Abstract
Mesenchymal stem cells (MSCs) have been shown to contribute to the recovery of tissues through homing to injured areas, especially to hypoxic, apoptotic, or inflamed areas and releasing factors that hasten endogenous repair. In some cases genetic engineering of the MSC is desired, since they are excellent delivery vehicles. We have derived MSCs from the human embryonic stem cell (hESC) line H9 (H9-MSCs). They expressed CD105, CD90, CD73, and CD146, and lacked expression of CD45, CD34, CD14, CD31, and HLA-DR, the hESC pluripotency markers SSEA-4 and Tra-1-81, and the hESC early differentiation marker SSEA-1. Marrow-derived MSCs showed a similar phenotype. H9-MSCs did not form teratoma in our initial studies, whereas the parent H9 line did so robustly. H9-MSCs differentiated into bone, cartilage, and adipocytes in vitro, and displayed increased migration under hypoxic conditions. Finally, using a hindlimb ischemia model, H9-MSCs were shown to home to the hypoxic muscle, but not the contralateral limb, by 48 h after IV injection. In summary, we have defined methods for differentiation of hESCs into MSCs and have defined their characteristics and in vivo migratory properties.
Introduction
Mesenchymal stem cells (MSCs, also known as marrow stromal cells) are adult, multipotent cells that are most often derived from bone marrow and adipose tissue and are capable of differentiating into osteoblasts, adipocytes, and chondrocytes.1 MSCs present a promising tool for cell therapy, and have been shown to contribute to the recovery of tissues in models of myocardial infarction,2 stroke model,3,4 meniscus injury,5 and hind limb ischemia models,6 among others. The leading theory of tissue repair and regeneration by adult MSCs is that the injected stem cells home to the injured area, in particular to hypoxic, apoptotic, or inflamed areas, and release paracrine factors that hasten endogenous repair.6 These secreted bioactive products can suppress the local immune system, enhance angiogenesis, inhibit fibrosis and apoptosis, and stimulate recruitment, retention, proliferation, and differentiation of tissue-residing stem cells.7
Paracrine effects exerted by MSCs are distinct from the classical model of direct differentiation of stem cells into the tissue to be regenerated. MSCs can, however, directly contribute to the repair of bone, muscle, tendon, and cartilage, and can be long-lived in various tissues when transplanted in the absence of acute injury.8–10 MSCs have been shown to be safe to date in human clinical trials. In some cases, where the patient lacks expression of a critical gene product, such as in osteogenesis imperfecta or lysososomal storage disorders, genetic engineering of the MSCs is desired. Using human embryonic stem cells (hESCs), a vector's integration site can be characterized and clones with benign integration sites can be expanded. Homologous recombination is also now feasible for hESCs or induced pluripotent stem cells, due to increased efficiencies, and clones with vectors targeted for gene correction can be expanded. Therefore, the generation of MSCs from hESCs that have had a therapeutic gene inserted into a safe integration site could potentially be considered as a future cellular therapy product, after further biosafety studies are conducted. However, there have been relatively few reports of MSC production from hESCs, and little characterization of their in vivo engraftment properties. The goal of the current studies was to gain a better understanding of the migratory properties of hESC-derived MSCs in vitro and in vivo.
hESCs have shown the ability to differentiate into cells of all three germ layers.11 Recently, MSCs have been generated from hESCs12 but in vivo analyses had not been done. Here we extend those studies to further characterize H9-MSCs, to rule out teratoma formation from the differentiated cells, and to examine the in vitro and in vivo migratory capacity of the H9-MSCs. In this study, we investigate the migratory characteristics and homing potential of hESC-derived MSCs in an effort to further understand their future therapeutic potential.
Materials and Methods
H9 cell culture
WiCell H9 (WA09) hESCs were cultured in knockout Dulbecco's Modified Eagle's Medium (DMEM)/F12 (Invitrogen) supplemented with 0.1 mM 2-mercaptoethanol (Invitrogen), 20% knockout serum replacer (Invitrogen), 0.1 mM minimum essential media-non essential amino acids (MEM-NEAA), 2 mM L-glutamine, and 4 ng/mL basic fibroblast growth factor with daily medium changes and maintained on a mouse embryonic fibroblast feeder layer (GlobalStem, Inc.).13 Cells were incubated at 37°C in 5.0% CO2 and passaged enzymatically with collagenase IV (Worthington Biochemical Corporation) approximately every 5 days.13 Pluripotency was confirmed by cell surface marker phenotype on a Beckman Coulter FC-500 flow cytometer with CXP acquisition software (Beckman Coulter, Inc.) using SSEA-4, Tra-1-81, and SSEA-1 antibodies (BD Biosciences).
