Abstract
Purpose
To review our clinical experience and determine if there are appropriate signs and symptoms to consider POLG sequencing prior to valproic acid (VPA) dosing in patients with seizures.
Methods
Four patients who developed VPA-induced hepatotoxicity were examined for POLG sequence variations. A subsequent chart review was used to describe clinical course prior to and after VPA dosing.
Results
Four patients of multiple different ethnicities, age 3–18 years, developed VPA-induced hepatotoxicity. All were given VPA due to intrac partial seizures. Three of the patients had developed epilepsia partialis continua. The time from VPA exposure to liver failure was between 2 and 3 months. Liver failure was reversible in one patient. Molecular studies revealed homozygous p.R597W or p.A467T mutations in two patients. The other two patients showed compound heterozygous mutations, p.A467T/p.Q68X and p.L83P/p.G888S. Clinical findings and POLG mutations were diagnostic of Alpers–Huttenlocher syndrome.
Conclusion
Our cases underscore several important findings: POLG mutations have been observed in every ethnic group studied to date; early predominance of epileptiform discharges over the occipital region is common in POLG-induced epilepsy; the EEG and MRI findings varying between patients and stages of the disease; and VPA dosing at any stage of Alpers–Huttenlocher syndrome can precipitate liver failure. Our data support an emerging proposal that POLG gene testing should be considered in any child or adolescent who presents or develops intractable seizures with or without status epilepticus or epilepsia partialis continua, particularly when there is a history of psychomotor regression.
Keywords: POLG, Idiosyncratic hepatotoxicity, Valproic acid, Alpers–Huttenlocher syndrome, Seizures
1. Introduction
Alpers1 described the syndrome that bears his name; refractory seizures, developmental regression, cortical blindness, and age of onset 3–7 years. The liver findings were subsequently noted by Huttenlocher et al.2 However, it was not until Bicknese et al.3 described valproic acid (VPA) toxicity in six children with Huttenlocher variant of Alpers syndrome that VPA-induced liver toxicity and Alpers syndrome were connected [Alpers–Huttenlocher syndrome is synonymous with Alpers syndrome]. Subsequently, Naviaux and Nguyen4 made the discovery that mutations within the mitochondrial DNA replicase, polymerase gamma 1 (POLG) was responsible for Alpers–Huttenlocher syndrome. Although now, POLG mutations, Alpers–Huttenlocher syndrome, and VPA-induced liver failure are known, there remains the question on how to predict patient populations where VPA exposure should be avoided.
The mechanism of POLG with VPA-induced liver failure is not clear. POLG together with deoxyguanosine kinase and MPV17, are the nuclear genes responsible for most cases of the hepatocerebral (OMIM 251880) form of mitochondrial DNA (mtDNA) depletion.5–7 Only the mutations in POLG and the mitochondrial DNA helicase TWINKLE have been associated with genetically proven VPA-induced liver failure.8 Human POLG is a heterotrimer, composed of one p140 catalytic subunit encoded by the POLG gene and two p55 accessory subunits encoded by the POLG2 gene.9,10 More than 150 pathogenic POLG mutations have been reported in patients with a broad spectrum of clinical phenotypes (http://tools.niehs.nih.gov/polg/).
In this study, we describe the finding of four cases including infant, child, adolescent, and young adult from different ethnic backgrounds who developed liver failure in response to VPA dosing. Regardless of the age of onset of seizures, administration of VPA precipitated liver failure within 2–3 months. Due to our increased understanding of this syndrome, we tested two other children for possible POLG mutations prior to exposure to VPA and they are still alive.
2. Methods
2.1. Patients and DNA
Blood samples of patients with clinical presentations suggestive of POLG deficiency were evaluated at the Mitochondrial Diagnostics Laboratory at Baylor College of Medicine. Total (nuclear and mitochondrial) DNA was extracted from peripheral blood leukocytes using commercially available DNA isolation kits (Gentra Systems Inc., Minneapolis, MN), according to the manufacturer’s protocols.
2.2. Sequencing analysis
Sequence-specific oligonucleotide primers linked to M13 universal primer sequences were designed to amplify the 22 coding exons and at least 50 nucleotides of each flanking intron of POLG gene. PCR products generated using Fast Start DNA polymerase (Roche, Indianapolis, IN) were purified on ExcelaPure 96-well UF PCR purification plates (Edge BioSystems, Gaithersburg, MD). Sequencing reactions were performed using the BigDye Terminator Cycle Sequencing kit (Version 3.1) and analyzed on an ABI3730XL automated DNA sequencer with Sequencing Analysis Software v5.1.1 (Applied Biosystems, Foster City, CA, USA). DNA sequences were analyzed using Mutation Surveyor Version 2.6.1 and the GenBank POLG sequence; ID NM_002693.1.
2.3. Clinical reports
2.3.1. Patient 1
This patient is an 18-year-old Hispanic male, who displayed normal development until 14 years of age when he was diagnosed with pulmonary tuberculosis (Table 1). He had a full recovery. His first seizures were complex partial seizures also occurred at 14 years of age. He failed antiepileptic therapy with oxcarbazepine and he was placed on VPA. In 2 months, he had bilateral foot drop, pes cavus, and mild ophthalmoplegia without obvious cognitive abnormality. Electromyography (EMG) testing demonstrated a peripheral neuropathy. Within 24 h of muscle biopsy he developed pancreatitis that progressed to multiple organ failure including kidneys, liver, lung, and pancreas. VPA was stopped. The muscle biopsy showed fiber size variation, ragged red fibers, and COX-negative fibers. Electron microscopy showed abnormal mitochondrial ultrastructure with increased lipid droplets. Although VPA was stopped, his liver function impairment progressed. Despite aggressive supportive care, he unfortunately died of sepsis and adult respiratory distress syndrome 27 days after the biopsy.
