epistasis, etc.), the genetic component VG of phenotypic variance VP consists of additive genetic (VA; due to additive allelic effects) and dominance genetic (VD, nonadditive allelic effects) variance. Together, these two variance components comprise broad sense heritability (denoted H2), while VA corresponds to narrow sense heritability (h2). The environmental component of phenotypic variance consists of shared/common environmental (VC, due to nongenetic causes of familial/twin resemblance), and nonshared/unique environmental variance (VE, due to causes of familial/twin differences, as well as measurement error that makes this component a necessary part of any twin data model). Intrapair covariance in MZ twins, who are genetically identical, is Cov(MZ) = VA + VD + VC, whereas in DZ twins, who are as similar genetically as any siblings, it is Cov(DZ) = 0.5Va + 0.25VD + VC. It is not possible, in twins raised together, to model simultaneously the contributions of shared environmental and dominance genetic components, because the former decreases and the latter increases differences between MZ and DZ covariances (e.g., Neale and Cardon 1992). Accordingly, the initial model to fit, which includes only one of these components, is selected based on whether the MZ correlation is less or greater than twice the DZ correlation. The former case corresponds to the presence of the shared environmental component, and the initial model tested is ACE (additive genetic, shared environment, and nonshared environment), whereas the latter case implies the presence of nonadditive (dominance) genetic effects, and the model is ADE (additive and dominance genetic, and nonshared environmental components). Variance–covariance matrices were used for structural equation model fitting. The χ2 statistics and Akaike Information Criterion (AIC) were used as fit indices. A nonsignificant χ2 value indicates a satisfactory fit, and a lower AIC suggests a more parsimonious model. Nested models (differing in that the elements present in one, more general, model are omitted from another) can be compared by evaluating significance of a χ2 value equal to the difference of the χ2 values of the more general model and a submodel, with the number of degrees of freedom equal to the difference between the respective numbers of degrees of freedom. The models were tested using Mx software (Neale et al. 2003).
Results
SUD diagnostic distributions
A lifetime diagnosis of SUD (related to illicit drugs) in the HAR group was observed significantly more often than in the LAR group (42.8 vs. 29.9%, P = 0.012), and the disorder had an earlier age of onset (mean ± SD, 16.2 ± 2.39 vs. 17.1 ± 2.20, P = 0.027). These differences confirm the assumption of the differences in SUD liability between sons of SUD-affected and nonaffected fathers, and were observed despite the fact that the LAR boys were on average a year older than the HAR group (maximal mean age reached 20.2 ± 4.18 and 18.8 ± 4.35; P < 0.0003), parallel with the difference in the number of assessments (4.4 vs. 3.9, P = 0.0001). The majority of SUD diagnoses were related to marijuana (95.0%); the rest of the diagnoses were abuse of/dependence on opiates (15.1%), cocaine (11.8%), and other illicit drugs.
A comorbid alcohol use disorder (AUD) was diagnosed in 65.5% of participants with a SUD, compared to 7.9% of the non-SUD group. AUD risk differences between HAR and LAR groups were not significant regardless of whether all non-AUD participants were taken into account (23.5 vs. 20.7%, respectively; P = 0.473), or only those who have been through at least five assessments (35.6 vs. 28.1%, P = 0.137).
Properties of the liability index
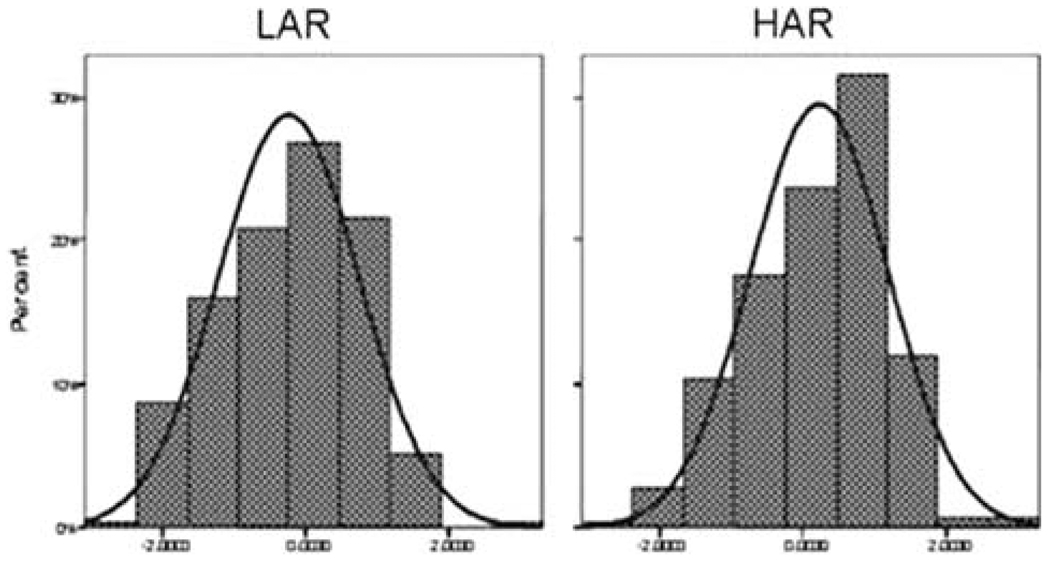
TLI has IRT-based marginal reliability of 0.93, accounting for 37% of variance. Figure 1 illustrates differences between the 10 and 12 years old sons of affected (SUD) fathers (high average risk group, HAR, N = 250) and sons of nonaffected fathers (low average risk, LAR; N = 250) in SUD liability as measured by TLI. The difference between mean TLI is significant (P = 1.2 × 10−7) and is approximately 0.5 SD (0.23 vs. −0.24, SDHAR ≈ SDLAR ≈ 1), consistent with theoretical expectations derived taking into account heritability of SUD liability (Vanyukov et al. 2003a) and suggesting concurrent validity of the index (its correspondence with the group, a known measure). Notably, the group difference is expected to increase as the target sample passes through the age of risk for SUD, since the index development procedure is grounded in the transmissibility of liability to lifetime SUD in adults.
