Abstract
We have developed and evaluated the reverse transcription (RT)-PCR detection of mRNA in cell culture to assay infectious adenoviruses (Ads) by using Ad type 2 (Ad2) and Ad41 as models. Only infectious Ads are detected because they are the only ones able to produce mRNA during replication in cell culture. Three primer sets for RT-PCR amplification of mRNA were evaluated for their sensitivity and specificity: a conserved region of late mRNA transcript encoding a virion structural hexon protein and detecting a wide range of human Ads and two primer sets targeting a region of an early mRNA transcript that specifically detects either Ad2 and Ad5 or Ad40 and Ad41. The mRNAs of infected A549 and Graham 293 cells were recovered from cell lysates with oligo(dT) at different time periods after infection and treated with RNase-free DNase to remove residual contaminating DNA, and then Ad mRNA was detected by RT-PCR assay. The mRNA of Ad2 was detected as early as 6 h after infection at 106 infectious units (IU) per cell culture and after longer incubation times at levels as low as 1 to 2 IU per cell culture. The mRNA of Ad41 was detected as soon as 24 h after infection at 106 IU per cell culture and at levels as low as 5 IU per cell culture after longer incubation times. To confirm the detection of only infectious viruses, it was shown that no mRNA was detected from Ad2 and Ad41 inactivated by free chlorine or high doses of collimated, monochromatic (254-nm) UV radiation. Detection of Ad2 mRNA exactly coincided with the presence of virus infectivity detected by cytopathogenic effects in cell cultures, but mRNA detection occurred sooner. These results suggest that mRNA detection by RT-PCR assay in inoculated cell cultures is a very sensitive, specific, and rapid method by which to detect infectious Ads in water and other environmental samples.
Human adenoviruses (Ads) are ubiquitous DNA viruses causing a variety of infectious diseases, such as respiratory disease, hemorrhagic cystitis, epidemic keratoconjunctivitis, and gastroenteritis (23). Fifty-one different serotypes of Ads have been identified on the basis of neutralization with type-specific animal antisera, and these serotypes can be classified into six subgroups (A to F) on the basis of their ability to agglutinate red blood cells (9). A number of Ads, including Ad type 2 (Ad2) and Ad5, are common etiologic agents worldwide for sporadic and epidemic episodes affecting the upper and lower respiratory tract, particularly in children (12, 30). Respiratory infections with Ads are associated with significant morbidity and mortality, especially among immunocompromised patients. Enteric Ad40 and Ad41 are potentially important waterborne viruses, are relatively resistant to sewage treatment, are identified as the second most common agents of gastroenteritis in children next to rotavirus in many studies (1, 3, 8), and are on the U.S. Environmental Protection Agency drinking water contaminant candidate list.
The standard method of detection of viral pathogens in environmental samples uses assays in mammalian cell culture. Although Ads are culturable in several cell lines, enteric Ad40 and Ad41 are difficult to culture and do not produce clear and consistent cytopathogenic effects (CPE) (28). Direct antigen detection by immunofluorescence techniques, enzyme immunoassay, or specific latex agglutination is used for clinical diagnosis of enteric Ad infection (14, 16, 25, 31) but is too insensitive for detection of the low concentrations of these viruses in environmental samples.
Molecular methods targeting nucleic acids are increasingly used for the detection of viruses and other waterborne microorganisms because of their high sensitivity, specificity, and speed. A PCR assay can detect nucleic acids of microorganisms that are difficult or impossible to cultivate, and a number of studies have used PCR assays to detect Ads in various environmental media, such as swimming pool water (19), coastal waters (15), river waters (5), sewage, and shellfish (21).
However, detection of Ads by PCR assay indicates only the presence of DNA of Ads and does not provide any information on infectivity, which is directly related to the human health risk (15, 26). Many microorganisms can be inactivated by natural (sunlight, high temperature, and other environmental stresses) or technological (e.g., chemical disinfectants or UV radiation) processes, but the inactivated microorganisms containing genomes or the genomes can continue to persist over relatively long periods of time in the environment (26). Components of the inactivated microorganisms, particularly the genomes, may be detected by current molecular techniques although they are not infectious. This is of great concern because some Ads have been shown to have a high particle to infectious unit ratio when cultured in permissive cells (4). The ability to detect only infectious viruses among many noninfectious viruses in environmental samples is important to predictions of public health risk in water and other environmental samples.
New molecular methods by which to detect only infectious microorganisms have been developed. Detection of bacterial mRNA by reverse transcription (RT)-PCR assay has been used to detect viable bacteria or the expression of bacterial virulence (pathogenicity) genes (18, 24). Integrated cell culture-PCR assay was developed to detect culturable viruses (6, 7, 22). This method combines cell culture and molecular detection of viral genomic nucleic acid. Cell culture prior to nucleic acid amplification increases the copy number of infectious viruses, which leads to higher sensitivity and an increased probability of their detectiion, even if they do not produce CPE. However, this method has the potential to detect the nucleic acid of inactivated viruses that may have been in the sample that was inoculated into cell cultures (26). Such carryover detection of nucleic acid of inactivated viruses could result in a false-positive result from samples containing no infectious viruses. However, only infectious Ads can enter cells and transcribe mRNA during replication. Therefore, detection of viral mRNA during cultivation is a definitive indication of the presence of infectious Ads. The objective of this study was to develop a new molecular method by which to detect viable Ads in environmental samples based on RT-PCR assay of their mRNA in cell culture.
MATERIALS AND METHODS
Ad culture and stock.
Ad2 strain ATCC VR-846 was obtained from the American Type Culture Collection (Manassas, Va.) and grown on A549 cells in Eagle's minimal essential medium (MEM) containing 2% fetal bovine serum. Ad41 strain ATCC VR-930 was cultured in Graham 293 cells in MEM containing 2% fetal bovine serum. The viruses were partially purified from infected cell lysates by chloroform extraction and stored frozen at −80°C until used for experiments. The infectivity titer of the Ad2 stock was estimated by most-probable-number analysis from observation of CPE on inoculated cell cultures. The infectivity titer of Ad41 was estimated by most-probable-number analysis with a PCR assay of viral DNA as the basis for detection in G293 cells. This nucleic acid endpoint was used to score infectivity because of inconsistent and ambiguous CPE production in cell culture. Infectivity is reported in infectivity units (IU) for both viruses.
Ad-specific primers.
