Abstract
Purpose of review
Acute anemic stress induces a physiological response that includes the rapid development of new erythrocytes. This process is referred to as stress erythropoiesis, which is distinct from steady state erythropoiesis. Much of what we know about stress erythropoiesis comes from the analysis of murine models. In this review, we will discuss our current understanding of the mechanisms that regulate stress erythropoiesis in mice and discuss outstanding questions in the field.
Recent findings
Stress erythropoiesis occurs in the murine spleen, fetal liver and adult liver. The signals that regulate this process are Hedgehog, bone morphogenetic protein 4 (BMP4), stem cell factor and hypoxia. Recent findings show that stress erythropoiesis utilizes a population of erythroid-restricted self-renewing stress progenitors. Although the BMP4-dependent stress erythropoiesis pathway was first characterized during the recovery from acute anemia, analysis of a mouse model of chronic anemia demonstrated that activation of the BMP4-dependent stress erythropoiesis pathway provides compensatory erythropoiesis in response to chronic anemia as well.
Summary
The BMP4-dependent stress erythropoiesis pathway plays a key role in the recovery from acute anemia and new data show that this pathway compensates for ineffective steady state erythropoiesis in a murine model of chronic anemia. The identification of a self-renewing population of stress erythroid progenitors in mice suggests that therapeutic manipulation of this pathway may be useful for the treatment of human anemia. However, the development of new therapies will await the characterization of an analogous pathway in humans.
Keywords: anemia, bone morphogenetic protein 4, erythropoiesis, Hedgehog, hypoxia
Introduction
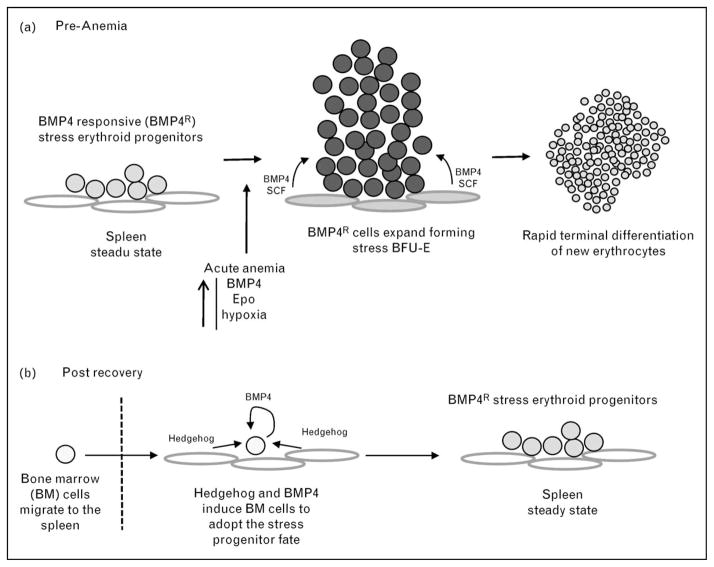
Steady state erythropoiesis is primarily homeostatic, producing new erythrocytes at a constant rate. For the most part, it is restricted to the bone marrow. In contrast, tissue hypoxia caused by anemia or during fetal development results in the activation of a physiological stress response designed to increase oxygen delivery to tissues. Increased erythropoiesis is a key component of this response [1]. At these times, stress erythropoiesis predominates, which is capable of rapidly generating large numbers of erythrocytes [2]. In the mouse, stress erythropoiesis occurs in the in adult spleen and liver [3–5] and during fetal development in the fetal liver [6]. Although early work in the field identified the murine spleen as the primary site of erythropoiesis during recovery from experimentally induced anemia, it was proposed that stress erythropoiesis represented an enhanced version of steady state erythropoiesis [7,8]. These studies suggested that as anemia progresses, tissue hypoxia increases and leads to the induction of erythropoietin (Epo) expression in the kidney [9]. The increase in serum Epo concentration drives the expansion and differentiation of bone marrow erythroid progenitors [10,11]. Some of these progenitors would then migrate to the spleen and finish their differentiation. This model is based on two ideas. The first idea is that Epo is the primary signal that drives stress erythropoiesis and the second is that the erythroid progenitors that respond to anemic stress are the same as the bone marrow steady state erythroid progenitors. Epo does play a key role in this process, primarily promoting the terminal differentiation of stress erythroid progenitors [12,13]. This role in stress erythropoiesis was recently reviewed in this series [1]. In this review, we will focus on earlier events in the response and propose a more comprehensive model for stress erythropoiesis, which is supported by recent work showing that stress erythropoiesis relies on a population of stress erythroid progenitor cells that are distinct from bone marrow steady state erythroid progenitors [2]. The development, expansion and differentiation of these progenitors is regulated in part by signals not previously associated with adult erythropoiesis: bone morphogenetic protein 4 (BMP4) and Hedgehog, which act in concert with signals previously associated with stress erythropoiesis – Epo, stem cell factor (SCF) and hypoxia.
