Abstract
HIF-1α is a nuclear factor important in the transcription of genes controlling angiogenesis including vascular endothelial growth factor (VEGF). Both hypoxia and oxidative stress are known mechanisms for the induction of HIF-1α. Oxidative stress and mitochondrial permeability transition (MPT) are mechanistically important in acetaminophen (APAP) toxicity in the mouse. MPT may occur as a result of oxidative stress and leads to a large increase in oxidative stress. We previously reported the induction of HIF-1α in mice with APAP toxicity and have shown that VEGF is important in hepatocyte regeneration following APAP toxicity. The following study was performed to examine the relative contribution of hypoxia versus oxidative stress to the induction of HIF-1α in APAP toxicity in the mouse. Time course studies using the hypoxia marker pimonidazole showed no staining for pimonidazole at 1 or 2 h in B6C3F1 mice treated with APAP. Staining for pimonidazole was present in the midzonal to periportal regions at 4, 8, 24 and 48 h and no staining was observed in centrilobular hepatocytes, the site of the toxicity. Subsequent studies with the MPT inhibitor cyclosporine A showed that cyclosporine A (CYC; 10 mg/kg) reduced HIF-1α induction in APAP treated mice at 1 and 4 h and did not inhibit the metabolism of APAP (depletion of hepatic non-protein sulfhydryls and hepatic protein adduct levels). The data suggest that HIF-1α induction in the early stages of APAP toxicity is secondary to oxidative stress via a mechanism involving MPT. In addition, APAP toxicity is not mediated by a hypoxia mechanism.
Keywords: hypoxia, acetaminophen, pimonidazole, mitochondria, glutathione, toxicity
INTRODUCTION
Acetaminophen (paracetamol, N-acetyl-p-aminophenol, APAP) overdose is one of the leading causes of acute liver failure in the United States. The role of metabolism in the mediation of toxicity has been recognized for 40 years (Jollow et al., 1973; Mitchell et al., 1973; Mitchell et al., 1973; Potter and Hinson, 1987). In addition, oxygen and nitrogen stress are considered to be important in the mediation of toxicity (Brand et al., 2004; Casteilla et al., 2001; Koop et al., 1992; Sies et al., 1992; Hinson et al., 1998). The relationships between the development of oxidative stress and subsequent adaptive responses that occur in the liver in APAP toxicity have not been well investigated. Unraveling the mechanisms that trigger endogenous hepatoprotective responses in toxin mediated liver injury can have implications for the future development of novel treatments for liver injury.
In previous work, we reported the induction of hypoxia inducible factor 1α (HIF-1α) in APAP toxicity in mice (James et al., 2006). HIF-1α is a transcription factor that is stabilized in response to low oxygen tension, leading to the transcription of a number of genes important in maintaining overall cellular homeostasis, including genes important in angiogenesis, metabolism, and gluconeogenesis. Vascular endothelial growth factor (VEGF) is a well known target of HIF-1α transcription. We previously reported the upregulation of VEGF in the late phases of APAP toxicity (Donahower et al., 2006; Donahower et al., 2010). A number of reports have shown that VEGF is important to the recovery of the liver in models of partial hepatectomy, hepatic ischemia reperfusion injury, carbon tetrachloride toxicity, and APAP toxicity (LeCouter et al., 2003; Taniguchi et al., 2001; Tsurui et al., 2005; Papastefanou et al., 2007).
