Summary
Background
In adults with HIV treated with antiretroviral drug regimens from within the three original drug classes (nucleoside or nucleotide reverse transcriptase inhibitors [NRTIs], non-NRTIs [NNRTIs], and protease inhibitors), virological failure occurs slowly, suggesting that long-term virological suppression can be achieved in most people, even in areas where access is restricted to drugs from these classes. It is unclear whether this is the case for children, the group who will need to maintain viral suppression for longest. We aimed to determine the rate and predictors of triple-class virological failure to the three original drugs classes in children.
Methods
In the Collaboration of Observational HIV Epidemiological Research Europe, the rate of triple-class virological failure was studied in children infected perinatally with HIV who were aged less than 16 years, starting antiretroviral therapy (ART) with three or more drugs, between 1998 and 2008. We used Kaplan-Meier and Cox regression methods to investigate the risk and predictors of triple-class virological failure after ART initiation.
Findings
Of 1007 children followed up for a median of 4·2 (IQR 2·4–6·5) years, 237 (24%) were triple-class exposed and 105 (10%) had triple-class virological failure, of whom 29 never had a viral-load measurement less than 500 copies per mL. Incidence of triple-class virological failure after ART initiation increased with time, and risk by 5 years after ART initiation was 12·0% (95% CI 9·4–14·6). In multivariate analysis, older age at ART initiation was associated with increased risk of failure (p=0·02). Of 686 children starting ART with NRTIs and either a NNRTI or ritonavir-boosted protease inhibitor, the rate of failure was higher than in adults with heterosexually transmitted HIV (hazard ratio 2·2 [95% CI 1·6–3·0, p<0·0001]).
Interpretation
Findings highlight the challenges of attaining long-term viral suppression in children who will be taking life-long ART. Early identification of children not responding to ART, adherence support, particularly for children and adolescents aged 13 years or older starting ART, and ART simplification strategies are all needed to attain and sustain virological suppression.
Funding
UK Medical Research Council award G0700832.
Introduction
Antiretroviral therapy (ART) has strikingly improved the prognosis for children with HIV, greatly reducing morbidity and mortality.1–3 However, a major challenge for treatment of these children is to minimise virological failure and development of drug resistance, so that treatment options continue to be available through adolescence and adulthood.
During the past decade, paediatric ART guidelines recommended an age-related CD4 percentage or count threshold for initiation of ART in infants and children.4 However, in view of results from the Children with HIV Early Antiretroviral Therapy (CHER) trial and other evidence,5,6 paediatric ART guidelines now unanimously advocate initiation of ART early in infancy because of the high risk of disease progression.7–9 Even if ART is not started early, vertically infected children face many more years of ART than do adults. A serious challenge for children with HIV, as for any chronic illness, is maintaining long-term adherence to treatment regimens, and thus virological suppression and prevention of emergence of resistance.10–14 However, experience with these children suggests that, with present treatment strategies, adherence rates are frequently less than optimum,15,16 which combined with the increased potential for inadequate drug dosing,17,18 contribute to appreciable risk of children acquiring multidrug-resistant HIV before adulthood.
As for adults, most paediatric ART regimens include drugs from one or more of the original three ART classes: nucleoside or nucleotide reverse transcriptase inhibitors (NRTI), non-NRTIs (NNRTI), and protease inhibitors. The availability of drugs from the new classes (integrase and entry inhibitors) remains limited by the lag in availability of appropriate formulations and pharmacokinetic data for children, and, in developing countries, high drug costs.18 Virological failure of the three original drug classes during childhood will severely limit future treatment options; therefore, the rate of triple-class virological failure should be monitored, to estimate the number of children transferring to adult care in probable need of treatment with new drugs.
We aimed to determine the rate and predictors of triple-class virological failure to the three original drug classes in children within the Collaboration of Observational HIV Epidemiological Research Europe (COHERE).19 The study forms part of the Pursuing Later Treatment Options II (PLATO II) project; an analysis of triple-class virological failure in adults from this project has been published.20
Methods
Study design
14 cohorts with paediatric data in COHERE submitted data in a standardised format21 to one of two regional coordinating centres, where error checks were done before data were merged. Children appearing in more than one cohort were identified, and duplicate records removed. This analysis (of data merged in 2008) was restricted to children perinatally infected with HIV aged less than 16 years who started ART from 1998 onwards with an initial regimen of two or more NRTIs and either a NNRTI or a protease inhibitor (ritonavir-boosted or unboosted), or three NRTIs only (children exposed to ART for the prevention of mother-to-child transmission before starting one of the above regimens were included). Unlike in the adult analysis,20 children starting initial regimens with unboosted protease inhibitors, more than two NRTIs with a NNRTI or protease inhibitor, and three NRTIs only were included to mirror the regimens commonly prescribed to children in the past decade.22 A second analysis was done to mirror the adult analysis. Children were followed up from the start of ART to their last viral-load measurement. Because the definition of virological failure used in this study required 4 months of use of a drug, children were included only if they had at least 4 months (122 days) of follow-up. Time spent off ART (after ART had been started) was included as follow-up. Children were said to have interrupted ART if they stopped all antiretrovirals.
Data extraction
The main outcome in this study was triple-class virological failure, defined as virological failure to at least two NRTIs, one NNRTI, and one protease inhibitor. We defined virological failure of a drug as one viral-load measurement of greater than 500 copies per mL after at least 4 months of continuous use of that drug, regardless of concomitant use of other drugs in that period. We did sensitivity analyses using two variations of this definition: first, 6 months instead of 4 months of continuous drug use; second, viral load above 500 copies per mL after 4 months continuous use had to be confirmed by a second viral load value greater than 500 copies per mL while still on or followed by discontinuation of the drug.