MSC generation and characterization
H9 hESCs were placed into feeder-free conditions using the Cellstart extracellular matrix (Invitrogen). They were maintained at 6 ng/mL basic fibroblast growth factor until the cells had stably adapted to the feeder-free environment, as evidenced by resuming growth. Once the hESCs were successfully converted to the feeder-free environment, MSC generation was initiated. Medium changes were reduced from once daily to once every 3 days. When the culture was 50% confluent with differentiated, adherent cells the hESC colonies were manually removed and the fibroblast-like cells were allowed to expand further. Medium changes continued at 3-day intervals until the cultures were 80%–90% confluent. At this point any visible colony structures were removed and the cells were passaged into α-MEM (Invitrogen) with 20% fetal bovine serum (FBS) with no extracellular matrix supplied. The MSC-like cells continued to expand and were cryopreserved. Additionally, cultures were characterized using an FC-500 flow cytometer with CXP acquisition software (Beckman-Coulter, Inc.) by immunostaining for CD14, CD31, CD45, HLA-DR (BD Biosciences), CD73, CD90, CD105 (eBioscience, Inc.), and CD146 (R&D Systems, Inc.).14 The cells were also karyotyped and the results were compared to the karyotype of the parental embryonic stem cell line H9.
Tumor formation assay
All rodent work was performed under an approved animal care protocol in the UC Davis Stem Cell Program immune-deficient mouse core. H9-MSCs were grown under standard MSC culture conditions, lifted, and counted. Approximately 1×106 cells were prepared in 30% Matrigel (BD Biosciences) in phosphate-buffered saline (PBS) for each 100 μL injection. Four NOD/SCID/IL2Rg−/− mice were injected subcutaneously on the left flank. The same procedure was followed with the parent H9 hESC line in 2 NOD/SCID/IL2Rg−/− mice, as positive controls. The mice injected with the H9 parental line were observed until tumors reached 1.5 cm in size at which point the mice were euthanized for tumor removal and analysis. The tumors formed were embedded and snap frozen in optimal cutting temperature (OCT) compound (Tissue-Tek®) and sectioned to 5 μm on a cryostat. The slides were then H&E stained, imaged, and then sent to the UC Davis Mutant Mouse Pathology Lab at the Center for Comparative Medicine for analysis. Mice injected with H9-MSCs were followed for 6 months in our standard “rule out teratoma assay,” euthanized, and subjected to rigorous autopsy to confirm biosafety, as we have described.8
Differentiation into osteogenic, adipogenic, and chondrogenic lineages
MSCs were differentiated into the osteogenic lineage by culture for 21 days at 70%–80% confluence in α-MEM containing 10% FBS, 1×L-glutamine, 0.2 mM ascorbic acid, 0.1 μM dexamethasone, and 10 mM β-glycerophosphate with medium changes every 3 days. For adipogenic differentiation MSCs were cultured at 70%–80% confluence on tissue culture-treated plastic for 15–21 days in α-MEM containing 10% FBS, 1×L-glutamine, 0.5 mM isobutylmethylxanthine, 50 μM indomethacin, and 0.5 μM dexamethasone with medium changes every 3 days.15 To differentiate into the chondrogenic lineage, 3.0×105 MSCs were cultured as a pellet at the bottom of a 15 mL conical tube for 21 days in α-MEM, 1% FBS, 1×L-glutamine, 0.5 μM dexamethasone, 0.2 mM ascorbic acid, and 10 ng/mL TGF-β3 with medium changes every 3 days.
Scratch test cell migration assay
A scratch test was performed to assess MSC migration as we have previously described.6 H9-MSCs were grown to 70%–80% confluence in a 100 mm tissue culture dish marked underneath with a black line. A 5 mm cell scraper was used to create a cell-free field with one edge along the black line. The cells were incubated at 37°C, 5.0% CO2. The dish was observed for any cell migration after 24 h on an Eclipse TE2000-S inverted, phase-contrast microscope (Nikon Instruments, Inc.). Pictures were taken with an Infinity 1 camera (Luminera Corporation). Migration was assessed by cell migration into the cell-free field.
Hindlimb ischemia model of stem cell migration to damaged tissue
All rodent work was performed under an approved animal care protocol in the UC Davis Stem Cell Program immune-deficient mouse core. Under anesthesia, NOD/SCID MPSVII and NOD/SCID/β-2-microglobulin-deficient mice were subjected to unilateral hind limb ischemia surgeries as we have previously described.6,16 The mice were shaved and prepped, and then the right femoral artery and vein were exposed and dissected from the femoral nerve, and the proximal portion of the femoral artery was ligated with 6-0 braided silk sutures. The distal portion of the saphenous artery and the remaining collateral arteries were ligated and removed from the hind limb. The wound was closed with 6-0 braided silk sutures. H9-MSCs (2.5×105) were injected into the tail vein 24 h after surgery. Care was taken to reduce the time from lifting the cells from the plate, washing, and injection, since cells can clump with time and can form emboli. Cells were injected within 1 h of harvesting from the plate, as we have previously described.6,16,17
Tissue harvest and imaging
Mice were euthanized on day 2 post-transplantation in deep anesthesia and perfused with PBS. The femoral muscle tissue was divided into an upper segment comprising the tissue proximal to the uppermost ligation site and extending approximately 2 mm toward the midline and a lower segment comprising the tissue extending 2 mm distal from the uppermost ligation site. The tissue was rinsed in ice cold PBS, embedded, and snap-frozen in OCT, and 5μm cryostat-sectioned were stained for the presence of β-Glucuronidase or human β-2-microglobulin-positive human cells as previously described.18,19 Images were acquired on a Zeiss Axioskop 2 Microscope running Axiovision software (Carl Zeiss, Inc.)