The role of hypoxia in the induction of HIF-1α has been well-characterized. During normoxia, the subunit HIF-1α is constitutively expressed and undergoes oxygen dependent proline hydroxylation whereby it is tagged for destruction through the ubiquitination system (Semenza, 2002). During hypoxia, proline hydroxylation is inhibited leading to the accumulation of the HIF-1α subunit in the cell where it binds to HIF-1β, another constitutively expressed subunit. The resulting heterodimeric complex translocates to the nucleus to activate genes that are important in maintaining cellular homeostasis including VEGF. Oxidative stress has also been identified as a mechanism for the induction of HIF-1α (Chandel et al., 2000; Duyndam et al., 2001; Pialoux et al., 2009; Bonello et al., 2007) and oxidative stress is mechanistically important in APAP toxicity (Albano et al., 1982; Hinson et al., 1998; Hinson et al., 2000). Evidence for the involvement of oxidative stress, including Fenton reactions and the involvement of superoxide, nitric oxide, and peroxynitrite, was recently reviewed (Hinson et al., 2009). Mitochondrial permeability transition (MPT) has also been implicated to be mechanistically important in APAP toxicity (Knight et al., 2001; Kon et al., 2004; Lemasters 1999; Reid et al., 2005). MPT results in the loss of mitochondrial membrane potential, leading to the failure of ATP synthesis, loss of cytochrome C and cell death (Lemasters et al., 1997). In support of this hypothesis, Jaeschke et al. (1990) reported the loss of ATP in liver homogenates in the early phases of APAP toxicity. ATP depletion may also occur as a result of hypoxia leading to anoxia and the loss of cell viability (LeMasters et al., 1981).
Our laboratory previously reported the induction of HIF-1α at 1 h in APAP toxicity in the mouse (James et al., 2006). This early time point is well before the onset of biochemical and histological changes indicating hepatocyte injury. In the following study, the relative contribution of hypoxia and oxidative stress via MPT to the induction of HIF-1α in APAP toxicity in the mouse was examined. We tested the hypothesis that HIF-1α induction in the early stages of APAP toxicity is secondary to oxidative stress. The MPT inhibitor cyclosporine A (CYC) was utilized to examine the relationship between oxidative stress and HIF-1α in mice with APAP toxicity. In addition, the potential relevance of hypoxia in the early stages of toxicity to the induction of HIF-1α was examined using pimonidazole, a marker of tissue hypoxia.
Materials and Methods
Drugs and Reagents
APAP was obtained from Sigma Chemical Co. (St. Louis, MO). CYC was obtained from Novartis (San Carlos, CA). Coomassie Plus Protein Assay Reagent was purchased from Pierce Chemical Co. (Rockford, IL). DTT (dithiothreitol; Cleland’s reagent) was obtained from Bio-Rad Laboratories (Hercules, CA). Gills Hematoxylin II and Permount were acquired from Fisher Scientific, Inc. (Pittsburgh, PA). Anti-HIF-1α monoclonal antibody was purchased from Novus Biologicals (Littleton, CO) and diluted 1:1000 immediately before use. Pimonidazole was purchased from Hypoxyprobe Inc. (Burlington, MA).
Experimental Animals
Six-week old male B6C3F1 mice (mean weight, 25.1 grams) were obtained from Harlan Sprague Dawley (Indianapolis, IN). All animal experimentation was in accordance with the criteria of the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences. Protocols for animal experimentation were approved by the institution’s Animal Care and Use Committee. Mice were acclimatized one week prior to the planned experiments and fed ad libitum. Animals were housed 3 per cage and maintained on a 12 h light/dark cycle. On the day prior to experiments, mice were fasted overnight and dosing studies began at 0800 the following morning. Food was returned to the mice 4 h after APAP. For the pimonidazole experiments, mice were injected with APAP (200 mg/kg IP) followed by pimonidazole hydrochloride (60 mg/kg IP; Hypoxyprobe, Burlington, MA) 1 h prior to animal sacrifice. Mice were sacrificed at the indicated times. In other studies, mice were administered APAP, immediately followed by CYC (50 mg/kg IP) and sacrificed at 1, 2, and 4 h after APAP. Other mice received APAP/vehicle and were sacrificed at the same time points. In another experiment, mice were administered APAP immediately followed by CYC (10 mg/kg or 20 mg/kg IP) and sacrificed at 30 or 60 minutes. Each experimental group included 3–6 mice. Animals were anesthetized with CO2 for blood sampling. Blood was removed from the retro-orbital plexus, allowed to coagulate at room temperature, centrifuged, and the serum was used for measurement of alanine aminotransferase (ALT). Mice were then euthanized in a CO2 atmosphere followed by cervical dislocation and removal of the livers. The time of CO2 narcosis was limited to less than 20 seconds to control for any potential effects of CO2 on HIF-1α induction. Pilot studies determined that neither saline injections nor short term (≤ 20 seconds) exposure to CO2 had a significant affect on HIF-1α induction. The livers were weighed and a portion was preserved in formalin for histological sections. The remaining livers were snap frozen in liquid nitrogen and stored at −80° C for additional analyses.