Table 1.
Summary of clinical course of patients exposed to VPA.
| Patient | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Gender/age (years) | Male/18 | Female/23 | Female/3.3 | Female/10 |
| Genotype allele 1/allele 2 | c.1789C>T/c.1789C>T | c.1399G>A/c.1399G>A | c.1399G>A/c.202C>T | c.T248C/c.G2662A |
| Amino acid change | p.R597W/p.R597W | p.A467T/p.A467T | p.A467T/p.Q68X | p.L83P/p.G888S |
| Ethnicity | Hispanic | Caucasian | Caucasian | Chinese |
| Health status before seizure onset | Normal developmental and growth status; pulmonary tuberculosis at 14-years and recovery | Sensory neuropathy, ataxia since 10 years | Celiac disease, mild developmental delay, ataxia | Mild elevation of hearing threshold at age 5-years |
| MRI of brain (hyperintensity on T2/FLAIR sequences) | Left cerebellar hemisphere, right cerebellar vermis, left thalamus, left centrum semiovale | (1) Right and left frontal lobe, left thalamus, bilateral parietal, right occipital | Left cerebellum | Semiovale, cerebellum periventricular white matter, right occipital |
| (2) Normal | ||||
| Age of seizure onset (year) | 14 | 15 | 2.8 | 9 |
| Seizure type | CPS | SPS and EPC myoclonus | CPS, EPC, visual hallucination, and myoclonus | CPS, EPC, status epilepticus |
| EEG | Generalized | Right occipital spikes, normal EEG, background slow, PLEDs | Left occipital spikes/right occipital spikes (varied with EEG), background slow | Background slow, left central and parietal spikes, left hemisphere periodic discharges, bilateral occipital spikes |
| Drug for seizure control | OXC, PHT, VPA | LTG stable for 7 years, VPA | OXC, VPA | PHT, CBZ, VPA, LTG, TOP, CLON |
| Duration (month) of add-on VPA before hepatotoxicity | 2 months | 3 months | 3 months | 2.5 months |
| Course after hepatotoxicity | Liver failure, renal failure and pancreatitis | Liver transplantation, progressive encephalopathy | Encephalopathy | Psychomotor regression, vegetative state |
| Lactate | 30 mmol/L (normal 0.5–1.7 mmol/L) | ND | Normal | Normal |
| Other phenotype | Ophthalmoplegia, peripheral neuropathy | Sensorineural hearing loss, dysmetria, intention tremor, hypotonia | Chronic constipation, cyclic vomiting, hypotonia, absent smooth pursuit | Leukodystrophy, right PCA infarct in MRI, cortical blindness at 9.5 years |
| Outcome | Expired at 18.5 years | Expired at 23.5 years | Expired at 3 years 4 months | Survived |
SPS, simple partial seizure; CPS, complex partial seizure; EPC, epilepsia partialis continua; MRI, magnetic resonance imaging; FLAIR, fluid attenuated inversion recovery; PCA, posterior cerebral artery; CBZ, carbamazepine; PHT, phenytoin; OXC, oxcarbazepine; VPA, valproic acid; LTG, Lamotrigine; CLON, clonazepam; TOP, topiramate; and PB, phenobarbital; PLEDs, periodic lateralized epileptiform discharges.
2.3.2. Patient 2
POLG mutations and brief symptoms list have been briefly described.11 Previously healthy until 10-years of age the patient developed drop attacks suggestive of ataxia or myoclonic seizures with electroencephalogram (EEG) showed photic convulsive responses and infrequent generalized discharges during slow wave sleep (Table 1). She was found to have a mild sensorineural hearing loss. At 15 years of age, she developed epilepsia partialis continua (EPC). EEG demonstrated right occipital epileptiform discharges. EMG examination demonstrated a moderate to severe symmetric sensory and motor axonal peripheral neuropathy of lower extremities. A brain MRI showed multiple hyperintense T2/FLAIR (fluid attenuated inversion recovery) changes in the right inferior frontal lobe, left medial frontal lobe, left thalamus, bilateral parietal, and occipital lobes with the most prominent lesion being the right occipital lobe. Repeated MRI 1 month later showed resolution of these changes. Muscle biopsy showed fiber type 2 predominance (60%), and scattered COX-negative fibers (6%). Electron transport chain (ETC) enzyme testing showed <15% of normal activity in complex III. Muscle and lymphocyte mtDNA was negative for common mitochondrial DNA mutations. Lamotrigine was started and she became seizure free and EEG normalized. She did extremely well clinically until age 21, when she developed increasing bouts of myoclonus. Initially, the myoclonus responded to levetiracetam but after several months myoclonus became more severe and VPA was added. Within 2 months she developed cognitive slowing, VPA was stopped, and she was hospitalized for increasing encephalopathy and myoclonus. At admission, she was found to be in liver failure. EEG demonstrated generalized slowing without epileptiform discharges. MRI of the brain was normal. One month after VPA was stopped, liver transplant was performed. Following transplant her liver function normalized but she developed periodic lateralized epileptiform discharges on EEG. Mechanical ventilation could not be weaned due to pulmonary edema and a right pneumothorax. Life support was withdrawn. She died within 2 days of transplant. Liver biopsy of her pre-transplanted liver showed extensive necrosis, bile stasis, bile ductular proliferation, moderate lymphoplasmacytic infiltration, and bridging fibrosis on Masson trichrome stain. The transplanted liver was normal at autopsy.