The genomic sequences of Ads were obtained from the GenBank database at the National Center for Biotechnology Information. The oligonucleotide primers used in this study are summarized in Table 1. The primers (primer pair AdC-E1AF-AdC-E1AR and primer pair AdF-E1AF-AdF-E1AR) complementary to E1A in the serotype-specific region (Ad groups C 2/5 and F 40/41) were designed from alignments prepared by the program BioEdit (version 5.0.9), with resulting amplicons being 260 and 280 bp for Ad2 and Ad41, respectively. In addition to serotype-specific primers, an Ad-specific primer pair (Hex1-Hex2) for detection of hexon gene sequences conserved among all recognized human Ad serotypes was used as described in a previous report, yielding an amplicon of 482 bp (32). DNA amplified by the Ad group-specific primer pair Hex1-Hex2 was subjected to a heminested PCR assay (with primer pair Hex1-Hex3), with the resulting amplicon being 443 bp, to achieve higher detection sensitivity. The sensitivities of primers were evaluated with viral genomic DNA heat released from serially diluted stock viruses at 95°C for 2 min.
TABLE 1.
Oligonucleotide primers for nucleic acid amplification of Ads
| Ad groups or types | Primer | Target genea | PCR | Sequence (5′→3′) | Amplicon size, bp (reference) |
|---|---|---|---|---|---|
| A-F | Hex1 | Hexon | First | TTCCCCATGGCICA(CT)AACAC | 482 (32) |
| Hex2 | First | CCCTGGTA(GT)CC(AG)AT(AG)TTGTA | |||
| Hex1 | Nested | TTCCCCATGGCICA(CT)AACAC | 443 | ||
| Hex3 | Nested | AGGAACCA(AG)TC(CT)TT(AG)GTCAT | |||
| 2/5 | AdC-E1AF | E1A | First | CCACCTACCCTTCACGAACT | 260 |
| AdC-E1AR | First | CTCGTGGCAGGTAAGATCG | |||
| 40/41 | AdF-E1AF | E1A | First | GGGAACTGGGATGACAT | 280 |
| AdF-E1AR | First | CCSTCTTCATAGCATTTC |
The target nucleic acids are both DNA and mRNA.
Nucleic acid purification.
mRNA was purified from inoculated A549 or G293 cells cultured in T25 flasks for predetermined numbers of days at 37°C. Cultured cell monolayers were inoculated with predetermined numbers of Ads in phosphate-buffered saline (PBS) and incubated with periodic gentle mixing for 1 h at 37°C. After the inoculum was removed, 10 ml of MEM containing 2% fetal bovine serum was added to the inoculated cell monolayer for culture at 37°C. After an incubation period for virus replication, the cell monolayer was washed with 5 ml of PBS and then treated with 5 ml of 0.25% trypsin (Sigma, St. Louis, Mo.) in PBS for 5 min. Cells were transferred to a polypropylene centrifuge tube and harvested by centrifugation at 300 × g for 5 min, and the supernatant was discarded. The recovered cell pellet was lysed with 3% β-mercaptoethanol and guanidinium isothiocyanate and then homogenized with a QIAshredder (QIAGEN, Valencia, Calif.). The mRNA in cell lysates was purified by oligo(dT) latex in accordance with the standard protocol of the Oligotex direct mRNA kit (QIAGEN) as a final volume of 60 μl. To remove any contaminant DNA, 10 μl of mRNA was incubated for 10 to 15 min with 2 μl of RQ1 RNase-free DNase, 2 μl of DNase buffer (Promega, Madison, Wis.), and 1 μl of RNase inhibitor (Promega) in a 20-μl volume. Residual DNase was inactivated by DNase stopping solution (Promega). A PCR assay was performed on the same concentrations of mRNA to check for any residual contaminating DNA, and the absence of DNA was confirmed by electrophoresis on a 2% agarose gel, followed by ethidium bromide staining. Viral DNA was extracted with a QIAamp viral kit in accordance with the standard protocol (QIAGEN).
PCR and RT-PCR assay conditions.
PCR assay was performed in 50-μl volumes containing 45 μl of reaction mixture (10 mM Tris-HCl [pH 9.0], 50 mM KCl, 0.1% Triton X-100 [Promega], 1.5 mM MgCl2, 200 μM each deoxynucleoside triphosphate, 0.6 μM each primer, 2.5 U of Taq DNA polymerase [Invitrogen, Carlsbad, Calif.]) per reaction and 5 μl of sample DNA extract. RT was performed on 5 μl of RNA extract and 4 μl of antisense mixture with heating at 94°C for 2 min to remove the secondary structure of RNA. The RT reaction was performed in 15-μl volumes with 10× PCR buffer (pH = 8.3) containing 50 pM primer, 3 mM MgCl2, 1 mM nucleotide mixture, 5 U of avian myeloblastosis virus reverse transcriptase (RTase; Promega), and 20 U of RNase inhibitor (Promega) at 42°C for 1 h, followed by inactivation of RTase at 94°C for 2 min. PCR was performed in 50-μl volumes containing 45 μl of reaction mixture (10× PCR buffer [Promega], which consisted of 10 mM Tris-HCl [pH 9.0], 1.5 mM MgCl2, 50 mM KCl, 200 μM each nucleotide, 30 pM each primer, and 2.5 U of Taq DNA polymerase [Invitrogen]) per reaction and 5 μl of sample per RT reaction. Amplification reactions were carried out in a thermocycler (PTC-200; MJ Research, Watertown, Mass.) with preliminary denaturation for 5 min at 94°C, followed by 40 cycles of denaturation at 94°C for 1 min, annealing at 45°C (E1A primers) or 50°C (hexon primers) for 1 min, primer extension at 72°C for 2 min, and a final extension at 72°C for 5 min. In all mRNA RT-PCRs, RT-PCR assays without RTase were also performed on the same concentrations of mRNA in order to prove that there was no DNA contamination in the mRNA extracts. Heminested PCR assay was performed in 50-μl volumes containing 49 μl of reaction mixture (10× PCR buffer [Promega] containing 10 mM Tris-HCl [pH 9.0], 1.5 mM MgCl2, 50 mM KCl, 200 μM each nucleotide, 0.6 μM each primer, and 2.5 U of Taq DNA polymerase [Invitrogen]) per reaction and 1 μl of either undiluted or 10-fold-diluted RT-PCR sample. Amplification reactions were carried out in a thermocycler with preliminary denaturation for 5 min at 94°C, followed by 40 cycles of denaturation at 94°C for 1 min, annealing at 50°C for 1 min, primer extension at 72°C for 2 min, and a final extension at 72°C for 5 min. Rigorous precautions were taken to preclude DNA contamination during the heminested PCR assay. These steps included a designated nested PCR area, UV irradiation of both the bench area and pipettes for at least 15 min, frequent changes of gloves, and many negative controls when heminested PCR samples were handled. Ten microliters of each reaction product was separated (1 h, 120V) on 2% agarose gels, stained with ethidium bromide, and visualized by UV transillumination.
Free-chlorine treatment.