Liver Histology
Hematoxylin and eosin staining was performed for histological examination of the liver samples. Sections were reviewed independently by three reviewers that were blinded to the experimental groups. The sections were scored for necrosis using 0 as absent and 4 as severe.
Metabolism and Toxicity Assays
Serum ALT levels were measured using an ACE Alera (Alfa Wassermann, West Caldwell, NJ). APAP covalently bound to protein in liver was measured by initial protease treatment of liver homogenates followed by high performance liquid chromatography-electrochemical analysis for APAP-cysteine as previously described (Muldrew et al., 2002). Results are expressed as nanomoles APAP-Cysteine per mg liver protein (Roberts et al., 1991). Measurement of non-protein sulfhydryls (a measure of glutathione) was performed utilizing standard protocols using Ellman’s reagent.
HIF-1α nuclear extraction
HIF-1α was measured by western blot as previously described (James et al., 2006). Liver tissue for HIF-1α was prepared using Active Motif’s (Carlsbad, CA) Nuclear Extract kit. A peroxidase-conjugated goat anti-mouse IgG secondary antibody (Santa Cruz Biotechnology Inc, Santa Cruz, CA) at 1:2000 dilution for 1 h at room temperature. Band detection was performed using ECL Plus detection (Amersham, Piscataway, NJ).
Immunohistochemical analysis for pimonidazole
Liver sections were deparaffinized with xylene and then rehydrated using decreasing diluents of ethanol and finally distilled water. A peroxidase blocking step was performed using 3% hydrogen peroxide for 15 minutes followed by antigen retrieval. FITC-Mab 1 (Hypoxyprobe Inc., Burlington, MA) was used as the primary antibody (1:400) for 30 minutes. Horse radish peroxidase was used as the secondary antibody linked to rabbit anti-FITC IgG at a dilution of 1:100 for 30 minutes.
