Abstract
In this review, we compare the reported values of Young's modulus (YM) obtained from indentation and tensile deformations of soft biological tissues. When the method of deformation is ignored, YM values for any given tissue typically span several orders of magnitude. If the method of deformation is considered, then a consistent and less ambiguous result emerges. On average, YM values for soft tissues are consistently lower when obtained by indentation deformations. We discuss the implications and potential impact of this finding.
Introduction
The rigid skeletal system of vertebrates provides support and protection for soft tissues that reside within and on the skeletal frame. These soft tissues range from large organs to small connective tissues with cells and extracellular matrices. They are continually stressed by a multitude of macro-, micro-, and nanoscopic forces, both internally and externally, and must be resilient enough to deform reversibly without damage and still maintain function. For decades it has been understood that changes in the macroscopic stiffness of tissues can indicate internal disease or injury. We now understand that changes in the bulk compliance of soft tissues can indicate the onset of conditions such as breast cancer,1–3 atherosclerosis,4–11 fibrosis,12 or glaucoma13 at a macroscopic level. At the micro- to nanoscopic level, bulk and local compliance influence a wide menu of fundamental cell behaviors, including cell morphology,14–17 proliferation,16,18–20 motility,15,21–25 differentiation,18,26–29 and response to therapeutic agents.30 It is therefore important to properly characterize the compliant biophysical state of tissues to understand how this biophysical attribute relates to proper function. However, a cursory review of the literature quickly reveals significant differences in reported values of the compliance of soft tissues. These variations can influence the understanding of tissue function and/or failure, interpretation of cellular responses to biophysical stimuli, or the rational design and use of biological simulants.31–35 The aim of this review is to determine the origins of these variations. We do not explore every confounding variable and material property for a given tissue; rather, the focus of this review is to delineate the extent to which the experimental method for measuring modulus affects the interpretation of a commonly quantified compliant descriptor, Young's modulus (YM), by specifically comparing probe indentation to tensile stretching measurements of soft biological tissues.
YM describes the ability of an elastic material to resist deformation to an applied stress. Unfortunately, reported values of YM for a given tissue can span several orders of magnitude. The human cornea is a good example, with reported modulus values ranging from 2.9 kPa36 to 19 MPa37 when measured by atomic force microscopy (AFM)38 tensile stretching,39,40 tonometry,41–43 or inflation/bulge testing,36,37,44–47 This wide variation in reported YM values is not limited to the cornea. Part of the variation in reported YM values stems from variation in controllable experimental variables. Examples include in vivo versus ex vivo measurements, tissue hydration state, time from death/tissue excision, temperature, storage medium, and the experimental method used. These experimental differences make direct comparison of results between studies difficult. Direct comparisons are also complicated by the various material properties that can be used to describe compliance. Again, the eye is an excellent example; intermixed with reports on intrinsic material properties such as bulk, shear, or YM are reports on properties such as ocular or scleral rigidity.48–50 Reliance on empirical values, such as ocular rigidity, can potentially complicate understanding and diagnosis of disease and should therefore be used with caution.
Variations in reported YM values for soft tissues may also stem from the application of elastic models to describe viscoelastic responses. YM is commonly used to try and quantify an intrinsic elastic property of soft, viscoelastic biomaterials. Strictly speaking, however, YM quantifies the response of a perfectly elastic material, limiting its use to metals and crystalline solids or to materials that possess significant regions of linear stress–strain behavior. These complications have been discussed in detail51 and contribute to confusion and variability on reported mechanical properties of soft tissues. As noted by Fung,51 YM values obtained by tensile measurements must be accompanied by a statement of the levels of stress and strain applied to the tissue to be of any quantitative value. The nonlinear response of soft tissues and the inherent difficulty in obtaining YM values by tensile deformation has also resulted in the formulation of nonlinear stress–strain models39,51,52 that do not attempt to define YM as the quantitative descriptive parameter that it is. However, given the aim of this review, which attempts to address the effect of experimental method on reported values of elastic modulus, its inclusion in this review is warranted.
For perfectly elastic materials, YM is defined as the ratio of applied stress to resultant strain and has units of measurement in N/m2.
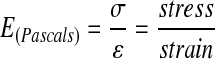 |
(1) |
Stress is the force divided by the area over which it is applied. Strain is a dimensionless quantity defined by the stress-induced change in length of a material divided by its unstressed length (ΔL/L). Soft tissues are not perfectly elastic materials or homogeneous, and they typically display both viscous and elastic properties that are dependent on time and typically display nonlinear stress–strain functions (nevertheless, nonlinear functions do not necessarily define a material as viscoelastic). For perfectly elastic materials a single YM value defines the response of material to deformation. For soft biological tissues, the resistance to deformation typically increases as the applied stress increases. Therefore, YM, defined by Equation 1, is not constant and depends on the specific applied stress, which is particularly important as tissues in vivo typically exist in a prestressed state.53 As the solution to Equation 1 is dependent on the applied stress, multiple YM values could be obtained for soft biological tissues. To avert this problem, soft biological tissues are typically assumed to behave as elastic solids if a significant linear regime of stress-to-strain exists in the limit of small strain response to applied stress.
Two methods are commonly used to deform soft tissues: probe indentation and tensile stretching. Both methods are employed to describe the compliant response of a material to an applied stress. They are, however, distinct. Indentation deformations are maximized at the point of indenter contact and radially diminish to zero with increasing distance from the probe, whereas tensile-induced strains span the bulk of the sample being stressed. If a tissue is not homogeneous from nanoscopic to macroscopic length scales, the measured compliance may depend on the experimental technique employed. This review summarizes previously published results and documents that differences in methods used are major contributors to the wide range of values reported for soft biological tissues. The results highlight that knowledge of the method used to measure YM is essential for correct interpretation of the data.
In this review, only those publications that report elastic modulus values are presented. The nonelastic properties of these viscoelastic tissues are not included here, as these descriptions of tissue mechanics would make the stated purpose of this review impossible. However, the time dependent, viscous properties of biological tissues are important and we direct readers to the following reviews and articles, which discuss these properties for many of the tissues reviewed here.54–62 In addition, values, such as the tangent modulus, obtained from regions of stress–strain curves that are outside of the elastic regime are not included in this review. Where possible, we compare YM values obtained when both values (by indentation and tensile stretching) for a given tissue have been reported (not all tissues we reviewed have reported values for both methods).
Indentation
There are a variety of instruments used to indent samples,63–66 ranging from AFMs, capable of applying piconewton loads, to nanoindentors, which can resolve nanonewton loads, to larger industrial indenters capable of applying micro to meganewton loads. No single indenter is ideally suited for every tissue type and ultimately the specific research objective will dictate which instrument is deemed appropriate. However, accurate determination of YM requires the specific apparatus be capable of sensitive detection of the initial point of contact between the indenter and the sample. The instrument must also have high resolution in the subsequent changes in load and indentation depth and fine control of the indentation velocity.
Under the assumption of elastic deformation, YM of a given biological material is typically determined by fitting the measured indentation depth as a function of indenter load during approach.67–70 YM values can also be obtained from the interpretation of unloading curves when the indenter is being withdrawn from the sample.71 The models used to fit these indentation curves are indenter geometry specific; therefore, indenters are objects that are, or can be, approximated as spheres, cones, or flat cylinders, as the contact mechanics for these geometries are well established.72–78 Table 1 lists the elastic model solutions for the common geometries used to determine YM. A full description of the elastic and viscous properties of a tissue would require additional measurement of its frequency-dependent response.79 It is instructive to generalize the equations listed in Table 1 to the following equation:
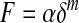 |
(2) |
Table 1.