Results
MSC generation and initial characterization by flow cytometry
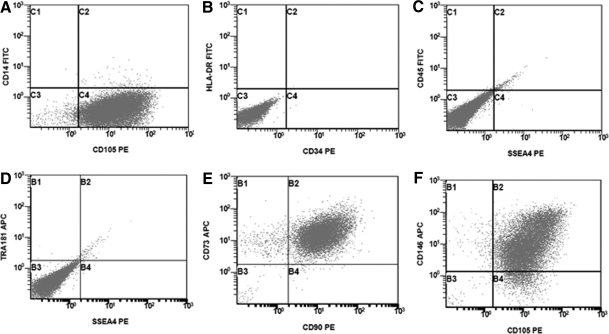
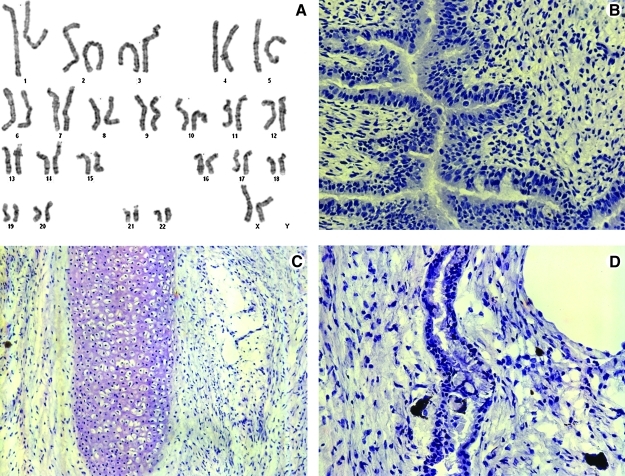
Six days after MSC differentiation was initiated by changing feeding schedules from daily to every 3 days, cells with a distinct fibroblast-like morphology began to appear around the edges of the colonies. At 12 days the cultures were nearly 50% confluent with such cells. Upon removal of cells that had the typical hESC colony appearance, the fibroblast-like cells began to grow more closely together until they were nearly confluent. After passaging to the MSC medium and regular tissue culture-treated plates, the cells began to expand as typical MSCs, although at an exaggerated rate for the initial passages. Morphology of the newly derived cells was consistent with known MSC morphology after three passages and they responded favorably to MSC culture conditions (Fig. 1). The presence of CD14, CD34, CD45, and HLA-DR as well as the ESC markers SSEA-4 and Tra-1-81 was not observed upon analysis by flow cytometry (Fig. 2A–D). The presence of the standard MSC markers CD73, CD90, CD105, and CD146 was confirmed (Fig, 2E, F). Cells were also karyotyped with a result of 46 XX (Fig. 3), which did not differ from the parental H9 line (data not shown).
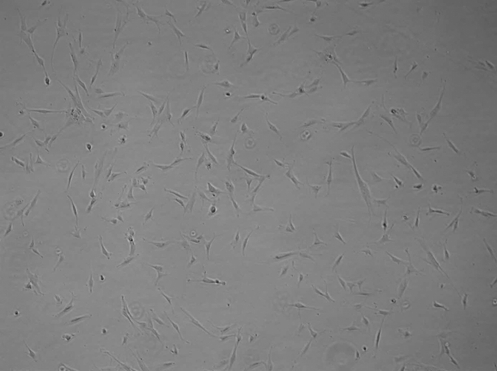
FIG. 1.
H9-MSC morphology. H9-MSCs were derived by decreasing the frequency of medium changes for H9 hESCs in feeder-free culture. As fibroblast-like cells began to fill the culture, colony structures were manually removed until the only cells remaining in culture were of fibroblast-like morphology. The residual hESCs were lost through sequential passaging. Pictured is a representative H9-MSC culture after four passages. hESCs, human embryonic stem cells; MSCs, mesenchymal stem cells.
FIG. 2.
Flow cytometry characterization of H9-MSCs. H9-MSCs were cultured in the complete medium, harvested, and analyzed for the commonly accepted cell surface marker expression profile for MSCs. The presence of CD14, CD34, CD45, and HLA-DR as well as the ESC markers SSEA-4 and Tra-1-81 was not observed (A–D). Additionally, the presence of CD73, CD90, CD105, and CD146 was confirmed (E, F).
FIG. 3.
H9-MSC karyotype and lack of teratoma formation. H9-MSCs were harvested and karyotyped. The karyotype matched that of the H9 parental cell line, without obvious translocations, deletions, or rearrangements (A). Teratoma were not formed from samples of H9-MSCs injected into NOD/SCID/IL2Rg−/− mice (n=4) but the parental line robustly generated teratoma with multilineage differentiation, as shown: (B) H9-derived endoderm; (C) H9-derived mesoderm; (D) H9-derived ectoderm.