To enable comparisons of the rate of triple-class virological failure in children with that of adults,20 and to represent rates of failure in children starting currently recommended regimens, we did a second analysis to restrict the population to children starting ART with an initial regimen of two or three NRTIs and either a NNRTI or a ritonavir-boosted protease inhibitor (ie, those for whom the initial ART regimen included an unboosted protease inhibitor, or three NRTIs, were excluded). Comparisons were made with adults heterosexually infected with HIV in COHERE (ie, excluding homosexual men), because this is the adult group that children with HIV are probably most similar to in terms of socioeconomic and migration status, and health seeking behaviour.20 Figure 1 shows the two inclusion criteria for initial ART regimens and definitions of triple-class virological failure for the main analysis and the comparison of children with adults.
Figure 1.
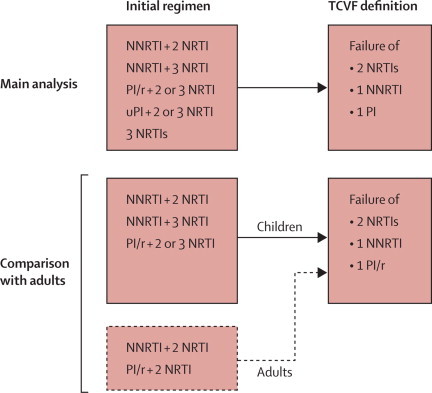
Inclusion criteria for initial regimens and definition of triple-class virological failure in the main analysis and the comparison of children with adults
TCVF=triple-class virological failure. NRTI=nucleoside or nucleotide reverse transcriptase inhibitors. NNRTI=non-NRTI. PI/r=ritonavir-boosted protease inhibitor. PI=protease inhibitor. uPI=unboosted protease inhibitor.
Statistical analysis
We used Kaplan-Meier and Cox regression methods to investigate the risk of triple-class virological failure after starting ART, and to compare the rate of failure in children with that in adults in the PLATO II project. Possible predictors of failure at the time of ART initiation were sex, age, year of ART initiation, initial drug regimen, previous ART exposure for prevention of mother-to-child transmission, AIDS diagnosis before ART, CD4 percentages and viral load (within 6 months before ART initiation). Analyses were done with SAS software (version 9.1).
For children with triple-class virological failure who had failed a ritonavir-boosted protease inhibitor, we examined data from resistance tests done as part of routine care after the start of ART and up to 4 months after triple-class virological failure for a subset of cohorts where data were available. Major mutations from the International AIDS Society USA, 2008, mutation list were used to define resistance.23 Thymidine-associated mutations in HIV reverse transcriptase were defined as M41L, D67N, K70R, L210W, T215Y, T215F, K219Q, and K219E.
Role of the funding source
The sponsors of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
A total of 1007 children were identified in the 14 COHERE cohorts. The UK and Ireland, Spanish, Dutch, and French cohorts contributed more than 90% of children, with a smaller proportion from Denmark, Italy, Belgium, and the European Collaborative Study. Table 1 shows the characteristics of children at the time of ART initiation. Just over a third of children initiated ART in each of the calendar periods 1998–2000 and 2001–03, and a quarter in 2004–07. Fewer than 10% had been exposed to ART as part of prevention of mother-to-child transmission programmes; 55 were exposed to zidovudine only, and 4 to NNRTIs. About 20% had a previous AIDS diagnosis. 560 (56%) children initiated ART on a NNRTI-based regimen—232 (41%) on efavirenz and 328 (59%) on nevirapine. Most children starting ART with a ritonavir-boosted protease inhibitor did so with lopinavir (94%; 118 of 126).
Table 1.
Characteristics of children at the time of antiretroviral therapy initiation
| Number of children (n=1007) | Median follow-up person-years | |
|---|---|---|
| Sex | ||
| Boy | 510 (51%) | 3·9 |
| Girl | 497 (50%) | 3·6 |
| Age at start of ART (years) | ||
| <2 | 350 (35%) | 4·1 |
| 2–4 | 202 (20%) | 4·3 |
| 5–9 | 265 (26%) | 4·0 |
| 10–15 | 190 (19%) | 2·9 |
| Median (IQR) | 4·2 (0·9–8·5) | .. |
| Year of ART initiation | ||
| 1998–2000 | 368 (37%) | 6·8 |
| 2001–2003 | 363 (36%) | 3·9 |
| 2004–2007 | 276 (27%) | 1·7 |
| Previous ART exposure for prevention of mother-to-child transmission* | ||
| No | 937 (93%) | 3·9 |
| Yes | 70 (7%) | 3·1 |
| Initial regimen | ||
| NNRTI + 2 NRTIs | 467 (46%) | 3·0 |
| NNRTI + 3 NRTIs | 93 (9%) | 3·9 |
| Ritonavir-boosted protease inhibitor + 2 or 3 NRTIs | 126 (13%) | 3·0 |
| Unboosted protease inhibitor + 2 or 3 NRTIs | 270 (27%) | 6·7 |
| 3 NRTIs | 51 (5%) | 4·8 |
| AIDS diagnosis before ART initiation | ||
| No | 793 (79%) | 3·7 |
| Yes | 214 (21%) | 4·1 |
| CD4 percentage at ART initiation | ||
| 0–9% | 193 (19%) | 3·8 |
| 10–19% | 254 (25%) | 3·2 |
| ≥20% | 236 (23%) | 4·3 |
| Unknown | 324 (32%) | 4·0 |
| Median (IQR) | 15·0 (9–24) | .. |
| Viral load at ART initiation (log10copies per mL) | ||
| 0–4·49 | 181 (18%) | 3·3 |
| 4·50-5·49 | 334 (33%) | 3·4 |
| ≥5·50 | 258 (26%) | 4·2 |
| Unknown | 234 (23%) | 4·1 |
| Median (IQR) | 5·1 (4·6–5·7) | .. |
ART=antiretroviral therapy. IQR=inter-quartile range. NRTI=nucleoside or nucleotide reverse transcriptase inhibitors. NNRTI=non-NRTI.