Sodium hypochlorite (6% household bleach) was used to make a 1-mg/liter concentration of free chlorine in PBS, and the concentration of free chlorine was measured by a standard DPD method (10, 26). The virus stock used for chlorination studies was further purified by washing in 0.01 M phosphate buffer with centrifugal ultrafiltration (Centriplus 100; Millipore Corp., Bedford, Mass.) and virion dispersing by filtration through a Tween 80-treated, 0.2-μm-porosity polycarbonate filter. Approximately 105 IU of Ad2 or 103 IU of Ad41 was seeded into 10 ml of 0.01 M phosphate buffer (pH = 7.5) and then exposed to a final concentration of 1 mg of free chlorine (pH = 7.5) per liter at 4°C for 1 to 100 min. Viruses with and without free-chlorine treatment were subjected to cultivation with either A549 or G293 cells, viral DNA detection by PCR assay, and mRNA detection by RT-PCR assay. Inoculated cell cultures were examined for CPE production for 7 to 10 days, and mRNA and DNA were extracted from cell cultures after 7 days.
UV disinfection.
A collimated-beam UV apparatus containing two 15-W, low-pressure mercury vapor germicidal lamps emitting nearly monochromatic UV radiation at 254 nm was used. The emitted UV light was directed through a circular opening to provide incident radiation to the surface of the test suspension in a cell culture petri dish (60 by 15 mm). The UV irradiance was measured with a radiometer (IL-500; International Light, Inc., Newburyport, Mass.) that had been factory calibrated in accordance with National Institute of Standards and Technology standards prior to the study. The measured incidence irradiance at the surface of the test liquid was corrected for any nonhomogeneity of irradiation across the surface area of the petri dish to provide the value for average incident irradiance. Approximately 5 × 105 IU of Ad2 and 103 IU of Ad41 were suspended in 5 ml of PBS and exposed to 254-nm UV light. The average irradiance in the mixed suspension was determined mathematically by the Beer-Lambert law over the sample depth, accounting for UV absorbance and incident average irradiance. The UV exposure doses were 0, 50, 100, and 250 mJ/cm2, which were calculated from the products of exposure time and UV irradiance.
Viruses with and without UV exposure were subjected to culture in either A549 or G293 cells, viral DNA detection by PCR assay, and mRNA detection by RT-PCR assay. Inoculated cell cultures were examined for CPE production for 7 to 10 days, and mRNA and DNA were extracted from cell cultures after 7 days.
Surface water sample concentrates.
Two 10-liter volumes of surface water were collected from a pond near Beaufort, N.C., and the Chattahoochee River in Cobb County, Ga., at different times of the year (January 2002 and March 2002, respectively) and had different turbidities. The sampled water was adjusted to pH 7.2 and supplemented with polyethylene glycol (Sigma) and NaCl to final concentrations of 8% and 0.3 M, respectively. Samples were incubated overnight at 4°C and then centrifuged at 6,706 × g for 30 min. The supernatants were carefully removed, and the pellets were resuspended in approximately 30 ml of Dulbecco's PBS and stored at −20°C until used. Four hundred microliters of water sample concentrate was seeded with 100 μl of 2 and 20 IU of Ad2 or with 0.1, 1, and 10 IU of Ad41, and then the samples in total volumes of 500 μl were inoculated onto 107 cells of A549 (Ad2) or G293 (Ad41) cells in T25 flasks. The flasks were incubated for 1 h at 37°C, the inoculum was then removed, and then 10 ml of MEM with 2% fetal bovine serum was added to each flask. Infected cells were incubated for 5 to 10 days at 37°C for virus replication and then examined for Ads by CPE, PCR, and mRNA RT-PCR assays.
RESULTS
Development and optimization of PCR and mRNA RT-PCR assays.
The PCR primers used in this study were evaluated to identify optimal conditions, and their detection sensitivities (lower limits of detection) are summarized in Table 2. When a PCR assay was performed under optimized conditions with heat-released Ad genomes, as little as 1 IU of Ad2 was detected with the AdC-E1AF-AdC-E1AR primer pair. Amounts as small as 10 and 0.1 IU of Ad2 were detected by the PCR assay with the Hex1-Hex2 primer pair and a subsequent heminested PCR assay with the Hex1-Hex3 primer pair, respectively. The PCR assay with the AdF-E1AF-AdF-E1AR primer pair detected as little as 0.01 IU of Ad41. The PCR assay with the Hex1-Hex2 primer pair and a subsequent heminested PCR assay with the Hex1-Hex3 primer pair detected as little as 1 and 0.01 IU of Ad41, respectively.
TABLE 2.
Sensitivities as lower detection limits of infectious Ads by conventional and nested PCR assays for different Ad DNA targets
| Ad type | Sensitivity (IU)
|
||
|---|---|---|---|
| PCR with E1A primersa | Hexon primersb
|
||
| PCR | Nested PCR | ||
| Ad2 | 1 | 10 | 0.1 |
| Ad41 | 0.01 | 1 | 0.01 |
PCR with the AdC-E1AF-AdC-E1AR primer pair for Ad2 and the AdF-E1AF-AdF-E1AR primer pair for Ad41.
PCR with the Hex1-Hex2 primer pair and nested PCR with the Hex1-Hex3 primer pair.
Detection kinetics of Ad2 and Ad41 infection at different MOIs by RT-PCR assay of mRNA.
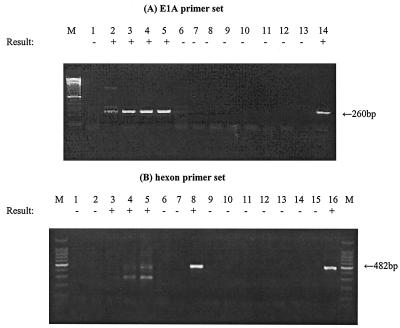
For mRNA extracts recovered from A549 cells infected with Ad2 at a multiplicity of infection (MOI) of 0.1 IU (106 IU per cell culture), E1A mRNA was detected as early as 6 h postinfection (p.i.) and hexon mRNA was detected as early as 24 h p.i. (Fig. 1). Both Ad mRNAs were detectable at subsequent intervals through 36 h p.i. An RT-PCR assay without RTase indicated that no viral DNA contamination remained in the mRNA extracts. For mRNA extracts recovered from G293 cells infected with Ad41 at an MOI of 0.1 (106 IU per cell culture), E1A mRNA was detected only after 7 days p.i. (Fig. 2). However, hexon mRNA was detected as early as 24 h p.i. and remained detectable through 7 days p.i. (Fig. 2). An RT-PCR assay without RTase indicated that no contaminating viral DNA remained in the mRNA extract.
FIG. 1.
Detection of Ad2 mRNA by RT-PCR assay at different times p.i. mRNA was extracted from A549 cells infected with Ad2 at an MOI of 0.1 IU/cell (106 IU in total per cell culture). (A) Lanes: 1 to 5, RT-PCR assay of mRNA extracted at 0, 6, 18, 24, and 36 h p.i.; 6, RT-PCR assay of cell control; 7 to 11, RT-PCR assay without RTase of mRNA extracted at 0, 6, 18, 24, and 36 h p.i.; 12, RT-PCR assay without RTase of cell control; 13, negative control; 14, positive control. (B) Lanes: 1 to 5, RT-PCR assay of mRNA extracted at 0, 6, 18, 24, and 36 h p.i.; 6, RT-PCR assay of cell control; 7, negative control; 8, positive control; 9 to 13, RT-PCR assay without RTase of mRNA extracted at 0, 6, 18, 24, and 36 h p.i.; 14, RT-PCR assay of cell control; 15, negative control; 16, positive control. A 100-bp DNA ladder was used as a molecular size marker (lane M). The detection sign (+ or −) was based on visual examination of the agarose gel for an amplicon of the correct size.