Fig. 1.
Liability index (TLI) distributions in HAR and LAR 10–12-year-old males
As logistic regression analysis indicates, TLI is predictive of SUD diagnoses of probands’ sons (OR = 1.81, P = 2.1 × 10−6; 95% CI: 1.415–2.303). Similarly, ROC curve analysis estimates an area under the curve (correct classification) of 0.661 (P = 5.6 × 10−7; 95% CI: 0.600– 0.722), with sensitivity of 0.66 and specificity of 0.61. In survival analysis, the index demonstrates a highly significant relationship with the rate of SUD development. A unit (1 SD) increase in TLI was related to a 70% yearly increase in SUD hazard (hazard ratio HR = 1.70; 95% CI: 1.40– 2.10, P = 1.8 × 10−7). The analysis using the risk group as the predictor variable results in a comparable hazard rate (HR = 1.70; 95% CI: 1.2–2.40; P = 0.003).
While the risk group (the paternal diagnosis of SUD) is predictive of SUD in the offspring sample, the predictive validity of TLI is underscored by its application within the risk group, which also supports its incremental validity as compared with prediction based on risk group assignment. The relationship between TLI and the SUD development rate is significant in the HAR group (HR = 2.06 [1.52– 2.78]; P = 2.8 × 10−6) and close to significance in the LAR group (HR = 1.29 [0.97–1.71]; P =0.078). The relationship is stronger for the most common SUD—cannabis use disorder—in the full sample (HR = 1.75 [1.43– 2.15]; P =8.7 × 10−8); it is also significant in both HAR and LAR groups (respectively, HR = 2.04 [1.50–2.76], P =4.4 × 10−6; and HR = 1.36 [1.01–1.83], P =0.040). In contrast, the relationship of TLI with the rate of alcohol use disorder development is weaker (HR = 1.29 [1.06– 1.58], P =0.011) in the total sample and the HAR group (HR = 1.55 [1.14–2.11], P =0.005), and nonpredictive in the LAR group (HR = 1.03 [0.78–1.35], P =0.882). This is consistent with the derivation of TLI as primarily a measure of liability to SUD related to illicit drugs, and with the discriminant validity of the index.
Bootstrap procedure for validating the results
In predicting SUD diagnosis, a 95% CI obtained from the bootstrap method (1.222–2.471) included the OR =1.81 which was estimated from the original sample. Furthermore, a 95% bootstrap CI (1.43–1.96) covered the HR = 1.70 which was estimated using the original sample. Thus, the range of variability of the estimates obtained from 1,000 samples and the original sample overlap.
Ethnic differences
There are no differences between the European- and African-American (EA and AA) subgroups in the frequencies of SUD (24 vs. 27% for a SUD diagnosis, P =0.453; and 22 vs. 27% for cannabis use disorder, P =0.269), or in the age of SUD onset (mean ± SD, 16.9 ± 2.36 in EA, and 16.4 ± 2.43 in AA, P =0.347). There are, however, TLI differences between the ethnic groups (−0.077 ± 1.002 vs. 0.254 ± 0.948, P =0.002). These differences could be in part accounted for by the larger proportion of AA recruited into the HAR than LAR group (13.2 vs. 8.7% of the sample, respectively, P =0.018). Nevertheless, regression analysis showed that a contribution of ethnic differences is above and beyond that due to the proportion difference: there was a significant F change (P =0.011, β = 0.113) when ethnicity was entered in the regression equation with TLI as the dependent variable after entering the risk group. The regression coefficient for the group drops only slightly when ethnicity is entered in the equation, from β = 0.24 to 0.23 (P =1.2 × 10−7 and 4.9 × 10−7, respectively), suggesting that both variables contribute to the liability variation independently. Interestingly, in both EA and AA groups, the frequency of SUD in children of non-SUD fathers (LAR) is substantial (20.6 and 26.2%).