Review of Theoretical Models
| Model | Theoretical F versus δ | Reference |
|---|---|---|
| I Purely elastic sphere compression |
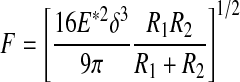 |
72,88,110 |
| II Rigid sphere indenter |
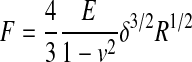 |
75 |
| III Rigid, flat-ended cylinder indenter |
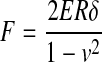 |
75 |
| IV Rigid cone indenter |
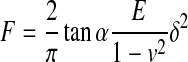 |
74,75 |
| V Rigid cone indenter |
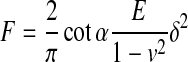 INCORRECT |
78 |
Note that the reduced modulus (E*) is used in Equation I and not in II–V, where it is assumed that the indenter is infinitely rigid:  .
.
E, Young's modulus; δ, indentation depth; R, radius; ν, Poisson's ratio; α, half angle opening.
where F is the force applied by the indenter, δ is the indentation depth, α and m are constants where the geometry of the indenter determines the value of m. The value of m is 1 for flat cylinders, 1.5 for spheres, and 2 for cones. Equation 2 is easily linearized (log–log plot), which allows for a quick and easy check to ensure that experimental data fits the correct power law for the indenter geometry used.
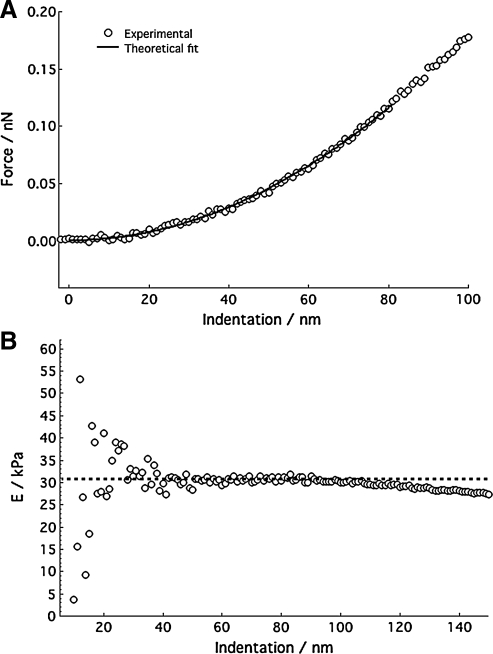
Using force versus indentation curves to determine YM can be complicated due to the viscoelastic nature of a given biological sample. It is at times difficult to determine the correct indentation depth over which the sample behaves as an elastic solid. This region must be defined so that YM can be accurately determined. For a perfectly elastic material, no energy is lost to the sample during indentation and both the loading and unloading curves will be coincident. The elastic regime of a viscoelastic material can therefore be experimentally determined by controlling the indentation velocity and depth to produce loading and unloading curves that fall on one another. This result is not always possible as adhesions between the probe and sample can occur that exceed either the load capacity or drive range of a given apparatus. This is particularly problematic when using AFMs, which use very weak springs and piezoelectric crystals with small drive ranges to move the indenter into a sample. A more quantitative solution to determine the elastic regime of a viscoelastic material can be obtained by noting that the models in Table 1 predict a constant value of E for any indentation depth. Therefore, the ratio of experimental values of force and indentation can be used to determine the range over which a specific model applies. For example, Figure 1A is a plot of indentation force versus depth on a polyacrylamide hydrogel prepared in our laboratory and indented using an AFM cantilever with an incorporated square pyramid tip. Figure 1B is a plot of 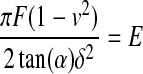 versus indentation depth, where F is the force, ν is Poisson's ratio, α is the half angle opening of the AFM tip and δ is the depth of penetration. In using this equation, we have assumed the square pyramid is a cone. This plot shows that the hydrogel behaved as an elastic solid with a constant E to ∼80 nm of indentation. Beyond 80 nm, E is no longer constant, indicating the material is no longer behaving as an elastic solid. When using an AFM, care must be taken to ensure that these deviations from linearity are not due to the common and often ignored, nonlinear response of cantilever deflection versus load for increasing loading conditions.80
versus indentation depth, where F is the force, ν is Poisson's ratio, α is the half angle opening of the AFM tip and δ is the depth of penetration. In using this equation, we have assumed the square pyramid is a cone. This plot shows that the hydrogel behaved as an elastic solid with a constant E to ∼80 nm of indentation. Beyond 80 nm, E is no longer constant, indicating the material is no longer behaving as an elastic solid. When using an AFM, care must be taken to ensure that these deviations from linearity are not due to the common and often ignored, nonlinear response of cantilever deflection versus load for increasing loading conditions.80
FIG. 1.
(A) Indentation force versus indentation depth of a polyacrylamide hydrogel with an overlay of the theoretic force based on Equation IV in Table 1. The appropriate indentation depth over which the fit was applied was determined from a plot of 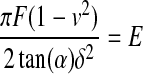 versus indentation depth, (B), which demonstrates that the gel behaves as an elastic solid up to ∼80 nm of indentation.
versus indentation depth, (B), which demonstrates that the gel behaves as an elastic solid up to ∼80 nm of indentation.
With very small indentations, the working end of the pyramid has been modeled as a sphere81 especially when the sharp tip has been made blunt.82–84 More typically, pyramidal indenters are assumed to be cones, especially in the case of cell indentation studies using AFMs.67,85 We emphasize the pyramidal indenter here, due to a citation error, which has occurred when referencing the rigid cone solution. The solution of the pyramidal indenter as a rigid cone has been presented in numerous publications. Frequently, Sneddon's 1965 publication78 is cited for the solution of a rigid cone indenter. However, the rigid cone solution published in Sneddon's 1965 publication is not consistent with his previous publications75,77 or the solution that preceded it.74 Although this error has been noted before,86 research articles continue to improperly cite Sneddon's 1965 article. This obviously becomes a problem when authors refer to Sneddon's 1965 article without also including the equation they used. We therefore recommend that Love74 or Sneddon75 be cited when referencing the use of the rigid cone solution.
Tensile stretching
Methods that involve tensile stretching offer a more direct and more economical approach for obtaining the material properties of a sample. Tests can be as simple as measuring the change in length (strain) of a sample when a mass is suspended from it (stress). Tensile measurements directly quantify the strain that is induced by a given stress and under the assumption of a linear elastic response, YM is determined from the slope of the stress–strain curve (Equation 1).
Typical stress–strain relationships observed for tensile measurements of soft biological tissues demonstrate that the resistance to deformation of the tissue increases with increasing stress. This nonlinear response means that the gradient of stress to strain is always increasing. Thus, the solution for E obtained by Equation 1 is always increasing. As mentioned earlier, YM values for soft tissues are typically based on the initial linear response. Measurement of tensile stretch will certainly lead to variation in reported values, as YM will be dependent on the stress that is applied. More importantly though, because the functional form of the stress–strain curve is nonlinear and demonstrates increasing resistance to deformation, the gradient of linear fits (E) to the initial response will always increase with increasing range of fit. This leads one to conclude that YM values measured by linear model fits using tensile measurements for soft biological tissues arguably represent an over estimate of the actual value. As noted by Fung,51 YM values, obtained by tensile measurements, must be accompanied by a statement on the levels of stress and strain applied to the tissue to be of any quantitative value.
Article inclusion criteria
The following are our inclusion criteria for values tabulated in Tables 2 and 3: (1) Cited articles stated they measured the elastic response of a tissue. (2) The cited articles used established models for defining the elastic modulus, which is dependent on tensile or indentation measurements. (3) To the best of our ability, we confirmed that the reported values corresponded with an elastic response. In the case of tensile measurements, this was primarily determined by confirming that the reported data displayed a linear stress–strain response (although a number of articles also used nonlinear models). If two elastic modulus values were reported, based on two separate linear regimes, we used the smaller elastic modulus value obtained at low strain. With the exception of “spinal cord and gray matter,” if tensile articles presented both instantaneous and relaxed elastic modulus values, we included the lower, relaxed elastic modulus value. For “spinal cord and gray matter,” all of the cited articles reported an instantaneous modulus using a model solution for hyperelastic materials.87 For indentation measurements, if multiple YM values were reported as a function of indentation depth, value inclusion was limited to data reported from the initial response of the sample. Review of indentation measurements has an additional complication in that the functional form of force versus indentation curves for elastic materials is always nonlinear. We therefore relied on representations of theoretical fits to the raw data if it was presented. Not all articles included the raw data that was used to define the reported value of YM, so we also relied on the written wording of the article and criteria 1 and 2. (4) When possible, we ensured that for each group of tissues, the cited articles were self-consistent in their measurement. For example, in the methods sections of the tensile reports on “tendon,” the authors described, in similar fashion, that the gradient of stress–strain curves were measured directly after the “toe” region, in the “elastic phase” or “linear region,” which they termed the elastic or YM of the sample. This task was not always possible though, especially for indentation measurements, as the model predictions are highly specific to the indentation method used, in which case we relied on criteria 1, 2, and 3. (5) We did not include YM values from diseased tissues.