Statistical Analysis
Results are expressed as means ± SE. A p value of 0.05 was considered significant for all analyses. Comparisons between multiple groups were performed by one-way analysis of variance followed by the Tukey HSD post-hoc test. Non-parametric analysis (Kruskal Wallis and Mann Whitney) were used for analysis of data that was not normally distributed. SPSS Version 10.0 (SPSS Inc., Chicago, IL) was used for all statistical analyses.
Results
Dose response of HIF-1α in APAP toxicity
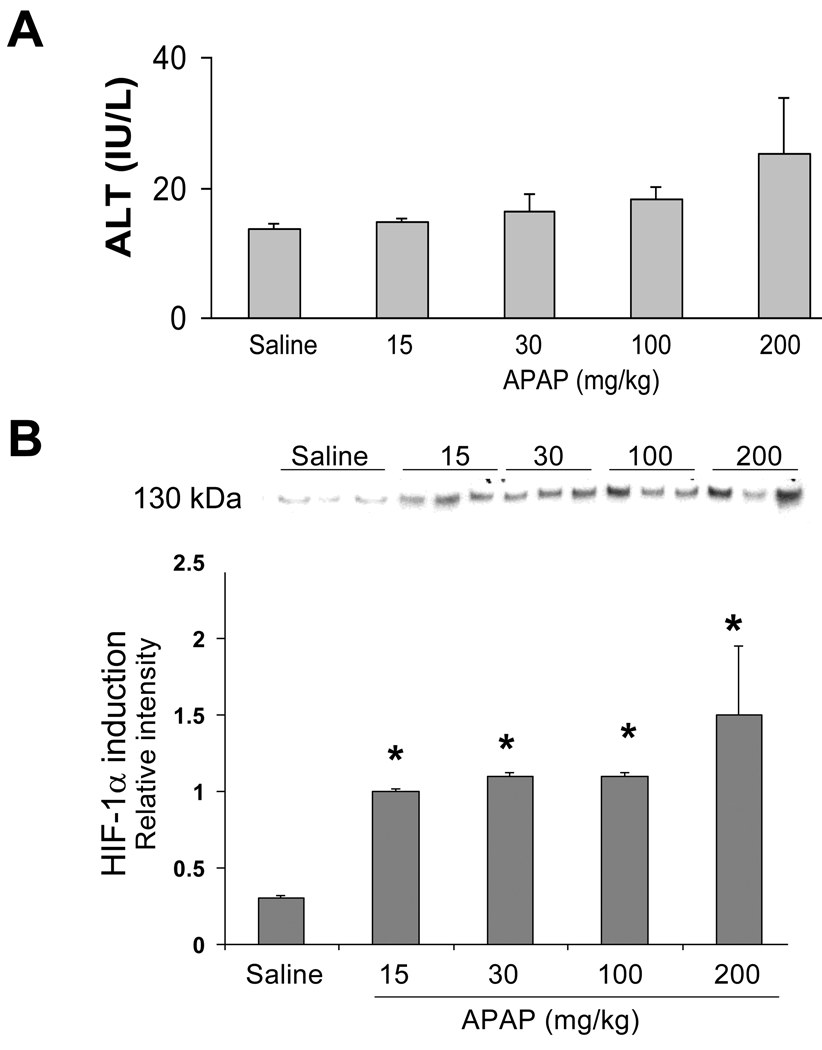
In previous work, we reported the induction of HIF-1α at 1 h in B6C3F1 male mice with APAP toxicity (James et al., 2006). To further examine HIF-1α induction in APAP toxicity in the mouse, B6C3F1 male mice were treated with APAP at 15, 30, 100, or 200 mg/kg by IP injection and sacrificed at 1 h. No significant changes were noted in serum ALT levels at 1 h as would be expected at this early time point (Fig. 1A). Western blot assays of nuclear extracts of mouse livers were performed to examine HIF-1α induction. As shown in Figure 1B, significant induction of HIF-1α was apparent at all doses of APAP (p<0.05).
Figure 1. Dose response examination of HIF-1α in acetaminophen toxicity.
B6C3F1 male mice were treated with saline or acetaminophen at 15, 30, 100 or 200 mg/kg IP and sacrificed at 1 h. A. ALT values were comparable in all groups of mice. B. Western blot assays of HIF-1α induction in hepatic nuclear extracts demonstrated that HIF-1α was increased in all APAP-treated groups of mice (*p<0.05).
Examination of the role of hypoxia in HIF-1α induction in APAP toxicity in the mouse