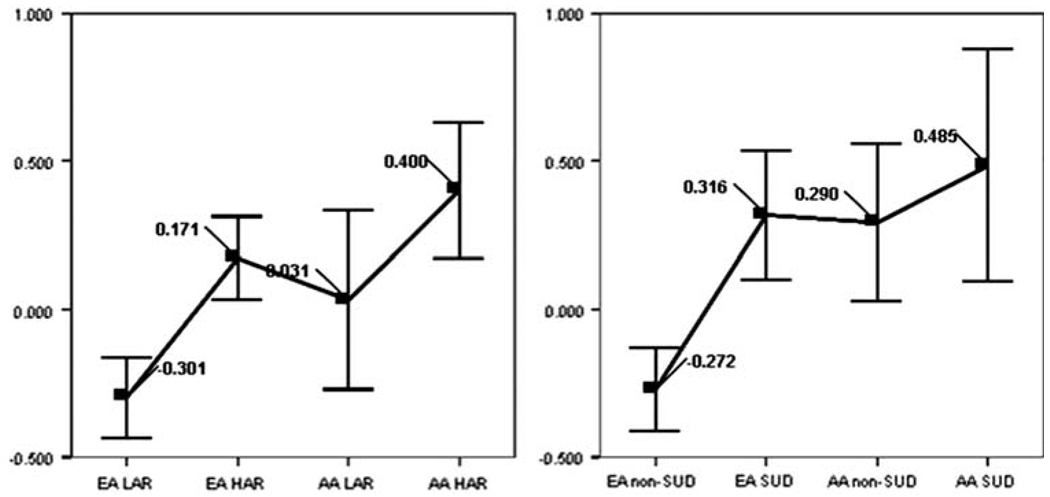
As presented in Table 1, there are ethnic differences in TLI within the risk groups. Comparison by ANOVA (uncorrected for multiple tests) showed AA scoring higher for the LAR group (P = 0.048) and a trend in the same direction for the HAR group (P = 0.104). There is no significant difference between HAR and LAR in AA. The ethnic variation and its pattern, illustrated in Fig. 2, become more pronounced in the comparisons of those who have and have not developed a SUD. Nonaffected AA youths score significantly higher than nonaffected EA (corrected P = 0.008) and do not significantly differ from either affected ethnic group. Nonaffected EA differ from both affected EA (P < 0.001) and affected AA (P = 0.002). Affected EA do not significantly differ from affected AA.
Table 1.
TLI statistics in the subgroups of the study
| Subgroup | N | Mean LI | SD |
|---|---|---|---|
| EAa LARb | 199 | −0.30 | 0.984 |
| EA HARc | 179 | 0.17 | 0.964 |
| AAd LAR | 42 | 0.03 | 0.969 |
| AA HAR | 64 | 0.40 | 0.911 |
| EA non-SUD | 174 | −0.27 | 0.930 |
| EA SUD | 90 | 0.32 | 1.047 |
| AA non-SUD | 45 | 0.29 | 0.888 |
| AA SUD | 29 | 0.49 | 1.038 |
European–Americans
Low average risk group (sons of control fathers)
High average risk group (sons of SUD-affected fathers)
African–Americans
Fig. 2.
Ethnic variation in TLI by risk group (left) and SUD outcome
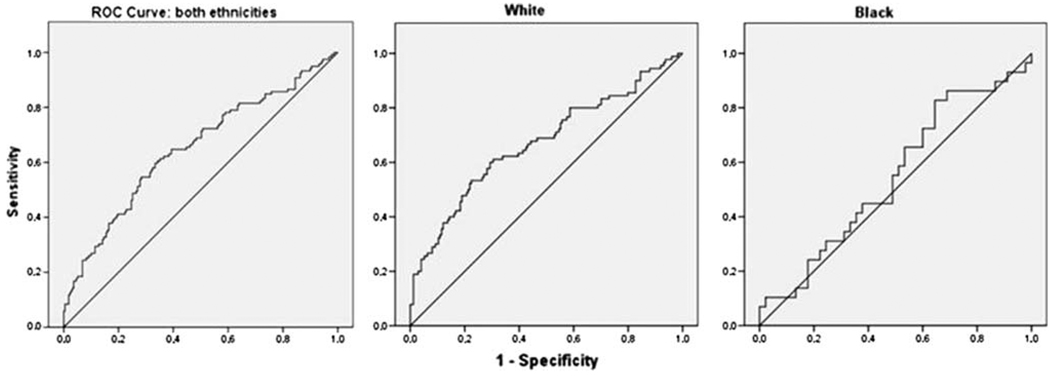
Accordingly, while ROC curve analysis within EA demonstrates the predictive utility of TLI, the area under the curve in AA does not differ significantly from 0.5 (Fig. 3). Similar ethnic differences are indicated by Cox regression analysis (Table 2). Whereas TLI predicts the rate of general SUD as well as cannabis use disorder development in European-Americans, it is not predictive in African-Americans.
Fig. 3.
ROC curve analysis of TLI in predicting SUD among European–Americans (left) and African–Americans
Table 2.