Table 2.
Young's Modulus of Soft Tissues, Measured by Indentation
|
Indentation data | |||
|---|---|---|---|
| Tissue | Range (kPa) | Average Young's modulus (kPa) | Reference |
| L&K | 0.6–4000 | ∼950 | 111–115 |
| L&K (outlier) | 4000 | 111 | |
| L&K (without outlier) | 0.6–760 | ∼190 | 112–115 |
| A&V | 6.5–21,000 | ∼3600 | 109,116–120 |
| A&V (outlier) | 21,000 | 109 | |
| A&V (without outlier) | 6.5–560 | ∼125 | 116–120 |
| Skin | 6–50,000 | ∼7700 | 112,121–127 |
| Skin (outliers) | 50,000–11,100 | 121,122 | |
| Skin (without outlier) | 6–222 | ∼85 | 112,123–127 |
| Cornea anterior base & Descemet's membrane | 7.5–50 | ∼29 | 38 |
| Sclera | No values | ||
| Breast tissue | 0.167–29 | ∼8 | 1,3,128,129 |
| Muscle | 2–12 | ∼7 | 120,130 |
| Spinal cord & gray matter | 0.2–7 | ∼3 | 66,131 |
| Tendon | No values | ||
L&K, liver & kidney; A&V, artery & vein.
Table 3.
Young's Modulus of Soft Tissues, Measured by Tensile Stretching
|
Tensile data | |||
|---|---|---|---|
| Tissue | Range (MPa) | Average Young's modulus (MPa) | Reference |
| Tendon | 43–1660 | ∼560 | 53,132–136 |
| Muscle | 480 | 480 | 137 |
| Skin | 21–39 | ∼30 | 135,138 |
| L&K | 1–15 | ∼10 | 139–141 |
| Cornea | 0.1–11.1 | ∼3.0 | 40,142–145 |
| Sclera | 0.6–4.9 | ∼2.7 | 146–150 |
| Spinal cord & gray matter | 0.4–3.6 | ∼2 | 151–154 |
| A&V | 0.6–3.5 | ∼2 | 155,156 |
| Breast tissue | No values | ||
Comparison of Indentation and Tensile Stretching Modulus Values
Tables 2 and 3 list YM values compiled from a number of soft biological tissues measured by indentation and tensile stretching, respectively. Tables 2 and 3 are arranged from largest to smallest average modulus. YM values for indentation have a range from ∼190 kPa for the organs located in the abdominal cavity, to around 3 kPa for spinal cord and gray matter. Tensile modulus values range from about 560 MPa for tendons to ∼2.0 MPa for spinal cord and gray matter. Some reported modulus values appeared to be clearly outside the median range. We suspect some of the values are outliers and have tabulated averages for both these outlier values included and excluded. For example, the indentation of arteries and veins had reported YM values that ranged from 6.5 to 21,000 kPa, giving an average YM around 3600 kPa. However, the single reported value of 21,000 kPa increases the average over 28-fold if included with the other values, potentially complicating interpretation of results. Table 4 compares the averaged result for the two methods. Comparisons between indentation and tensile measurements in Table 4 do no include the suspected outliers noted in Tables 2 and 3.
Table 4.
Comparison of Indentation and Tensile Measurements of Young's Modulus
|
Indentation versus tensile | ||
|---|---|---|
| Tissue | Indentation (kPa) | Tensile (MPa) |
| Skin | ∼85 | ∼30 |
| L&K | ∼190 | ∼10 |
| Spinal cord & gray matter | ∼3 | ∼2 |
| Muscle | ∼7 | ∼480 |
| Tendon | No values | ∼560 |
| Breast tissue | ∼8 | No values |
| A&V | ∼125 | ∼2 |
| Sclera | No values | ∼2.7 |
| Cornea | ∼29 | ∼3.0 |
The values are the averages without the suspected outliers. Of the tabled citations on tissue mechanics, eight reports did not clearly state the tissue hydration condition, and the rest were measured in “wet” or “hydrated” (such as skin) conditions. Of those eight, we considered only one as an indentation outlier.109 The results in this table are therefore not changed by consideration of this variable.
To put these values in context, YM of a 25% aqueous solution of gelatin is reported to be ∼30 kPa88 (sphere–sphere compression), polydimethylsiloxane (PDMS) silicone rubber ∼800 kPa89 (rheometry), and tissue culture polystyrene to be ∼3 GPa90 (indentation). In assembling this review, there were some tissues that could not be directly compared due to a lack of published YM values in either tensile or indentation measurement. We have included these tissues to highlight possible research areas of interest. Other materials of interest to biological systems include polymeric hydrogels.91–95 In general, hydrogels can be formulated from a variety of polymers with modulus values that span from very compliant to extremely rigid. A particularly interesting biological simulant, Matrigel™,31 initially derived from a mouse tumor cell line is used as a three dimensional platform for tissue cultured cells and has a reported YM of around 1 kPa.35
Discussion
One can conclude from the information and data presented that the values of YM for soft biological tissues depends on the method by which it is obtained. Additionally, tensile measurements consistently result in larger YM values compared with indentation measurements. The difference in YM between these two methods is an experimental confirmation that soft biological tissues are not homogeneous over all length scales. Indentation measurements are localized to the region of indenter contact in the order of millimeters to nanometers depending on probe size/geometry. Tensile measurements induce macroscopic deformations that span the bulk of a tissue with the entire specimen stretched. Understanding the differences is straightforward with muscles or ligaments. These tissues are better suited to resist deformation from a given tensile stress in the direction of fiber orientation as compared to a localized indentation that might be perpendicular to the fiber orientation. It is less clear, however, why tensile measurements consistently result in larger YM values for every soft tissue reviewed here. As discussed earlier, the discrepancy may be due, in small part, to the application of Equation 1 to tensile measurements of tissues that display increasing resistance to deformation with increasing stress, resulting in modulus values that are greater than the actual YM. This may not account for the entire difference, as tensile measurements of YM can be several orders of magnitude greater than the indentation measurement. One hypothesis that might contribute to the difference is due to the combined response of extracellular matrices, individual cells, longer-ranged protein polymers like collagen, actin and elastin, and the effect of constrained water, which lead to an increased resistance to deformation for macroscopic tensile measurements. These effects would not be observed in indentation measurements as the indenter induces sample deformation on the local environment only, both in terms of the indenter geometry and the depth of penetration. This is particularly relevant in relation to the water constrained within the interstitial spaces of the tissue. Indentations, especially in the case of AFMs or nanoindenters, typically perturb the tissue on the same scale as the constituents that make up the tissue. The local volume of water around the indenter therefore contributes very little to the resistance to deformation, as the tissue surrounding the indenter is under very little stress and is therefore capable of accommodating these small fluctuations in water content. Tensile measurements, on the other hand, stress the bulk of all the constituents of the tissue. Trapped water, which is incompressible, will significantly increase the tissue's resistance to deformation during an applied tensile stress. The significant differences in reported YM suggest that indentation and tensile measurements are inherently measuring different properties of the same tissue and that the scale of tissue perturbation is the dominant factor. For example, a YM value that includes the effect of constrained water may best represent the behavior of a tissue, in vivo, to a macroscopic load.
