Abstract
URobotics (Urology Robotics) is a program of the Urology Department at the Johns Hopkins Medical Institutions dedicated to the development of new technology for urologic surgery (http://urology.jhu.edu/urobotics). The program is unique in that it is the only academic engineering program exclusively applied to urology. The program combines efforts and expertise from the medical and engineering fields through a close partnership of clinical and technical personnel. Since its creation in 1996, the URobotics lab has created several devices, instruments, and robotic systems, several of which have been successfully used in the operating room. This article reviews the technology developed in our laboratory and its surgical applications, and highlights our future directions.
INTRODUCTION
The economic advantages, increased precision, and improved quality demonstrated by industrial robots stimulated the application of robots for health care delivery. The utilization of robots in surgery was pioneered in the 1980s in the fields of neurosurgery and orthopedic surgery.1–3 Surgical robotics has since expanded to other surgical applications, including urology.4,5
Robotic devices to assist urologists with transurethral resection of the prostate, percutaneous renal access, laparoscopy, and brachytherapy are currently under development or already in clinical use. Some systems are now commercially available. The goal of the URobotics program is to contribute to the development of this field of urologic technology.
The development of surgical robots is highly demanding, compared to other fields, due to the enhanced safety, sterilization, compactness, operating-room (OR) requirements, compatibility with medical imaging equipment, and special ergonomics required. Testing and evaluation of surgical robots is a laborious process involving several non-clinical stages and endorsements before clinical assessment. Moreover, robotics for soft-tissue operations, such as the urologic systems, should adapt to the deformability and mobility of the operated organ. Although these difficulties delayed the evolution of surgical robotics until the late 1980s, recent research has allowed the development of several purpose-designed systems.6 This article presents a brief review of the technology developed in our laboratory, its applications, and our future goals.
The URobotics Lab
The URobotics Lab is located at the Johns Hopkins Bayview Medical Center, in close proximity to the Urology Clinic, radiology facilities, and the ORs. The URobotics program has extensive dedicated facilities, including offices, laboratories, and a machine shop fully equipped for robotic design and manufacturing (Fig. 1). The laboratory concentrates mainly on the development of robotic systems and other hardware components required for robotic, image-guided, and telerobotic surgery. All systems developed have been constructed in our facilities.
Fig. 1.
The URobotics workshop. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com]
SYSTEMS AND TECHNOLOGY
The PAKY Needle Driver
PAKY (Percutaneous Access of the Kidney) is a radiolucent needle driver used to guide and actively drive a trocar needle in X-ray-guided percutaneous procedures (Fig. 2). Its design and construction allow for unobstructed visualization of the anatomical target and radiological guidance of the needle.7,8 An electric motor is used to automate needle insertion. The driver is based on a new mechanical transmission, the “Friction Transmission with Axial Loading” developed in our laboratory.9 The driver is constructed of acrylic plastic, making it inexpensive to manufacture as a sterile disposable part. A novel feature of the insertion device is that it grasps the barrel of the needle, not the needle head. This significantly reduces the unsupported length of the needle during insertion, thus minimizing the lateral flexure of the unsupported needle under insertion loading.
Fig. 2.
The PAKY needle driver: in close-up (a) and in surgical use for image-guided percutaneous renal access (b). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com]
The PAKY needle driver is connected to a passive positioning arm mounted on the operating room table. A custom rigid side rail is mounted on the OR fluoroscopic table to provide a sturdy base for the operation. This is critical to maintain the needle trajectory under the insertion force. Needle insertion is actuated by a variable-speed DC motor, which the surgeon regulates via a joystick control.
PAKY has been successfully used in numerous clinical cases.10,11 The system was used in the OR in conjunction with a C-arm portable fluoroscopy unit (Fig. 2). Superimposed Needle Registration,7 a registration and targeting procedure that mimics the surgical technique of experienced urologists, has been adopted for operational use.