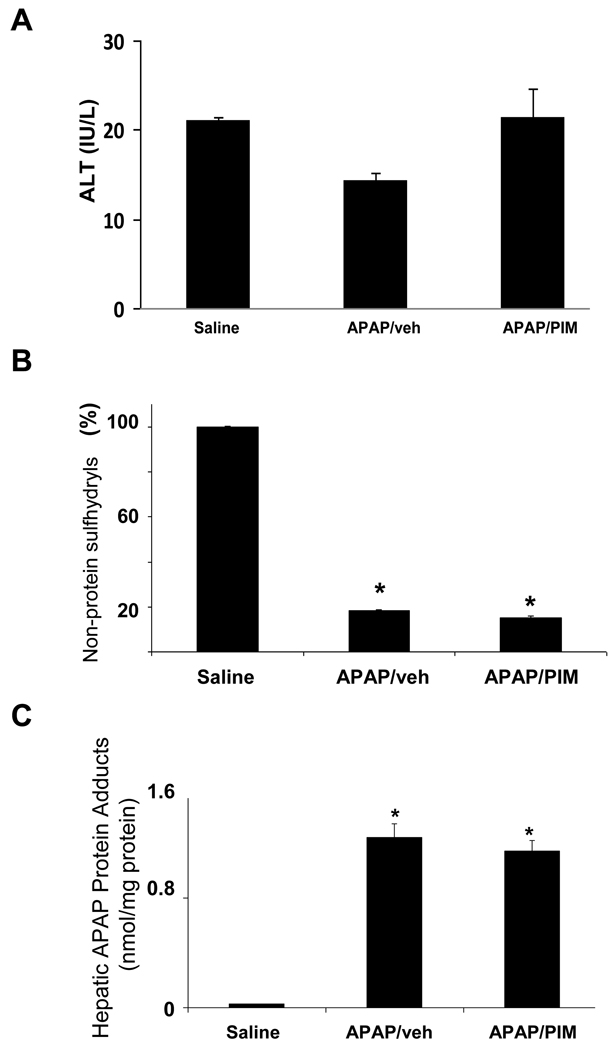
Pimonidazole is reductively activated in hypoxic conditions and is a commonly used marker of cellular hypoxia (Begg et al., 2001; Copple et al., 2003; Raleigh et al., 1998; Varia et al., 1998). In order to examine the possible role of hypoxia in the induction of HIF-1α in APAP toxicity, mice were treated with APAP (200 mg/kg IP) followed by pimonidazole (60 mg/kg IP). Other mice received APAP followed by vehicle IP. The mice were sacrificed at 1 h. To verify that pimonidazole had no affect on the metabolism of APAP, serum ALT, hepatic non-protein sulfhydryls and APAP protein adduct measurements were determined. Figure 2A demonstrates that ALT values were comparable between the mice, as would be expected at this early time point in toxicity. Hepatic non-protein sulfhydryls were depleted to comparable levels in both groups of APAP treated mice (Fig. 2B). In addition, measurement of hepatic APAP protein adducts was comparable between the two APAP treatment groups. Thus, the compound pimonidazole had no inhibitory affect on APAP metabolism that would preclude its use in the mouse model of APAP toxicity.
Figure 2. Effect of pimonidazole on acetaminophen (APAP) toxicity and metabolism.
B6C3F1 mice were treated with APAP (200 mg/kg IP) followed by pimonidazole (PIM) IV and sacrificed at 1 h. A. Serum ALT values. ALT values were comparable in all groups of mice. B. Hepatic non-protein sulfhydryls. Hepatic non-protein sulfhydryls were depleted to comparable levels in the APAP/veh and APAP/PIM mice, compared to the saline mice (*p<0.05). C. APAP protein adduct levels in liver. APAP protein adducts were reduced in the APAP/veh and the APAP/PIM levels (*p<0.05) and the reduction was comparable between the two groups.