The strong dependence of YM on experimental method can significantly affect our interpretation of tissue function, disease status, or how cells respond to biophysical cues. A clear distinction exists between macroscopic, microscopic, and sub-microscopic responses to external stimuli. For example, it has been shown that cellular behavior is influenced by the compliance of the substrate on which the cell resides.15,16,18,20,23,29,96–101 To accurately relate these laboratory observations back to in vivo cell function, compliance of the tissue and the matrix with which a cell interacts must be characterized. These values can be obtained with the AFM or possibly nanoindenters, but certainly not by macroscopic tensile measurements. The design of larger implants such as artificial joints or cartilage, however, may be better served by macroscopic tensile measurements. The results of this review clearly demonstrate that the rational design of engineered tissues and biological simulants for use in the laboratory or clinic must reflect the heterogeneous physical properties of a soft tissue. Therefore, measurements of YM obtained by both tensile and indentation methods are relevant in the design and fabrication of such devices.
Of considerable interest is the modeling of tissue mechanics through mathematical models such as finite element analysis, which is used to better understand how tissues may respond to a given stress.102–108 These models are complex but can be understood generally by assuming that tissues behave as perfectly elastic bodies and incorporate experimentally obtained material properties, such as YM, to describe tissue function. As noted in the introduction, YM values of soft tissues can be obtained only if one assumes that the tissue behaves as an elastic body under small stress conditions. However, the tabled data in this review demonstrate that this assumption does not lead to a single YM value independent of experimental method. At least two values obtained from the different measurement techniques exist for each soft tissue reviewed here (highlighting our previous argument that the term YM must be very clearly defined when describing the material property of a soft, viscoelastic biological tissue). Therefore, research articles using theoretical models must state how YM was determined, justify why a single value was used, or explicitly account for the heterogeneous nature of soft tissues. For example, it would not be appropriate to use tensile measures of YM in mathematical models designed to understand tissue mechanics at the micro- through nanoscopic scale.
The successful design of tissues engineered for improved function or replacement of native tissues requires the combined knowledge of biology, chemistry, and materials science, as they must interface with complex biochemical and biophysical environments in vivo. Increasingly, we understand that the biophysical environment plays a crucial role in the success of these engineered tissues. This review highlights and re-enforces an important attribute of soft tissues that must be considered when trying to model or engineer replacements; the response of soft tissues to an applied stress should not be considered independent of experimental method and that engineered tissues must reflect the heterogeneous material properties of native tissues.
In summary, this review has shown that soft biological tissues do not have a single YM value independent of experimental method, and that modulus values for a single tissue can span several orders of magnitude. If the method of deformation is delineated then the data provided can be better interpreted in context. On average, YM values of soft tissues are consistently lower when obtained by local indentation as compared to bulk tensile deformations. The scientific objective of a given research proposal therefore dictates which method is most appropriate.
Acknowledgments
The authors wish to thank the NIH for providing funding for this review under the following grants: R01HL079012, R01E4016134, R01EY19475, RC2AR058971, and P30EY12576. Supported in part by an unrestricted grant from Research to Prevent Blindness and a grant by National Glaucoma Research, a program of the American Health Assistance Foundation.
Disclosure Statement
No competing financial interests exist.
References
- 1.Samani A. Plewes D. An inverse problem solution for measuring the elastic modulus of intact ex vivo breast tissue tumours. Phys Med Biol. 2007;52:1247. doi: 10.1088/0031-9155/52/5/003. [DOI] [PubMed] [Google Scholar]
- 2.Krouskop T.A. Wheeler T.M. Kallel F. Garra B.S. Hall T. Elastic moduli of breast and prostate tissues under compression. Ultrason Imaging. 1998;20:260. doi: 10.1177/016173469802000403. [DOI] [PubMed] [Google Scholar]
- 3.Paszek M.J. Zahir N. Johnson K.R. Lakins J.N. Rozenberg G.I. Gefen A., et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Farrar D.J. Green H.D. Bond M.G. Wagner W.D. Gobbee R.A. Aortic pulse wave velocity, elasticity, and composition in a nonhuman primate model of atherosclerosis. Circ Res. 1978;43:52. doi: 10.1161/01.res.43.1.52. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa M. Watanabe Y. Rheological properties of the thoracic aorta in normal and whhl rabbits. Biorheology. 1988;25:147. doi: 10.3233/bir-1988-251-222. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi K. Ide K. Matsumoto T. Aortic walls in atherosclerotic rabbits—mechanical study. J Biomech Eng. 1994;116:284. doi: 10.1115/1.2895732. [DOI] [PubMed] [Google Scholar]
- 7.Imura T. Yamamoto K. Satoh T. Mikami T. Yasuda H. Arteriosclerotic change in the human abdominal aorta in vivo in relation to coronary heart disease and risk factors. Atherosclerosis. 1988;73:149. doi: 10.1016/0021-9150(88)90036-6. [DOI] [PubMed] [Google Scholar]
- 8.Richter H.A. Mittermayer C. Volume elasticity, modulus of elasticity and compliance of normal and arteriosclerotic human aorta. Biorheology. 1984;21:723. doi: 10.3233/bir-1984-21505. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto T. Abe H. Ohashi T. Kato Y. Sato M. Local elastic modulus of atherosclerotic lesions of rabbit thoracic aortas measured by pipette aspiration method. Physiol Meas. 2002;23:635. doi: 10.1088/0967-3334/23/4/304. [DOI] [PubMed] [Google Scholar]
- 10.Claridge M.W. Bate G.R. Hoskins P.R. Adam D.J. Bradbury A.W. Wilmink A.B. Measurement of arterial stiffness in subjects with vascular disease: are vessel wall changes more sensitive than increase in intima-media thickness? Atherosclerosis. 2009;205:477. doi: 10.1016/j.atherosclerosis.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 11.Wuyts F.L. Vanhuyse V.J. Langewouters G.J. Decraemer W.F. Raman E.R. Buyle S. Elastic properties of human aortas in relation to age and atherosclerosis: a structural model. Phys Med Biol. 1995;40:1577. doi: 10.1088/0031-9155/40/10/002. [DOI] [PubMed] [Google Scholar]
- 12.Yeh W.C. Li P.C. Jeng Y.M. Hsu H.C. Kuo P.L. Li M.L., et al. Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med Biol. 2002;28:467. doi: 10.1016/s0301-5629(02)00489-1. [DOI] [PubMed] [Google Scholar]