Tumor formation assay
H9-MSC and the parent H9 hESC line were assayed for tumorigenic potential by the UC Davis Adult and Embryonic Stem Cell Core and the UC Davis Immune Deficient Mouse Core under an approved animal care protocol. The mice injected with H9 hESCs were euthanized at 4 months with prominent tumors at the site of injection. Necropsy confirmed the presence of a large subcutaneous tumor in each mouse. After sectioning and H&E staining, endoderm, mesoderm, and ectoderm were clearly visible (Fig. 3B–D). The mice injected with H9-MSCs displayed no visible signs of tumor at the site of injection at 4 months. As a precaution they were euthanized at 6 months to allow for additional time for tumor development, and a complete autopsy with surveillance of all tissues was conducted as we have described.8 Upon necropsy there were no tumors found (data not shown). These findings were confirmed by the UC Davis Mutant Mouse Pathology Lab.
Osteogenic, chondrogenic, and adipogenic lineage differentiation of H9-MSCs
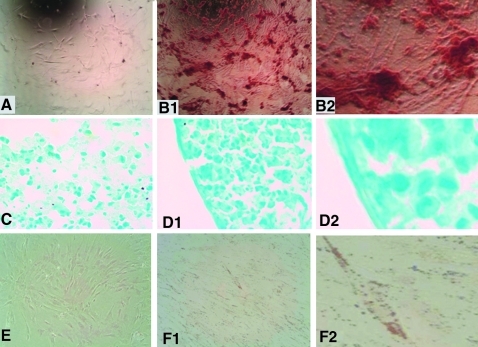
H9-MSCs were cultured in the osteogenic differentiation medium for 21 days. The cells were fixed in the plate and stained for mineralized plaques with Alizarin Red. The control culture (Fig. 4A) showed no mineral deposits, whereas the culture in the supplemented differentiation medium showed bright staining of mineralized plaques (Figs. 4B1 and 4B2).
FIG. 4.
Trilineage differentiation of H9-MSCs. H9-MSCs were cultured in the osteogenic differentiation medium for 21 days. The cells were fixed in the plate and stained for mineralized plaques with Alizarin Red. The control culture (A) showed no mineral deposits, whereas the culture in the supplemented differentiation medium showed bright staining of mineralized plaques (B1). (B2) provides a higher magnification view of (B1). Additionally, H9-MSCs were cultured as pellets in the chondrogenic medium for 21 days. The cell pellets were removed, embedded in Optimal Cutting Temperature (OCT) compound, and sectioned. The sections were stained with Alcian Blue for the presence of cartilaginous substrate. The control pellet (C) displayed little dark staining compared to the pellet grown in the chondrogenic medium (D1), which showed bright staining and a more compact morphology. (D2) provides a higher magnification view of (D1) and highlights the more compact association of the cells. H9-MSCs were also cultured in the adipogenic medium for 21 days. The cells were fixed in their plate and stained for the presence of fat vacuoles with oil red O. The control culture showed no positive staining for fat vacuoles (E). The culture maintained in the adipogenic differentiation medium displayed staining for the presence of lipids (F1). (F2) provides a higher magnification view of (F1).
Additionally, H9-MSCs were cultured as pellets in the chondrogenic medium for 21 days. The cell pellets were removed, embedded in OCT, and sectioned. The sections were stained with Alcian Blue for the presence of cartilaginous substrate. The control pellet (Fig. 4C) stained only weakly, as compared to the pellet grown in the chondrogenic medium (Figs. 4D1 and 4D2), which showed bright staining and a more compact morphology.
To further test their capacity for adipose lineage differentiation, H9-MSCs were cultured in the adipogenic medium for 21 days. The cells were fixed in their plate and stained for the presence of fat vacuoles with oil red O. The control culture (Fig. 4E) showed no positive staining for fat vacuoles. The culture maintained in the adipogenic differentiation medium (Figs. 4F1 and 4F2) displayed positive staining for the presence of fat vacuoles. These data confirm that H9-MSCs can undergo lineage differentiation along the same lineages as bone marrow and adipose-derived MSCs from adult sources, as we have previously described.16,17
Demonstration of hypoxia-induced migration of H9-MSCs in culture
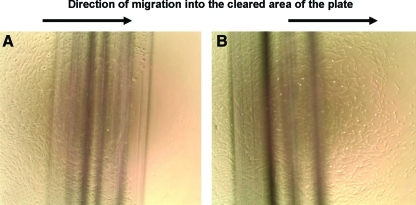
We have previously shown enhanced migration of MSCs from adult sources (BM and adipose tissue) when cultured in hypoxic conditions.6,16 In the next set of experiments we sought to determine whether the H9-MSCs would also display enhanced migration under hypoxia. H9-MSCs were plated in a 10 cm tissue culture dish and allowed to grow to 70%–80% confluence. A cell scraper was used to remove the cells in a 5 mm strip along a black line marked on the dish. The cells were then incubated in normoxic or hypoxic conditions (5.0% CO2, room air vs. 3.0% O2 achieved by nitrogen flush in hypoxic incubator) for 24 h. Cells cultured in normoxia did not migrate significantly, as we have previously described (Fig. 5A).6,16 After 24 h incubation in hypoxic conditions, numerous H9-MSCs had migrated over the black line into the area that had been cleared of cells (Fig. 5B). These data are similar to those that we have previously published for adult MSCs.6,16 Based upon these data, we sought to determine whether or not these ESC-derived cells could migrate to areas of tissue injury, as we have demonstrated for adult stem cells.6,16,18,20–22
FIG. 5.