Time course of pimonidazole expression in APAP toxicity in the mouse
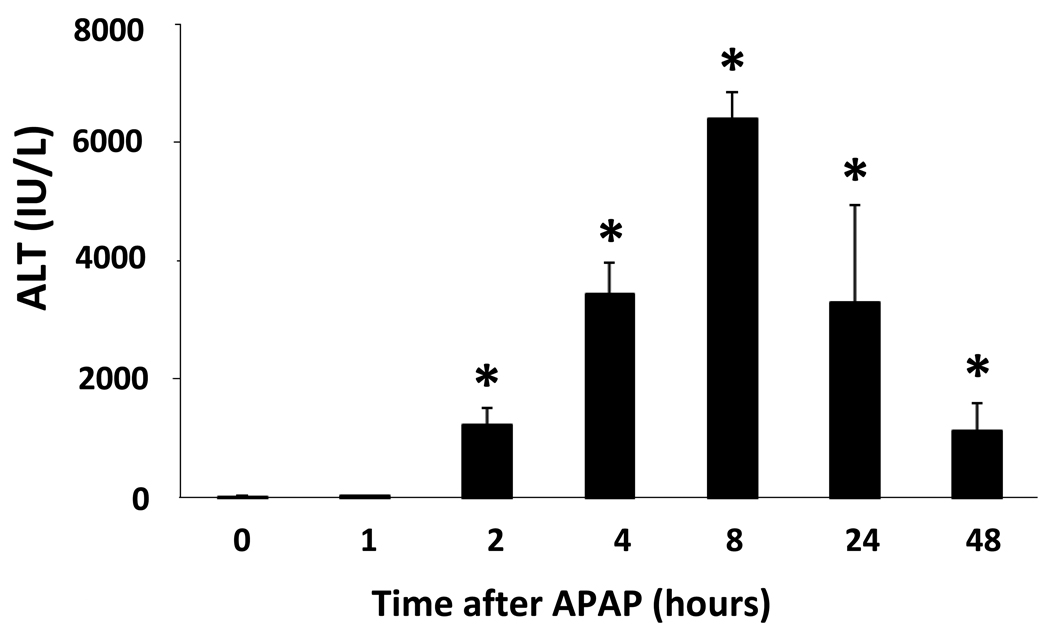
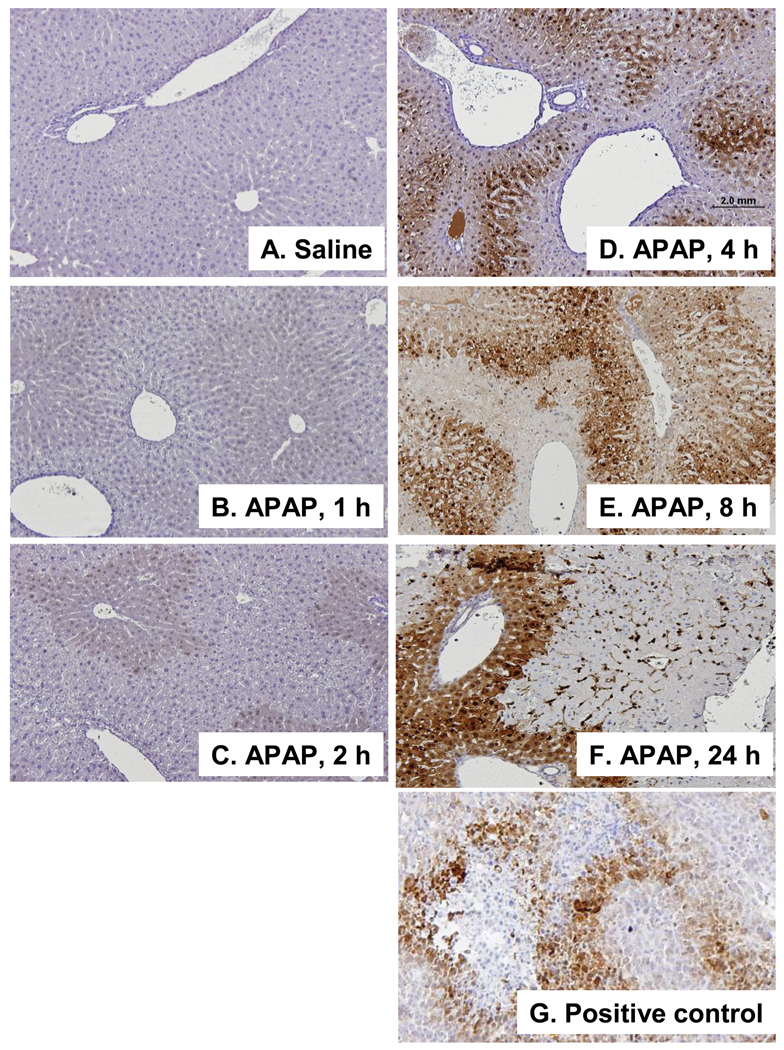
To examine the temporal sequence and regional distribution of hypoxia in APAP toxicity, B6C3F1 male mice were treated with APAP (200 mg/kg IP) and sacrificed at 1, 2, 4, 8, 24, and 48 h after APAP administration. One hour prior to animal sacrifice, the mice were treated with pimonidazole 60 mg/kg IP. Figure 3 demonstrates the time course of toxicity in the mice and shows that ALT values were significantly increased above saline at 2, 4, 8, 24 and 48 h. Sections from the APAP and saline treated mice were stained for pimonidazole. Liver sections from an FSall tumor in a C3 mouse served as the positive control (Fig 4F). Analysis of immunohistochemical assays for pimonidazole showed no staining in the saline mice (Fig. 4A) or the APAP mice at 1 or 2 h (Fig. 4B; Fig. 4C). At 4 h, mild to moderate staining for pimonidazole was noted in the midzonal regions of the liver (Fig. 4D). The intensity and distribution of the midzonal staining was increased at 8 h and staining of the endothelial cells lining the sinusoids in the midzonal regions was also noted at this time point. By the 24 h time point, there was enhanced staining that encompassed the midzonal to periportal regions (Fig. 4E). These same findings persisted at 48 h (data not shown). Overall, the staining was initially present in the midzonal regions and progressed to the periportal regions over time and there was sparing of the centrilobular regions. Collectively, the immunohistochemical data suggest that hypoxia was not an operant mechanism in the early stages (1–2 h) of toxicity.