2.3.3. Patient 3
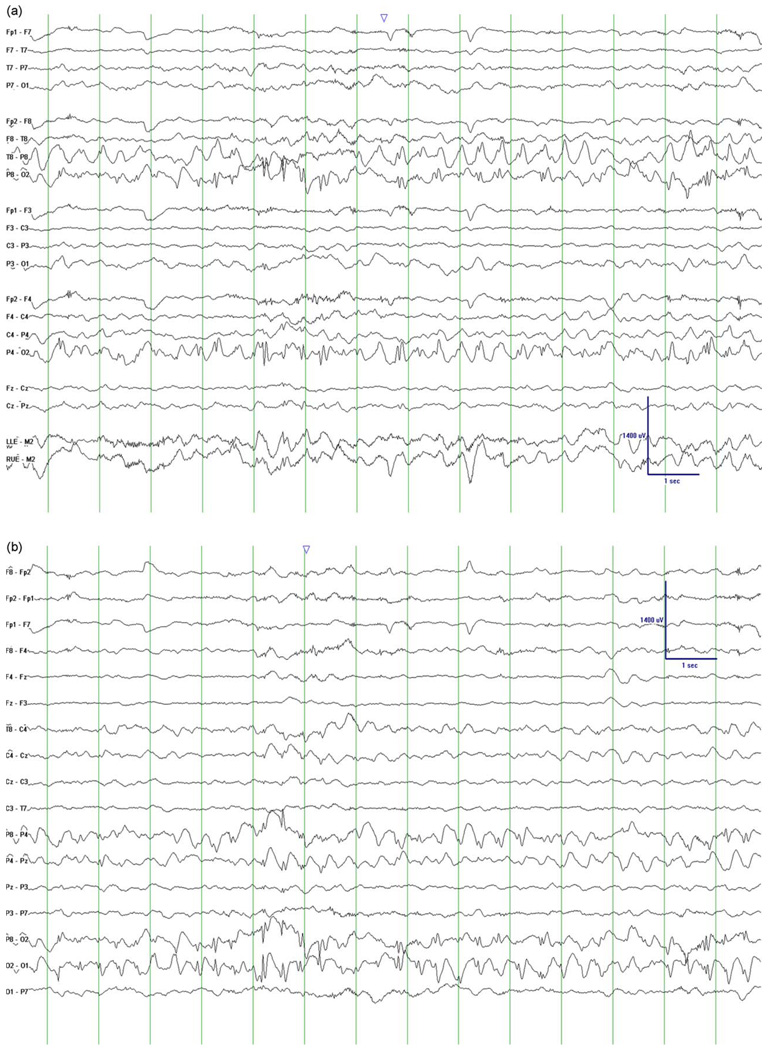
The patient was initially brought to medical attention due to frequent vomiting (projectile) episodes beginning during infancy. POLG mutations and brief symptom summary have been previously reported.11 The mother had Crohn’s disease and the father’s family had multiple members with Celiac and thyroid disease (autoimmune). At 18 months of age, she developed myoclonic jerks and was noted to be mildly ataxic with developmental delay (Table 1). An EEG was performed and demonstrated occipital epileptiform discharges. Due to formula intolerance and family history, at 33 months of age she was evaluated for possible Celiac disease. She had elevated endomysial IgA and transglutamase IgA titers and persistent eosinophilia. On small bowel biopsy, she showed features consistent with but not diagnostic of Celiac disease. MRI scan showed two foci of hyperintense T2/FLAIR lesions in the medial aspect of the left cerebellar hemisphere. MRI gradient echo sequences did not detect calcifications. A gluten free diet had no effect on formula tolerance, seizures or ataxia. Subsequently, she developed EPC with left hand clonic movements and eye deviation to the left with visual hallucination of seeing spiders. EPC was effectively treated with lorazepam, fosphenytoin, and phenobarbital. She developed myoclonus. The EEG during this time continued to show occipital epileptiform discharges (Fig. 1a and b). The posterior dominant rhythm was slow. Seizures continued, and VPA was started at age 3 years. Due to increasing obtundation and seizures, she was admitted to the hospital 3 months after starting VPA. On admission, liver failure was noted. VPA was stopped. MRI was repeated showing increased T2/FLAIR signal in the periventricular white matter, bilateral caudate, and thalami. The previous cerebellar lesions had resolved. The child’s parents decided to take her home on hospice and she expired within 3 weeks.
Fig. 1.
This EEG epoch demonstrates occipital lobe epileptiform discharges of patient 3 at age 3 years. (a) Bipolar montage and predominant right occipital discharges. (b) Same epoch but in a transverse montage. This view shows that the predominant discharge is right occipital. The background rhythm is slow for age. Interestingly, in other EEGs, the occipital discharge was predominantly left occipital.