Antiretroviral therapy (ART) for prevention of mother-to-child transmission was defined as one or two antiretrovirals initiated within 7 days of birth and stopped up to 60 days after, or three or more antiretrovirals initiated within 7 days of birth and stopped up to 60 days after, and then no antiretrovirals for at least 90 days.
Children were followed up for a median of 4·2 (IQR 2·4–6·5) years and 932 (93%) of 1007 for at least 12 months, with a median of 90 (56–112) days between viral-load measurements. 258 (26%) children interrupted ART at some time, of whom 158 (61%) restarted ART; most follow-up (93%) was of children on ART. Half (518 [51%] of 1007) had first-line virological failure, with about 58% (95% CI 54–62) failure by 5 years after initiation of ART. Most children (470 [91%] of 518) had not started a third drug class at first virological failure; 206 (44%) of 470 started a third drug class before the end of follow-up, with 55% (50–61) doing so by 5 years after first virological failure.
By the end of follow-up, 335 (33%) of 1007 children had started a third drug class, of whom 105 (10% overall and 44% of those with triple-class exposure) had developed triple-class virological failure. Nine (1%) died without such treatment failure. Incidence of triple-class virological failure during each year after ART initiation increased sharply in the first 2 years, from 0·5 per 100 person-years (0·2–1·2) in the first year after ART initiation to 2·6 per 100 person-years (1·5–3·6) in the second year, and then rose more gradually up to the sixth year (4·0 per 100 person-years [2·1–7·0]; figure 2). By 5 years after ART initiation the estimated cumulative proportion of children who had triple-class virological failure was 12·0% (9·4–14·6), and by 8 years it was 20·3% (15·9–24·7). Redefinition of virological failure to 6 months of continuous drug use rather than 4 months gave an estimated cumulative proportion of children with triple-class virological failure by 5 years of 9·4% (7·1–11·8%), and modification of the definition to require confirmation of virological failure gave a 5-year proportion of 9·5% (7·2–11·8).
Figure 2.
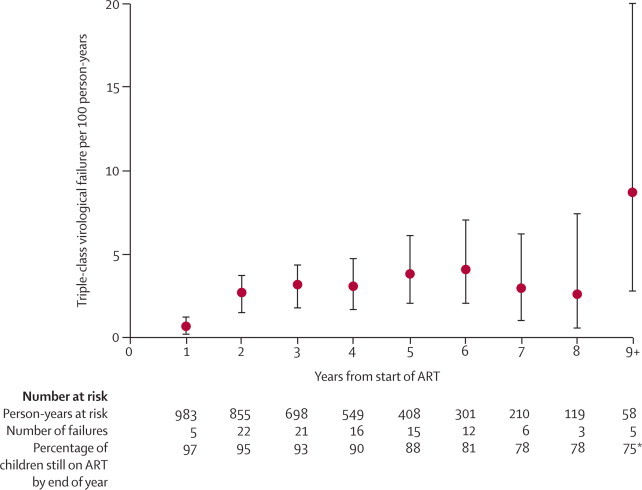
Incidence per 100 person-years (95% CI) of triple-class virological failure in children with HIV by duration of antiretroviral therapy
*At end of year 9.
Older age (10–15 years) at the time of ART initiation was associated with an increased risk of failure (table 2). Children with a previous AIDS diagnosis had a non-significant trend towards an increased risk of triple-class virological failure (table 2). We recorded no significant difference in the risk of failure by sex, year of ART initiation, type of initial ART regimen, previous ART exposure for prevention of mother-to-child transmission, or CD4 percentage or viral load at ART initiation.
Table 2.