Liability index Cox regression analysis results for ethnic groups
| Diagnosis (depend. variable) | Ethnicity | B | SE | Wald | df | P | Hazard ratio (95% CI) |
|---|---|---|---|---|---|---|---|
| SUDa | EA | 0.57 | 0.120 | 22.520 | 1 | 2.1 × 10−6 | 1.77 (1.398–2.341) |
| AA | 0.23 | 0.221 | 1.119 | 1 | 0.290 | 1.26 (0.819–1.948) | |
| CUDb | EA | 0.61 | 0.125 | 23.643 | 1 | 1.2 × 10−6 | 1.84 (1.4374–2.346) |
| AA | 0.23 | 0.221 | 1.119 | 1 | 0.290 | 1.26 (0.819–1.948) |
Substance use disorder (any illicit substance)
Cannabis use disorder
Twin analysis
Correlation analysis in twins demonstrates high familiaity of the TLI. In the entire twin sample, intra-pair correlation among MZ pairs was slightly greater than double that of DZ twins, 0.80 (P ≪ 0.001) vs. 0.39 (P < 0.01). The Table 3 data, however, suggest differences in the correlation coefficients between males and females. In MZ twins, the correlation was slightly higher among females (albeit significantly at P =0.03). In DZ pairs, the difference between male and female DZ correlations was not significant. TLI was also lower (P =0.006) in female pairs (mean of intrapair averages ± SD was −0.115 ± 0.874 vs. 0.226 ± 0.970 in males). These latter differences are expected, inasmuch as the risk for SUD, measured largely by indicators of behavior dysregulation, is likely to be lower in girls, while the variances do not differ between sexes. The selection of the initial model was ADE, due to rmz > 2rdz. Table 4 presents the results of model fitting and comparison of nested models. Whereas the full model fits well, the AE model fits slightly better and is more parsimonious. The E model fails. The data suggest that twin similarity and heritability in this sample are entirely due to additive genetic effects, h2 = H2 = 0.79.
Table 3.
Intrapair twin TLI correlations
| Zygosity | Males (N) | Females (N) |
|---|---|---|
| MZ | 0.85 (110) | 0.73 (73) |
| DZ | 0.27 (33) | 0.62 (16) |
Table 4.
Structural equation model fitting
| Sample | Model | χ2 | df | P | AIC | Δχ2 | Δdf | PΔ | Model parameters (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Full | a2 | d2 | e2 | ||||||||
| ADE | 3.592 | 3 | 0.309 | −2.408 | 0.79 (0.00–0.84) | 0.00 (0.00–0.81) | 0.20 (0.16–0.27) | ||||
| AE | 3.592 | 4 | 0.464 | −4.408 | 0.000 | 1 | – | 0.79 (0.73–0.84) | 0.21 (0.16–0.27) | ||
| E | 197.249 | 5 | <0.001 | 189.657 | 193.851 | 2 | <0.001 | 1.00 (1.00–1.00) | |||
| Male | a2 | d2 | e2 | ||||||||
| ADE | 1.159 | 3 | 0.763 | −4.841 | 0.25 (0.00–0.89) | 0.60 (0.00–0.89) | 0.14 (0.11–0.20) | ||||
| AE | 2.199 | 4 | 0.699 | −5.801 | 0.699 | 1 | 0.308 | 0.85 (0.80–0.89) | 0.15 (0.11–0.20) | ||
| E | 145.564 | 5 | <0.001 | 135.564 | 144.405 | 2 | <0.001 | 1.00 (1.00–1.00) | |||
| Female | a2 | c2 | e2 | ||||||||
| ACE | 3.392 | 3 | 0.335 | −2.608 | 0.07 (0.00–0.77) | 0.65 (0.00–0.80) | 0.28 (0.19–0.40) | ||||
| CE | 3.471 | 4 | 0.482 | −4.529 | 0.079 | 1 | 0.779 | 0.71 (0.60–0.80) | 0.29 (0.20–0.40) | ||
| E | 65.697 | 5 | <0.001 | 55.697 | 62.305 | 2 | <0.001 | 1.00 (1.00–1.00)) | |||
Whereas the small number of DZ twins does not ensure confidence in the DZ correlation coefficients in the male and female subsamples, the preliminary analysis data are suggestive of sex differences in the phenotypic variance composition. The comparison of MZ and DZ correlations within the subsamples prefers the initial ADE model in males and the ACE model in females. As presented in Table 4, in males, the AE model, while obviously more parsimonious, does not significantly improve the fit compared to the full ADE model. Dropping the dominance component from the model does not appear justified, considering that rmz is substantially higher than 2rdz, but the DE model, while estimating D to be as high as the A component in the AE model (data not shown), is not quite biologically plausible. Therefore, the results, estimating heritability (broad sense) at 0.85, likely contributed by both additive and nonadditive genetic components, do not allow nonambiguous model selection based on these data.
Discussion
The importance of defining a trait for research has been clear at least since Mendel’s discoveries, which became possible as a result of his selecting unambiguously defined characters (traits) with distinct variants (phenotypes) and readily identifiable patterns of inheritance. Studying complex (multifactorial) traits, individual variation in which results from contribution of several or many genes as well as environmental factors, each contributing a small proportion of variance, remains difficult even when the trait is directly observable and measurable (e.g., stature). Even more complicated is studying many complex (particularly psychiatric) disorders, liabilities to which are not only quantitative rather than discrete traits, but also are unob–servable (latent).
We have proposed a method of indexing liability (Vanyukov et al. 2003a; Vanyukov and Tarter 2000) that does not rely on the symptoms of the disorder. The target of the investigation was common transmissible liability to addictions that are consequent to use of illicit drugs. Liabilities to SUD related to illicit substances share virtually all genetic variance (Kendler et al. 2003b), and comprise a group that is genetically distinct from, albeit highly correlated with, the group related to licit substances (Kendler et al. 2007).