Bone morphogenetic protein 4 regulates the expansion of stress erythroid progenitors resident in the spleen during the recovery from acute anemia
Our work on stress erythropoiesis started with the analysis of the murine flexed-tail (f) mutation. f/f mutant mice exhibit a fetal–neonatal anemia that resolves about 2 weeks after birth [14–19]. Despite having near normal steady state erythropoiesis, adult f/f mice are slow to recover from phenylhydrazine (PHZ)-induced acute hemolytic anemia [20]. The delayed recovery observed in f/f mice is caused by a defect in the expansion of erythroid progenitors (BFU-E) in the spleen [2]. Bone marrow erythropoiesis is unaffected. Careful analysis of erythroid progenitors showed that spleen BFU-E differed from bone marrow BFU-E. They form larger colonies, much faster (5 vs. 7 days) than their bone marrow counterparts. Furthermore, unlike bone marrow BFU-E, which require both Epo and a burst-promoting signal, spleen BFU-E formed colonies in media containing only Epo. This observation is similar to human fetal BFU-E that can also form colonies with Epo alone [21], which is consistent with the idea that spleen erythroid progenitors are distinct from bone marrow erythroid progenitors and may be more similar to fetal erythroid progenitors. Cloning of the f mutant locus showed that it encoded Smad5 [2,22], a receptor activated Smad that functions downstream of the receptors for BMPs 2, 4 and 7 [23]. Subsequent analysis showed that BMP4 induced the expansion of BFU-E in the spleen during the recovery from acute anemia. Together, these data suggested a new model for stress erythropoiesis in which BMP4 drives the expansion of a population of specialized stress erythroid progenitors that are resident in the spleen, which we termed ‘stress BFU-E’. These progenitors exhibit ideal properties of a stress erythroid progenitor in that they rapidly produce large numbers of mature erythrocytes at times of acute stress.
In addition to BMP4, two other signals drive the expansion of stress erythroid progenitors in the spleen. Early work on two murine mutants, dominant white spotting (W) and steel (Sl), which encode the Kit receptor [24,25] and its ligand SCF [26–28], respectively, demonstrated a role for the Kit/SCF signaling pathway in stress erythropoiesis. Although this early work suggested that the defect in stress erythropoiesis in W and Sl mutant mice was a corollary of the macrocytic anemia present in these mice [29], analysis of W mutant mice showed a defect in the expansion of stress BFU-E in the spleen during the recovery from anemia [30]. Kit receptor signaling activates numerous downstream pathways and plays a wide-ranging role in regulating hematopoiesis. Recent work dissecting the response downstream of Kit showed that specific signaling pathways regulated by the phosphorylation of tyrosine 567 of Kit receptor are required for the recovery from acute anemia [31•]. Although BMP4-dependent and SCF-dependent signals are required for the expansion of stress BFU-E, they are not sufficient to recapitulate in vitro the expansion of stress BFU-E observed in vivo during the recovery from acute anemia. The full response requires hypoxic (1% O2) culture conditions. Hypoxia potentiates the response of stress erythroid progenitors to BMP4 and alters the response to SCF. Under normal atmospheric O2 (20%) conditions SCF modestly increases the size of the bursts, but in the presence of low O2 (1%) SCF significantly increases the number of stress BFU-E and greatly increases the burst size [30]. The mechanism by which hypoxia alters the response of stress BFU-E to SCF and BMP4 is not understood. Further insights into this mechanism could aid in the development of drugs that could potentially improve stress erythropoiesis in clinical situations. One key aspect of the regulation of BMP4-dependent stress erythropoiesis pathway is this requirement for hypoxia. It functions at two levels to limit stress erythropoiesis to times of anemic stress. Not only is hypoxia required for the maximal response of stress progenitors to BMP4 and SCF [30], hypoxia also regulates the induction of BMP4 expression in the liver and spleen [32]. Hypoxia-inducible factor-2α regulates BMP4 expression by binding to two response elements in the BMP4 gene.