FIG. 2.
Detection of Ad41 mRNA by RT-PCR assay at different times p.i. mRNA was extracted from G293 cells infected with Ad41 at an MOI of 0.1 IU/cell (106 IU in total per cell culture). (A) Lanes: 1 to 6, RT-PCR assay of mRNA extracted at 0, 6, 12, 24, and 36 h and 7 days p.i.; 7, RT-PCR assay of cell control; 8, negative control; 9, positive control, 10 to 15, RT-PCR assay without RTase of mRNA extracted at 0, 6, 12, 24, and 36 h and 7 days p.i.; 16, RT-PCR assay without RTase of cell control; 17, negative control; 18, positive control. (B) Lanes: 1 to 6, RT-PCR assay of mRNA extracted at 0, 6, 12, 24, and 36 h and 7 day p.i.; 7, RT-PCR assay of cell control; 8, negative control; 9, positive control, 10 to 15 RT-PCR assay without RTase of mRNA extracted at 0, 6, 12, 24, and 36 h and 7 days p.i.; 16, RT-PCR assay without RTase of cell control; 17, negative control; 18, positive control. A 100-bp DNA ladder was used as a molecular size marker (lane M). The detection sign (+ or −) was based on visual examination of the agarose gel for an amplicon of the correct size.
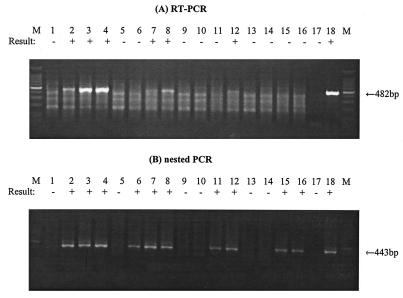
Figure 3 shows mRNA detection at different times p.i. for cell cultures inoculated with different amounts of Ad41. Approximately 107 cells of G293 were infected with different amounts (5 × 104, 5 × 102, 50, and 5 IU) of Ad41. Infected cells were harvested at 1, 2, 3, and 5 days p.i., and then mRNA extracts from infected cells were tested by an RT-PCR assays with the Hex1-Hex2 primer pair with and without RTase. When cells were infected with 5 × 104, 500, and 50 IU, hexon mRNA was detected as early as 1, 3, and 5 days p.i. by mRNA RT-PCR assay, respectively. Hexon mRNA was not detectable by the first-round RT-PCR assay when cells were infected with only 5 IU. However, greater detection sensitivities were obtained when a heminested PCR assay was performed. In cells infected with 5 × 104 and 5 × 102 IU of Ad41, hexon mRNA was detected within 1 day by the heminested RT-PCR assay. Hexon mRNA also was detected by heminested RT-PCR assay as early as 3 days p.i. in cells infected with 5 or 50 IU of Ad41.
FIG. 3.
Detection of Ad41 mRNA by RT-PCR assay and subsequent nested PCR assay with hexon primer sets at different MOIs of Ad41 and different times p.i. (A) Lanes: 1 to 4, RT-PCR assay of mRNA from G293 cells infected with 5 × 104 IU of Ad41 at 0, 1, 3, and 5 days p.i.; 5 to 8, RT-PCR assay of mRNA from G293 cells infected with 5 × 102 IU of Ad41 at 0, 1, 3, and 5 days p.i.; 9 to 12, RT-PCR assay of mRNA from G293 cells infected with 50 IU at 0, 1, 3, and 5 days p.i.; 13 to 16, RT-PCR assay of mRNA from G293 cells infected with 5 IU at 0, 1, 3, and 5 days p.i.; 17, negative control; 18, positive control. (B) Lanes: 1 to 4, nested PCR assay of mRNA from G293 cells infected with 5 × 104 IU of Ad41 at 0, 1, 3, and 5 days p.i.; 5 to 8, nested PCR assay of mRNA from G293 cells infected with 5 × 102 IU of Ad41 at 0, 1, 3, and 5 days p.i.; 9 to 12, nested PCR assay of mRNA from G293 cells infected with 50 IU at 0, 1, 3, and 5 days p.i.; 13 to 16, nested PCR assay of mRNA from G293 cells infected with 5 IU at 0, 1, 3, and 5 days p.i.; 17, negative control; 18, positive control. A 100-bp DNA ladder was used as a molecular size marker (lane M). The detection sign (+ or −) was based on visual examination of the agarose gel for an amplicon of the correct size.
Lower sensitivity limit for detection of Ad2 and Ad41 infection by mRNA RT-PCR assay.
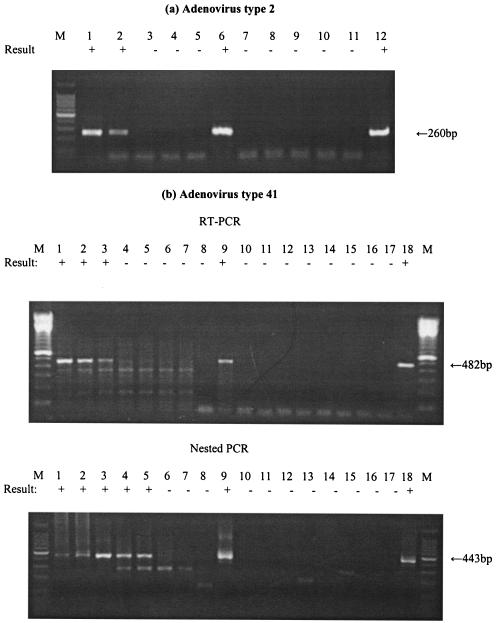
Figure 4 shows the results as RT-PCR products of Ad mRNA at different levels of viral inoculation into cell cultures. The RT-PCR assay detected as little as 2 IU of Ad2 E1A mRNA after 7 days p.i. (Fig. 4a). Six different samples containing from 103 to 10−3 IU of Ad41 were inoculated onto cell cultures, and mRNA was extracted after 7 days p.i. Ad41 hexon mRNA was detected at levels as low as 10 IU after 7 days p.i. by RT-PCR assay. The heminested PCR assay detected hexon mRNA for an inoculum level as low as 0.1 IU of Ad41 (Fig. 4b).
FIG. 4.