Predictors of triple class virological failure
|
Univariate analyses |
Multivariate analyses |
|||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p | Hazard ratio | 95% CI | p | |
| Sex | ||||||
| Boy | 1·0 | .. | 0·96 | 1·0 | .. | 0·92 |
| Girl | 1·0 | 0·7–1·5 | .. | 1·0 | 0·7–1·5 | .. |
| Age at start of ART (years) | ||||||
| <2 | 1·2 | 0·7–2·0 | 0·03 | 1·2 | 0·7–2·0 | 0·02 |
| 2–4 | 0·9 | 0·5–1·7 | .. | 0·9 | 0·5–1·7 | .. |
| 5–9 | 1·0 | .. | .. | 1·0 | .. | .. |
| 10–15 | 2·1 | 1·2–3·7 | .. | 2·3 | 1·2–4·1 | .. |
| Year of ART initiation | ||||||
| 1998–2000 | 1·0 | .. | 0·46 | 1·0 | .. | 0·83 |
| 2001–2003 | 0·7 | 0·5–1·2 | .. | 0·9 | 0·5–1·5 | .. |
| 2004–2007 | 0·9 | 0·4–2·0 | .. | 1·0 | 0·4–2·4 | .. |
| Initial regimen | ||||||
| NNRTI+2 NRTIs | 1·0 | .. | 0·46 | 1·0 | .. | 0·24 |
| NNRTI+3 NRTIs | 0·7 | 0·3–1·6 | .. | 0·7 | 0·3–1·6 | .. |
| Ritonavir-boosted protease inhibitor + 2 or 3 NRTIs | 0·5 | 0·2–1·4 | .. | 0·4 | 0·1–1·3 | .. |
| Unboosted protease inhibitor + 2 or 3 NRTIs | 1·1 | 0·7–1·7 | .. | 1·1 | 0·7–1·7 | .. |
| 3 NRTIs | 0·8 | 0·3–2·1 | .. | 0·8 | 0·3–2·0 | .. |
| Previous ART exposure for prevention of mother-to-child transmission | ||||||
| No or unknown | 1·0 | .. | 0·91 | .. | .. | 0·64 |
| Yes | 1·0 | 0·4–2·3 | .. | 1·3 | 0·5–3·4 | .. |
| AIDS diagnosis before ART initiation | ||||||
| No | 1·0 | .. | 0·18 | 1·0 | .. | 0·12 |
| Yes | 1·3 | 0·9–2·1 | .. | 1·4 | 0·9–2·3 | .. |
| CD4 percentage at ART initiation | ||||||
| 0–9% | 1·0 | .. | 0·61 | 1·0 | .. | 0·73 |
| 10–19% | 0·9 | 0·5–1·7 | .. | 1·0 | 0·6–1·9 | .. |
| ≥20% | 0·8 | 0·4–1·5 | .. | 0·9 | 0·5–1·8 | .. |
| Unknown | 1·1 | 0·7–1·9 | .. | 1·3 | 0·7–2·4 | .. |
| Viral load at ART initiation (log10copies per mL) | ||||||
| 0–4·49 | 0·65 | 0·3–1·2 | 0·58 | 0·5 | 0·3–1·0 | 0·24 |
| 4·50–5·49 | 1·0 | .. | .. | 1·0 | .. | .. |
| ≥5·50 | 1·0 | 0·6–1·6 | .. | 0·9 | 0·5–1·6 | .. |
| Unknown | 1·0 | 0·6–1·6 | .. | 0·7 | 0·4–1·3 | .. |
ART=antiretroviral therapy. NRTI=nucleoside or nucleotide reverse transcriptase inhibitors. NNRTI=non-NRTI.
Of the children with triple-class virological failure, 29 of 105 never had a viral load measurement of less than 500 copies per mL. Compared with the 76 who had at least one viral load measurement less than 500 copies per mL before developing treatment failure, these children started ART at a younger age (median 1·8 [IQR 0·5–4·4] vs 6·1 [1·2–10·1] years, p=0·008) and were triple-class exposed more quickly after starting ART (2·1 [1·4–3·8] vs 3·3 [1·9–4·6] years, p=0·018).
Of the 686 children starting ART with an initial regimen of two or three NRTIs and either a NNRTI or a ritonavir-boosted protease inhibitor, 39 (6%) had triple-class virological failure (defined as virological failure of two NRTIs, one NNRTI, and ritonavir-boosted protease inhibitor). The trend in the incidence of triple-class virological failure during each year after the start of ART was similar to the main analysis (data not shown), and by 5 years after ART initiation the estimated cumulative proportion of children who had such failure according to this narrow definition was 8·2% (95% CI 5·1–11·2). This proportion was significantly higher than that of adults heterosexually infected with HIV in the PLATO II project—4·2% (3·8–4·6), HR 2·2 (1·6–3·0, p<0·0001), adjusted for AIDS diagnosis before ART initiation and year of ART initiation. To explore the extent to which this raised risk of triple-class virological failure in children compared with adults was driven by treatment failure in older children and adolescents aged 10–15 years at ART initiation, we repeated this analysis, adjusted for AIDS diagnosis before ART initiation and year of ART initiation, for children aged 9 years or younger compared with adults; the HR was 1·7 (1·1–2·5, p=0·011).
Mutation data from resistance tests were available for 40 (77%) children who had triple-class virological failure (81 tests). These tests detected mutations against NRTI in 26 of 36 children, mutations against NNRTIs in 28 of 29, and mutations against protease inhibitors in 0 of 15, while they took ART drugs of the corresponding class. M184V (17%), M41L (6), T215Y (6), and D67N (5) were the most common NRTI mutations; nine children had at least one thymidine-associated mutation. K103N (14), Y181C (13), and Y188L (6) were the most common NNRTI mutations.
Discussion
12% of children had triple-class virological failure after 5 years on ART, and about a fifth had such treatment failure by 8 years on ART. Although these risks are low, and highlight the great success of antiretroviral treatment in children, they raise concerns about the proportion of children starting ART who are likely to maintain viral suppression for life, despite the potential availability of newer drugs from other classes (panel). The rate of triple-class virological failure was highest in the first 2 years after ART initiation, rising gradually thereafter. After 6 years on ART, the number of children with failure was small and the confidence intervals were wide, but the apparent decline in the rate of triple-class virological failure could also mirror early dropout of children who adhered most poorly to drug regimens, which is in line with our finding that about a quarter of children with triple-class virological failure had never achieved virological suppression. This small group started ART at an earlier age and became triple-class exposed more quickly, raising the issue of adequate dosing in young children and presumably relating to very poor adherence by their caregivers.
Panel. Research in context.
Systematic review
Although reports of paediatric cohort studies have described triple-class virological failure among small numbers of children, no large cohort or cohort collaboration has undertaken a formal assessment of the incidence or consequences of triple-class failure and no randomised trials of children with triple-class failure have been done.