Hedgehog signaling regulates the maintenance of the stress progenitor pool in the spleen
Acute anemia leads to the complete mobilization of stress erythroid progenitors in the spleen, so there must be a mechanism by which new stress erythroid progenitors are replenished following recovery [33•]. Although bone marrow erythroid progenitors do not respond to BMP4, SCF and hypoxia dependent signals in a manner similar to spleen stress progenitors, transplant experiments showed that bone marrow progenitor cells that migrate to the spleen adopt the stress erythroid progenitor fate. This observation suggested that the spleen microenvironment contained a signal that induced cells to become stress erythroid progenitors. This situation is similar to chondrocyte development during murine embryogenesis. In this case, BMP4 induces somitic mesoderm cells to differentiate into chondrocytes, but prior to differentiation Hedgehog signaling is required to make these cells competent to respond to BMP4 [34]. Similarly, the development of new stress erythroid progenitors in the spleen requires Hedgehog signaling to induce bone marrow progenitor cells to adopt the stress progenitor fate, which allows them to respond to BMP4, SCF and hypoxia during the recovery from acute anemia. When Hedgehog signaling is blocked by the deletion of the Hedgehog receptor smoothened (Smo) in adult mice, stress erythroid progenitors fail to develop in the spleen following recovery from acute anemia. Furthermore, culturing bone marrow cells with Hedgehog induces the development of stress BFU-E. These experiments established a role for Hedgehog signaling in stress erythropoiesis [33•]. The focus of current work is to identify the targets of Hedgehog signaling that specify the stress erythroid fate. During chondrocyte development Sonic Hedgehog induces the expression of two transcription factors Nkx3.2 and Sox9, which promote cartilage development in a BMP4-dependent manner [35,36]. It is not clear if these factors or other members of the Nkx or Sox families function in stress erythropoiesis.
Bone morphogenetic protein 4-dependent stress erythropoiesis utilizes a self-renewing population of erythroid-restricted stress progenitors
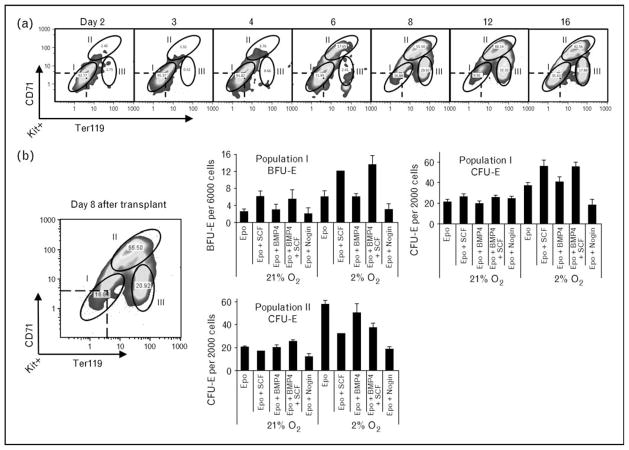
On the basis of these earlier studies, a model for stress erythropoiesis emerged in which stress erythroid progenitors are resident in the spleen. Anemic/hypoxic stress induces the expression of BMP4, which acts in concert with SCF, hypoxia and Epo to promote the rapid expansion and differentiation of new erythrocytes. In the postrecovery period, bone marrow cells migrate into the spleen and in response to Hedgehog adopt the stress erythroid fate, which replenishes the pool of stress erythroid progenitors (Fig. 1). Murine adult bone marrow steady state erythroid progenitors are characterized by the expression of CD71 and Ter119 cells [11,37]. In contrast, spleen stress progenitors, expanded in vitro, were Kit+CD71+Ter119+, which is consistent with the idea that stress erythroid progenitors are distinct from steady state erythroid progenitors [30]. Further analysis of these progenitors required a new experimental system, bone marrow transplant (BMT), which takes advantage of the observation that in order to avoid lethal anemia, new erythrocytes must be made prior to engraftment of donor stem cells. These erythrocytes are produced by short-term radioprotective cells, which also produce platelets and neutrophils in the immediate posttransplant period [38,39]. BMT assays done with donor cells that were defective in BMP4 signaling exhibited a defect specifically in erythroid recovery with no defects in the generation of platelets and neutrophils. Therefore, erythroid short-term radioprotection is BMP4-dependent stress erythropoiesis [40••]. One of the advantages of the BMT assay is that donor cells can be followed by using different alleles of CD45 cells in congenic donors and recipients. Analysis of donor-derived cells in the spleen showed that the Kit+CD71+Ter119+ population of stress erythroid progenitors could be resolved into three distinct populations (Fig. 2). Fluorescence-activated cell sorting analysis showed that the three populations referred to as populations I, II and III had distinct erythroid potential. Population I cells, which are present in the spleens of untreated mice, contained all the stress BFU-E. Population II cells gave rise to CFU-E. Whereas population III cells did not form colonies and were approximately 50% benzidine positive, which is consistent with these cells being the most mature. Analysis of the three populations during recovery suggests a temporal relationship in which population I cells differentiate into population II cells, which in turn generate population III cells. Further analysis of population I yielded the surprising result that a large percentage of these cells were also Sca1 positive. The presence of hematopoietic stem cell (HSC) markers [41–45], Kit and Sca1, on the surface of population I cells suggested that they might be multi-potential and have the potential to self-renew. Surprisingly, transplanting purified population I cells into lethally irradiated secondary recipients rescued erythropoiesis, but without contribution to other lineages. Furthermore, donor-derived cells isolated from the spleens of secondary transplants were able to rescue erythropoiesis in tertiary transplants with similar efficiency to that observed in the secondary transplants. Analysis of secondary transplants showed that donor population I cells generated an initial wave of erythropoiesis that maintained the survival of the recipient mice. Recipient HSCs that had survived the radiation treatment then repopulated the mice and replaced the donor-derived erythrocytes. Surprisingly, treatment of these mice with PHZ to induce anemia led to the generation of donor-derived erythrocytes, which suggests that the donor population I cells have established a durable stress erythroid compartment in the recipient mice that is capable of responding to subsequent anemic challenges. Taken together, these data demonstrate that erythroid short-term radioprotection utilizes self-renewing erythroid-restricted stress progenitor cells. These results also raise several interesting questions. What signals regulate the self-renewal of population I cells and what signals regulate the commitment to differentiation? Furthermore, what is the mechanism that restricts these progenitors to the erythroid lineage?