- 13.Last J.A. Tingrui P. Yuzhe D. Reilly C.M. Keller K. Acott T.S., et al. Elastic modulus determination of normal, glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. doi: 10.1167/iovs.10-6342. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou S.Y. Cheng C.M. LeDuc P.R. Composite polymer systems with control of local substrate elasticity and their effect on cytoskeletal and morphological characteristics of adherent cells. Biomaterials. 2009;30:3136. doi: 10.1016/j.biomaterials.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 15.Isenberg B.C. Dimilla P.A. Walker M. Kim S. Wong J.Y. Vascular smooth muscle cell durotaxis depends on substrate stiffness gradient strength. Biophys J. 2009;97:1313. doi: 10.1016/j.bpj.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao S.W. Yu T.B. Guan Z. De novo design of saccharide-peptide hydrogels as synthetic scaffolds for tailored cell responses. J Am Chem Soc. 2009;131:17638. doi: 10.1021/ja907097t. [DOI] [PubMed] [Google Scholar]
- 17.Subramanian A. Lin H.Y. Crosslinked chitosan: its physical properties and the effects of matrix stiffness on chondrocyte cell morphology and proliferation. J Biomed Mater Res A. 2005;75:742. doi: 10.1002/jbm.a.30489. [DOI] [PubMed] [Google Scholar]
- 18.Leipzig N.D. Shoichet M.S. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009;30:6867. doi: 10.1016/j.biomaterials.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Hadjipanayi E. Mudera V. Brown R.A. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J Tissue Eng Regen Med. 2009;3:77. doi: 10.1002/term.136. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh K. Pan Z. Guan E. Ge S. Liu Y. Nakamura T., et al. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials. 2007;28:671. doi: 10.1016/j.biomaterials.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C.C. Hsieh P.C.H. Wang G.M. Chen W.C. Yeh M.L. The influence of surface morphology and rigidity on the substrata on cell motility. Mater Lett. 2009;63:1872. [Google Scholar]
- 22.Pelham R.J., Jr. Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y.M. Ogawa R. Kakugo A. Osada Y. Gong J.P. Dynamic cell behavior on synthetic hydrogels with different charge densities. Soft Matter. 2009;5:1804. [Google Scholar]
- 24.Wong J.Y. Velasco A. Rajagopalan P. Pham Q. Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir. 2003;19:1908. [Google Scholar]
- 25.Gray D.S. Tien J. Chen C.S. Repositioning of cells by mechanotaxis on surfaces with micropatterned young's modulus. J Biomed Mater Res A. 2003;66A:605. doi: 10.1002/jbm.a.10585. [DOI] [PubMed] [Google Scholar]
- 26.Kloxin A.M. Benton J.A. Anseth K.S. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials. 2010;31:1. doi: 10.1016/j.biomaterials.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moussallem M.D. Olenych S.G. Scott S.L. Keller T.C., 3rd Schlenoff J.B. Smooth muscle cell phenotype modulation and contraction on native and cross-linked polyelectrolyte multilayers. Biomacromolecules. 2009;10:3062. doi: 10.1021/bm9007309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerjee A. Artha M. Choudhary S. Ashton R.S. Bhatia S.R. Schaffer D.V., et al. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials. 2009;30:4695. doi: 10.1016/j.biomaterials.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Discher D.E. Janmey P. Wang Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 30.McKee C.T. Wood J.A. Shah N.M. Fischer M.E. Reilly C.M. Murphy C.J., et al. The effect of biophysical attributes of the ocular trabecular meshwork associated with glaucoma on the cell response to therapeutic agents. Biomaterials. 2011;32:2417. doi: 10.1016/j.biomaterials.2010.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleinman H.K. Martin G.R. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Semler E.J. Ranucci C.S. Moghe P.V. Mechanochemical manipulation of hepatocyte aggregation can selectively induce or repress liver-specific function. Biotechnol Bioeng. 2000;69:359. doi: 10.1002/1097-0290(20000820)69:4<359::aid-bit2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 33.Zaman M.H. Trapani L.M. Sieminski A.L. Mackellar D. Gong H. Kamm R.D., et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci U S A. 2006;103:10889. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alcaraz J. Xu R. Mori H. Nelson C.M. Mroue R. Spencer V.A., et al. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 2008;27:2829. doi: 10.1038/emboj.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soofi S.S. Last J.A. Liliensiek S.J. Nealey P.F. Murphy C.J. The elastic modulus of Matrigel as determined by atomic force microscopy. J Struct Biol. 2009;167:216. doi: 10.1016/j.jsb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hjortdal J.O. Regional elastic performance of the human cornea. J Biomech. 1996;29:931. doi: 10.1016/0021-9290(95)00152-2. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz N.J. Mackay R.S. Sackman J.L. A theoretical and experimental study of the mechanical behavior of the cornea with application to the measurement of intraocular pressure. Bull Math Biophys. 1966;28:585. [Google Scholar]
- 38.Last J.A. Liliensiek S.J. Nealey P.F. Murphy C.J. Determining the mechanical properties of human corneal basement membranes with atomic force microscopy. J Struct Biol. 2009;167:19. doi: 10.1016/j.jsb.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nash I.S. Greene P.R. Foster C.S. Comparison of mechanical properties of keratoconus and normal corneas. Exp Eye Res. 1982;35:413. doi: 10.1016/0014-4835(82)90040-9. [DOI] [PubMed] [Google Scholar]
- 40.Jayasuriya A.C. Ghosh S. Scheinbeim J.I. Lubkin V. Bennett G. Kramer P. A study of piezoelectric and mechanical anisotropies of the human cornea. Biosens Bioelectron. 2003;18:381. doi: 10.1016/s0956-5663(02)00144-6. [DOI] [PubMed] [Google Scholar]
- 41.Sjontoft E. Edmund C. Invivo determination of Young modulus for the human cornea. Bull Math Biol. 1987;49:217. doi: 10.1007/BF02459699. [DOI] [PubMed] [Google Scholar]
- 42.Hamilton K.E. Pye D.C. Young's modulus in normal corners and the effect on applanation tonometry. Optom Vis Sci. 2008;85:445. doi: 10.1097/OPX.0b013e3181783a70. [DOI] [PubMed] [Google Scholar]
- 43.Liu J. Roberts C.J. Influence of corneal biomechanical properties on intraocular pressure measurement—quantitative analysis. J Cataract Refract Surg. 2005;31:146. doi: 10.1016/j.jcrs.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 44.Elsheikh A. Wang D.F. Pye D. Determination of the modulus of elasticity of the human cornea. J Refract Surg. 2007;23:808. doi: 10.3928/1081-597X-20071001-11. [DOI] [PubMed] [Google Scholar]
- 45.Woo S.L. Kobayashi A.S. Schlegel W.A. Lawrence C. Nonlinear material properties of intact cornea and sclera. Exp Eye Res. 1972;14:29. doi: 10.1016/0014-4835(72)90139-x. [DOI] [PubMed] [Google Scholar]
- 46.Jue B. Maurice D.M. The mechanical properties of the rabbit and human cornea. J Biomech. 1986;19:847. doi: 10.1016/0021-9290(86)90135-1. [DOI] [PubMed] [Google Scholar]
- 47.Elsheikh A. Alhasso D. Rama P. Assessment of the epithelium's contribution to corneal biomechanics. Exp Eye Res. 2008;86:445. doi: 10.1016/j.exer.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Johnson M.W. Han D.P. Hoffman K.E. The effect of scleral buckling on ocular rigidity. Ophthalmology. 1990;97:190. doi: 10.1016/s0161-6420(90)32606-4. [DOI] [PubMed] [Google Scholar]
- 49.White O.W. Ocular elasticity? Ophthalmology. 1990;97:1092. doi: 10.1016/s0161-6420(90)32455-7. [DOI] [PubMed] [Google Scholar]