2.3.4. Patient 4
This 10-year-old Chinese girl had unremarkable growth and development except a mild elevation of hearing threshold, detected at 5 years of age. This patient has been previously described.12 Briefly, seizures started at 9 years of age and were multifocal partial seizures that often progressed to status epilepticus. EEG showed frequent epileptiform discharges over central, parietal, and occipital regions with slowing of the background (Table 1). Repeated EEG 7 months later demonstrated generalized slowing, periodic slow and spike wave over the left hemisphere, and almost continuous spike and wave discharges over the bi-occipital regions. Due to intractability to multiple medications, VPA was started. VPA was stopped after 2.5 months due to liver failure. Since the initial report,12 her liver function gradually improved and now has returned to near normal functioning. Clinically, she has demonstrated development regression, losing the ability to walk, sit, or speak and eventually progressed to a vegetative state. She remained in a vegetative state until death at age 13.
2.3.5. Patient 5
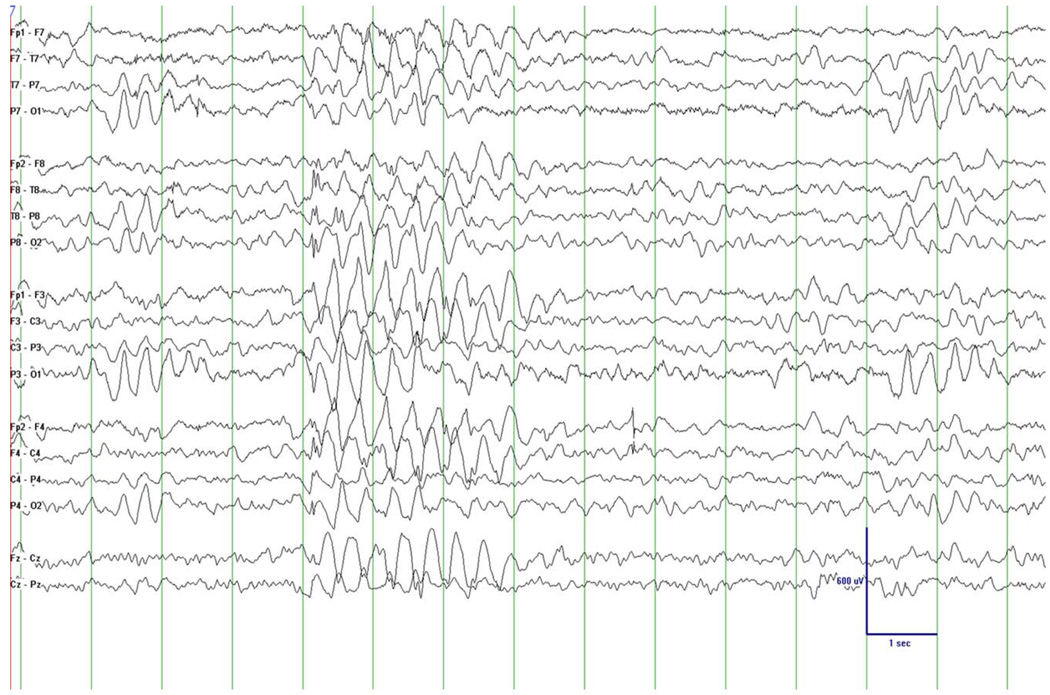
Patient 5 (Table 2) did not receive VPA and will be discussed later. The 11-year-old boy was normal developing until 2.5 years, when he developed gait ataxia with repeated episodes of ketotic hypoglycemia in the context of cyclic vomiting, requiring multiple hospitalizations. He developed seizures at 6-years of age and seizures have been intractable to antiseizure medications. An EEG at 10 years of age demonstrated generalized epileptiform discharges suggesting generalized epilepsy (Fig. 2). Muscle biopsy was performed for a suspicion of mitochondrial disease. ETC results were normal and histochemical studies of the muscle demonstrated scattered COX-negative fibers. POLG sequencing demonstrated compound heterozygous mutations, p.A467T and p.G848S. Currently at 11-years of age, he has severe gastrointenstinal dysmotility, intractable generalized seizures, ophthalmoplegia, myoclonus, bilateral eyelid ptosis, axial hypotonia, muscle weakness and fatigue, and cognitive regression. He has just begun to develop signs and symptoms of liver failure.
Table 2.
Summary of clinical course of patients not exposed to VPA.u.
| Patient | ||
|---|---|---|
| 5 | 6 | |
| Gender/age (years) | Male/11 | Female/2 |
| Genotype allele 1/allele 2 | c.1399G>A/c.2542G>A | c.2542G>A/c.2243G>C |
| Amino acid change | A467T/G848S | G848S/W748S |
| Ethnicity | Caucasian | Caucasian |
| Health status before seizure onset | Cyclic vomiting, episodic hypoglycemia | Normal |
| MRI of brain (hyperintensity on T2/FLAIR sequences) | Normal | Normal |
| Age of seizure onset (years) | 6 | 4 |
| Seizure type | Generalized | CPS, EPC |
| EEG | Generalized spikes, background slow | Right occipital |
| Drug for seizure control | CBZ, OXC, LTG, ZNS, LEV, TPM | LEV, OXC |
| Duration (month) of add-on VPA before hepatotoxicity | VPA not given | VPA not given |
| Course after hepatotoxicity | N/A | N/A |
| Lactate | Normal | Normal |
| Other phenotype | GI dysmotility, eyelid ptosis,ophthalmoplegia, sensorineural hearning loss, absent smooth pursuit eye movements | Truncal ataxia, intention tremor |
| Outcome | Alive | Alive |
EEG, encephalogram; MRI, magnetic resonance imaging; FLAIR, fluid attenuated inversion recovery; GI, gastrointestinal; CPS, complex partial seizure; GTC, general tonic seizure; SE, status epilepticus; VPA, valproic acid; CBZ, carbamazepine; PHT, phenytoin; OXC, oxcarbazepine; LTG, Lamotrigine; CLON, clonazepam; TPM, topiramate; LEV, levetiracetam; GAB, gabapentin; and FOS, fosphenytoin.