Figure 1. Model of the bone morphogenetic protein 4 stress erythropoiesis pathway.
(a) Activation by acute anemia. (b) Postrecovery replenishment of stress progenitors. BFU-E, burst forming unit-erythroid; BM, bone marrow; BMP4, bone morphogenetic protein 4; Epo, erythropoietin; SCF, stem cell factor.
Figure 2. Three distinct populations of stress erythroid progenitors develop in the spleen during the recovery from bone marrow transplant.
(a) Flow cytometry analysis of spleen cells on the indicated days after transplant. The cells are gated on Kit+ cells and the expression of CD71 and Ter119 is analyzed. (b) Populations I, II and III were sorted on day 8 after transplant and plated for burst forming unit-erythroid (BFU-E) and colony forming unit-erythroid (CFU-E). Population II cells did not give rise to BFU-E and Population III cells failed to form colonies of any type. BMP4, bone morphogenetic protein 4; Epo, erythropoietin; SCF, stem cell factor. Adapted from [40••].
Activation of the bone morphogenetic protein 4-dependent stress erythropoiesis pathway alleviates the anemia in a mouse model of anemia induced by chronic inflammation
Both acute hemolytic anemia caused by PHZ injection and anemia following BMT are excellent experimental models of acute anemia. In clinical settings, chronic anemia and anemia associated with chronic inflammation, also known as the anemia of chronic disease (ACD), are much greater problems [46,47]. Because acute anemia leads to the complete mobilization of stress progenitors in the spleen, it was unclear how this pathway would function in chronic anemia. However, recent work by Millot et al. [48•] addressed this question and identified a role for stress erythropoiesis in the response to chronic anemia. They used a murine model for chronic inflammation: the zymosan-induced general inflammation (ZIGI) mouse, which causes severe generalized inflammation that leads to long lasting anemia [49]. Bone marrow erythropoiesis is suppressed in the ZIGI mice, but treatment with Epo partially alleviates the anemia. However, Epo does not increase bone marrow erythropoiesis, but rather induces stress erythropoiesis in the spleen. Bone marrow BFU-Es are inhibited by interferon-γ (IFNγ), which is highly expressed in inflammatory disease. In contrast, spleen stress BFU-Es were not affected by IFNγ treatment. Epo induces the expression of BMP4 by spleen macrophages. Whether this induction is direct or indirect is not clear. The increased expression of BMP4 leads to an expansion of stress BFU-E and increased erythropoiesis. Of interest, these experiments show that unlike acute anemia in which BMP4-dependent stress erythropoiesis is induced by acute hypoxia, in this model of chronic anemia the pathway is inhibited despite the severe anemia. Epo injection is required to activate the pathway. The differential response of the BMP4-dependent stress erythropoiesis pathway to acute vs. chronic anemia is also highlighted by analysis of mice carrying a conditional allele of the Epo gene [50•]. Deletion of the Epo gene in adult mice leads to severe Epo-deficient anemia. Despite the anemia, EpoΔ/Δ mice recovered from PHZ-induced acute anemia with the same kinetics as controls. On the basis of these observations, further studies will be needed to understand the mechanisms that regulate stress erythropoiesis in chronic anemia.
Is there an equivalent bone morphogenetic protein 4-dependent pathway in human stress erythropoiesis?