Based on the transmissibility of SUD liability, supported by family studies and heritability data on SUD diagnosis, we used that paternal categorical liability phenotype as reference for selecting children’s characteristics related to, and thus indicating, common SUD liability [discussed in detail previously (Vanyukov and Tarter 2000; Vanyukov et al. 2003a)]. These characteristics were then used to construct an index of transmissible SUD liability. This report addresses the reliability and validity of this instrument, as well as its predictive ability for the disorder diagnosis and the rate of SUD development. While the risk group per se is a good predictor of SUD, TLI enables evaluation of risk on a continuous scale in the absence of parental data and, most importantly, regardless of parental diagnosis and its availability (within the risk group). Interestingly, whereas TLI’s relationship with the most common (and frequently first to develop) cannabis use disorder is significant in both HAR and LAR groups, the effect is weaker in the LAR group (and nonsignificant in that group for “any SUD”). This is expected, however, because (1) the precision of TLI in the less deviant area of the liability distribution is lower (TLI indicators are largely indicators of behavioral deviance); and, perhaps more importantly, (2) the sample has not yet passed the age of risk for SUD, whereas it is expected that the lower TLI values are associated not only with a lower SUD risk but also with later disorder onset. At this juncture, the disorder is more likely to have already developed in individuals with higher liability, whereas those with lower liability (one of the manifestations of which is a lower onset age) have not yet realized their risk. This decreases the strength of the relationship between the TLI and the disorder in individuals with lower liability until the sample has passed through the age of risk.
The TLI relationship with alcohol use disorder is relatively weak, which can be accounted for by the specificity of liabilities to alcoholism versus SUD related to illicit drugs. Whereas the data unsurprisingly show high comorbidity of AUD with SUD, the AUD risk differences between the HAR and LAR groups were not significant. These data, in conjunction with the group differences for SUD frequency, are consistent with some degree of specificity of familial liability transmission for disorders related to illicit versus licit substances. Of greater interest is the finding that the relationship of TLI with the risk for cannabis dependence disorder is somewhat stronger than that for “any SUD.” Cannabis is most often the first illicit drug used and thus the first for SUD onset. Nevertheless, some individuals start their drug use with hard drugs (opiates, cocaine) (Tarter et al. 2006), leading to earlier (or solely, in six individuals in this sample) onset of non-cannabis SUD. Whereas TLI reflects transmissible liability, it is possible that the relatively late hard-drug-related disorder onset occurring first is substantially contributed by non-transmissible, individual factors such as variation in accessibility of cannabis in early/mid-adolescence, resulting in a somewhat lower effect of TLI on time-to-any SUD, as opposed to the rate of specifically cannabis-related SUD development.
An important finding of this study is the ethnic variation in the magnitude, risk group differences, and predictive ability of TLI. In summary, this variation appears to be due to that in AA neither the risk group nor the presence/ absence of SUD discriminate between TLI values, which are as high in nonaffected AA subjects as they are in the affected ones. In effect, TLI does not reflect the assessed SUD liability variation in the AA groups. In contrast, the distributional properties of TLI among the EA individuals follow expectations ensuing from the studies of intergen-erational transmission of SUD liability (risk group differences), further verified in relation to the diagnostic outcome. Since TLI items were selected in a predominantly EA sample, it is possible that item selection in an AA sample (provided a sufficient number were available) could have resulted in a different index, specific to the AA population. On the other hand, it is possible that the transmissibility (including heritability) of SUD liability in the AA population is substantially lower than in the EA population, and the phenotypic variation is largely due to environmental non-familial contribution. There are few if any studies, however, that have addressed this issue specifically in AA twins. Most of the relevant genetic research (e.g., Kendler et al. 2003b; Button et al. 2006) has been conducted on samples with mixed ethnicity, predominantly EA, often without reporting the ethnic composition of the sample. As the items constructing TLI are largely indicators of behavioral dysregulation/undercontrol (see Appendix), variation in the AA group’s SUD risk appears to be determined by other factors. The phenotypic distribution of behavioral regulation in the AA group is shifted relative to the EA group—mostly from the “low risk” tail. The mechanisms of this shift require elucidation. The restriction of the sample to males only and the incomplete transition of the sample through the age of risk for SUD are limitations of the study. Taking into account the ethnic differences, it is possible that the index derived is fully applicable only to European–Americans.
A limitation of the study is that, because the SUD diagnosis in the fathers was assessed when they were adults, TLI reflects mechanisms that result in the disorder with the onset age range predominantly covering the modal age of risk (the fathers’ mean SUD onset age ± SEM, 20.3 ± 0.34; median 18, and the range is from 11 to 48 years). The age of the children’s sample is substantially lower than their fathers’ when the latter manifested the disorder (16.4 ± 0.16; median 16, range 9–25). The children, therefore, have not yet fully realized the risk indexed by TLI. This suggests that as the children’s sample becomes older, the predictive ability of the index may improve. In addition, the repetition de novo of the full index derivation procedure at each assessment time point (as sufficient samples accumulate to run IRT item calibration analyses for the follow-up visits), and/or testing differences in item properties (differential item functioning, DIF) may both verify the item set and detect items that are age- or ethnicity-specific in any of their parameters.