Fig. 2.
This EEG epoch shows generalized epileptiform discharges in a bipolar montage (patient 5). This epoch demonstrates a large amplitude spike and wave discharge (large delta discharge followed by a notched spike) at 2–2.5 Hz. There is also an intermittent slow that is maximal bi-occipital with left occipital predominance. The background rhythm is slow for age.
2.3.6. Patient 6
Patient 6 (Table 2) did not receive VPA and will be discussed later. The 4-year-old girl began having seizures at the age of 2 years, with EPC arising from the right occipital head region. The seizures were described as eye jerking/nystagmus to the left and right. Seizures were treated with levetiracetam and oxcarbazepine. She became seizure free after several days of combination therapy, However, there remained frequent spike and wave discharges in the right occipital region on EEG. Otherwise her development has been on tract. On neurological examination there is a mild wide-based gait with truncal ataxia. She also has an intention tremor. The MRI of the brain was normal. POLG sequencing detected heterozygous mutations p.G848S and p.W748S mutations.
3. Results
Only one (patient 2) of the four patients (with liver failure) described above was suspected of having a mitochondrial disorder at the time of VPA dosing. She had a deficiency of complex III noted on electron transport chain analysis. All patients were on polytherapy for seizure control. The entire mitochondrial genome of patient 1 was sequenced but deleterious mutations were not found. None of the patients were thought to have Alpers–Huttenlocher syndrome. The sentinel finding of VPA-induced liver failure triggered mutational analysis of POLG in patients 1–4.
Gene sequencing revealed a missense variant, c.1789C>T (p.R597W), in patient 1. In patient 2, a common homozygous POLG mutation, c.1399G>A (p.A467T), was detected. Patient 3 was compound heterozygous for a missense mutation, c.1399G>A (p.A467T) and a nonsense mutation, c.202C>T (p.Q68X). Patient 4 was compound heterozygous for two missense variants, c.248T>C (p.L83P) and c.2662G>A (p.G888S).
All patients had confirmed pathogenic mutations in POLG gene. Our four patients are of diverse ethnic background and the age of disease onset varies from infants to adolescents to young adults. Early growth and development appeared normal until the first seizures at 9 and 14 years of age for patients 4 and 1. Patient 2 had early ataxia with myoclonus and mild sensorineural hearing loss. Myoclonus was essentially well controlled with lamotrigine. At 22-years of age, VPA was started. Although she was found to have a complex III defect, she was homogygous for the p. A467T mutation and at autopsy had pathological findings of liver and brain (data not presented) consistent with Alpers–Huttenlocher syndrome. Complex IV defects have been found in Alpers–Huttenlocher patients13 and our patient 2 would suggest that complex III defects are also compatible with Alpers–Huttenlocher syndrome. Patient 3 presented in infancy with projectile vomiting. Each patient developed intractable complex partial seizures, with 3 patients developing EPC and/or status epilepticus. In all patients VPA was administered for seizure control, ultimately leading to liver failure and a genetic diagnosis. The time to liver failure was short in each, approximately 3 months after VPA exposure. Patient 2 underwent an unsuccessful liver transplant. Patients 1–3 died of acute problems from liver failure or transplant, while patient 4 eventually died from complications of liver failure.
4. Discussion
The incidence of fatal VPA-induced hepatotoxicity decreases with increasing age. In adults, the incidence is about 1:40,000.14 In children under 2 years receiving polytherapy of VPA with other antiepileptic medications, liver toxicity can be as high as 1/600.14 VPA is the medication of choice for generalized seizure15,16 and efficacious in focal seizures as noted in many studies and expert opinion articles.17–19 The efficacy of VPA in controlling both generalized and focal seizures in the context of VPA-induced hepatic failure makes clinical acumen of its use extremely important. Thinking that VPA-induced liver failure is an age-related disorder, many clinicians do not hesitate to use VPA in patients >2 years with uncontrolled seizures. Furthermore, VPA is the medication of choice in another mitochondrial disease syndrome, myoclonus, epilepsy with ragged red fibers (MERRF).20 Our data, together with the broad age range of patients with reported VPA-induced liver failure with POLG mutations, suggests that VPA exposure is related to POLG genotype and disease, in particular Alpers–Huttenocher syndrome.21–27
The onset of seizures may give clues to the presence of POLG mutations and Alpers–Huttenlocher syndrome. Several reports have shown that patients with POLG mutations and Alpers–Huttenlocher syndrome present with syndromic epilepsy with occipital lobe predilection.5,21,23–25 In addition, our patient population (Table 1) and recent literature demonstrates seizure semiology consistent with occipital lobe epileptic discharges; focal clonic motor seizures, secondarily generalized tonic–clonic seizures, hallucinations and nystagmus.23–25,28 However, early studies have not consistently shown occipital lobe discharges, with some describing generalized discharges, hypsarrhythmia or multifocal discharges.28,29 Tulinius and Hagne28 suggested that timing of the disease progression was likely responsible for EEG variation. The predominance of reports with POLG proven Alpers–Huttenlocher syndrome strongly suggests that the majority of patients presenting with seizures have predominantly occipital lobe discharges. Often discharges are seen in the context of EPC or status epilepticus.23–25 In one small study, five patients presented with convulsive status epilepticus.21 Three of four of our patients would compliment focal EEG findings within the occipital region and EPC in the course of their disease. However, not all patients having Alpers–Huttenlocker have focal EEG patterns. Patients 2 and 5 had generalized spikes, more reminisce of the report by Boyd et al.29 One of our patients (patient 2) demonstrated that normal EEG findings are also possible during the course of the syndrome. We agree with Tulinius and Hagne28 that EEG may change during the course of this disorder. Most patients, at the time of seizure onset will display a focal pattern with posterior head distribution. Some may have generalized discharges, but this is likely uncommon early, but may be seen later in the course of the disorder (patients 2 and 5). As demonstrated in our patients, most, if not all, will have abnormal slowing of the background frequency suggesting encephalopathy. However, the EEG may also be normal as seen in patient 2.