Sensitivity of detection of Ad2 and Ad41 mRNAs. An RT-PCR assay of Ad2 mRNA was performed with the E1A primer set, and an RT-PCR assay of Ad41 mRNA was performed with the hexon primer set. mRNA was recovered from infected cells after 5 to 7 days of incubation. (A) Lanes: 1 to 3, RT-PCR assay of Ad2 mRNA from A549 cells infected with 20, 2, and 0.2 IU; 4, RT-PCR assay of mRNA from noninfected A549 cells; 5, negative control; 6, positive control; 7 to 9, PCR assay of Ad2 mRNA from cells infected with 20, 2, and 0.2 IU; 10, PCR assay of mRNA in noninfected A549 cells; 11, negative control; 12, positive control. (B) Lanes: 1 to 7, RT-PCR assay or subsequent nested PCR assay of Ad41 mRNA from G293 cells infected with 103, 102, 10, 1, 0.1, 0.01, and 0.001 IU at 7 days p.i.; 8, negative control; 9, positive control; 10 to 16, RT-PCR assay without RTase or subsequent nested PCR assay of mRNA from G293 cells infected with 103, 102, 10, 1, 0.1, 0.01, and 0.001 IU at 7 days p.i.; 17, negative control; 18, positive control. A 100-bp DNA ladder was used as a molecular size marker (lane M). The detection sign (+ or −) was based on visual examination of the agarose gel for an amplicon of the correct size.
Ability of mRNA RT-PCR assay to distinguish between infectious and inactivated Ads exposed to free chlorine or UV radiation.
Ad2 and Ad41 in buffered water were treated with a 1-mg/liter dose of free chlorine for 1, 30, and 100 min and then inoculated into cell cultures. After 7 days p.i., mRNA was extracted from inoculated cells and subjected to an RT-PCR assay. As shown in Tables 3 and 4, only untreated Ad2 produced a CPE in A549 cells; chlorinated viruses produced no CPE in these cells. No Ad2 mRNA was detected by the E1A RT-PCR assay in cells inoculated with free-chlorine-treated viruses, but such mRNA was detected in cells inoculated with control viruses not treated with free chlorine. Ad DNA was detected by PCR assay in the virus inoculum even after the maximum dose of free-chlorine treatment. After 7 days p.i., no CPE was observed in any G293 cells inoculated with Ad41. As with Ad2, no hexon mRNA was detected by RT-PCR assay in G293 cells inoculated with free-chlorine-treated viruses, but hexon mRNA was detected by RT-PCR assay in cells inoculated with control viruses not treated with free chlorine. For both Ad2 and Ad41 not treated with free chlorine, increases in Ad DNA concentrations were observed by PCR assay after 7 days p.i. of cell cultures relative to the Ad DNA concentrations in initially inoculated cultures.
TABLE 3.
Results of culture, PCR, and mRNA RT-PCR of Ad2 disinfected by different Ct doses of free chlorine
| Method | Result obtained with free-chlorine dose as Ct (mg · min/liter)a of:
|
|||
|---|---|---|---|---|
| 0 | 1 | 30 | 100 | |
| CPEb | + | − | − | − |
| DNA detection (PCR) | + | + | + | + |
| Increased viral DNA during culture (PCR) | + | − | − | − |
| mRNA detectionc (RT-PCR) | + | − | − | − |
Ct dose = concentration (milligrams per liter) × contact time (minutes) in phosphate buffer (pH = 7.2) at 4°C. Approximately 105 IU of Ad2 was exposed to free chlorine (1 mg/liter) for a predetermined time and then used to inoculate A549 cells.
CPE on A549 cells after 7 days.
mRNA was extracted from infected A549 cells at 5 to 7 days p.i. and subjected to RT-PCR with the AdC-E1AF-AdC-E1AR primer pair.
TABLE 4.
Results of culture, PCR, and mRNA RT-PCR of Ad41 disinfected by differents Ct doses of free chlorine
| Method | Result obtained with free-chlorine dose as Ct (mg · min/liter)a of:
|
|||
|---|---|---|---|---|
| 0 | 1 | 30 | 100 | |
| CPEb | − | − | − | − |
| DNA detection (PCR) | + | + | + | − |
| Increased viral DNA during culture (PCR) | + | − | − | − |
| mRNA detectionc (RT-PCR) | + | − | − | − |
Ct dose = concentration (milligrams per liter) × contact time (minutes) in phosphate buffer (pH = 7.2) at 4°C. Approximately 103 IU of Ad41 was exposed to free chlorine (1 mg/liter) for a predetermined time and then used to inoculate Graham 293 cells.
CPE on Graham 293 cells after 7 days.
mRNA was extracted from infected G293 cells at 7 days p.i. and subjected to RT-PCR with the Ad1-Ad2 primer pair.
Ad2 and Ad41 in PBS were exposed to different doses (0, 50, 100, and 250 mJ/cm2) of collimated, monochromatic (254 nm) UV radiation. After 7 days p.i., mRNA was extracted from inoculated cells and subjected to RT-PCR assay. As shown in Tables 5 and 6, Ad2 either left untreated or exposed to lower doses (50 and 100 mJ/cm2) of UV light produced a CPE in A549 cells; Ad2 exposed to the highest UV dose produced no CPE in these cells. After 7 days p.i., no CPE was observed in any G293 cells inoculated with Ad41. Ad DNA was detected by PCR assay in the virus inoculum even after exposure to the maximum dose of UV light. No mRNA was detected in cell cultures inoculated with Ads exposed to the highest UV dose (250 mJ/cm2), whereas mRNA was detected in cell cultures inoculated with untreated virus (zero UV dose) and virus treated with the lower UV doses (50 and 100 mJ/cm2). For both Ad2 and Ad41 left untreated or treated at the lower UV doses (50 and 100 mJ/cm2), increases in Ad DNA concentrations were observed by PCR assay after 7 days p.i. of cell cultures relative to Ad DNA concentrations in initially inoculated cultures.
TABLE 5.
Results of culture, PCR, and mRNA RT-PCR of Ad2 disinfected by different doses of 254-nm UV radiation
| Method | Result obtained with UV dose (mJ/cm2)a of:
|
|||
|---|---|---|---|---|
| 0 | 50 | 100 | 250 | |
| CPEb | + | + | + | − |
| DNA detection (PCR) | + | + | + | + |
| Increased viral DNA during culture (PCR) | + | + | + | − |
| mRNA detectionc (RT-PCR) | + | + | + | − |
Dose = UV irradiance (milliwatts per square centimeter) × exposure time (seconds) in PBS (pH = 7.2) at room temperature. Approximately 5 × 105 IU of Ad2 was exposed to 254-nm UV light.
CPE on A549 cells after 7 days.
mRNA was extracted from infected cells at 5 to 7 days p.i. and subjected to RT-PCR with the AdC-E1AF-AdC-E1AR primer pair.
TABLE 6.