A scratch test assay shows hypoxia-induced migration of H9-MSCs in culture. H9-MSCs were plated in a 10 cm tissue culture dish and allowed to grow to 70%–80% confluence. A cell scraper was used to remove the cells in a 5 mm strip along a black line marked on the dish. The cells were then incubated in normoxic (5.0% CO2/ambient O2) versus hypoxic conditions (5.0% CO2, 3.0% O2) for 24 h. After 24 h of incubation, there was little migration in normoxic conditions (A), while in hypoxic conditions, numerous H9-MSCs had migrated over the black line into the area that had been cleared of cells (B). Color images available online at www.liebertonline.com/tea
Homing of H9-MSCs to areas of ischemic injury
We sought to determine whether the human H9-MSCs could engraft in the ischemic muscle tissue of mice with surgically induced hindlimb ischemia. The femoral arteries of NOD/SCID MPSVII mice were ligated to cause the limb to become ischemic, as we have described.6,16,20
Via tail vein injection, 5.0×105 H9-MSCs were transplanted 24 h after induction of the ischemia, using our previously described methods for bone marrow and adipose-derived MSCs. A total of eight mice were transplanted with the H9-MSCs. These mice were compared to four negative controls for staining that had the hindlimb ischemia (HLI) surgery but only PBS injection without cells. Control animals that had the hindlimb ischemia surgery but did not have cells injected were used to rule out any potential false-positive antibody staining of apoptotic cells in damaged tissues. For both MSCs and hESCs injected systemically, clumping of cells can cause emboli. Since MSCs will rapidly begin entering anoikis-death by detachment, when integrins are dis-engaged from the culture plate, we worked very quickly from the time of cell harvest to the injection. We also ensured that no visible small cell clumps were injected. Prefiltration of cells before injection, using a syringe filter, can help with this technique. In prior works using human MSCs, we have performed intraventricular injection into the heart that evades this problem.6,16 In the current studies we sought to evaluate the homing/lodgment of hESC-derived MSCs at the site of injury in a short time-frame after intravenous injection, which is the most clinically relevant route of administration.
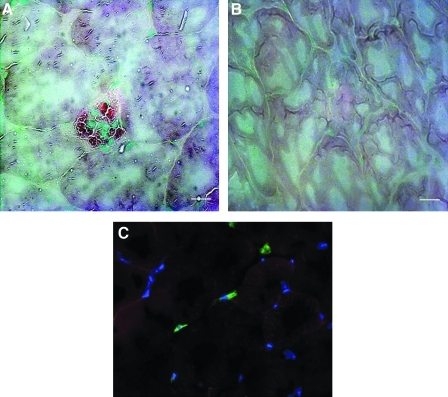
Experimental and control mice were killed 24 h postinjection and tissues were harvested, frozen in OCT cutting substrate, and sectioned on a cryostat. The tissue sections were stained for the presence of beta-glucuronidase, which is not present in the tissues of NOD/SCID MPSVII mice, which are null for the enzyme.17,22,23 In the ischemic muscle tissue, the H9-MSCs can be observed around a damaged vessel (Fig. 6A, red enzymatic stain). The contralateral, nonischemic muscle showed no signs of engraftment as verified by the absence of the red substrate stain (Fig. 6B). Controls without cell injection also showed no staining. Figure 6C shows a tissue section obtained from the same model of hindlimb ischemia induced in a NOD/SCID β-2-microglobulin knockout mouse. The human cells were observed in the ischemic muscle using antibody staining specific for human β-2-microglobulin (green). Again MSCs were located in close proximity to blood vessels in the ischemic tissue but were not observed in the contralateral limb (data not shown). These data demonstrate that hESC-derived MSCs can migrate in vivo and can home to or lodge in sites of ischemic injury, properties that are known to be characteristic of adult MSCs.18,20,24,25
FIG. 6.
H9-MSCs engraft in the ischemic muscle tissue of a mouse with a surgically induced ischemic hindlimb. The femoral artery of a NOD/SCID MPSVII mouse was ligated to cause the limb to become ischemic. About 5.0×105 H9-MSCs were injected via the tail vein 24 h after induction of the ischemia. The mouse was sacrificed 24 h postinjection and its tissues were harvested. The tissue was frozen and sectioned on a cryostat. The tissue sections were stained for the presence of beta-glucuronidase, which is not present in the mouse tissue. In the ischemic muscle tissue, the H9-MSCs can be observed around a damaged vessel (A, red enzymatic stain). The contralateral, nonischemic muscle showed no signs of engraftment (B). In a tissue section obtained from the same model of hindlimb ischemia induced in a NOD/SCID β-2-microglobulin knockout mouse (C), the human cells were observed in the ischemic muscle using antibody staining specific for human β-2-microglobulin (green). Scale bar is 50 μm.