In one study, epileptiform discharges were high amplitude delta frequency waves with superimposed polyspikes.21 However, this was not the case in our population (Figs. 1 and 2). This would suggest that although the EEG is very helpful in location and frequency of epileptiform discharges, discharges can be variable and clinical acumen is required interpreting findings with diagnostic conclusions.
Three of our patients (patients 2–4) had EPC sometime in their disease, with another patient (patient 4) having a status epilepticus event. Patient 1 did not have documented episodes of either EPC or status epilepticus. The literature and our findings would suggest that most patients with Alpers–Huttenlocher syndrome have explosive seizure onset, usually presenting with occipital lobe semiologies and frequent bouts of EPC and/or status epilepticus during the course of their disease. Patients with other mitochondrial diseases such as Leigh syndrome, MELAS, mitochondrial transfer RNA mutations and electron transport chain disorders have been associated with EPC.30–32 Therefore, careful evaluations for other possible mitochondrial diseases33–35 and other etiologies of EPC need to be investigated.
Most of the other patients with POLG mutations and VPA-induced liver toxicity were reported from Caucasian of Northern European countries and had similar POLG mutations.23–25 Our patients are of diverse ethnicities including Hispanic, Caucasian, northern European, and Chinese. This emphasizes that POLG mutations and VPA hepatotoxicity can be pan-ethnic. Furthermore, VPA-induced hepatotoxicity can happen at any age or stage of the disorder.
Screening of the five most common mutations, p.A467T, p.W748S, p.G848S, p.T914P, and p.T251I + p.P587L in POLG gene would detect both mutant alleles in about half of cases with recessive POLG-related disease and one mutant allele in about one third of cases.11 However, our experience and the literature22,36 suggests that other mutations exist and therefore, screening for these most frequent mutations may not adequately screen for POLG-related disorder in a particular patient. Therefore, full gene sequencing of POLG is currently the most efficacious method to use before VPA dosing.
Review of the cases with POLG mutations and liver failure induced by VPA revealed that most patients had unremarkable clinical history before seizure onset. Often the early clues to a particular mitochondrial DNA depletion syndrome are elusive and therefore, more caution is required. Any ethnicity should be suspected. Early development is likely to be normal. The initial symptoms may be explosive seizures that have an occipital lobe predominance and difficult to control with medications. Seizures may occur at any age during infancy through adolescence. The EEG pattern will often be focal discharges from the occipital lobe region and seizure semiology will be compatible with occipital lobe epilepsy. There likely will be frequent bouts of EPC and status epilepticus. If there are MRI/MRS abnormalities present, they will likely be represented by T2/FLAIR hyperintensity signals within the thalamus, occipital region, and/or cerebellum (Table 1).21,28 MRS imaging may show the presence of lactate peaks.21,33 Progressive psychomotor regression is almost universally found when first symptoms appear. In this clinical scenario, POLG gene sequencing should be performed. Using these principles, we have performed POLG gene sequencing prior to VPA dosing based on clinical suspicion (Table 2). Patients 5 and 6 were found to harbor compound heterozygous mutations described with Alpers–Huttenlocher syndrome. Both patients are currently alive.
Currently, sequencing the POLG gene remains the best diagnostic test to prevent VPA-induced liver failure and patient death. Although open to debate, it is our suggestion that POLG gene testing should be standard before giving VPA for seizure treatment in this genetic mitochondrial DNA depletion epilepsy syndrome. Our patients 5 and 6 are alive because our clinical acumen induced our decision to sequence the POLG gene.
Acknowledgements
RPS is supported in part by the Mitochondrial Research Guild of Seattle Children’s Hospital. The authors would like to thank the patients and families for allowing us to participate in the health care.
Abbreviations
- POLG
polymerase gamma
- VPA
valproic acid
- MELAS
mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes
- MERRF
myoclonus, epilepsy with ragged red fibers
- MRI
magnetic resonance imaging
- MRS
proton (1H) magnetic resonance spectroscopy
- FLAIR
fluid attenuated inversion recovery
- MPV17
mitochondrial inner membrane protein
- TWINKLE
gene encoding mitochondrial helicase
- PCR
polymerase chain reaction
Footnotes
Conflicts of Interest
None of the authors has any conflict of interests to disclose.