Interpretation
Our study shows that the rate of development of triple-class virological failure in children with HIV in Europe is low, which supports the high efficacy of these drugs in children, but the rate is higher than in adults. These children will be receiving antiretroviral therapy for an entire lifetime, and these findings highlight the challenges in attainment of long-term viral suppression. Early identification of non-responders, adherence support, especially for older children and young people aged 13 years or older starting antiretroviral therapy, and simplification of antiretroviral strategies are all needed to attain and sustain virological suppression.
Restriction of the analysis to children starting ART with two NRTIs and either a NNRTI or a boosted protease inhibitor and use of the same definition of treatment failure showed that the overall rate of failure in children was over two-times higher than in adults with heterosexually transmitted HIV in COHERE. This increased rate of triple-class virological failure could be explained by lower virological suppression rates in children than in adults,19 absence of alternative regimens, adherence issues related to taste and formulation, a tendency for switches in regimen for children being delayed because of the need to manage adherence problems before switching treatment and hence switching regimen at high viral loads,24 and social factors, especially those that affect adolescents.
As in the adult analysis,20 adolescents and young adults had the highest risk of triple-class virological failure. Drug adherence is a challenge for children and young people with any chronic disease. For those with HIV infection, there are additional factors, including coming to terms with disclosure of their HIV status, secrecy and guilt among adult family members, and dealing with HIV alongside their own sexual development. Fear of stigma increases their isolation and tendency towards denial, all of which might adversely affect drug adherence. Although there are several studies of adherence to ART in children and young adults with HIV, most are small, heterogeneous—including both caregiver and child adherence measurements—and nearly all have been cross-sectional.25 Murphy and colleagues16 reported a decline in adherence by teenagers over time associated with duration of ART, and Kekitiinwa and colleagues26 described poor responses to ART in newly diagnosed young people aged 10 years or older in the UK and Ireland.
However, when adolescents aged 10–15 years were excluded from the comparison with adults, the HR declined from 2·2 to 1·7, but remained significant. This finding suggests that factors relating to young children, whose drugs are mainly given by caregivers, are also important and need to be considered. Few adherence studies have differentiated child and caregiver adherence or studied changes in adherence longitudinally with age. In addition to adherence, other complexities in treatment of children with HIV include dosing and pharmacokinetics of drugs, and palatability and tolerability of formulations, all of which vary substantially with age.27 All these factors might adversely affect virological response and contribute to poorer responses in children than in adults.
We recorded no association between drug classes used in the initial regimen and triple-class virological failure, supporting the use of regimens containing either NNRTIs or protease inhibitors as first-line ART in accordance with the current guidelines.7–9 Although our study was a non-randomised comparison, the PENPACT-1 trial28 of ART initiation with protease inhibitor versus NNRTI combination ART regimens, in children with HIV-1 infection who have not received ART, showed no significant virological, immunological, or clinical differences between these two randomised arms at 4 years after ART initiation; of note, PENPACT-1 included both boosted and unboosted protease inhibitors, as in the present study. Our results also suggest that the rate of triple-class virological failure was higher in children who had been diagnosed with AIDS before starting ART, compared with those without an AIDS diagnosis at ART initiation, supporting the current recommendations of ART initiation at higher CD4 counts than previously used, and before clinical disease progression.7–9 However, virological failure was not associated with CD4 percentage or viral load at ART initiation, unlike in adults with HIV,20 and could be due in part to low power, but also to the longer time taken to attain a virological set-point after primary infection in children (about 5 years)29 than in adults. No association between virological failure and use of ART for prevention of mother-to-child transmission was seen, although numbers were small.
Virological failure to a drug class does not necessarily mean that no drugs from that class can successfully suppress the virus in the future, especially if no resistance mutations occurred. For children with resistance test data, we found that most children on a NRTI or a NNRTI had resistance mutations, implying loss of future drug options from these classes. By contrast, protease-inhibitor resistance was not seen, which is consistent with results from the linked adult study and a randomised trial in children.30,31 Because boosted protease inhibitors are now the standard of care for children with HIV,7–9 resistance to them is probably unusual in children failing a boosted protease-inhibitor regimen.32
Our results have implications for developing and developed countries. Increasingly, drugs from the original three classes are widely available, and our results provide encouragement that viral load suppression can be achieved and maintained for many years with use of these drugs. In adults, adherence to ART has proved to be at least as high in sub-Saharan Africa as it is in North America, supporting the fact that outcomes reported in developed countries are relevant for developing countries.33 However, regardless of setting, there will be a need for availability of newer drugs if children with HIV infection are to maintain viral suppression for sufficient durations to enable them to live as long as their uninfected peers. If options do become exhausted then it becomes important to understand the immunological and clinical consequences of long-term virological failure on ART in children, as has been studied in adults.34 Such analyses are beyond the scope of this study and, in fact, there have not been large enough numbers of children in the PLATO II project in this situation to allow reliable conclusions to be drawn.
The rate of virological failure of the three original drug classes seen in this study shows the challenge of maintaining lifelong viral suppression in children who start ART much earlier in life than do adults. Further detailed analysis is needed to compare rates of switching to second-line ART and viral suppression on second-line ART between adults and children, and to compare the development of resistance, and to repeat this analysis once further follow-up time has accrued; there is continued need for strategies to promote optimum drug adherence in children, caregivers, and young people to minimise the likelihood of triple-class virological failure, and for development of suitable new drugs and formulations to optimise the treatment of children with treatment failure. Fixed drug combinations and simplification of strategies could be important ways to maintain treatment options while children move through adolescence and reach adulthood.