Figure 3. Time course of APAP toxicity.
B6C3F1 mice were treated with APAP (200 mg/kg IP) followed by pimonidazole (PIM) IP and sacrificed at the indicated times. Serum ALT values were increased at 2, 4, 8, and 24 and 48 h compared to saline treated mice (*p<0.05).
Figure 4. Time course of pimonidazole binding in APAP toxicity in the mouse.
A. Saline control. B. Mouse at 1 h indicating no staining for pimonidazole. C. Mouse at 2 h indicating no staining for pimonidazole. D. Mouse at 4 h indicating mild to moderate staining in the midzonal regions and sparing of staining in the regions around the CV. E. Mouse at 8 h showing wider distribution of pimonidazole staining. F. Mouse at 24 h showing intense staining for pimonidazole in the midzonal and periportal regions. G. Positive control (F Sall tumor). All images are 10X magnification.
Effect of Cyclosporine A on the early stages of APAP toxicity
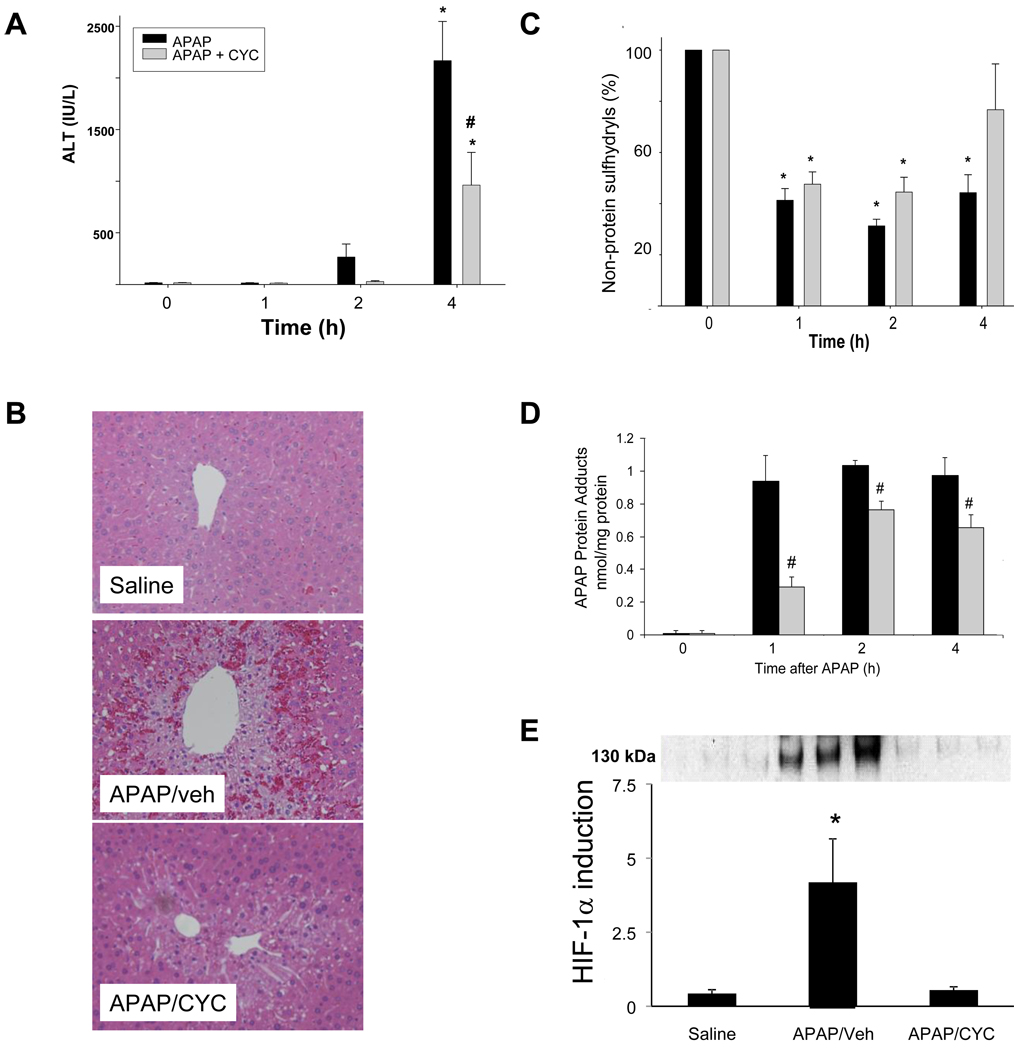
CYC, an MPT inhibitor, was previously reported to reduce APAP toxicity and the mechanism was postulated to be blockade of MPT and oxidative stress in vivo (Masubuchi et al., 2005). To test the hypothesis that HIF-1α induction is secondary to oxidative stress through the mechanism of MPT, mice were treated with CYC and APAP. Mice received 50 mg/kg IP CYC (Masubuchi et al., 2005) or vehicle (VEH) immediately after APAP and the mice were sacrificed at 1, 2, and 4 h. As shown in Figure 5A, ALT was increased above the saline treated mice in both APAP groups at 4 h. However, ALT levels were lower in the mice that received APAP/CYC compared to the APAP/veh mice. Consistent with the ALT results, mice receiving APAP/CYC had reduced necrosis scores compared to the APAP/veh mice (Table 1A; Fig. 5B). To assess the effect of CYC on the metabolism of APAP, liver samples were analyzed for hepatic non-protein sulfhydryls and APAP protein adducts, respectively. As demonstrated in Figure 5C, hepatic non-protein sulfhydryls were depleted to comparable