The results of the twin data analysis, while preliminary and thus warranting caution, are consistent with a high familiality of the index, supporting the methodology of TLI derivation as a measure of transmissible SUD liability. Moreover, the data suggest that the transmissibility of the TLI in males is entirely due to genetic factors. The heritability estimates are high, consistent with the results obtained in studies with categorical SUD diagnoses, which showed, as in this research, no contribution of shared environment (e.g., Kendler et al. 2007). Sex differences in the composition of the TLI phenotypic variance are nevertheless possible, as suggested by sex-specific correlations in this study and the entirely environmental composition of intrapair similarity estimated in females, and require further investigation in a larger twin sample, currently under preparation. With this caveat in mind, the data suggest the utility of the index as a quantitative phenotypic measure of SUD risk. At least in the males, for whom the TLI was originally developed, its estimated high heritability suggests its applicability in genetic (e.g., association) studies in the populations before the development, or otherwise in the absence of, any SUD symptoms (children; asymptomatic).
As the target population grows older and is exposed to drugs, drug use and related behavioral and psychiatric information can be incorporated into the index or used for its refinement, depending on the purpose of its construction. Finally, as this population passes the age of risk, its own, instead of parental, SUD diagnostic/severity information can be used as reference for selecting constructs and items to measure liability on a continuous scale. Such selection will include not only disorder symptoms but also “normal” psychological items, thus ensuring precision measurement in nonaffected and asymptomatic individuals. Application of IRT and related methods can ensure that the scale of this measurement is the same at different ages and in different populations. The relationships of putative etiological factors (including genetic and environmental characteristics and intermediate traits) with the liability indices can be examined to study the mechanisms of the determination of, and changes in, the risk for SUD during individual development and subsequent to intervention. The method described herein and in the previous papers may serve as a model for quantitative indexing of liabilities to other complex disorders, particularly those with relative late onset.
Acknowledgments
The authors are indebted to the staff of the Center for Education and Drug Abuse Research, University of Pittsburgh, for their effort and dedication. This study was supported by the National Institute on Drug Abuse grants P50DA005605, R01DA011922, R01DA019157, K02DA018701, K02DA017822, and the National Institute on Alcohol Abuse and Alcoholism grant K02AA00296
Appendix
See Table 5.
Table 5.
Liability index items
| Item text | Respondent | Response categories |
Source (reference) |
|---|---|---|---|
| Characteristics of child prior to age 13 | Parent | 1 = Yes 2 = No |
Tarter Childhood History Questionnaire (Tarter et al. 1977) |
| 1. Lying | |||
| 2. Stealing | |||
| 3. Impulsive | |||
| 4. Did you often annoy people on purpose to get even? | Child | 0 = No 1 = Yes |
K-SADS-E (Orvaschel and Puig-Antich 1987) |
| 5. Did you often do things to annoy people like grabbing another child’s hat? |
|||
| 6. Did you blurt out answers to questions before they had been completed or did you get into trouble because you would rush into things without thinking? |
|||
| 7. Were things so bad that you were thinking a lot about death or that you would be better off dead? |
|||
| 8. Did he often do things to annoy people like grabbing another child’s hat? |
Parent | 0 = No 1 = Yes |
K-SADS-E (Orvaschel and Puig-Antich 1987) |
| 9. Did he often annoy people on purpose to get even? | |||
| 10. Did he have difficulty staying in line in the supermarket or waiting for his turn while he was playing with other children? |
|||
| 11. Did he blurt out answers to questions before they had been completed or did he get into trouble because he would rush into things without thinking? |
|||
| 12. Did he get into trouble a lot for talking out of turn in school or talking without the teacher calling on him or for bothering people? |
|||
| 13. Did he get into trouble because he would do things without thinking about them first, for example running into the street without looking? |
|||
| 14. Does your child skip classes or school without an excuse? | |||
| 15. I interrupt on people when they are speaking | Child | 0 = Never true 1 = Occasionally true 2 = Mostly true 3 = Always true |
Dysregulation Inventory (Mezzich et al. 2001) |
| 16. He/she interrupts on people when they are speaking | Parent | 0 = Never true 1 = Occasionally true 2 = Mostly true 3 = Always true |
Dysregulation Inventory (Mezzich et al. 2001) |