Acknowledgments
Acknowledgments
The PLATO II project is funded by UK Medical Research Council award G0700832. The COHERE study group has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under EuroCoord grant agreement number 260694; Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (ANRS), France; HIV Monitoring Foundation, Netherlands; and the Augustinus Foundation, Denmark. The group has also received project specific funding from the UK Medical Research Council and the Swiss Bridge Foundation. A list of the funding sources of participating cohorts can be found on the regional coordinating centre websites.
Contributors
All members of the PLATO II analysis and writing group participated in discussions about the design of the study, the choice of statistical analyses and interpretation of the findings, and were involved in the preparation and review of the final report. Additionally, Rebecca Lodwick and Andrew Phillips were responsible for undertaking all analyses; Rebecca Lodwick acted as guarantor for the analyses and had full access to the data set. Hannah Castro, Ali Judd, Diana Gibb, Karina Butler, Rebecca Lodwick, and Andrew Phillips were responsible for the study concept and design. Ali Judd, Di Gibb, Ard van Sighem, Jose T Ramos, Josiane Warsawski, Claire Thorne, Antoni Noguera-Julian, Niels Obel, Dominique Costagliola, Pat A Tookey, and Genevieve Chene acquired data for the study. Hannah Castro, Ali Judd, Di Gibb, Karina Butler, Rebecca Lodwick, and Andrew Phillips analysed and interpreted the data and drafted the report. All authors critically reviewed the report. Céline Colin, Jesper Kjaer, and Jesper Grarup provided administrative, technical, and material support.
PLATO II project
Analysis and writing group—Hannah Castro, Ali Judd, Diana M Gibb, Karina Butler, Rebecca K Lodwick, Ard van Sighem, Jose T Ramos, Josiane Warsawski, Claire Thorne, Antoni Noguera-Julian, Niels Obel, Dominique Costagliola, Pat A Tookey, Céline Colin, Jesper Kjaer, Jesper Grarup, Genevieve Chene, Andrew Phillips.
Project team—Andrea Antinori (ICC), Antonella Castagna (San Raffaele Cohort), Dominique Costagliola (ANRS CO4 FHDH), Alessandro Cozzi-Lepri (ICONA), Andrea De Luca (ICONA), Stephane De Wit (Brussels St Pierre Cohort), Maria Dorrucci (CASCADE), Xavier Duval (ANRS CO8 COPILOTE), Federico García (Co-RIS), Jade Ghosn (ANRS CO6 PRIMO), Huldrych Günthard (SHCS), Ali Judd (CHIPS), Bruno Ledergerber (SHCS), Sergio Lo Caputo (Italian Master Cohort), Rebecca Lodwick (statistician), Antoni Noguera-Julian (CoRISPE-cat), Bernard Masquelier (ANRS CO3 AQUITAINE), Laurence Meyer (ANRS CO2 SEROCO), Amanda Mocroft (EuroSIDA), Cristina Mussini (Modena Cohort), Niels Obel (Danish HIV Cohort Study), Dimitrios Paraskevis (AMACS), Roger Paredes (EuroSIDA), Santiago Pérez-Hoyos (GEMES-Haemo), Andrew Phillips (PLATO II project leader; UK HIV Drug Resistance Database/UK CHIC), Deenan Pillay (UK HIV Drug Resistance Database/UK CHIC), Daniel Podzamczer (PISCIS), José T Ramos (Madrid Cohort), Peter Reiss (ATHENA), Christoph Stephan (Frankfurt HIV Cohort), Ramón Teira (VACH), Claire Thorne (ECS), Pat A Tookey (NSHPC), Carlo Torti (Italian Master Cohort), Giota Touloumi (AMACS), Ard van Sighem (ATHENA), Josiane Warsawski (ANRS CO1 EPF).
COHERE steering committee
Contributing cohorts—Robert Zangerle (AHIVCOS), Giota Touloumi (AMACS), Josiane Warszawski (ANRS CO1 EPF/ANRS CO11 OBSERVATOIRE EPF), Laurence Meyer (ANRS CO2 SEROCO), François Dabis (ANRS CO3 AQUITAINE), Murielle Mary Krause (ANRS CO4 FHDH), Jade Ghosn (ANRS CO6 PRIMO), Catherine Leport (ANRS CO8 COPILOTE), Frank de Wolf (ATHENA), Peter Reiss (ATHENA), Maria Prins (CASCADE), Heiner C Bücher (CASCADE), Caroline Sabin (UK CHIC), Diana Gibb (CHIPS), Gerd Fätkenheuer (Cologne Bonn), Julia Del Amo (CoRIS), Antoni Noguera-Julian (CoRISPE-cat), Niels Obel (Danish HIV Cohort), Claire Thorne (ECS), Amanda Mocroft (EuroSIDA), Ole Kirk (EuroSIDA), Christoph Stephan (Frankfurt), Santiago Pérez-Hoyos (GEMES-Haemo), Andrea Antinori (ICC), Antonella d'Arminio Monforte (ICONA), Pier-Angelo Tovo (ITLR), Maurizio de Martino (ITLR), Norbert H Brockmeyer (KOMPNET), José T Ramos (Madrid Cohort), Manuel Battegay (MoCHIV), Patrick Francioli (SHCS), Cristina Mussini (Modena Cohort), Dolors Carnicer-Pont (NENEXP), Pat A Tookey (NSHPC), Jordi Casabona (PISCIS), Jose M Miró (PISCIS), Antonella Castagna (San Raffaele), Stephane de Wit (St. Pierre Cohort), Tessa Goetghebuer (St Pierre Paediatric Cohort), Carlo Torti (Italian Master Cohort), Ramon Teira (VACH), Myriam Garrido (VACH).