Discussion
The generation and characterization of MSCs from hESCs has been previously demonstrated.12,26,27 This report expands upon the above studies by simplifying the differentiation process, providing teratoma and karyotype analysis for biosafety, and by providing an assessment of the cells ability to migrate under hypoxia in vitro and to areas of tissue damage in vivo. As a possible tool for regenerative therapies, the ability of MSCs to migrate to damaged tissue is a highly important factor to consider. This migration makes it possible to deliver therapeutic effects, either innate or engineered, to areas needing the most direct benefit in some injuries. A previous study28 investigated the transplantation of hESC-derived MSCs in a model of hindlimb ischemia. However, their model utilized animals with immune suppression induced by daily injections of cyclosporin A, a significant disadvantage to utilizing animals that are genetically immune deficient. Additionally, the investigators injected the MSCs directly into the site of injury, foregoing the ability to study their migratory properties in an in vivo model of hindlimb ischemia. Thus, our study establishes for the first time that MSCs generated from hESCs retain the migratory properties of MSCs derived from adult tissues, and that they recapitulate migratory properties that we have previously reported for adult stem cells.6,16,20
It has been shown that the culture of adult MSCs in a hypoxic environment in vitro enhances their migratory properties in vivo.6,16,29,30 In the current study we demonstrate a similar in vitro response to hypoxic culture conditions (3% O2) by use of a simple scratch test assay of cell migration. Cells grown in such conditions migrated into a cell-free area created by a cell scraper when cultured in hypoxic conditions versus cells in normoxic conditions (∼21% O2), as shown in Figure 5. The demonstration of this response in vitro reveals another important characteristic of hESC-derived MSCs in that, at an in vitro level, the cells have matured enough to demonstrate characteristic migratory responsiveness to hypoxic conditions. This response has been shown to play an important role in the behavior of these cells in the in vivo models of injury. This finding confirms that hESC-derived MSCs display a property that will be crucial when considering their potential in the treatment of ischemic injuries.
The characterization of the H9-MSCs was based on previously described methods14 and demonstrated that the cells derived from the differentiation protocol we used met the criteria by which adult MSCs are commonly determined. Their characterization also included an analysis for the expression of specific markers of hESC pluripotency. SSEA-4 and Tra-1-81 are expressed on hESCs and are known to be indicators of pluripotency.11 The hESC-derived MSCs showed no significant expression of either marker (Fig. 2). Although it has been previously reported that a subpopulation of bone marrow-derived MSCs also express SSEA-4,31 our study showed a marked lack of SSEA-4 expression in the majority of cells in the expanded bone marrow-derived MSC populations tested, and the H9-MSCs also lacked it. The hESC-derived MSCs were also shown to differentiate into each of the three lineages: adipocyte, chondrocyte, and osteoblast. The retention of these properties of adult tissue-derived MSCs demonstrates that the cells generated through the differentiation protocol that we have described here are consistent with MSCs derived from adult tissues by the criteria commonly accepted for their identification. In addition, the H9-MSCs had the same karyotype as the parental H9 line and lacked in vivo teratoma formation. We have previously reported the lack of tumorigenicity from genetically engineered human bone marrow-derived MSCs, through extensive testing, and in future studies will perform the same high levels of preclinical testing for the H9-MSCs.8 Our current studies were done to establish methods and are not an exhaustive biosafety study, as should be done before considering cellular therapies. Additional biosafety measures that will need to be explored in the future, among others, are maintenance of normal karyotype through multiple passages, larger numbers of cells studied in teratoma assay, and sensitive quantitative polymerase chain reaction to rule out retention of any pluripotent stem cells. These and other variables will be important data to be obtained before envisioning cellular therapy using hESC-derived MSCs.
In the studies presented in the current report we demonstrate that not only can cells be generated from hESCs that display the phenotype of MSCs in terms of the classically established criteria for their identification, but also that these cells retain the migratory properties and basic responsiveness to hypoxia that have been demonstrated in MSCs derived from adult tissues. These factors, in previous works, have been shown to be important to the responsiveness of the cells as potential therapeutic agents. Our work forms a useful foundation upon which can be based a more thorough investigation of the clinically relevant properties of MSCs derived from hESCs. The results of these studies suggest that hESC-derived MSCs can display the same therapeutic properties as have been observed in studies involving adult tissue-derived MSCs and repair of ischemic damage. Whether the same properties can be achieved from human-induced pluripotent stem cells is currently under investigation.
This system could be useful for production of large numbers of MSCs from ESCs or induced pluripotent stem cells with safe harbor integration sites to deliver factors or to correct a genetic deficiency. Using hESCs, which have almost unlimited expansion capabilities, a vector's integration site can be characterized and clones with benign integration sites can be greatly expanded and fully characterized. Homologous recombination is also now feasible for hESCs or induced pluripotent stem cells, due to increased efficiencies, and clones with vectors targeted for gene correction can be expanded. Therefore, to reduce risk from random insertion of transgenes, further characterization, biosafety profiling, and examination of the in vivo characteristics of hESC-derived MSCs are warranted, even though MSCs are a cell type that is easily expanded from somatic tissues.