Z-Stage PAKY
Z-Stage PAKY is a modified version of the PAKY needle driver adapted to implement a CT/MRI registration method in addition to the existing needle driving capabilities of PAKY (Fig. 3). The driver was constructed in our laboratory in collaboration with the NSF Engineering Research Center for Computer Integrated Surgical Systems and Technology (CISST) at Hopkins and the University of Tokyo. It utilizes the same friction transmission principle as the PAKY driver, and also presents a Z-shaped Brown-Roberts-Wells12,13 localizer frame. The Z-Stage PAKY allows for the localization of the procedure needle from a single CT/MR slice.14 Phantom tests performed under CT showed that the device provides a fast and accurate registration method for cross-sectional image-guided stereotaxis. The device is Zone 1 compatible with MR and CT scanners, being entirely constructed of acrylic plastic and using CT/MR contrast. In February 2001, the driver was successfully used in conjunction with the RCM robot for a CT-guided kidney biopsy procedure.
Fig. 3.
The Z-Stage PAKY needle driver supported by the RCM robot in a CT-scanner phantom test. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com]
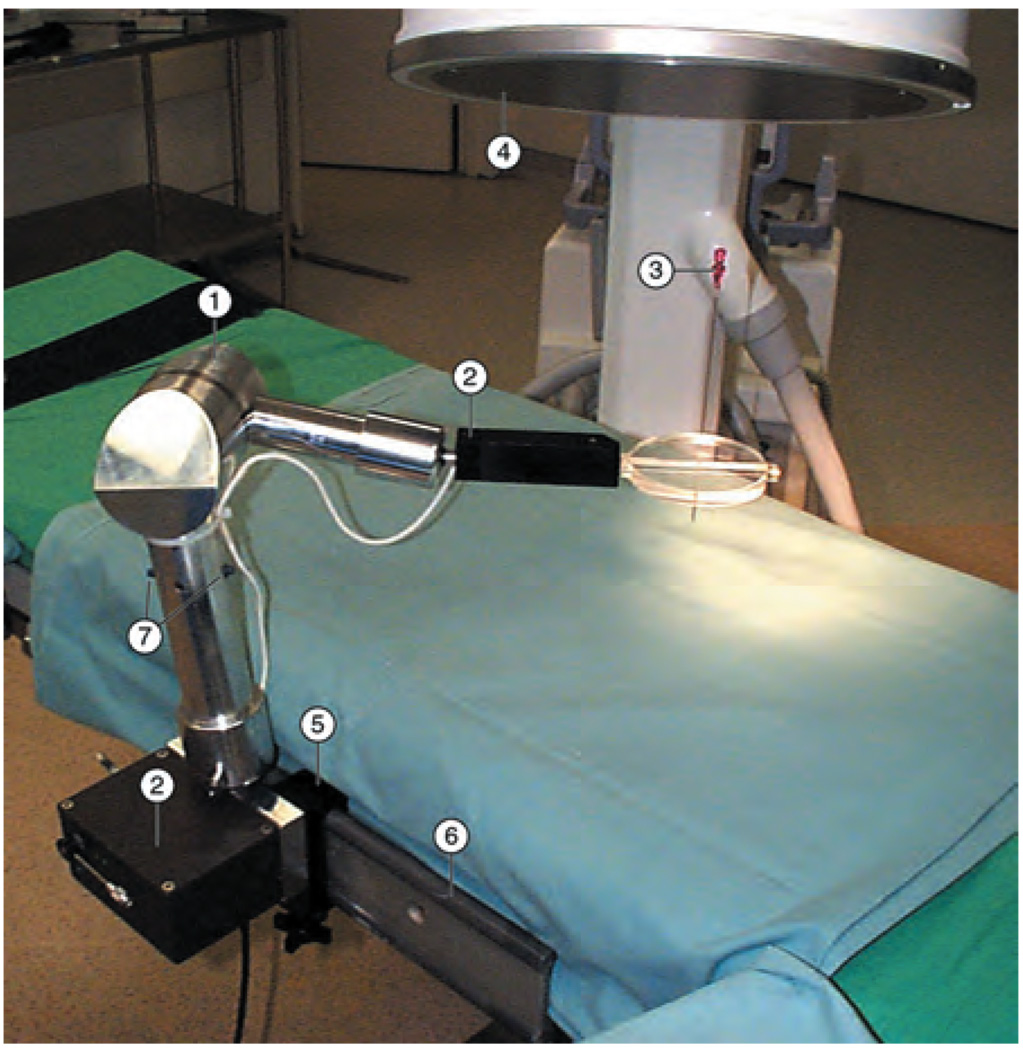
The RCM Robot
The RCM (Remote Center of Motion) robot is a compact robot for surgical applications that implements a fulcrum point located distal to the mechanism.15,16 The robot presents a compact design: it may be folded into a 171 × 69 × 52-mm box, and weighs only 1.6 kg. The robot can precisely orient a surgical instrument in space while maintaining the location of one of its points. This kinematic architecture makes it suitable for minimally invasive applications, as well as trocar/needle orientation in percutaneous procedures. RCM accommodates various end-effectors. We used the RCM in conjunction with the PAKY needle driver (Fig. 4) for performing image-guided percutaneous renal access.16 The robot was successfully used at the Johns Hopkins Medical Institutions for numerous surgical procedures.11,17 The robot orients and inserts the needle using X-ray fluoroscopy guidance from a C-arm imager, as controlled by the surgeon. The robot was also used in several telesurgical cases.18 In the first case, the robot was located in Rome, Italy, while the surgeon performed percutaneous access from our institution in Baltimore, MD. Most recently, the RCM was used with a special laparoscopic instrument driver for organ retraction (Fig. 14).