European AIDS Treatment Group—Nikos Dedes.Executive committee—Ian Weller (Chair, University College London), Jordi Casabona (PISCIS), Dominique Costagliola (FHDH), Antonella d'Arminio-Monforte (ICONA), Bruno Ledergerber (SHCS), Maria Prins (CASCADE), Frank de Wolf (ATHENA), Jesper Grarup (Head of Copenhagen Regional Coordinating Centre), Genevieve Chene (Head, Bordeaux Regional Co-ordinating Centre).
Regional co-ordinating centres—Fideline Collin-Filleul, Céline Colin, Christine Schwimmer (Bordeaux RCC cohorts), Michelle Ellefson, Jesper Kjaer, Maria Paulsen (Copenhagen RCC cohorts).
Project leaders and statistical analysis—Julia Bohlius, Vincent Bouteloup, Heiner C Bucher, Alessandro Cozzi-Lepri, François Dabis, Antonella d'Arminio Monforte, Frank de Wolf, Maria Prins, Matthias Egger, Hansjakob Furrer, Ole Kirk, Olivier Lambotte, Charlotte Lewden, Rebecca Lodwick, Sophie Matheron, Laurence Meyer, Jose Miro, Amanda Mocroft, Roger Paredes, Andrew Phillips, Massimo Puoti, Joanne Reekie, Caroline Sabin, Colette Smit, Jonathan Sterne, Rodolphe Thiebaut, Claire Thorne, Linda Wittkop.
Conflicts of interest
We declare that we have no conflicts of interest.
Correspondence to: Dr Ali Judd, Medical Research Council Clinical Trials Unit, 222 Euston Road, London NW1 2DA, UK a.judd@ctu.mrc.ac.uk
References
- 1.Judd A, Doerholt K, Tookey PA, for the Collaborative HIV Paediatric Study (CHIPS) and National Study of HIV in Pregnancy and Childhood (NSHPC) Morbidity, mortality, and response to treatment by children in the United Kingdom and Ireland with perinatally acquired HIV infection during 1996-2006: planning for teenage and adult care. Clin Infect Dis. 2007;45:918–924. doi: 10.1086/521167. [DOI] [PubMed] [Google Scholar]
- 2.Brady MT, Oleske JM, Williams PL, for the Pediatric AIDS Clinical Trials Group 219/219C Team Declines in mortality rates and changes in causes of death in HIV-1 infected children during the HAART era. J Acquir Immune Defic Syndr. 2010;53:86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.deMartino M, Tovo PA, Balducci M, for the Italian Register for HIV Infection in Children and the Italian National AIDS Registry Reduction in mortality with availability of antiretroviral therapy for children with perinatal infection. JAMA. 2000;284:190–197. doi: 10.1001/jama.284.2.190. [DOI] [PubMed] [Google Scholar]
- 4.Sharland M, Blanche S, Castelli G, Ramos J, Gibb DM, for the PENTA Steering Committee PENTA guidelines for the use of antiretroviral therapy 2004. HIV Med. 2004;5(suppl 2):61–86. doi: 10.1111/j.1468-1293.2004.00227.x. [DOI] [PubMed] [Google Scholar]
- 5.Violari A, Cotton MF, Gibb DM. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goetghebuer T, Haelterman E, Le Chenadec J. Effect of early antiretroviral therapy on the risk of AIDS/death in HIV infected infants: the European Infant Collaborative Study. AIDS. 2009;23:597–604. doi: 10.1097/QAD.0b013e328326ca37. [DOI] [PubMed] [Google Scholar]
- 7.Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children Guidelines for the use of antiretroviral agents in pediatric HIV infection. Feb 23, 2009; pp 1–139. http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pdf (accessed April 21, 2010). [DOI] [PubMed]
- 8.Welch S, Sharland M, Lyall EG, PENTA Steering Committee PENTA 2009 guidelines for the use of antiretroviral therapy in paediatric HIV-1 infection. HIV Med. 2009;10:591–613. doi: 10.1111/j.1468-1293.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 9.WHO Antiretroviral therapy for HIV infection in infants and children: Towards universal access. Recommendations for a public health approach. 2010. http://www.who.int/hiv/pub/paediatric/infants2010/en/index.html (accessed Aug 12, 2010). [PubMed]
- 10.Paterson DL, Swindells S, Mohr J. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 11.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146:564–573. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 12.Bangsberg DR, Kroetz DL, Deeks SG. Adherence-resistance relationships to combination HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2007;4:65–72. doi: 10.1007/s11904-007-0010-0. [DOI] [PubMed] [Google Scholar]
- 13.Deeks SG. Determinants of virological response to antiretroviral therapy: implications for long-term strategies. Clin Infect Dis. 2000;30(suppl 2):177–184. doi: 10.1086/313855. [DOI] [PubMed] [Google Scholar]
- 14.Reddington C, Cohen J, Baldillo A. Adherence to medication regimens among children with human immunodeficiency virus infection. Pediatr Infect Dis. 2000;19:1148–1153. doi: 10.1097/00006454-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Martin S, Elliott-DeSorbo DK, Wolters PL. Patient, caregiver and regimen characteristics associated with adherence to highly active antiretroviral therapy among HIV-infected children and adolescents. Pediatr Infect Dis. 2007;26:61–67. doi: 10.1097/01.inf.0000250625.80340.48. [DOI] [PubMed] [Google Scholar]