References
- 1.Alpers BJ. Diffuse progressive degeneration of the gray matter of the cerebrum. Archives of Neurology and Psychiatry. 1931;25:469–505. [Google Scholar]
- 2.Huttenlocher PR, Solitare GB, Adams G. Infantile diffuse cerebral degeneration with hepatic cirrhosis. Archives of Neurology. 1976;33:186–192. doi: 10.1001/archneur.1976.00500030042009. [DOI] [PubMed] [Google Scholar]
- 3.Bicknese AR, May W, Hickey WF, Dodson WE. Early childhood hepatocerebral degeneration misdiagnosed as valproate hepatotoxicity. Annals of Neurology. 1992;32:767–775. doi: 10.1002/ana.410320610. [DOI] [PubMed] [Google Scholar]
- 4.Naviaux RK, Nguyen KV. POLG mutations associated with Alpers’ syndrome and mitochondrial DNA depletion. Annals of Neurology. 2004;55:706–712. doi: 10.1002/ana.20079. [DOI] [PubMed] [Google Scholar]
- 5.McFarland R, Hudson G, Taylor RW, Green SH, Hodges S, McKiernan PJ, et al. Reversible valproate hepatotoxicity due to mutations in mitochondrial DNA polymerase gamma (POLG1) Archives of Disease in Childhood. 2008;93:151–153. doi: 10.1136/adc.2007.122911. [DOI] [PubMed] [Google Scholar]
- 6.Spinazzola A, Viscomi C, Fernandez-Vizarra E, Carrara F, D’Adamo P, Calvo S, et al. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nature Genetics. 2006;38:570–575. doi: 10.1038/ng1765. [DOI] [PubMed] [Google Scholar]
- 7.Mandel H, Szargel R, Labay V, Elpeleg O, Saada A, Shalata A, et al. The deoxyguanosine kinase gene is mutated in individuals with depleted hepatocerebral mitochondrial DNA. Nature Genetics. 2001;29:337–341. doi: 10.1038/ng746. [DOI] [PubMed] [Google Scholar]
- 8.Hakonen AH, Isohanni P, Paetau A, Herva R, Suomalainen A, Lonnqvist T. Recessive Twinkle mutations in early onset encepalopathy with mtDNA depletion. Brain. 2007;130:3032–3040. doi: 10.1093/brain/awm242. [DOI] [PubMed] [Google Scholar]
- 9.Yakubovskaya E, Lukin M, Chen Z, Berriman J, Wall JS, Kobayashi R, et al. The EM structure of human DNA polymerase gamma reveals a localized contact between the catalytic and accessory subunits. EMBO Journal. 2007;26:4283–4291. doi: 10.1038/sj.emboj.7601843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrodeguas JA, Theis K, Bogenhagen DF, Kisker C. Crystal structure and deletion analysis show that the accessory subunit of mammalian DNA polymerase gamma, Pol gamma B, functions as a homodimer. Molecular Cell. 2001;7:43–54. doi: 10.1016/s1097-2765(01)00153-8. [DOI] [PubMed] [Google Scholar]
- 11.Wong L-J, Naviaux RK, Brunetti-Pierri N, Zhang Q, Schmitt ES, Truong C, et al. Molecular and clinical genetics of mitochondrial diseases due to POLG mutations. Human Mutation. 2008;29:E150–E172. doi: 10.1002/humu.20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao X, Wu Y, Wong L-JC, Zhang Y, Xiong H, Chou P-C, et al. Alpers syndrome with prominent white matter changes. Brain and Development. 2008;30:295–300. doi: 10.1016/j.braindev.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Castro-Gabo M, Gonzalez-Conde V, Fernandez-Seara MJ, Rodrigo-Saez E, Fernandex-Cebrian S, Alonso-Martin A, et al. Early mitochondrial encephalomyopathy due to complex IV deficiency consistent with Alpers–Huttenlocker syndrome report of two cases. Review Neurology. 1999;29:912–917. [PubMed] [Google Scholar]
- 14.Dreifuss FE, Santilli N, Langer DH, Sweeney KP, Moline KA, Menander KB. Valproic acid hepatic fatalities: a retrospective review. Neurology. 1987;37:379–385. doi: 10.1212/wnl.37.3.379. [DOI] [PubMed] [Google Scholar]
- 15.Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalized and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1016–1026. doi: 10.1016/S0140-6736(07)60461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French JA, Kanner AM, Bautista J, Abou-Khalil B, Browne T, Harden CL, et al. Efficacy and tolerability of the new antiepileptic drugs I: treatment of new onset epilepsy: report of the therapeutics and technology assessment subcommittee and quality standards subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2004;62:1252–1260. doi: 10.1212/01.wnl.0000123693.82339.fc. [DOI] [PubMed] [Google Scholar]
- 17.Coppola G. Treatment of partial seizures in childhood: an overview. CNS Drugs. 2004;18:133–156. doi: 10.2165/00023210-200418030-00001. [DOI] [PubMed] [Google Scholar]
- 18.Saneto RP, Kotagal P, Rothner AD, Baker J, Kotagal LL. Valproic acid use in pedatric partial epilepsy after initial medication failure. Journal of Pediatric Neurology. 2004;2:199–203. [Google Scholar]