Results of culture, PCR, and mRNA RT-PCR of Ad41 disinfected by different doses of 254-nm UV radiation
| Method | Result obtained with UV dose (mJ/cm2)a of:
|
|||
|---|---|---|---|---|
| 0 | 50 | 100 | 250 | |
| CPEb | − | − | − | − |
| DNA detection (PCR) | + | + | + | + |
| Increased viral DNA during culture (PCR) | + | + | + | − |
| mRNA detectionc (RT-PCR) | + | + | + | − |
Dose = UV irradiance (milliwatts per square centimeter) × exposure time (seconds) in PBS (pH = 7.2) at room temperature. Approximately 103 IU of Ad41 was exposed to 254-nm UV light.
CPE on Graham293 cells after 7 days.
mRNA was extracted from infected G293 cells at 7 days p.i. and subjected to RT-PCR with the Ad1-Ad2 primer pair.
Validation of mRNA RT-PCR assay for Ads in environmental water sample concentrates.
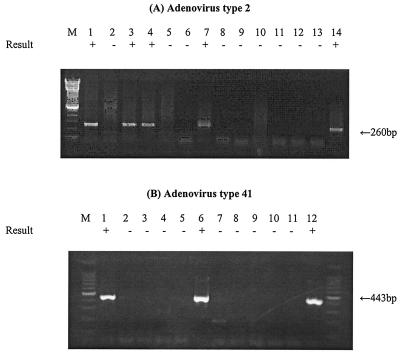
Figure 5 shows the results of experiments to determine the ability of the mRNA RT-PCR assay to detect infectious Ads in environmental water sample concentrates. Environmental water sample concentrates seeded with relatively low numbers of Ads were inoculated into cell cultures that were then incubated for 7 days, and then mRNA was extracted from the cultured cells and subjected to the RT-PCR assay. As shown in Fig. 5, the RT-PCR assay of Ad2 E1A mRNA detected as few as 20 IU in the North Carolina water sample concentrate and as few as 2 IU in the Georgia water sample concentrate. The heminested RT-PCR assay of Ad41 hexon mRNA detected as few as 10 IU in the Georgia water sample concentrate. Therefore, low levels of infectious Ad2 and Ad41 in concentrate from surface water samples could be detected by mRNA RT-PCR assay of inoculated cell cultures after 7 days of incubation. Detection of Ad2 mRNA exactly coincided with the presence of virus infectivity, as detected by CPE in cell cultures. No clear CPE was observed after 7 days of incubation in any G293 cells inoculated with Ad41.
FIG. 5.
RT-PCR assay detection of Ad mRNA from cells inoculated with environmental water concentrates seeded with Ads. mRNA was recovered from infected cells after 5 to 7 days of incubation. (A) Lanes: 1 and 2, RT-PCR assay of Ad2 mRNA from A549 cells infected with 20 and 2 IU in North Carolina water concentrate; 3 and 4, RT-PCR assay of Ad2 mRNA from cells infected with 20 and 2 IU in Georgia water concentrate; 5, RT-PCR assay of mRNA in noninfected A549 cells; 6, negative control; 7, positive control; 8 and 9, PCR assay of Ad2 mRNA from A549 cells infected with 20 and 2 IU in North Carolina water concentrate; 10 to 11, PCR assay of Ad2 mRNA from cells infected with 20 and 2 IU in Georgia water concentrate; 12, PCR assay of mRNA in noninfected A549 cells; 13, negative control; 14, positive control. (B) Lanes: 1 to 3, RT-PCR assay of Ad41 mRNA from G293 cells infected with 10, 1, and 0.1 IU in Georgia water concentrate; 4, RT-PCR assay of mRNA in noninfected G293 cells; 5, negative control; 6, positive control; 7 to 9, PCR assay of Ad41 mRNA from G293 cells infected with 10, 1, and 0.1 IU in Georgia water concentrate; 10, PCR assay of mRNA in noninfected G293 cells; 11, negative control; 12, positive control. The detection sign (+ or −) was based on visual examination of the agarose gel for an amplicon of the correct size.
DISCUSSION
The results of this study indicate that the Ad mRNA RT-PCR assay is an effective, sensitive, and rapid method by which to detect low levels of infectious Ads in water and clearly distinguish them from noninfectious viruses. Previous studies on the prevalence of Ads in surface and drinking water examined virus presence by DNA PCR assay. To overcome the potential for false-positive results in the PCR assay due to the amplification of DNA from noninfectious viruses, we have developed a method by which to detect infectious Ads based on RT-PCR amplification of viral mRNA. Because only infectious viruses can produce mRNA in cell cultures, this assay is specific for infectious viruses. The findings of this study are particularly applicable for quantitative detection of infectious enteric Ads (Ad40 and Ad41) because these viruses do not consistently produce clear CPE in cell cultures. To our knowledge, this is the first report of an unambiguous detection method for infectious DNA viruses in environmental samples with mRNA as the target for RT-PCR amplification.
Although detection of viable bacterial pathogens by RT-PCR assay of mRNA has been previously reported (18, 24), there are some important differences between bacterial and viral pathogens in the RT-PCR assay detection of mRNA as a measure of infectivity or viability. Detection of bacterial mRNA by RT-PCR assay is closely related to the kinetics of mRNA disappearance after loss of viability and culturability. Bacterial mRNA has been detected in dead Escherichia coli, including detection by RT-PCR assay (24), and therefore it is not an absolute indicator of viability or culturability when there is slow degradation of mRNA in bacteria or recently killed bacteria are present. However, viral mRNA does not pre-exist in the Ads before infection. Only when an infectious virus enters a host cell and transcribes mRNA during replication will viral mRNAs be present in inoculated cell cultures.
The results of this study also show that Ads inactivated by either free chlorine or UV can still be detected by PCR assay, but because they do not produce detectable mRNA in cell culture, they are not detected by mRNA RT-PCR assay. These results indicate that naked viral DNA or DNA fragments are not detected, and therefore, such DNA probably does not transfect cells and produce viral mRNA. Because Ads inactivated by both chemical (free chlorine) and physical (UV radiation) agents were not detected by mRNA RT-PCR assay, the developed method detects only infectious viruses and not those inactivated by disinfection processes.
Rapid and sensitive detection of Ads by RT-PCR assay of mRNA requires (i) rapid transcription of virion DNA after infection, (ii) sensitive RT-PCR amplification of target mRNA, and (iii) abundant mRNA expression during viral replication. The replication cycle of Ads is known to start when viral DNA enters the nucleus, about 30 min after virus adsorption (11, 17). The mRNA of Ad2 at late time periods in the replication cycle accounts for 20 to 30% of the total RNA synthesis in the cell. The copy number of Ad2 early mRNA transcripts reaches a steady state of approximately 500 to 1,000 copies per cell. Late mRNA transcripts reach a steady state of 2,000 to 5,000 copies per cell at 18 h p.i. and 20,000 to 50,000 copies per cell at 32 h p.i.