- 16.Murphy DA, Belzer M, Durako SJ, Sarr M, Wilson CM, Muenz LR. Adolescent Medicine HIV/AIDS Research Network. Longitudinal antiretroviral adherence among adolescents infected with human immunodeficiency virus. Arch Pediatr Adolesc Med. 2005;159:764–770. doi: 10.1001/archpedi.159.8.764. [DOI] [PubMed] [Google Scholar]
- 17.Menson EN, Walker AS, Sharland M, for the Collaborative HIV Paediatric Study Steering Committee Underdosing of antiretrovirals in UK and Irish children with HIV as an example of problems in prescribing medicines to children, 1997–2005: cohort study. BMJ. 2006;332:1183–1187. doi: 10.1136/bmj.332.7551.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Academy of Pediatrics Committee on Pediatric AIDS. Section on International Child Health. Havens PL, Gibb DM. Increasing antiretroviral drug access for children with HIV infection. Pediatrics. 2007;119:838–845. doi: 10.1542/peds.2007-0273. [DOI] [PubMed] [Google Scholar]
- 19.Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Study Group Response to combination antiretroviral therapy: variation by age. AIDS. 2008;22:1463–1473. doi: 10.1097/QAD.0b013e3282f88d02. [DOI] [PubMed] [Google Scholar]
- 20.The Pursuing Later Treatment Options II (PLATO II) Project Team for the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Triple-class virologic failure in HIV-infected patients undergoing antiretroviral therapy for up to 10 years. Arch Intern Med. 2010;170:410–419. doi: 10.1001/archinternmed.2009.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kjaer J, Ledergerber B. HIV cohort collaborations: proposal for harmonization of data exchange. Antivir Ther. 2004;9:631–633. [PubMed] [Google Scholar]
- 22.Walker AS, Doerholt K, Sharland M, Gibb DM, for the Collaborative HIV Paediatric Study (CHIPS) Steering Committee Response to highly active antiretroviral therapy varies with age: the UK and Ireland Collaborative HIV Paediatric Study. AIDS. 2004;18:1915–1924. doi: 10.1097/00002030-200409240-00007. [DOI] [PubMed] [Google Scholar]
- 23.Johnson VA, Brun-Vezinet F, Clotet B. Update of the Drug Resistance Mutations in HIV-1. Top HIV Med. 2008;16:138–145. [PubMed] [Google Scholar]
- 24.Lee KJ, Lyall H, Walker AS, Sharland M, Judd A, Gibb DM. Wide disparity in switch to second-line therapy in HIV infected children in CHIPS. Eighth International Congress in Drug Therapy in HIV Infection; Glasgow, UK; Nov 12–16, 2006. PL2.4 (abstr).
- 25.Butler L, Bain Brickely D, Chan J, Kennedy G, Rutherford G. Rates and determinants of adherence to antiretroviral therapy (ART) in infants, children and adolescents: a systematic review. XVIII International AIDS Conference; Vienna, Austria; July 18–23, 2010. MOAB0205 (abstr).
- 26.Kekitiinwa A, Lee KJ, Walker AS. Differences in factors associated with initial growth, CD4, and viral load responses to ART in HIV-infected children in Kampala, Uganda, and the United Kingdom/Ireland. J Acquir Immune Defic Syndr Hum Retrovirol. 2008;49:384–392. doi: 10.1097/QAI.0b013e31818cdef5. [DOI] [PubMed] [Google Scholar]
- 27.Giaquinto C, Morelli E, Fregonese F. Current and future antiretroviral treatment options in paediatric HIV infection. Clin Drug Investig. 2008;28:375–397. doi: 10.2165/00044011-200828060-00005. [DOI] [PubMed] [Google Scholar]
- 28.Melvin A, on behalf of the PENPACT-1 Study Team. PENPACT-1 (PENTA 9/PACTG 390): A randomised trial of protease inhibitor (PI) vs non-nucleoside reverse transcriptase inhibitor (NNRTI) combination antiretroviral (ART) regimens and viral load (VL) treatment switching strategies in HIV-1-infected ART-naive children age >30 days and <18 years. XVIII International AIDS Conference; Vienna, Austria; July 18–23, 2010. THLBB104 (abstr).
- 29.Paediatric European Network for Treatment of AIDS (PENTA) HIV-1 viral load and CD4 cell count in untreated children with vertically acquired asymptomatic or mild disease. Paediatric European Network for Treatment of AIDS (PENTA) AIDS. 1998;12:1–8. (abstr). [PubMed] [Google Scholar]
- 30.The PLATO II Project Team for the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Group; Drug resistance associated mutations in patients who during the course of treatment develop virological failure to the three original classes of antiretroviral drugs. European AIDS Clinical Society Conference; Cologne, Germany; Nov 11–14, 2009. PE 3.5/5 (abstr).
- 31.PENPACT-1 (PENTA 9/PACTG 390) Study Team First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. Lancet Infect Dis. 2011 doi: 10.1016/S1473-3099(10)70313-3. published online Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyd K, Walker AS, Dunn DT et al. The prevalence of darunavir associated mutations in PI-naïve and pi-experienced HIV-1 Infected children in the uk. CROI; San Francisco, USA; Feb 16–19, 2010. 851 (abstr).
- 33.Mills EJ, Nachega JB, Buchan I. Adherence to antiretroviral therapy in sub-Saharan Africa and North America—a meta-analysis. JAMA. 2006;296:679–690. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 34.Ledergerber B, Lundgren JD, Walker AS, PLATO Collaboration Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364:51–62. doi: 10.1016/S0140-6736(04)16589-6. [DOI] [PubMed] [Google Scholar]