Fig. 4.
RCM and PAKY performing X-ray-guided percutaneous renal access. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com]
Fig. 14.
Teleoperated Karl Storz laparoscopy equipment and the BW-RCM robot with the Lap Driver supporting a tissue retractor. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com]
The system is presently used at Hopkins for percutaneous renal access, offering an unquestionable improvement in needle placement accuracy and procedure time, while reducing the radiation exposure to patient and urologist.11 Presently, PAKY is under continued evaluation, and new C-arm-based image-guidance algorithms are under development. Under CT guidance, the system has been used for kidney and spine percutaneous procedures. 19
The GREY Arm
The GREY Arm was developed in our URobotics laboratory as a suitable mechanism for positioning and supporting compact surgical robots (such as the RCM) and instrumentation in the proximity of the operative field. Often, the accuracy of surgical instrumentation and surgical procedures relies heavily on the ability of the supporting device to provide a sturdy base under the payload and dynamics of the instrument. The GREY Arm (Fig. 5) is a compact and sturdy passive mechanical arm equipped with a central braking system, which can be easily manipulated at desired locations and firmly locked in place. The arm presents serial link architecture with six degrees of freedom, comprising two links and three joints: spherical-rotational-spherical.
Fig. 5.
The GREY Arm supporting the PAKY needle driver. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com]
The novelty of the arm lies in the special design of the braking mechanism that simultaneously locks all the joints using a single electric motor.20–22 The arm design is simple and safe. One of its safety features is the power fail-safe design rendered by the normally locked braking mechanism being unlocked by a low-voltage electrical actuator. The arm is designed for stand-alone use in the OR as an independent module.
The Smart Needle™
The Smart Needle (YIM) is a specially designed needle that can be used to acquire measurements of electrical impedance at the tip of the needle (Fig. 6). Such measurements may provide localized information regarding the nature of the tissue at the needle tip, such as the presence of cancer.23,24 We have initially used the needle sensor for the confirmation of needle insertion into the renal collecting system in image-guided percutaneous procedures.25,26 This used the electrical impedance discrimination between the urine in the collecting system and the collection system itself.
Fig. 6.
The Smart Needle sensor of electrical impedance. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com]
The Ball-Worm Transmission
This technology has been developed in our laboratory to fulfill the need for implementing a simple and small nonbacklash (no play between the input and output shafts, precise) rotational transmission.27 Such mechanisms are required for actuating revolute joints of precision surgical manipulators, such as the robots described elsewhere. An appropriate mechanism suitable for precise miniaturized medical robotics has not been commercially available or reported in the literature.
The ball-worm is a miniature transmission of rotational motion between two shafts with nonintersecting axes using rolling elements. It represents a combination of two mechanical principles: the worm transmission and the rolling of spherical balls. The key feature of the ball-worm technology is the substitution of sliding friction with rolling friction by the addition of a finite number of rolling elements, spherical balls, which are recirculated between the worm and the worm gear. Several major advantages result from this scheme: non-backlash, kinematic precision, high efficiency, increased power-transmission capability, no lubrication requirement, and the possibility of construction from materials that are not necessarily friction paired (as in the classic worm mechanism). In addition, the design allows the miniaturization of the transmission assembly.