- 50.Purslow P.P. Karwatowski W.S. Ocular elasticity. Is engineering stiffness a more useful characterization parameter than ocular rigidity? Ophthalmology. 1996;103:1686. doi: 10.1016/s0161-6420(96)30446-6. [DOI] [PubMed] [Google Scholar]
- 51.Fung Y.C.B. Elasticity of soft tissues in simple elongation. Am J Physiol. 1967;213:1532. doi: 10.1152/ajplegacy.1967.213.6.1532. [DOI] [PubMed] [Google Scholar]
- 52.Ehlers W. Karajan N. Markert B. An extended biphasic model for charged hydrated tissues with application to the intervertebral disc. Biomech Model Mechanobiol. 2009;8:233. doi: 10.1007/s10237-008-0129-y. [DOI] [PubMed] [Google Scholar]
- 53.Shadwick R.E. Elastic energy storage in tendons: mechanical differences related to function and age. J Appl Physiol. 1990;68:1033. doi: 10.1152/jappl.1990.68.3.1033. [DOI] [PubMed] [Google Scholar]
- 54.Wineman A. Nonlinear viscoelastic solids—a review. Math Mech Solids. 2009;14:300. [Google Scholar]
- 55.Fatemi M. Greenleaf J.F. Imaging the viscoelastic properties of tissue. Imaging of complex media with acoustic and seismic waves. Top Appl Phys. 2002;84:257. [Google Scholar]
- 56.Salacinski H.J. Goldner S. Giudiceandrea A. Hamilton G. Seifalian A.M. Edwards A., et al. The mechanical behavior of vascular grafts: a review. J Biomater Appl. 2001;15:241. doi: 10.1106/NA5T-J57A-JTDD-FD04. [DOI] [PubMed] [Google Scholar]
- 57.Taylor D.C. Dalton J.D., Jr. Seaber A.V. Garrett W.E., Jr. Viscoelastic properties of muscle-tendon units. The biomechanical effects of stretching. Am J Sports Med. 1990;18:300. doi: 10.1177/036354659001800314. [DOI] [PubMed] [Google Scholar]
- 58.Nasseri S. Bilston L.E. Phan-Thien N. Viscoelastic properties of pig kidney in shear, experimental results and modelling. Rheol Acta. 2002;41:180. [Google Scholar]
- 59.Christensen M.S. Hargens C.W., 3rd Nacht S. Gans E.H. Viscoelastic properties of intact human skin: instrumentation, hydration effects, and the contribution of the stratum corneum. J Invest Dermatol. 1977;69:282. doi: 10.1111/1523-1747.ep12507500. [DOI] [PubMed] [Google Scholar]
- 60.Bisplinghoff J.A. McNally C. Manoogian S.J. Duma S.M. Dynamic material properties of the human sclera. J Biomech. 2009;42:1493. doi: 10.1016/j.jbiomech.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 61.Kiss M.Z. Daniels M.J. Varghese T. Investigation of temperature-dependent viscoelastic properties of thermal lesions in ex vivo animal liver tissue. J Biomech. 2009;42:959. doi: 10.1016/j.jbiomech.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Green M.A. Bilston L.E. Sinkus R. In vivo brain viscoelastic properties measured by magnetic resonance elastography. NMR Biomed. 2008;21:755. doi: 10.1002/nbm.1254. [DOI] [PubMed] [Google Scholar]
- 63.Santos N.C. Castanho M.A. An overview of the biophysical applications of atomic force microscopy. Biophys Chem. 2004;107:133. doi: 10.1016/j.bpc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Briscoe B.J. Fiori L. Pelillo E. Nano-indentation of polymeric surfaces. J Phys D. 1998;31:2395. [Google Scholar]
- 65.Ebenstein D.M. Pruitt L.A. Nanoindnetation of biological materials. Nano Today. 2006;1:26. [Google Scholar]
- 66.Saxena T. Gilbert J.L. Hasenwinkel J.M. A versatile mesoindentation system to evaluate the micromechanical properties of soft, hydrated substrates on a cellular scale. J Biomed Mater Res A. 2009;90:1206. doi: 10.1002/jbm.a.32178. [DOI] [PubMed] [Google Scholar]
- 67.Radmacher M. Studying the mechanics of cellular processes by atomic force microscopy. Cell mechanics, book series. Method Cell Biol. 2007;83:347. doi: 10.1016/S0091-679X(07)83015-9. [DOI] [PubMed] [Google Scholar]
- 68.Cheng Y.-T. Cheng C.-M. Scaling, dimensional analysis, and indentation measurements. Mater Sci Eng R. 2004;44:91. [Google Scholar]
- 69.Bowen W.R. Lovitt R.W. Wright C.J. Application of atomic force microscopy to the study of micromechanical properties of biological materials. Biotechnol Lett. 2000;22:893. [Google Scholar]
- 70.Lin D.C. Horkay F. Nanomechanics of polymer gels and biological tissues: a critical review of analytical approaches in the hertzian regime and beyond. Soft Matter. 2008;4:669. doi: 10.1039/b714637j. [DOI] [PubMed] [Google Scholar]
- 71.Oliver W.C. Pharr G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7:1564. [Google Scholar]
- 72.Hertz H. Uber die beruhrung fester elastischer korper. J Reine Angew Mathematik. 1882;92:156. [Google Scholar]
- 73.Boussinesq J. Application des Potentiels a Petude de l'equilibre et du Mouvement des Solides Elastiques. Paris: Gauthier-Villars; 1885. [Google Scholar]
- 74.Love A.E.H. Boussingesq's problem for a rigid cone. Q J Math. 1939;10:161. [Google Scholar]
- 75.Harding J.W. Sneddon I.N. The elastic stresses produced by the indentation of the plane surface of a semi-infinite elastic solid by a rigid punch. Proc Camb Philol Soc. 1945;41:16. [Google Scholar]
- 76.Sneddon I.N. Boussinesq problem for a flat-ended cylinder. Proc Camb Philol Soc. 1946;42:29. [Google Scholar]
- 77.Sneddon I.N. Boussinesqs problem for a rigid cone. Proc Camb Philol Soc. 1948;44:492. [Google Scholar]
- 78.Sneddon I.N. The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int J Eng Sci. 1965;3:47. [Google Scholar]
- 79.Mahaffy R.E. Shih C.K. MacKintosh F.C. Kas J. Scanning probe-based frequency-dependent microrheology of polymer gels and biological cells. Phys Rev Lett. 2000;85:880. doi: 10.1103/PhysRevLett.85.880. [DOI] [PubMed] [Google Scholar]
- 80.Thormann E. Pettersson T. Claesson P.M. How to measure forces with atomic force microscopy without significant influence from nonlinear optical lever sensitivity. Rev Sci Instrum. 2009;80:093701. doi: 10.1063/1.3194048. [DOI] [PubMed] [Google Scholar]
- 81.Tsui T.Y. Oliver W.C. Pharr G.M. Thin Films: Stresses and Mechanical Properties VI. Pittsburg, PA: Mater. Res. Soc. Symp. Proc.; 1997. [Google Scholar]
- 82.Rico F. Roca-Cusachs P. Gavara N. Farre R. Rotger M. Navajas D. Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;72:021914. doi: 10.1103/PhysRevE.72.021914. [DOI] [PubMed] [Google Scholar]
- 83.Costa K.D. Yin F.C. Analysis of indentation: implications for measuring mechanical properties with atomic force microscopy. J Biomech Eng. 1999;121:462. doi: 10.1115/1.2835074. [DOI] [PubMed] [Google Scholar]
- 84.Na S. Sun Z. Meininger G.A. Humphrey J.D. On atomic force microscopy and the constitutive behavior of living cells. Biomech Model Mechanobiol. 2004;3:75. doi: 10.1007/s10237-004-0051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Domke J. Radmacher M. Measuring the elastic properties of thin polymer films with the atomic force microscope. Langmuir. 1998;14:3320. [Google Scholar]
- 86.Clifford C.A. Seah M.P. Quantification issues in the identification of nanoscale regions of homopolymers using modulus measurement via afm nanoindentation. Appl Surf Sci. 2005;252:1915. [Google Scholar]
- 87.Ogden R.W. Large deformations of isotropic elasticity-correlation of theory and experiment for incompressible rubberlike solids. Proc R Soc Lon Ser A. 1972;326:565. [Google Scholar]
- 88.Johnson K.L. Kendall K. Roberts A.D. Surface energy and contact of elastic solids. Proc R Soc Lond A Math. 1971;324:301. [Google Scholar]
- 89.Lotters J.C. Olthuis W. Veltink P.H. Bergveld P. The mechanical properties of the rubber elastic polymer polydimethylsiloxane for sensor applications. J Micromech Microeng. 1997;7:145. [Google Scholar]