Experiments using the ZIGI mouse model suggest that manipulation of the BMP4-dependent stress erythropoiesis pathway could be used as a potential treatment for ACD. However, any discussion about potential therapies is purely speculative because an analogous pathway has not been identified in humans. The analysis of stress erythropoiesis in humans is difficult because direct experiments to examine the mechanisms that regulate stress erythropoiesis are not possible and analysis of erythropoiesis in patients with severe anemia can be complicated by indirect effects of disease pathology. Despite these difficulties, some general themes have been identified in human stress erythropoiesis. Human stress erythropoiesis often exhibits properties of fetal erythropoiesis. Fetal erythrocyte characteristics and antigens as well as the expression of fetal hemoglobin (HbF) are observed during the recovery from erythropoietic stress [51,52]. These characteristics are observed following BMT [53–55], in acute anemia syndromes like transient erythro-blastopenia of childhood [56,57] and in some patients with thalassemia and sickle cell anemia [58–62]. Similar effects are observed in nonhuman primates treated with PHZ [63–65]. A few studies have reported observations that support a possible connection between the murine BMP4-dependent stress erythropoiesis and the stress erythropoiesis in humans. Analysis of peripheral blood and bone marrow progenitors from sickle cell anemia patients identified a population of progenitor cells that express CD34 and glycophorin A (GPA) on their surface. These cells also express CD71 and KIT. These progenitor cells give rise to a higher proportion of HbF+ F cells than normal bone marrow progenitors. The production of F cells is also increased when cells are grown at 5% O2 compared with ambient 20% O2 [66,67]. The murine bone marrow population that gives rise to stress BFU-E in the spleen following BMT is the short-term reconstituting HSCs, which are CD34+Kit+Sca1+Lin− cells [45]. It is interesting to speculate that the CD34+GPA+CD71+Kit+ cells may be the human equivalent of the murine stress progenitors we observed during the recovery from BMT; however, further work will be needed to establish any connection between these ‘stress’ progenitors observed in patients and the BMP4-dependent stress erythropoiesis pathway.
Conclusion
The identification of the BMP4-dependent stress erythropoiesis pathway and the characterization of the stress erythroid progenitor cell populations and the signals that regulate this process are important step in understanding how anemic stress alters erythropoiesis. In the murine system, this model will serve as a conceptual framework for the reanalysis of previous work and as a guide for future studies. Although no data exists to demonstrate that human stress erythropoiesis utilizes similar signals, the work done in mice has established an excellent starting point for future experiments using human progenitors. The goal of this work is to increase our understanding of human stress erythropoiesis to such an extent that targets for therapeutic intervention could be identified and tested in the clinic.
Acknowledgments
Work in our laboratory has been funded by the NIH/NIDDK (RO1DK080040-01) (R.P.F.) and the National Blood Foundation (R.F.P.). We would like to thank the members of the Paulson laboratory for the excellent discussions concerning stress erythropoiesis. In the past 18 months, there have been many excellent studies that have included analysis of the recovery from acute anemia. Unfortunately due to space considerations, we were unable to cite all of our colleagues’ work. We apologize for this omission.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 197).
- 1.Socolovsky M. Molecular insights into stress erythropoiesis. Curr Opin Hematol. 2007;14:215–224. doi: 10.1097/MOH.0b013e3280de2bf1. [DOI] [PubMed] [Google Scholar]
- 2.Lenox L, Perry J, Paulson R. BMP4 and Madh5 regulate the erythroid response to acute anemia. Blood. 2005;105:2741–2748. doi: 10.1182/blood-2004-02-0703. [DOI] [PubMed] [Google Scholar]
- 3.Lenox LE, Shi L, Hegde S, et al. Extramedullary erythropoiesis in the adult liver requires BMP-4/Smad5-dependent signaling. Exp Hematol. 2009;37:549–558. doi: 10.1016/j.exphem.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ploemacher RE, van Soest PL. Morphological investigation on phenylhydrazine-induced erythropoiesis in the adult mouse liver. Cell Tissue Res. 1977;178:435–461. doi: 10.1007/BF00219567. [DOI] [PubMed] [Google Scholar]