The design has been implemented and manufactured in our laboratory using computer-controlled machine tools: the HAAS SL-20 Turning Center and the HAAS VF-1 Vertical Machining Center (Fig. 1). In the prototype (Fig. 7), the overall size of the assembly, including the bearings, is 54 × 28 × 67 mm. During testing, the transmission exhibited no perceptible backlash and minimal friction. Two main advantages of this technology are the kinematic precision and miniaturized construction, which makes this transmission appropriate for precise surgical operations.
Fig. 7.
Photograph of the prototype ball-worm transmission. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com]
The BW-RCM
The BW-RCM (Ball-Worm RCM) is the new generation of the RCM robot implemented on the ball-worm technology. This version also augments redundant encoding and index marks on both axes. These significantly enhance the safety, kinematic performance, and rigidity of the mechanism. A photograph of the first BW-RCM with a new version of the PAKY driver and a new supporting arm is presented in Figure 8.
Fig. 8.
BW-RCM Robot, PAKY needle driver, and supporting arm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com]
Laser-Based CT/MR Registration
A simple method for robot registration in CT and MR imaging systems was developed19 for using the PAKY-RCM system under CT guidance. The method uses the laser markers readily available on any CT scanner and does not require imaging, thus eliminating radiation exposure. Its accuracy is inherited from the laser positioning system. This approach does not require additional hardware, laser alignment being performed on the instrument used in the clinical application. Moreover, robotic guidance allows radiological interventions to be performed on scanners without fluoro-CT capability. Unlike the manual approach, the method allows performance of oblique insertions, for which the skin entry point and the target are located in different slices.
The implementation is realized using the latest version of the PAKY-BW-RCM robot. The system was successfully used for eleven CT-guided biopsy and radiofrequency ablation procedures on the kidney and spine (Fig. 9), and for placement of nephrostomy tubes. Further investigation will explore its application to other organs and procedures.
Fig. 9.
Robotic CT-guided spine radiofrequency ablation. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com]
Laparoscopy Simulators and Tests of Dexterity
For laparoscopy training and evaluation, we have developed two training devices and a set of experiments providing quantifiable scales of dexterity.28 For the purpose of simplifying the process of laparoscopy training, we adopted a step-by-step strategy that decouples the major components of difficulty in performing instrument maneuvers. An inverted manipulation training device for laparoscopy under direct (3D) vision was developed. For the beginner, performing laparoscopy maneuvers in a common “pelvic trainer box” under monitor vision is a difficult task. By contrast, we developed a first-step trainer (Fig. 10) that allows the trainee to accommodate to the use of laparoscopic instruments and perform “through-the-hole” inverted-motion manipulation tasks while directly observing the site.
Fig. 10.
Inverted manipulation trainer under 3D vision (Step 1 Trainer). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com]
With this trainer, laparoscopic instruments are inserted as usual through the trocar ports, thus allowing various motion-training exercises to be conducted.
After becoming familiar with laparoscopic instrumentation and gaining inverted manipulation skills under direct vision, the trainee is presented with the challenge of performing the same operations inside an opaque box by observing a regular laparoscopic view. This uses the inverted manipulation training under 2D vision. The training device (Fig. 11) is similar to the existing, commercially available pelvic trainer boxes, except for the fact that it uses ball-joint trocar entry ports, as in the Step 1 trainer.
Fig. 11.
Inverted manipulation trainer under 2D vision (Step 2 Trainer). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com]
The trainer is used to gain manipulation skills under monitor vision, especially with regard to depth perception and hand-eye coordination. Animal organs may also be included in the box and used for grasping, cutting, coagulating, suturing tissues, and other surgical maneuvers.
A third-step laparoscopic trainer is currently under development: the high-fidelity synthetic torso for training and evaluation of urologic laparoscopic skills. By using a realistic anatomical setting, the trainer creates a more natural bridge between “in-box laparoscopy” (Step 2 or any other pelvic trainer) and the real surgical case. It has the potential to reduce the necessary live-animal training, and could potentially add training elements lacking in the current box and animal-training sequence.
For the purpose of quantifiably assessing the learning curve in laparoscopy, we designed six tests of laparoscopic dexterity.28 Each test is performed using a specially designed testing device (Fig. 12).
Fig. 12.