| 17. Excitable, impulsive best describes the child | Teacher | 0 = Not at all 1 = Just a little 2 = Pretty much 3 = Very much |
Conners Teacher Questionnaire (Conners 1969) |
| The behavior of the child is best described as … | Teacher | 0 = Not at all 1 = Just a little 2 = Pretty much 3 = Very much |
Disruptive Behavior Disorders Scale (Pelham et al. 1992) |
| 18. …often engages in physically dangerous activities without considering possible consequences (not for the purpose of thrill-seeking), e.g., runs into street without looking |
|||
| 19. …has difficulty awaiting turn in games or group situations | |||
| 20. …often blurts out answers to questions before they have been completed |
|||
| 21. …often interrupts or intrudes on others, e.g., butts into other children’s games |
|||
| Describes your child now or within the past 6 months… | Parent | 0 = Not true, 1 = Somewhat or Sometimes true 2 = Very true or often true |
Child Behavior Checklist (Achenbach and Edelbrock 1983) |
| 22. Impulsive or acts without thinking | |||
| 23. Destroys things belonging to his/her family or others | |||
| 24. Disobedient at school | |||
| 25. Steals at home | |||
| 26. Bites fingernails | |||
| 27. Picks nose, skin or other parts or body | |||
| Describes the pupil now or within the past 2 months… | Teacher | 0 = Not true 1 = Somewhat or Sometimes true 2 = Very true or often true |
Teacher’s Report Form of the Child Behavior Checklist (Achenbach 1991) |
| 28. Impulsive or acts without thinking | |||
| 29. Talks out of turn | |||
| 30. Aches or pains (not stomach or headaches) (without known medical causes) |
|||
| 31. Headaches (without known medical causes | |||
| 32. Deliberately harms self or attempts suicide | |||
| 33. I move a great deal in my sleep | Child | 1 = Usually false 2 = More false than true 3 = More true than false 4 = Usually true |
Dimensions of Temperament Survey—Revised (Lerner et al. 1982) |
| 34. I don’t move around much at all in my sleep. (reverse-coded) | |||
| 35. I get hungry about the same time each day. (reverse-coded) | |||
| 36. I usually eat the same amount each day. (reverse-coded) | |||
| 37. I eat about the same amount at supper from day to day. (reverse-coded) |
|||
| 38. My appetite seems to stay the same day after day. (reverse- coded) |
|||
| 39. My child moves a great deal in his/her sleep | Parent | 1 = Usually false 2 = More false than true 3 = More true than false 4 = Usually true |
Dimensions of Temperament Survey—Revised (Lerner et al. 1982) |
| 40. In the morning, my child is still in the same place as he/she was when he/she fell asleep. (reverse-coded) |
|||
| 41. My child doesn’t move around much at all in his/her sleep. (reverse-coded) |
|||
| 42. It takes my child a long time to get used to a new thing in the home. (reverse-coded) |
|||
| 43. It takes my child a long time to adjust to new schedules. (reverse-coded) |
|||
| 44. Changes in plans make my child restless. (reverse-coded) | |||
| 45. My child resists changes in routine. (reverse-coded) |
Contributor Information
Michael M. Vanyukov, Email: mmv@pitt.edu, Center for Education and Drug Abuse Research (CEDAR), Department of Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh, 711 Salk Hall, Pittsburgh, PA 15261, USA; Department of Psychiatry, University of Pittsburgh Medical School, Pittsburgh, PA 15213, USA; Department of Human Genetics, University of Pittsburgh School of Public Health, Pittsburgh, PA 15261, USA.
Levent Kirisci, Center for Education and Drug Abuse Research (CEDAR), Department of Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh, 711 Salk Hall, Pittsburgh, PA 15261, USA; Department of Psychiatry, University of Pittsburgh Medical School, Pittsburgh, PA 15213, USA.
Lisa Moss, Department of Human Genetics, University of Pittsburgh School of Public Health, Pittsburgh, PA 15261, USA.
Ralph E. Tarter, Center for Education and Drug Abuse Research (CEDAR), Department of Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh, 711 Salk Hall, Pittsburgh, PA 15261, USA Department of Psychiatry, University of Pittsburgh Medical School, Pittsburgh, PA 15213, USA.
Maureen D. Reynolds, Center for Education and Drug Abuse Research (CEDAR), Department of Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh, 711 Salk Hall, Pittsburgh, PA 15261, USA
Brion S. Maher, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, VA 23219, USA
Galina P. Kirillova, Center for Education and Drug Abuse Research (CEDAR), Department of Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh, 711 Salk Hall, Pittsburgh, PA 15261, USA
Ty Ridenour, Center for Education and Drug Abuse Research (CEDAR), Department of Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh, 711 Salk Hall, Pittsburgh, PA 15261, USA.
Duncan B. Clark, Center for Education and Drug Abuse Research (CEDAR), Department of Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh, 711 Salk Hall, Pittsburgh, PA 15261, USA Department of Psychiatry, University of Pittsburgh Medical School, Pittsburgh, PA 15213, USA.