- 5.Ploemacher RE, van Soest PL, Vos O. Kinetics of erythropoiesis in the liver induced in adult mice by phenylhydrazine. Scand J Haematol. 1977;19:424–434. doi: 10.1111/j.1600-0609.1977.tb01497.x. [DOI] [PubMed] [Google Scholar]
- 6.Porayette P, Paulson RF. BMP4/Smad5 dependent stress erythropoiesis is required for the expansion of erythroid progenitors during fetal development. Dev Biol. 2008;317:24–35. doi: 10.1016/j.ydbio.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hara H, Ogawa M. Erythropoietic precursors in mice with phenylhydrazine-induced anemia. Am J Hematol. 1976;1:453–458. doi: 10.1002/ajh.2830010410. [DOI] [PubMed] [Google Scholar]
- 8.Hara H, Ogawa M. Erythropoietic precursors in mice under erythropoietic stimulation and suppression. Exp Hematol. 1977;5:141–148. [PubMed] [Google Scholar]
- 9.Wang GL, Semenza GL. Molecular basis of hypoxia-induced erythropoietin expression. Curr Opin Hematol. 1996;3:156–162. doi: 10.1097/00062752-199603020-00009. [DOI] [PubMed] [Google Scholar]
- 10.Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15:146–155. doi: 10.1016/j.tcb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Wojchowski DM, Menon MP, Sathyanarayana P, et al. Erythropoietin-dependent erythropoiesis: new insights and questions. Blood Cells Mol Dis. 2006;36:232–238. doi: 10.1016/j.bcmd.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Pop R, Sadegh C, et al. Suppression of Fas-FasL coexpression by erythropoietin mediates erythroblast expansion during the erythropoietic stress response in vivo. Blood. 2006;108:123–133. doi: 10.1182/blood-2005-11-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Socolovsky M, Murrell M, Liu Y, et al. Negative autoregulation by FAS mediates robust fetal erythropoiesis. PLoS Biol. 2007;5:e252. doi: 10.1371/journal.pbio.0050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bateman A, Cole R. Colony forming cells in the livers of prenatal flexed (f/f) anaemic mice. Cell Tissue Kinet. 1972;5:165–173. doi: 10.1111/j.1365-2184.1972.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 15.Cole R, Regan T. Haematopoietic progenitor cells in the prenatal conegenitally anaemic ‘Flexed-tail’ (f/f) mice. Br J Haematol. 1976;33:387–394. doi: 10.1111/j.1365-2141.1976.tb03555.x. [DOI] [PubMed] [Google Scholar]
- 16.Gruneberg H. The anaemia of the flexed-tail mice (Mus musculus L.) II: Siderocytes J Genet. 1942;44:246–271. [Google Scholar]
- 17.Gruneberg H. The anaemia of the flexed-tail mouse (Mus musculus L.). I: Static and dynamic haematology. J Genet. 1942;43:45–68. [Google Scholar]
- 18.Hunt H, Premar D. Flexed-tail a mutation in the house mouse. Anat Rec. 1928;41:117. [Google Scholar]
- 19.Mixter R, Hunt H. Anemia in the flexed tailed mouse, Mus musculus. Genetics. 1933;18:367–387. doi: 10.1093/genetics/18.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman D, Russell E, Levin E. Enzymatic studies of the hemopoietic defect in flexed mice. Genetics. 1969;61:631–642. doi: 10.1093/genetics/61.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valtieri M, Gabbianelli M, Pelosi E, et al. Erythropoietin alone induces erythroid burst formation by human embryonic but not adult BFU-E in unicellular serum-free culture. Blood. 1989;74:460–470. [PubMed] [Google Scholar]
- 22.Hegde S, Lenox LE, Lariviere A, et al. An intronic sequence mutated in flexed-tail mice regulates splicing of Smad5. Mamm Genome. 2007;18:852–860. doi: 10.1007/s00335-007-9074-9. [DOI] [PubMed] [Google Scholar]
- 23.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 24.Chabot B, Stephenson DA, Chapman VM, et al. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 25.Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 26.Copeland NG, Gilbert DJ, Cho BC, et al. Mast cell growth factor maps near the steel locus on mouse chromosome 10 and is deleted in a number of steel alleles. Cell. 1990;63:175–183. doi: 10.1016/0092-8674(90)90298-s. [DOI] [PubMed] [Google Scholar]
- 27.Huang E, Nocka K, Beier DR, et al. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- 28.Zsebo KM, Williams DA, Geissler EN, et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- 29.Harrison D, Russell E. The response of W/Wv and Sl/Sld anaemic mice to hematopoietic stimuli. Br J Haematol. 1972;22:155–167. doi: 10.1111/j.1365-2141.1972.tb08797.x. [DOI] [PubMed] [Google Scholar]