Devices for laparoscopic dexterity testing. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com]
These tests and trainers have also been used to evaluate the performance of robot-aided versus manual laparoscopy. A set of experiments has been performed with the Zeus (Computer Motion, Inc., Goleta, CA) teleoperation system to compare its performance with the manual approach (Fig. 13). The experiments showed no improvement in surgeon performance using the system.28
Fig. 13.
Dexterity evaluation and force-feedback experiments with the Zeus teleoperation system. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com]
Telesurgery
We have extensive experience in performing telementoring and telesurgical applications between our facilities at Hopkins and various hospitals around the world.8,13,14 Our most recent system uses four ISDN lines to perform high-quality video and audio communications, remote control of the electrocautery machine and a rack of Karl Storz laparoscopy instruments (Fig. 14), and simultaneous teleoperation of two robotic systems. The first robotic system is the AESOP (Automated Endoscopic System for Optimal Positioning), which is a laparoscopic camera holder from Computer Motion, Inc., Goleta, CA. The second robot has been developed in our laboratory, consisting of the BW-RCM robot and a special driver for laparoscopic instruments, the Lap Driver (Fig. 14). The Lap Driver could be used for positioning and orienting a laparoscopic tissue retractor or other laparoscopic instrument. The RCM-Lap Driver system presents similar functionality with the AESOP robot in a miniaturized size, with steadier positioning and more precise targeting and tracking capabilities.
FUTURE DIRECTIONS
The development of MR-compatible robotic systems is a very challenging engineering task. MR scanners use magnetic fields of very high density, on the order of one tesla. Ferromagnetic materials, which are normally used for robot construction, undergo very-high-intensity forces when exposed to such fields. Concurrently, MR fields present variable components, resulting in the induction of electricity in conductive elements, the creation of electrical interference, and overheating. In addition, electromagnetic motors commonly used for robotic actuation would also interfere with the MR field. Novel engineering principles and methods should be developed to overcome these problems.31 This task demands a quantum leap in the current technology of mechatronic devices, and clearly leads medical robotics into the next millennium.32,33
Our near-future research will mainly concentrate on the development of a high-mobility, precision, miniature robot that could operate within the fields of classic (closed-bore) as well as interventional (open) MR scanners. For telesurgery, we will continue to increase the complexity of our existing system by adding and improving the existing robotic components and also perfecting the teleoperation protocols. The goal is to obtain a complete teleoperation system, which would allow the remote surgeon to independently perform the operation.
CONCLUSION
Robotic applications offer a wide range of possibilities for improving current surgical techniques, as well as allowing the development of new procedures which could not be performed without the aid of surgical robots. Among many other advantages, these new instruments potentially improve the precision of manipulation compared to manual procedures, and provide improved mapping between the patient and his volumetric image as given by sophisticated imaging equipment. This requires the development of miniature, extremely high-dexterity robots that are able to operate inside the image scanners, including open as well as conventional closed-bore CT and MR imagers. With continued improvements in hardware and software, the application of surgical robotics will only expand. Physician acceptance of these systems will ultimately depend on their ability to advance surgical performance, improve patient safety, and reduce cost. Surgical robotics has the potential to open new horizons for the surgical practice. Our URobotics program is dedicated to being part of this development process.
ACKNOWLEDGMENT
The work presented in this article was partially supported by grant No. 1R21CA088232-01A1 from the National Cancer Institute (NCI). The content of this article is solely the responsibility of the author and does not necessarily represent the official views of NCI.