Compared to Ad2, Ad41 is more difficult to culture, and the copy numbers of Ad41 mRNAs are much lower than those of Ad2 (29). However, Ad41 has been successfully propagated in several cell lines, including G293, a line of human embryo kidney cells transformed with the Ad5 E1 region (13, 28). Ad41 is known to infect G293 cells in accordance with a single-hit model, which means that only one virus is sufficient to infect G293 cells productively (27). This single-hit model suggests that an mRNA RT-PCR assay should be able todetect a single infectious Ad41 particle in a G293 cell culture. Our results are generally consistent with these biological characteristics of Ad41 because very low levels (as little as 5 IU) of virus in the inocula were detected by mRNA RT-PCR assay of cell culture extracts. The results of this study indicated that mRNA RT-PCR assay of Ad41 requires longer incubation times p.i. and has a sensitivity lower than that of Ad2 mRNA RT-PCR assay detection. However, with a heminested PCR assay, it was possible to detect mRNA from cells infected with only 5 IU of Ad41 as early as 3 days p.i. The heminested PCR assay can also increase specificity, as well as sensitivity, in viral nucleic acid detection because of the need to recognize in succession two different target sequences of nucleic acid. However, stringent quality control is required for a nested PCR assay because even a small amount of contamination with PCR products can lead to false-positive results.
In the system developed to detect Ads by RT-PCR assay of mRNA, two primer pairs were used, one pair targeting an early transcript (E1A) and the other targeting a late transcript (hexon). The primers targeting the E1A region were designed to be subgenus specific, whereas those targeting the hexon region were designed to be genus specific. Transcription of the E1A gene is known to persist throughout infection, whereas the hexon gene is transcribed preferentially at late times after infection and reaches higher copy numbers during infection. Therefore, the E1A primers should have the advantage of rapid detection because of rapid expression, and the hexon primers should have the advantage of better sensitivity because of a higher copy number during infection. Our data show that both the E1A and hexon genes are increasingly expressed for up to 7 days. The mRNA RT-PCR assay with the E1A primers was more sensitive and rapid for Ad2, while the hexon primers was more sensitive for Ad41. This is because RT-PCR assay sensitivity depends on both the sensitivity of the RT-PCR assay per target mRNA and the number of target mRNAs present. However, both primer pairs show adequate sensitivity for detection of low copy numbers of mRNA in infected cells.
Detection of pathogenic viruses in environmental samples presents several important challenges: (i) low concentrations of viruses, (ii) the presence of various chemicals in samples that may inhibit nucleic acid extraction or nucleic acid amplification, (iii) high ratios of particles to infectious viruses, and (iv) small sample volumes (5 to 50 μl) for PCRs compared to the large volumes of environmental sample concentrates (typically 10 to 50 ml). The use of cell culture to amplify infectious viruses prior to RT-PCR assay can address many of these issues. Cell cultures increase the concentrations of infectious viruses and viral nucleic acids and reduce the effects of PCR inhibitors in the samples. In addition, the sample material from cultured cells can be reduced to a volume of 60 μl during the process of mRNA extraction, which additionally concentrates the samples approximately 10- to 100-fold.
Our data indicated a somewhat lower mRNA RT-PCR assay detection sensitivity when Ads were suspended in environmental water sample concentrates compared to PBS. These data suggest that inhibitors of virus infection in cell culture or inhibitors that interfere with virus recovery from inoculated cell cultures are likely present in environmental water concentrates. Various particles, dissolved solutes, and different conditions of ionic strength in water concentrates can potentially influence virus adsorption to particles, inhibit virus infection of cells, or reduce mRNA production in infected cells. Any of these effects could lead to reduced mRNA RT-PCR assay sensitivity for Ads in inoculated cell cultures.
As expected, an increase in viral DNA occurred during cultivation that coincided with mRNA detection by RT-PCR assay. This is because an infectious virus will transcribe mRNA and replicate its DNA genome during cultivation. Detection of DNA increases during cultivation can be a method of detecting the presence of infectious viruses. Viral DNA was extracted from only 140 μl of cultured cell material, but mRNA was extracted from the entire 10-ml volume of cell culture material. Therefore, the ability to detect a DNA increase might have been reduced compared to the ability to detect mRNA. The sensitivity of virion nucleic acid detection is especially important when cell cultures were inoculated with only a few slowly replicating Ads like Ad41. A second passage of the inoculated cell culture material can increase virus detection sensitivity (2). In this study, the mRNA of Ad41 was detected even when cells were infected with only 0.1 IU of infectious virus. This result may be due to the greater detection sensitivity of the mRNA RT-PCR assay than detection of an increase in virion DNA by PCR assay. It should be noted that the titer of the Ad41 stock used in these studies was estimated by increased viral DNA production in inoculated cell cultures. If an assay based on PCR amplification of DNA underestimates virus infectivity in cell cultures, then a more sensitive method by which to detect infectious virus, such as an RT-PCR assay of mRNA, would give higher titers of virus infectivity.
In future studies, this newly developed method needs to be tested with field isolates of Ads because not all strains are known to grow well in G293 and other cell cultures (20). In addition, other cell lines, such as Caco-2, Hep-2, and PLC/PRF/5, need to be tested for the detection of Ad40 and Ad41 mRNAs because these viruses do not grow especially well in G293 cells.
In summary, we have developed a new method for sensitive detection of viable Ads by RT-PCR assay of mRNA. The method is potentially capable of detecting low numbers of infectious Ads in complex environmental media such as concentrates from drinking water samples within 3 days. This method has the potential for widespread application in the detection of infectious Ads not only in water but also in wastewater, foods, and air.
Acknowledgments
This study was supported by grants from the American Water Works Association Research Foundation (AWWARF RFP no. 2591) and the Water Environment Research Federation (98-HHE-2).
We thank Dean Erdman for providing the sequence of a heminested PCR primer and Jan Vinjé for helpful advice on the primer design.