- 30.Perry J, Harandi O, Paulson R. BMP4, SCF and hypoxia cooperatively regulate the expansion of murine stress erythroid progenitors. Blood. 2007;109:4494–4502. doi: 10.1182/blood-2006-04-016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Agosti V, Karur V, Sathyanarayana P, et al. A KIT juxtamembrane PY567-directed pathway provides nonredundant signals for erythroid progenitor cell development and stress erythropoiesis. Exp Hematol. 2009;37:159–171. doi: 10.1016/j.exphem.2008.10.009. This study carefully dissects the roles of different signaling pathways downstream of the Kit receptor to identify the essential role for tyrosine 567-dependent signaling in stress erythropoiesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu DC, Paulson RF. Hypoxia regulates BMP4 expression in the murine spleen during the recovery from acute anemia. PloS One. 2010;5:e11303. doi: 10.1371/journal.pone.0011303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Perry JM, Harandi OF, Porayette P, et al. Maintenance of the BMP4-dependent stress erythropoiesis pathway in the murine spleen requires hedgehog signaling. Blood. 2009;113:911–918. doi: 10.1182/blood-2008-03-147892. This study establishes an essential function for Hedgehog signaling in the development of new stress erythroid progenitors following recovery from acute anemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murtaugh L, Chyung J, Lassar A. Sonic Hedgehog promotes somitic chondrogenesis by altering the cellular response to BMP signaling. Genes Dev. 1999;13:225–237. doi: 10.1101/gad.13.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murtaugh LC, Zeng L, Chyung JH, et al. The chick transcriptional repressor Nkx3.2 acts downstream of Shh to promote BMP-dependent axial chondrogenesis. Dev Cell. 2001;1:411–422. doi: 10.1016/s1534-5807(01)00039-9. [DOI] [PubMed] [Google Scholar]
- 36.Zeng L, Kempf H, Murtaugh LC, et al. Shh establishes an Nkx3.2/Sox9 autoregulatory loop that is maintained by BMP signals to induce somitic chondrogenesis. Genes Dev. 2002;16:1990–2005. doi: 10.1101/gad.1008002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Socolovsky M, Gross AW, et al. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003;102:3938–3946. doi: 10.1182/blood-2003-05-1479. [DOI] [PubMed] [Google Scholar]
- 38.Jones RJ, Collector MI, Barber JP, et al. Characterization of mouse lympho-hematopoietic stem cells lacking spleen colony-forming activity. Blood. 1996;88:487–491. [PubMed] [Google Scholar]
- 39.Jones RJ, Wagner JE, Celano P, et al. Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature. 1990;347:188–189. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- 40••.Harandi OF, Hedge S, Wu DC, et al. Murine erythroid short-term radio-protection requires a BMP4-dependent, self-renewing population of stress erythroid progenitors. J Clin Invest. 2010;120:4507–4519. doi: 10.1172/JCI41291. This study describes the identification of three populations of stress erythroid progenitors that expand in the spleen during the recovery from acute anemia. The most immature of these progenitors is a self-renewing erythroid-restricted stress progenitor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmes C, Stanford WL. Concise review: stem cell antigen-1 – expression, function, and enigma. Stem Cells. 2007;25:1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 42.Li CL, Johnson GR. Rhodamine123 reveals heterogeneity within murine Lin−, Sca-1+ hemopoietic stem cells. J Exp Med. 1992;175:1443–1447. doi: 10.1084/jem.175.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nibley WE, Pohlmann SJ, Spangrude GJ. Patterns of organ-specific engraftment by stem cell subsets and committed progenitors. Stem Cells. 1997;15 (Suppl 1):31–39. doi: 10.1002/stem.5530150806. [DOI] [PubMed] [Google Scholar]
- 44.Nibley WE, Spangrude GJ. Primitive stem cells alone mediate rapid marrow recovery and multilineage engraftment after transplantation. Bone Marrow Transplant. 1998;21:345–354. doi: 10.1038/sj.bmt.1701097. [DOI] [PubMed] [Google Scholar]
- 45.Osawa M, Hanada K, Hamada H, et al. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 46.Price EA, Schrier SL. Unexplained aspects of anemia of inflammation. Adv Hematol. 2010;2010:508739. doi: 10.1155/2010/508739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 48•.Millot S, Andrieu V, Letteron P, et al. Erythropoietin stimulates spleen BMP4-dependent stress erythropoiesis and partially corrects anemia in a mouse model of generalized inflammation. Blood. 2010;116:6072–6081. doi: 10.1182/blood-2010-04-281840. This study shows that activation of the BMP4-dependent stress erythropoiesis pathway by Epo injection alleviates the anemia of a mouse model of ACD. [DOI] [PubMed] [Google Scholar]