REFERENCES
- 1.Buckingham RA, Buckingham RO. Robots in operating theatres. BMJ. 1995;311:1479. doi: 10.1136/bmj.311.7018.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cadeddu JA, Stoianovici D, Kavoussi LR. Robotics in urologic surgery. Urology. 1997;49(4):501–507. doi: 10.1016/s0090-4295(96)00561-4. [DOI] [PubMed] [Google Scholar]
- 3.Cadeddu JA, Stoianovici D, Kavoussi LR. Telepresence and robotics: urology in the 21st century. Contemp Urol. 1997;9(10):86–97. [Google Scholar]
- 4.Stoianovici D, Cadeddu JA, Kavoussi LR. AUA Update Series Vol. XVIII. American Urological Association; 1999. Urologic applications of robotics; pp. 194–200. [Google Scholar]
- 5.Stoianovici D. Robotic surgery. World J Urol. 2000;18(4):289–295. doi: 10.1007/pl00007078. [DOI] [PubMed] [Google Scholar]
- 6.Cadeddu JA, Stoianovici D, Kavoussi LR. The use of robotics in urological surgery. Urol Int. 1997;4(3):11–14. [Google Scholar]
- 7.Stoianovici D, Cadeddu JA, Demaree RD, Basile HA, Taylor RH, Whitcomb LL, Sharpe WN, Kavoussi LR. An efficient needle injection technique and radiological guidance method for percutaneous procedures. In: Troccaz J, Grimson E, Mösges R, editors. Lecture Notes in Computer Science 1205; Proceedings of the First Joint Conference on Computer Vision, Virtual Reality and Robotics in Medicine and Medical Robotics and Computer-Assisted Surgery (CVRMed-MRCAS’97); March 1997; Grenoble, France. Berlin: Springer; 1997. pp. 295–298. [Google Scholar]
- 8.Stoianovici D, Cadeddu JA, Demaree RD, Basile HA, Taylor RH, Whitcomb LL, Kavoussi LR. A novel mechanical transmission applied to percutaneous renal access. Proceedings of the ASME Dynamic Systems and Control Division. 1997:401–406. DSC-61. [Google Scholar]
- 9.Stoianovici D, Kavoussi LR, Whitcomb LL, Taylor RH, Cadeddu JA, Basile HA, Demaree RD. Friction transmission with axial loading and a radiolucent surgical needle drive. No. 60/038,115. Provisional U.S. Patent of Invention. 1996 Filed as regular U.S. utility and PCT application by Johns Hopkins University on February 20, 1998.
- 10.Cadeddu JA, Bzostek A, Schreiner S, Barnes AC, Roberts WW, Anderson JH, Taylor RH, Kavoussi LR. A robotic system for percutaneous renal access. J Urol. 1997;158:1589. [PubMed] [Google Scholar]
- 11.Cadeddu JA, Stoianovici D, Chen RN, Moore RG, Kavoussi LR. Stereotactic mechanical percutaneous renal access. J Endourol. 1998;12(2):121–126. doi: 10.1089/end.1998.12.121. [DOI] [PubMed] [Google Scholar]
- 12.Brown RA. A stereotactic head frame for use with CT body scanners. Invest Radiol. 1979;14:300–304. doi: 10.1097/00004424-197907000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Brown RA, Roberts TS, Osborne AG. Stereotaxic frame and computer software for CT-directed neurosurgical localization. Invest Radiol. 1980;15:308–312. doi: 10.1097/00004424-198007000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Susil RC, Anderson JH, Taylor RH. A single registration method for CT guided interventions. In: Taylor C, Colchester A, editors. Lecture Notes in Computer Science 1679; Proceedings of the Second International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI’99); September 1999; Cambridge, England. Berlin: Springer; 1999. pp. 798–808. [Google Scholar]
- 15.Stoianovici D, Whitcomb LL, Anderson JH, Taylor RH, Kavoussi LR. A modular surgical robotic system for image guided percutaneous procedures. In: Wells WM, Colchester A, Delp S, editors. Lecture Notes in Computer Science 1496; Proceedings of the First International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI’98); October 1998; Cambridge, MA. Berlin: Springer; 1998. pp. 404–410. [Google Scholar]
- 16.Stoianovici D, Cadeddu JA, Whitcomb LL, Taylor RH, Kavoussi LR. A robotic system for precise percutaneous needle insertion. Proceedings of the Thirteenth Annual Meeting of the Society for Urology and Engineering; May 1998; San Diego, CA. p. 4. [Google Scholar]
- 17.Bishoff JT, Stoianovici D, Lee BR, Bauer J, Taylor RH, Whitcomb LL, Cadeddu JA, Chan D, Kavoussi LR. RCM-PAKY: clinical application of a new robotic system for precise needle placement. J Endourol. 1998;12:S82. [Google Scholar]