- 90.Tsukruk V.V. Huang Z. Chizhik S.A. Gorbunov V.V. Probing of micromechanical properties of compliant polymeric materials. J Mater Sci. 1998;33:4905. [Google Scholar]
- 91.Lutolf M.P. Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 92.Hoffman A.S. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54:3. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 93.Jeong B. Kim S.W. Bae Y.H. Thermosensitive sol-gel reversible hydrogels. Adv Drug Deliv Rev. 2002;54:37. doi: 10.1016/s0169-409x(01)00242-3. [DOI] [PubMed] [Google Scholar]
- 94.Griffith L.G. Polymeric biomaterials. Acta Mater. 2000;48:263. [Google Scholar]
- 95.Jen A.C. Wake M.C. Mikos A.G. Review: hydrogels for cell immobilization. Biotech Bioeng. 1996;50:357. doi: 10.1002/(SICI)1097-0290(19960520)50:4<357::AID-BIT2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 96.Wong J.Y. Leach J.B. Brown X.Q. Balance of chemistry, topography, and mechanics at the cell-biomaterial interface: issues and challenges for assessing the role of substrate mechanics on gel response. Surf Sci. 2004;570:119. [Google Scholar]
- 97.Irwin E.F. Saha K. Rosenbluth M. Gamble L.J. Castner D.G. Healy K.E. Modulus-dependent macrophage adhesion and behavior. J Biomater Sci Polym Ed. 2008;19:1363. doi: 10.1163/156856208786052407. [DOI] [PubMed] [Google Scholar]
- 98.Saha K. Keung A.J. Irwin E.F. Li Y. Little L. Schaffer D.V., et al. Substrate modulus directs neural stem cell behavior. Biophys J. 2008;95:4426. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leach J.B. Brown X.Q. Jacot J.G. Dimilla P.A. Wong J.Y. Neurite outgrowth and branching of pc12 cells on very soft substrates sharply decreases below a threshold of substrate rigidity. J Neural Eng. 2007;4:26. doi: 10.1088/1741-2560/4/2/003. [DOI] [PubMed] [Google Scholar]
- 100.Brown X.Q. Ookawa K. Wong J.Y. Evaluation of polydimethylsiloxane scaffolds with physiologically-relevant elastic moduli: interplay of substrate mechanics and surface chemistry effects on vascular smooth muscle cell response. Biomaterials. 2005;26:3123. doi: 10.1016/j.biomaterials.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 101.Discher D.E. Mooney D.J. Zandstra P.W. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Asejczyk-Widlicka M. Srodka D.W. Kasprzak H. Pierscionek B.K. Modelling the elastic properties of the anterior eye and their contribution to maintenance of image quality: the role of the limbus. Eye (Lond) 2007;21:1087. doi: 10.1038/sj.eye.6702464. [DOI] [PubMed] [Google Scholar]
- 103.Amini R. Barocas V.H. Anterior chamber angle opening during corneoscleral indentation: the mechanism of whole eye globe deformation and the importance of the limbus. Invest Ophthalmol Vis Sci. 2009;50:5288. doi: 10.1167/iovs.08-2890. [DOI] [PubMed] [Google Scholar]
- 104.Srodka W. Pierscionek B.K. Effect of material properties of the eyeball coat on optical image stability. J Biomed Opt. 2008;13:054013. doi: 10.1117/1.2975844. [DOI] [PubMed] [Google Scholar]
- 105.Taylor Z. Miller K. Reassessment of brain elasticity for analysis of biomechanisms of hydrocephalus. J Biomech. 2004;37:1263. doi: 10.1016/j.jbiomech.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 106.Cheng T. Gan R.Z. Experimental measurement and modeling analysis on mechanical properties of tensor tympani tendon. Med Eng Phys. 2008;30:358. doi: 10.1016/j.medengphy.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 107.Chanthasopeephan T. Desai J.P. Lau A.C. Measuring forces in liver cutting: New equipment and experimental results. Ann Biomed Eng. 2003;31:1372. doi: 10.1114/1.1624601. [DOI] [PubMed] [Google Scholar]
- 108.Holzapfel G.A. Gasser T.C. Ogden R.W. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elast. 2000;61:1. [Google Scholar]
- 109.Akhtar R. Schwarzer N. Sherratt M.J. Watson R.E.B. Graham H.K. Trafford A.W., et al. Nanoindentation of histological specimens: mapping the elastic properties of soft tissues. J Mater Res. 2009;24:638. doi: 10.1557/JMR.2009.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Johnson K.L. Contact Mechanics. Cambridge: Cambridge University Press; 1985. [Google Scholar]
- 111.Carter F.J. Frank T.G. Davies P.J. McLean D. Cuschieri A. Measurements and modelling of the compliance of human and porcine organs. Med Image Anal. 2001;5:231. doi: 10.1016/s1361-8415(01)00048-2. [DOI] [PubMed] [Google Scholar]
- 112.Constantinides G. Kalcioglu Z.I. McFarland M. Smith J.F. Van Vliet K.J. Probing mechanical properties of fully hydrated gels and biological tissues. J Biomech. 2008;41:3285. doi: 10.1016/j.jbiomech.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 113.Chen E.J. Novakofski J. Jenkins W.K. OBrien W.D. Young's modulus measurements of soft tissues with application to elasticity imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 1996;43:191. [Google Scholar]
- 114.Barnes S.L. Lyshchik A. Washington M.K. Gore J.C. Miga M.I. Development of a mechanical testing assay for fibrotic murine liver. Med Phys. 2007;34:4439. doi: 10.1118/1.2795665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tay B.K. Kim J. Srinivasan M.A. In vivo mechanical behavior of intra-abdominal organs. IEEE Trans Biomed Eng. 2006;53:2129. doi: 10.1109/TBME.2006.879474. [DOI] [PubMed] [Google Scholar]
- 116.Ebenstein D.M. Pruitt L.A. Nanoindentation of soft hydrated materials for application to vascular tissues. J Biomed Mater Res A. 2004;69:222. doi: 10.1002/jbm.a.20096. [DOI] [PubMed] [Google Scholar]
- 117.Jacot J.G. Dianis S. Schnall J. Wong J.Y. A simple microindentation technique for mapping the microscale compliance of soft hydrated materials and tissues. J Biomed Mater Res A. 2006;79:485. doi: 10.1002/jbm.a.30812. [DOI] [PubMed] [Google Scholar]
- 118.Lundkvist A. Lilleodden E. Siekhaus W. Kinney J. Pruitt L. Balooch M. Viscoelastic properties of healthy human artery measured in saline solution by afm-based indentation technique. In: Gerberich W.W., editor; Gao H., editor; Sundgren J.-E., editor; Baker S.P., editor. Thin Films: Stresses and Mechanical Properties VI. Pittsburgh: Mater Res Soc; 1997. [Google Scholar]
- 119.Oie T. Murayama Y. Fukuda T. Nagai C. Omata S. Kanda K., et al. Local elasticity imaging of vascular tissues using a tactile mapping system. J Artif Organs. 2009;12:40. doi: 10.1007/s10047-008-0440-5. [DOI] [PubMed] [Google Scholar]
- 120.Engler A.J. Richer L. Wong J.Y. Picart C. Discher D.E. Surface probe measurements of the elasticity of sectioned tissue, thin gels and polyelectrolyte multilayer films: correlations between substrate stiffness and cell adhesion. Surf Sci. 2004;570:142. [Google Scholar]
- 121.Yuan Y.H. Verma R. Measuring microelastic properties of stratum corneum. Colloid Surface B. 2006;48:6. doi: 10.1016/j.colsurfb.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 122.Kendall M.A.F. Chong Y.-F. Cock A. The mechanical properties of skin epidermis in relation to targeted gene and drug delivery. Biomaterials. 2007;28:4968. doi: 10.1016/j.biomaterials.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 123.Leung S.-F. Zheng Y. Choi C.Y.K. Mak S.S.S. Chiu S.K.W. Zee B., et al. Quantitative measurement of post-irradiation neck fibrosis based on the Young modulus. Cancer. 2002;95:656. doi: 10.1002/cncr.10700. [DOI] [PubMed] [Google Scholar]