- 49.Lasocki S, Millot S, Andrieu V, et al. Phlebotomies or erythropoietin injections allow mobilization of iron stores in a mouse model mimicking intensive care anemia. Crit Care Med. 2008;36:2388–2394. doi: 10.1097/CCM.0b013e31818103b9. [DOI] [PubMed] [Google Scholar]
- 50•.Zeigler BM, Vajdos J, Qin W, et al. A mouse model for an erythropoietin-deficiency anemia. Dis Mod Mech. 2010;3:763–772. doi: 10.1242/dmm.004788. This study describes the development of a mouse model of Epo-deficient anemia and shows that activation of the BMP4-dependent stress erythropoiesis pathway in response to acute anemia is unaffected by mutation of the Epo gene. [DOI] [PubMed] [Google Scholar]
- 51.Alter BP. Fetal erythropoiesis in stress hematopoiesis. Exp Hematol. 1979;7 (Suppl 5):200–209. [PubMed] [Google Scholar]
- 52.Stamatoyannopoulos G, Veith R, Galanello R, et al. Hb F production in stressed erythropoiesis: observations and kinetic models. Ann N Y Acad Sci. 1985;445:188–197. doi: 10.1111/j.1749-6632.1985.tb17188.x. [DOI] [PubMed] [Google Scholar]
- 53.Galanello R, Barella S, Maccioni L, et al. Erythropoiesis following bone marrow transplantation from donors heterozygous for beta-thalassaemia. Br J Haematol. 1989;72:561–566. doi: 10.1111/j.1365-2141.1989.tb04324.x. [DOI] [PubMed] [Google Scholar]
- 54.Meletis J, Papavasiliou S, Yataganas X, et al. ‘Fetal’ erythropoiesis following bone marrow transplantation as estimated by the number of F cells in the peripheral blood. Bone Marrow Transplant. 1994;14:737–740. [PubMed] [Google Scholar]
- 55.Weinberg RS, Schofield JM, Lenes AL, et al. Adult ‘fetal-like’ erythropoiesis characterizes recovery from bone marrow transplantation. Br J Haematol. 1986;63:415–424. doi: 10.1111/j.1365-2141.1986.tb07518.x. [DOI] [PubMed] [Google Scholar]
- 56.Link MP, Alter BP. Fetal-like erythropoiesis during recovery from transient erythroblastopenia of childhood (TEC) Pediatr Res. 1981;15:1036–1039. doi: 10.1203/00006450-198107000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Papayannopoulou T, Vichinsky E, Stamatoyannopoulos G. Fetal Hb production during acute erythroid expansion. I: Observations in patients with transient erythroblastopenia and postphlebotomy. Br J Haematol. 1980;44:535–546. doi: 10.1111/j.1365-2141.1980.tb08707.x. [DOI] [PubMed] [Google Scholar]
- 58.Bank A. Regulation of human fetal hemoglobin: new players, new complexities. Blood. 2006;107:435–443. doi: 10.1182/blood-2005-05-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forget BG. Molecular basis of hereditary persistence of fetal hemoglobin. Ann N Y Acad Sci. 1998;850:38–44. doi: 10.1111/j.1749-6632.1998.tb10460.x. [DOI] [PubMed] [Google Scholar]
- 60.Frenette PS, Atweh GF. Sickle cell disease: old discoveries, new concepts, and future promise. J Clin Invest. 2007;117:850–858. doi: 10.1172/JCI30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Panigrahi I, Agarwal S. Genetic determinants of phenotype in beta-thalassemia. Hematology. 2008;13:247–252. doi: 10.1179/102453308X316031. [DOI] [PubMed] [Google Scholar]
- 62.Thein SL. Genetic modifiers of the beta-haemoglobinopathies. Br J Haematol. 2008;141:357–366. doi: 10.1111/j.1365-2141.2008.07084.x. [DOI] [PubMed] [Google Scholar]
- 63.DeSimone J, Biel M, Heller P. Maintenance of fetal hemoglobin (HbF) elevations in the baboon by prolonged erythropoietic stress. Blood. 1982;60:519–523. [PubMed] [Google Scholar]
- 64.DeSimone J, Biel SI, Heller P. Stimulation of fetal hemoglobin synthesis in baboons by hemolysis and hypoxia. Proc Natl Acad Sci U S A. 1978;75:2937–2940. doi: 10.1073/pnas.75.6.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DeSimone J, Heller P, Adams JG. Hemopoietic stress and fetal hemoglobin synthesis: comparative studies in vivo and in vitro. Blood. 1979;54:1176–1181. [PubMed] [Google Scholar]
- 66.Luck L, Zeng L, Hiti AL, et al. Human CD34(+) and CD34(+)CD38(−) hematopoietic progenitors in sickle cell disease differ phenotypically and functionally from normal and suggest distinct subpopulations that generate F cells. Exp Hematol. 2004;32:483–493. doi: 10.1016/j.exphem.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 67.Mathias LA, Fisher TC, Zeng L, et al. Ineffective erythropoiesis in beta-thalassemia major is due to apoptosis at the polychromatophilic normoblast stage. Exp Hematol. 2000;28:1343–1353. doi: 10.1016/s0301-472x(00)00555-5. [DOI] [PubMed] [Google Scholar]