Ischemic injuries comprise a significant subset of the injuries that could possibly be treated using MSCs. Cardiac, bowel, brain, skin, kidney, and critical limb ischemia could all benefit from the potential of MSCs to home to areas of tissue damage and secrete paracrine factors to aid in angiogenesis and cellular repair. The generation of these cells, especially in a mouse feeder-free culture system, is especially important as more work is done using in vivo animal disease models to investigate the potential of MSCs in the treatment of various ischemic disorders. The ability to generate these cells from hESCs and in the future from iPSC would provide for a source of cells to make such therapies readily available in nearly unlimited numbers.
Conclusion
MSCs were derived from the H9 hESC line and were characterized based on the commonly accepted criteria for defining MSCs. They displayed the cell surface phenotype characteristic of MSCs derived from adult tissues as determined by flow cytometry. Additionally, these cells were found to have a karyotype matching the known karyotype for H9 hESCs. The in vivo assays done to determine potential tumorigenesis from these expanded derivatives displayed no generation of tumors or teratoma in immune-deficient mice, although more extensive testing must be done before considering cellular therapies. The cells' potential utility for regenerative medicine uses was found to be intact due to their ability to form bone, cartilage, and fat. In addition, they were found to be responsive to hypoxia through increased migration and were able to home to areas of ischemic damage. Further investigation of these cells will more fully assess their regenerative potential and in vivo biosafety.
Author Contributions
William Gruenloh: Conception and design, collection and/or assembly of data, data analysis and interpretation, article writing, and final approval of article.
Amal Kambal: Conception and design, collection and/or assembly of data, data analysis and interpretation, article writing, and final approval of article.
Claus Sondergaard: Collection and/or assembly of data, data analysis and interpretation, and final approval of article.
Jeannine McGee: Collection and/or assembly of data, and final approval of article.
Catherine Nacey: Collection and/or assembly of data, and final approval of article.
Stefanos Kalomoiris: Collection and/or assembly of data, and final approval of article.
Karen Pepper: Collection of data and final approval of article.
Scott Olson: Collection and/or assembly of data, data analysis and interpretation, and final approval of article.
Fernando Fierro: Conception and design, collection and/or assembly of data, data analysis and interpretation, article writing, and final approval of article.
Jan A. Nolta: Conception and design, Collection and/or assembly of data, data analysis and interpretation, article writing, and final approval of article.
Acknowledgments
The authors thank Dr. Alexander Borowsky in the UC Davis Center for Comparative Medicine for reading the teratoma slides and WiCell for providing the H9 cell line, purchased under MTA (J.N.). This work was supported by the UC Davis Stem Cell program start-up funding from the Deans' Office (J.N.) and the Department of Surgery (C.S.S.), UC Davis Health Sciences Campus, and the National Institutes of Health (NIH), National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK #2R01DK61848 and 2R01DK53041 [J.N.]), and National Heart, Lung and Blood Institute (NHLBI #RO1HL073256 (J.N.). The California Institute for regenerative Medicine (CIRM) provided funding for batches of human bone marrow-derived MSCs that were used in comparisons with H9-MSCs when in excess from directly funded studies (CIRM TR1-01257 [J.N.]). Funding bodies supported salaries, equipment, mice, and supplies needed for the collection and analysis of the data.
Disclosure Statement
No competing financial interests exist.
References
- 1.Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 2.Laflamme M.A. Murry C.E. Regenerating the heart. Nat Biotechnol. 2005;23:845. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 3.Chen J. Li Y. Katakowski M. Chen X. Wang L. Lu D. Lu M. Gautam S.C. Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 4.Li Y. Chen J. Zhang C.L. Wang L. Lu D. Katakowski M. Gao Q. Shen L.H. Zhang J. Lu M. Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 5.Murphy J.M. Fink D.J. Hunziker E.B. Barry F.P. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 6.Rosova I. Dao M. Capoccia B. Link D. Nolta J.A. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salem H.K. Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem cells (Dayton, Ohio) 2010;28:585. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer G. Dao M.A. Case S.S. Meyerrose T. Wirthlin L. Zhou P. Wang X. Herrbrich P. Arevalo J. Csik S. Skelton D.C. Walker J. Pepper K. Kohn D.B. Nolta J.A. In vivo biosafety model to assess the risk of adverse events from retroviral and lentiviral vectors. Mol Ther. 2008;16:1308. doi: 10.1038/mt.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dao M.A. Pepper K.A. Nolta J.A. Long-term cytokine production from engineered primary human stromal cells influences human hematopoiesis in an in vivo xenograft model. Stem Cells. 1997;15:443. doi: 10.1002/stem.150443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nolta J. Genetic Engineering of Mesenchymal Stem Cells. New York: Springer-Verlag; 2009. [Google Scholar]