REFERENCES
- 1.Avery, R. M., A. P. Shelton, G. M. Beards, O. O. Omotade, O. C. Oyejide, and D. O. Olaleye. 1992. Viral agents associated with infantile gastroenteritis in Nigeria: relative prevalence of adenovirus serotypes 40 and 41, astrovirus, and rotavirus serotypes 1 to 4. J. Diarrhoeal Dis. Res. 10:105-108. [PubMed] [Google Scholar]
- 2.Blackmer, F., K. A. Reynolds, C. P. Gerba, and I. L. Pepper. 2000. Use of integrated cell culture-PCR to evaluate the effectiveness of poliovirus inactivation by chlorine. Appl. Environ. Microbiol. 66:2267-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt, C. D., H. W. Kim, W. J. Rodriguez, J. O. Arrobio, B. C. Jeffries, E. P. Stallings, C. Lewis, A. J. Miles, M. K. Gardner, and R. H. Parrott. 1985. Adenoviruses and pediatric gastroenteritis. J. Infect. Dis. 151:437-443. [DOI] [PubMed] [Google Scholar]
- 4.Brown, M., H. L. Wilson-Friesen, and F. Doane. 1992. A block in release of progeny virus and a high particle-to-infectious unit ratio contribute to poor growth of enteric adenovirus types 40 and 41 in cell culture. J. Virol. 66:3198-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castignolles, N., F. Petit, I. Mendel, L. Simon, L. Cattolico, and C. Buffet-Janvresse. 1998. Detection of adenovirus in the waters of the Seine River estuary by nested-PCR. Mol. Cell. Probes 12:175-180. [DOI] [PubMed] [Google Scholar]
- 6.Chapron, C. D., N. A. Ballester, J. H. Fontaine, C. N. Frades, and A. B. Margolin. 2000. Detection of astroviruses, enteroviruses, and adenovirus types 40 and 41 in surface waters collected and evaluated by the information collection rule and an integrated cell culture-nested PCR procedure. Appl. Environ. Microbiol. 66:2520-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung, H., L. A. Jaykus, and M. D. Sobsey. 1996. Detection of human enteric viruses in oysters by in vivo and in vitro amplification of nucleic acids. Appl. Environ. Microbiol. 62:3772-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz, J. R., P. Caceres, F. Cano, J. Flores, A. Bartlett, and B. Torun. 1990. Adenovirus types 40 and 41 and rotaviruses associated with diarrhea in children from Guatemala. J. Clin. Microbiol. 28:1780-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Jong, J. C., A. G. Wermenbol, M. W. Verweij-Uijterwaal, K. W. Slaterus, P. Wertheim-Van Dillen, G. J. Van Doornum, S. H. Khoo, and J. C. Hierholzer. 1999. Adenoviruses from human immunodeficiency virus-infected individuals, including two strains that represent new candidate serotypes Ad50 and Ad51 of species B1 and D, respectively. J. Clin. Microbiol. 37:3940-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eaton, A. D., L. S. Clesceri, and A. E. Greenberg. 1995. Standard methods, 19th ed. American Public Health Association, Washington, D.C.
- 11.Flint, S. J., and P. A. Sharp. 1976. Adenovirus transcription. V. Quantitation of viral RNA sequences in adenovirus 2-infected and transformed cells. J. Mol. Biol. 106:749-774. [DOI] [PubMed] [Google Scholar]
- 12.Foy, H. M. 1997. Adenoviruses, p. 119-138. In A. S. Evans and K. R. A. (ed.), Viral infections of humans: epidemiology and control, 4th ed. Plenum Medical Book Company, New York, N.Y.
- 13.Graham, F. L., J. Smiley, W. C. Russel, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-72. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann, J. E., D. M. Perron-Henry, and N. R. Blacklow. 1987. Antigen detection with monoclonal antibodies for the diagnosis of adenovirus gastroenteritis. J. Infect. Dis. 155:1167-1171. [DOI] [PubMed] [Google Scholar]
- 15.Jiang, S., R. Noble, and W. Chu. 2001. Human adenoviruses and coliphages in urban runoff-impacted coastal waters of Southern California. Appl. Environ. Microbiol. 67:179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson, M. E., I. Uhnoo, L. Svensson, C. A. Pettersson, and G. Wadell. 1985. Enzyme-linked immunosorbent assay for detection of enteric adenovirus 41. J. Med. Virol. 17:19-27. [DOI] [PubMed] [Google Scholar]
- 17.Lucas, J. J., and H. S. Ginsberg. 1971. Synthesis of virus-specific ribonucleic acid in KB cells infected with type 2 adenovirus. J. Virol. 8:203-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McIngvale, S. C., D. Elhanafi, and M. A. Drake. 2002. Optimization of reverse transcriptase PCR to detect viable Shiga-toxin-producing Escherichia coli. Appl. Environ. Microbiol. 68:799-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papapetropoulou, M., and A. C. Vantarakis. 1998. Detection of adenovirus outbreak at a municipal swimming pool by nested PCR amplification. J. Infect. 36:101-103. [DOI] [PubMed] [Google Scholar]
- 20.Perron-Henry, D. M., J. E. Herrmann, and N. R. Blacklow. 1988. Isolation and propagation of enteric adenoviruses in HEp-2 cells. J. Clin. Microbiol. 26:1445-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pina, S., M. Puig, F. Lucena, J. Jofre, and R. Girones. 1998. Viral pollution in the environment and in shellfish: human adenovirus detection by PCR as an index of human viruses. Appl. Environ. Microbiol. 64:3376-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds, K. A., C. P. Gerba, and I. L. Pepper. 1996. Detection of infectious enteroviruses by an integrated cell culture-PCR procedure. Appl. Environ. Microbiol. 62:1424-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shenk, T. E. 2001. Adenoviridae: the viruses and their replication, p. 2265-2300. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 24.Sheridan, G. E., C. I. Masters, J. A. Shallcross, and B. M. MacKey. 1998. Detection of mRNA by reverse transcription-PCR as an indicator of viability in Escherichia coli cells. Appl. Environ. Microbiol. 64:1313-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh-Naz, N., W. J. Rodriguez, A. H. Kidd, and C. D. Brandt. 1988. Monoclonal antibody enzyme-linked immunosorbent assay for specific identification and typing of subgroup F adenoviruses. J. Clin. Microbiol. 26:297-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sobsey, M. D., D. A. Battigelli, G. A. Shin, and S. Newland. 1998. RT-PCR amplification detects inactivated viruses in water and wastewater. Water Sci. Technol. 38:91-94. [Google Scholar]
- 27.Tiemessen, C. T., and A. H. Kidd. 1990. Adenovirus 41 growth in semi-permissive cells shows multiple-hit kinetics. Arch. Virol. 110:239-245. [DOI] [PubMed] [Google Scholar]
- 28.Tiemessen, C. T., and A. H. Kidd. 1995. The subgroup F adenoviruses. J. Gen. Virol. 76(Pt. 3):481-497. [DOI] [PubMed] [Google Scholar]
- 29.Tiemessen, C. T., M. J. Nel, and A. H. Kidd. 1996. Adenovirus 41 replication: cell-related differences in viral gene transcription. Mol. Cell. Probes 10:279-287. [DOI] [PubMed] [Google Scholar]
- 30.Videla, C., G. Carballal, A. Misirlian, and M. Aguilar. 1998. Acute lower respiratory infections due to respiratory syncytial virus and adenovirus among hospitalized children from Argentina. Clin. Diagn. Virol. 10:17-23. [DOI] [PubMed] [Google Scholar]
- 31.Walpita, P., and S. Darougar. 1989. Double-label immunofluorescence method for simultaneous detection of adenovirus and herpes simplex virus from the eye. J. Clin. Microbiol. 27:1623-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu, W., M. C. McDonough, and D. D. Erdman. 2000. Species-specific identification of human adenoviruses by a multiplex PCR assay. J. Clin. Microbiol. 38:4114-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]