References
- Achenbach TM. Manual for the teacher’s report form and 1991 profile. Burlington: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- Achenbach TM, Edelbrock C. Manual for the child behavior checklist and revised child behavior profile. Burlington: T.M. Achenbach; 1983. [Google Scholar]
- Button TM, Hewitt JK, Rhee SH, Young SE, Corley RP, Stallings MC. Examination of the causes of covariation between conduct disorder symptoms and vulnerability to drug dependence. Twin Res Hum Genet. 2006;9:38–45. doi: 10.1375/183242706776402993. [DOI] [PubMed] [Google Scholar]
- Conners CK. A teacher rating scale for use in drug studies with children. Am J Psychiatry. 1969;126:152–156. doi: 10.1176/ajp.126.6.884. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An introduction to the bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- Embretson SE, Reise SP. Item response theory for psychologists. Mahwah: Lawrence Erlbaum Associates Inc; 2000. [Google Scholar]
- Falconer DS. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann Hum Genet. 1965;29:51–76. [Google Scholar]
- Jenkins EA, Maher BS, Marazita ML, Tarter R, Ganger JB, Watt-Morse M, Vanyukov MM. Pittsburgh registry of infant multiplets (PRIM): an update. Twin Res Hum Genet. 2006;9:1006–1008. doi: 10.1375/183242706779462921. [DOI] [PubMed] [Google Scholar]
- Kandel D. Stages in adolescent involvement in drug use. Science. 1975;190:912–914. doi: 10.1126/science.1188374. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003a;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003b;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry. 2007;64:1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- Kirisci L, Vanyukov M, Dunn M, Tarter R. Item response theory modeling of substance use: an index based on 10 drug categories. Psychol Addict Behav. 2002;16:290–298. [PubMed] [Google Scholar]
- Kirisci L, Tarter RE, Reynolds M, Vanyukov M. Individual differences in childhood neurobehavior disinhibition predict decision to desist substance use during adolescence and substance use disorder in young adulthood: a prospective study. Addict Behav. 2006;31:686–696. doi: 10.1016/j.addbeh.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Kirisci L, Tarter R, Mezzich A, Ridenour T, Reynolds M, Vanyukov M. Prediction of cannabis use disorder between boyhood and young adulthood: clarifying the phenotype and environtype. Am J Addict. 2009;18:36–47. doi: 10.1080/10550490802408829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Rounsaville BJ. Sensitivity of psychiatric diagnosis based on the best estimate procedure. Am J Psychiatry. 1992;149:1225–1227. doi: 10.1176/ajp.149.9.1225. [DOI] [PubMed] [Google Scholar]
- Lerner RM, Palermo M, Spiro A, Nesselroade AL. Assessing the dimensions of temperamental individuality across the life span: the dimensions of temperament survey. Child Dev. 1982;53:149–157. [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O’Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Mezzich AC, Tarter RE, Giancola PR, Kirisci L. The dysregulation inventory: a new scale to assess the risk for substance use disorder. J Child Adolesc Subst Abuse. 2001;10:35–43. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht: Kluwer Academic Publishers; 1992. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: statistical modeling 6th edn. Richmond, VA 23298: Department of Psychiatry; 2003. VCU Box 900126. [Google Scholar]
- Nichols RC, Bilbro WC. Diagnosis of twin zygosity. Acta Genet Stat Med. 1966;16:265–275. doi: 10.1159/000151973. [DOI] [PubMed] [Google Scholar]
- Nunnally JC, Bernstein IH. Psychometric theory. 3rd edn. New York: McGraw-Hill; 1994. [Google Scholar]
- Orvaschel H, Puig-Antich J. Schedule for affective disorder and schizophrenia for school age children, epidemiological version: Kiddie-SADS-E (K-SADS-E) 4th edn. Pittsburgh: Western Psychiatric Institute and Clinic; 1987. [Google Scholar]
- Orvaschel H, Puig-Antich J, Chambers W, Tabrizi MA, Johnson R. Retrospective assessment of prepubertal major depression with the Kiddie-SADS-E. J Am Acad Child Psychiatry. 1982;21:392–397. doi: 10.1016/s0002-7138(09)60944-4. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. J Am Acad Child Adol Psychiatry. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Rice JP, Cloninger CR, Reich T. General causal models for sex differences in the familial transmission of multifactorial traits: an application to human spatial visualizing ability. Soc Biol. 1980;26:36–47. doi: 10.1080/19485565.1980.9988401. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Williams J, Miriam G. Instruction manual for the structured clinical interview for DSM-III-R. New York: New State Psychiatric Institute; 1987. [Google Scholar]
- Strassberg M, Peters K, Marazita M, Ganger J, Watt-Morse M, Tarter R, Murrelle L, Vanyukov M. Pittsburgh registry of infant multiplets (PRIM) Twin Res. 2002;5:499–501. doi: 10.1375/136905202320906363. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Vanyukov MM. Theoretical and operational framework for research into the etiology of substance use disorders. J Child Adolesc Subst Abuse. 2001;10:1–12. [Google Scholar]
- Tarter R, McBride H, Buonpane N, Schneider D. Differentiation of alcoholics: childhood history of minimal brain dysfunction, family history and drinking pattern. Arch Gen Psychiatry. 1977;34:761–768. doi: 10.1001/archpsyc.1977.01770190023002. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Vanyukov M, Kirisci L, Reynolds M, Clark DB. Predictors of marijuana use in adolescents before and after licit drug use: examination of the gateway hypothesis. Am J Psychiatry. 2006;163:2134–2140. doi: 10.1176/ajp.2006.163.12.2134. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Tarter RE. Genetic studies of substance abuse. Drug Alcohol Depend. 2000;59:101–123. doi: 10.1016/s0376-8716(99)00109-x. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Kirisci L, Tarter RE, Simkevitz HF, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 2. A measurement approach. Neurosci Biobehav Rev. 2003a;27:517–526. doi: 10.1016/j.neubiorev.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Vanyukov M, Moss H, Tarter R. Research designs in family studies. In: Sloboda Z, Bukowski WJ, editors. Handbook of drug abuse prevention: theory science and practice. New York: Kluwer Academic/Plenum Publishers; 2003b. pp. 497–519. [Google Scholar]
- Vanyukov MM, Tarter RE, Kirisci L, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 1. Common mechanisms and manifestations. Neurosci Biobehav Rev. 2003c;27:507–515. doi: 10.1016/j.neubiorev.2003.08.002. [DOI] [PubMed] [Google Scholar]