- 11.Thomson J.A. Itskovitz-Eldor J. Shapiro S.S. Waknitz M.A. Swiergiel J.J. Marshall V.S. Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 12.Trivedi P. Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp Hematol. 2008;36:350. doi: 10.1016/j.exphem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez R. Wesselschmidt R.L. Schwartz P.H. Loring J.F. Human Embryonic Stem Cell Culture. In: Loring J.F., editor; Wesselschmidt R.L., editor; Schwartz P.H., editor. Human Stem Cell Manual: A Laboratory Guide. New York: Elsevier, Inc.; 2007. pp. 3–17. [Google Scholar]
- 14.Dominici M. Le Blanc K. Mueller I. Slaper-Cortenbach I. Marini F. Krause D. Deans R. Keating A. Prockop D. Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa R. Mizuno H. Watanabe A. Migita M. Hyakusoku H. Shimada T. Adipogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice-including relationship of sex differences. Biochem Biophys Res Commun. 2004;319:511. doi: 10.1016/j.bbrc.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Rosová I. Link D. Nolta J. Small interfering RNA-mediated decreases in c-Met levels affect the differentiation potential of human mesenchymal stem cells and reduce their capacity for tissue repair. Tissue Eng. 2010;16:2627. doi: 10.1089/ten.tea.2009.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyerrose T. De Ugarte D. Hofling A. Herrbrich P.E. Cordonnier T.D. Shultz L.D. Eagon J.C. Wirthlin L. Sands M.S. Hedrick M.A. Nolta J.A. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells. 2007;25:220. doi: 10.1634/stemcells.2006-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sondergaard C.S. Hess D.A. Maxwell D.J. Weinheimer C. Rosova I. Creer M.H. Piwnica-Worms D. Kovacs A. Pedersen L. Nolta J.A. Human cord blood progenitors with high aldehyde dehydrogenase activity improve vascular density in a model of acute myocardial infarction. J Transl Med. 2010;8:24. doi: 10.1186/1479-5876-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou P. Hohm S. Olusanya Y. Hess D.A. Nolta J. Human progenitor cells with high aldehyde dehydrogenase activity efficiently engraft into damaged liver in a novel model. Hepatology. 2009;49:1992. doi: 10.1002/hep.22862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capoccia B.J. Robson D.L. Levac K.D. Maxwell D.J. Hohm S.A. Neelamkavil M.J. Bell G.I. Xenocostas A. Link D.C. Piwnica-Worms D. Nolta J.A. Hess D.A. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood. 2009;113:5340. doi: 10.1182/blood-2008-04-154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maxwell D.J. Bonde J. Hess D.A. Hohm S.A. Lahey R. Zhou P. Creer M.H. Piwnica-Worms D. Nolta J.A. Fluorophore-conjugated iron oxide nanoparticle labeling and analysis of engrafting human hematopoietic stem cells. Stem Cells. 2008;26:517. doi: 10.1634/stemcells.2007-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyerrose T.E. Roberts M. Ohlemiller K.K. Vogler C.A. Wirthlin L. Nolta J.A. Sands M.S. Lentiviral-transduced human mesenchymal stem cells persistently express therapeutic levels of enzyme in a xenotransplantation model of human disease. Stem Cells. 2008;26:1713. doi: 10.1634/stemcells.2008-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofling A.A. Vogler C. Creer M.H. Sands M.S. Engraftment of human CD34+ cells leads to widespread distribution of donor-derived cells and correction of tissue pathology in a novel murine xenotransplantation model of lysosomal storage disease. Blood. 2003;101:2054. doi: 10.1182/blood-2002-08-2597. [DOI] [PubMed] [Google Scholar]
- 24.Bexell D. Scheding S. Bengzon J. Toward brain tumor gene therapy using multipotent mesenchymal stromal cell vectors. Mol Ther. 2010;18:1067. doi: 10.1038/mt.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caplan A.I. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barberi T. Willis L.M. Socci N.D. Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:e161. doi: 10.1371/journal.pmed.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lian Q. Lye E. Suan Yeo K. Khia Way Tan E. Salto-Tellez M. Liu T.M. Palanisamy N. El Oakley R.M. Lee E.H. Lim B. Lim S.K. Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cells. 2007;25:425. doi: 10.1634/stemcells.2006-0420. [DOI] [PubMed] [Google Scholar]
- 28.Laurila J. Laatikainen L. Castellone M. Trivedi P. Heikkila J. Hinkkanen A. Hematti P. Laukkanen M. Human embryonic stem cell-derived mesenchymal stromal cell transplantation in a rat hind limb injury model. Cytotherapy 1. 2009;11:726. doi: 10.3109/14653240903067299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinnaird T. Stabile E. Burnett M.S. Shou M. Lee C.W. Barr S. Fuchs S. Epstein S.E. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 30.Rochefort G.Y. Delorme B. Lopez A. Herault O. Bonnet P. Charbord P. Eder V. Domenech J. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24:2202. doi: 10.1634/stemcells.2006-0164. [DOI] [PubMed] [Google Scholar]
- 31.Gang E.J. Bosnakovski D. Figueiredo C.A. Visser J.W. Perlingeiro R.C. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]