- 124.Ling H.Y. Choi P.C. Zheng Y.P. Lau K.T. Extraction of mechanical properties of foot plantar tissues using ultrasound indentation associated with genetic algorithm. J Mater Sci Mater Med. 2007;18:1579. doi: 10.1007/s10856-007-3025-6. [DOI] [PubMed] [Google Scholar]
- 125.Pailler-Mattei C. Bec S. Zahouani H. In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med Eng Phys. 2008;30:599. doi: 10.1016/j.medengphy.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 126.Mak A.F.T. George H.W. Liu B.S. Lee B.D. Biomechanical assessment of below-knee residual limb tissue. J Rehabil Res Dev. 1994;31:188. [PubMed] [Google Scholar]
- 127.Zheng Y.P. Choi Y.K.C. Wong K. Chan S. Mak A.F.T. Biomechanical assessment of plantar foot tissue in diabetic patients using an ultrasound indentation system. Ultrasound Med Biol. 2000;26:451. doi: 10.1016/s0301-5629(99)00163-5. [DOI] [PubMed] [Google Scholar]
- 128.Samani A. Bishop J. Luginbuhl C. Plewes D.B. Measuring the elastic modulus of ex vivo small tissue samples. Phys Med Biol. 2003;48:2183. doi: 10.1088/0031-9155/48/14/310. [DOI] [PubMed] [Google Scholar]
- 129.Han L. Noble J.A. Burcher M. A novel ultrasound indentation system for measuring biomechanical properties of in vivo soft tissue. Ultrasound Med Biol. 2003;29:813. doi: 10.1016/s0301-5629(02)00776-7. [DOI] [PubMed] [Google Scholar]
- 130.Chen E.J. Novakofski J. Jenkins W.K. O'Brien W.D. Young's modulus measurements of soft tissues with application to elasticity imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 1996;43:191. [Google Scholar]
- 131.Elkin B.S. Azeloglu E.U. Costa K.D. Morrison B., 3rd Mechanical heterogeneity of the rat hippocampus measured by atomic force microscope indentation. J Neurotrauma. 2007;24:812. doi: 10.1089/neu.2006.0169. [DOI] [PubMed] [Google Scholar]
- 132.Azizi E. Halenda G.M. Roberts T.J. Mechanical properties of the gastrocnemius aponeurosis in wild turkeys. Integr Comp Biol. 2009;42:51. doi: 10.1093/icb/icp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cui L. Maas H. Perreault E.J. Sandercock T.G. In situ estimation of tendon material properties: differences between muscles of the feline hindlimb. J Biomech. 2009;42:679. doi: 10.1016/j.jbiomech.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.de Almeida F.M. Tomiosso T.C. Nakagaki W.R. Gomes L. Matiello-Rosa S.M. Pimentel E.R. Effects of passive stretching on the biochemical and biomechanical properties of calcaneal tendon of rats. Connect Tissue Res. 2009;50:279. [PubMed] [Google Scholar]
- 135.Vogel H.G. Influence of maturation and age on mechanical and biochemical parameters of connective tissue of various organs in the rat. Connect Tissue Res. 1978;6:161. doi: 10.3109/03008207809152626. [DOI] [PubMed] [Google Scholar]
- 136.Wren T.A. Yerby S.A. Beaupre G.S. Carter D.R. Mechanical properties of the human Achilles tendon. Clin Biomech (Bristol, Avon) 2001;16:245. doi: 10.1016/s0268-0033(00)00089-9. [DOI] [PubMed] [Google Scholar]
- 137.Buchanan C.I. Marsh R.L. Effects of long-term exercise on the biomechanical properties of the Achilles tendon of guinea fowl. J Appl Physiol. 2001;90:164. doi: 10.1152/jappl.2001.90.1.164. [DOI] [PubMed] [Google Scholar]
- 138.Ozyazgan I. Liman N. Dursun N. Gunes I. The effects of ovariectomy on the mechanical properties of skin in rats. Maturitas. 2002;43:65. doi: 10.1016/s0378-5122(02)00181-0. [DOI] [PubMed] [Google Scholar]
- 139.Herbert L.A. Chen W.C. Hartmann A. Garancis J.C. Mechanical properties of the dog renal capsule. J Appl Physiol. 1976;40:164. doi: 10.1152/jappl.1976.40.2.164. [DOI] [PubMed] [Google Scholar]
- 140.Hollenstein M. Nava A. Valtorta D. Snedeker J.G. Mazza E. Mechanical characterization of the liver capsule and parenchyma. Lect Notes Comput Sci. 2006;4072:150. [Google Scholar]
- 141.Snedeker J.G. Niederer P. Schmidlin F.R. Farshad M. Demetropoulos C.K. Lee J.B., et al. Strain-rate dependent material properties of the porcine and human kidney capsule. J Biomech. 2005;38:1011. doi: 10.1016/j.jbiomech.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 142.Jayasuriya A.C. Scheinbeim J.I. Lubkin V. Bennett G. Kramer P. Piezoelectric and mechanical properties in bovine cornea. J Biomed Mater Res A. 2003;66A:260. doi: 10.1002/jbm.a.10536. [DOI] [PubMed] [Google Scholar]
- 143.Reichel E. Miller D. Blanco E. Mastanduno R. The elastic modulus of central and perilimbal bovine cornea. Ann Ophthalmol. 1989;21:205. [PubMed] [Google Scholar]
- 144.Wollensak G. Iomdina E. Long-term biomechanical properties of rabbit cornea after photodynamic collagen crosslinking. Acta Ophthalmol. 2009;87:48. doi: 10.1111/j.1755-3768.2008.01190.x. [DOI] [PubMed] [Google Scholar]
- 145.Wollensak G. Spoerl E. Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-a-induced cross-linking. J Cataract Refract Surg. 2003;29:1780. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 146.Downs C.J. Frasncis Suh J.-K. Thomas K.A. Belleza A.J. Burgoyne C.F. Hart R.T. Viscoelastic characterization of peripapillary sclera: material properties by quadrant in rabbit and monkey eyes. Trans ASME. 2003;125:124. doi: 10.1115/1.1536930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Girard M. Suh J.-K.F. Hart R.T. Burgoyne C.F. Downs J.C. Effects of storage time on the mechanical properties of rabbit perpapillary sclera after enucleation. Curr Eye Res. 2007;32:465. doi: 10.1080/02713680701273792. [DOI] [PubMed] [Google Scholar]
- 148.Schultz D.S. Lotz J.C. Lee S.M. Trinidad M.L. Stewart J.M. Structural factors that mediate scleral stiffness. Invest Ophthalmol Vis Sci. 2008;49:4232. doi: 10.1167/iovs.08-1970. [DOI] [PubMed] [Google Scholar]
- 149.Downs J.C. Suh J.K. Thomas K.A. Bellezza A.J. Hart R.T. Burgoyne C.F. Viscoelastic material properties of the peripapillary sclera in normal and early-glaucoma monkey eyes. Invest Ophthalmol Vis Sci. 2005;46:540. doi: 10.1167/iovs.04-0114. [DOI] [PubMed] [Google Scholar]
- 150.Phillips J.R. McBrien N.A. Form deprivation myopia: elastic properties of sclera. Ophthalmic Physiol Opt. 1995;15:357. [PubMed] [Google Scholar]
- 151.Maikos J.T. Elias R.A. Shreiber D.I. Mechanical properties of dura mater from the rat brain and spinal cord. J Neurotrauma. 2008;25:38. doi: 10.1089/neu.2007.0348. [DOI] [PubMed] [Google Scholar]
- 152.Velardi F. Fraternali F. Angelillo M. Anisotropic constitutive equations and experimental tensile behavior of brain tissue. Biomech Model Mechanobiol. 2006;5:53. doi: 10.1007/s10237-005-0007-9. [DOI] [PubMed] [Google Scholar]
- 153.Bilston L.E. Thibault L.E. The mechanical properties of the human cervical spinal cord in vitro. Ann Biomed Eng. 1996;24:67. doi: 10.1007/BF02770996. [DOI] [PubMed] [Google Scholar]
- 154.Miller K. Chinzei K. Mechanical properties of brain tissue in tension. J Biomech. 2002;35:483. doi: 10.1016/s0021-9290(01)00234-2. [DOI] [PubMed] [Google Scholar]
- 155.Assoul N. Flaud P. Chaouat M. Letourneur D. Bataille I. Mechanical properties of rat thoracic and abdominal aortas. J Biomech. 2008;41:2227. doi: 10.1016/j.jbiomech.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 156.Reddy K.G. Age-related cross-linking of collagen is associated with aortic wall matrix stiffness in the pathogenesis of drug-induced diabetes in rats. Microvasc Res. 2004;68:132. doi: 10.1016/j.mvr.2004.04.002. [DOI] [PubMed] [Google Scholar]