- 19.Dean JC, Penry JK. Valproate monotherapy in 30 patients with partial seizures. Epilepsia. 1988;29:140–144. doi: 10.1111/j.1528-1157.1988.tb04409.x. [DOI] [PubMed] [Google Scholar]
- 20.Berkovic SF. Progressive myoclonus epilepsies. In: Pellock JM, Dodson WE, Bourgeois BFD, editors. Pediatric epilepsy diagnosis and therapy. 2nd ed. New York: Demos Medical Publishing; 2001. pp. 233–242. [Google Scholar]
- 21.Wolf NI, Rahman S, Schmitt B, Taanman J-W, Duncan AJ, Harting I, et al. Status epilepticus in children with Alpers’ disease caused by POLG1 mutations: EEG and MRI findings. Epilepsia. 2009;50:1596–1607. doi: 10.1111/j.1528-1167.2008.01877.x. [DOI] [PubMed] [Google Scholar]
- 22.Chinnery PF, Zeviani M. 155th ENMC workshop: polymerase gamma and disorders of mitochondrial DNA synthesis, 21–23 September 2007, Naarden, The Netherlands. Neuromuscular Disorders. 2008;18:259–267. doi: 10.1016/j.nmd.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Engelsen BA, Bernt A, Tzoulis C, Karlsen B, Lillebo A, Laegreid LM, et al. POLG1 mutations cause a syndromic epilepsy with occipital lobe predilection. Brain. 2008;131:818–828. doi: 10.1093/brain/awn007. [DOI] [PubMed] [Google Scholar]
- 24.Uusimaa J, Hinttala R, Rantala H, Paivarinta M, Herva R, Roytta M, et al. Homozygous W748S mutation in the POLG1 gene in patients with juvenile-onset Alpers syndrome and status epilepticus. Epilepsia. 2008;49:1038–1045. doi: 10.1111/j.1528-1167.2008.01544.x. [DOI] [PubMed] [Google Scholar]
- 25.Wiltshire E, Davidzon G, DiMauro S, Akman HO, Sadleir L, Haas L, et al. Juvenile Alpers disease. Archives of Neurology. 2008;65:121–124. doi: 10.1001/archneurol.2007.14. [DOI] [PubMed] [Google Scholar]
- 26.Galimberti CA, Diegoli M, Sartori I, Uggetti C, Brega A, Tartara A, et al. Brain pseudoatrophy and mental regression on valproate and a mitochondrial DNA mutation. Neurology. 2006;67:1715–1717. doi: 10.1212/01.wnl.0000242882.58086.9a. [DOI] [PubMed] [Google Scholar]
- 27.Lam CW, Lau CH, Williams JC, Chan YW, Wong L-JC. Mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS) triggered by valproate therapy. European Journal of Pediatrics. 1997;156:562–564. doi: 10.1007/s004310050663. [DOI] [PubMed] [Google Scholar]
- 28.Tulinius MH, Hagne I. EEG findings in children and adolescents with mitochondrial encephalomyopathies: a study of 25 cases. Brain and Development. 1991;13:167–173. doi: 10.1016/s0387-7604(12)80024-6. [DOI] [PubMed] [Google Scholar]
- 29.Boyd SG, Harden A, Egger J, Pampliglione G. Progressive neuronal degeneration of childhood with liver disease (“Alpers’ disease”): characteristic neurophysiological features. Neuropediatrics. 1986;17:75–80. doi: 10.1055/s-2008-1052505. [DOI] [PubMed] [Google Scholar]
- 30.Saneto RP, Cohen BH, Hoppel CL. Epilepsia partialis continua associated with mitochondrial electron transport chain dysfunction. Annals of Neurology. 2002;52 Suppl. 1:S150. [Google Scholar]
- 31.Elia M, Musumeci SA, Ferri R, Colamaria V, Azan G, Greco D, et al. Leigh syndrome and partial defect of cytochrome c oxidase associated with epilepsia partialis continua. Brain and Development. 1996;18:207–211. doi: 10.1016/0387-7604(95)00126-3. [DOI] [PubMed] [Google Scholar]
- 32.Schuelke M, Bakker M, Stoltenburg G, Sperner J, von Voers A. Epilepsia partialis continua associated with a homoplasmic mitochondrial tRNA Ser(UCN) mutation. Annals of Neurology. 1998;44:700–704. doi: 10.1002/ana.410440420. [DOI] [PubMed] [Google Scholar]
- 33.Saneto RP, Friedman S, Shaw DWW. Neuroimaging and mitochondrial disease. Mitochondrion. 2008;8:396–413. doi: 10.1016/j.mito.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haas RH, Parika S, Falk MJ, Saneto RP, Wolf NI, Darin N, et al. The in-depth evaluation of suspected mitochondrial disease: The Mitochondrial Medicine Society’s Committee on diagnosis. Molecular Genetics and Metabolism. 2008;94:16–37. doi: 10.1016/j.ymgme.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas RH, Parikh S, Falk MJ, Saneto RP, Wolf N, Darin N, et al. Mitochondrial disease: a practical approach for primary care physicians. Pediatrics. 2007;120:1326–1333. doi: 10.1542/peds.2007-0391. [DOI] [PubMed] [Google Scholar]
- 36.Stewart JD, Tennant S, Powell H, Pyle A, Blakely EL, He L, et al. Novel POLG1 mutations associated with neuromuscular and liver phenotype in adults and children. Journal of Medical Genetics. 2009;46:209–214. doi: 10.1136/jmg.2008.058180. [DOI] [PubMed] [Google Scholar]