- 18.Stoianovici D, Lee BR, Bishoff JT, Micali S, Whitcomb LL, Taylor RH, Kavoussi LR. Robotic telemanipulation for percutaneous renal access. J Endourol. 1998;12:S201. [Google Scholar]
- 19.Patriciu A, Solomon S, Kavoussi LR, Stoianovici D. Robotic kidney and spine percutaneous procedures using a new laser-based CT registration method. Presented at the Fourth International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI 2001); October 2001; Utrecht, The Netherlands. [Google Scholar]
- 20.Lerner GA, Stoianovici D, Whitcomb LL, Kavoussi LR. A passive positioning device for surgical robots and instrumentation. Provisional application #DM-3489 for U.S. Patent of Invention filed by Johns Hopkins University. 1998 [Google Scholar]
- 21.Lerner G, Stoianovici D, Whitcomb LL, Kavoussi LR. A steady positioning system for surgical instrumentation. Fourteenth Annual Meeting of the Society for Urology and Engineering; May 1999; Dallas, TX: 1999. p. 1. [Google Scholar]
- 22.Lerner G, Stoianovici D, Whitcomb LL, Kavoussi LR. A passive positioning and supporting device for surgical robots and instrumentation. In: Taylor C, Colchester A, editors. Lecture Notes in Computer Science 1679; Proceedings of the Second International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI’99); September 1999; Cambridge, England. Berlin: Springer; 1999. pp. 1052–1061. [Google Scholar]
- 23.Lee BR, Roberts WW, Smith DG, Ko HW, Epstein JI, Lecksell K, Partin AW. Bioimpedance: novel use of a minimally invasive technique for cancer localization in the intact prostate. Prostate. 1999;39(3):213–218. doi: 10.1002/(sici)1097-0045(19990515)39:3<213::aid-pros10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Stoianovici D, Kavoussi LR, Allaf M, Jackman S. A surgical needle probe for electrical impedance measurements. U.S. Patent of Invention #DM-3375 filed by Johns Hopkins University. 1998 [Google Scholar]
- 25.Allaf ME, Stoianovici D, Jackman SV, Kavoussi LR. A novel system for the confirmation of percutaneous renal access by electrical impedance measurements. J Endourol. 1998;12:S82. doi: 10.1089/end.1998.12.121. [DOI] [PubMed] [Google Scholar]
- 26.Stoianovici D, Allaf ME, Jackman SV, Kavoussi LR. The “YIM” needle: Confirming percutaneous access through urinary impedance. Proceedings of the Thirteenth Annual Meeting of the Society for Urology and Engineering; May 1998; San Diego, CA. p. 5. [Google Scholar]
- 27.Stoianovici D, Kavoussi LR. Ball-worm transmission. Regular US utility and PCT patent application by the Johns Hopkins University (#DM-3512) 1999 [Google Scholar]
- 28.Roberts WW, Iordachita I, Patriciu A, Mazilu D, Jarrett TW, Kavoussi LR, Stoianovici D. Quantifiable tests of laparoscopic dexterity: robotic versus manual laparoscopy. Presented at the Sixteenth Annual Meeting of the Society for Urology and Engineering; June 2001; Anaheim, CA. [Google Scholar]
- 29.Kavoussi LR, Moore RG, Partin AW, et al. Telerobotic assisted laparoscopic surgery: initial laboratory and clinical experience. Urology. 1994;44:15. doi: 10.1016/s0090-4295(94)80003-0. [DOI] [PubMed] [Google Scholar]
- 30.Kavoussi LR, Moore RG, Adams JB, Partin AW. Comparison of robotic versus human laparoscopic camera control. J Urol. 1995;154:2134. [PubMed] [Google Scholar]
- 31.Chinzei K, Kikinis R, Jolesz FA. MR compatibility of mechatronic devices: design criteria. In: Taylor C, Colchester A, editors. Lecture Notes in Computer Science 1679; Proceedings of the Second International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI’99); September 1999; Cambridge, England. Berlin: Springer; 1999. pp. 1020–1030. [Google Scholar]
- 32.Hynynen K, Darkazanli A, Unger E, Schenck JF. MRI guided noninvasive ultrasound surgery. Med Phys. 1992;20:107–116. doi: 10.1118/1.597093. [DOI] [PubMed] [Google Scholar]
- 33.Masamune K, Kobayashi E, Masutani Y, Suzuki M, Dohi T, Iseki H, Takakura K. Development of an MRI-compatible needle insertion manipulator for stereotactic neurosurgery. J Image Guid Surg. 1995;1(4):242–248. doi: 10.1002/(SICI)1522-712X(1995)1:4<242::AID-IGS7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]