levels at 1 and 2 h in the APAP/veh mice and the APAP/CYC mice. At 4 h, hepatic non-protein sulfhydryls rebounded to levels that approached baseline in the APAP/CYC group, but remained low in the APAP/veh group. Furthermore, hepatic levels of APAP protein adducts were increased in the APAP/veh and the APAP/CYC mice at 1, 2, and 4 h. However, levels of hepatic APAP protein adducts were lower in the mice that received APAP/CYC (Fig. 5D). Thus the data demonstrated that CYC at a dose of 50 mg/kg reduced APAP toxicity by inhibiting the metabolism of APAP.
Figure 5. Effect of cyclosporine A (50 mg/kg) on acetaminophen metabolism and toxicity in mice.
Mice were treated with APAP followed by cyclosporine A (50 mg/kg) and sacrificed at the indicated times. A. Mean (±SE) values of serum ALT, an indicator of hepatic toxicity. ALT was significantly increased in the APAP/veh and APAP/CYC mice at 4 h compared to the saline mice (*p ≤ 0.05). Mean ALT was lower at 4 h in the APAP/CYC mice (#p<0.05) compared to the APAP/veh mice. B. H&E stained sections (10X) of representative saline, APAP/veh, and APAP/CYC mice at 4 h, indicated decreased necrosis in the APAP/CYC mice. C. Hepatic non-protein sulfhydryls. The APAP/veh and APAP/CYC mice had comparable amounts of non-protein sulfhydryl depletion, compared to the saline mice, *p ≤ 0.05. D. Hepatic levels of APAP protein adducts (mean ± SE). Adduct values were significantly increased in APAP/veh and APAP/CYC mice (*p<0.05) compared to the saline mice. However, the APAP/CYC mice had APAP protein adduct levels that were significantly lower (#p<0.05) than the APAP/veh mice. E. HIF-1α induction in nuclear extracts for APAP treated livers at 1 h. The three lanes are representative examples of the mice treated with saline, APAP/veh, or APAP/CYC. Densitometry analysis of western blots demonstrated that HIF-1α induction was reduced in the APAP/CYC mice (*p<0.05), compared to the APAP/veh mice.
Table 1.
Necrosis Scores at 4 hours
| Mean | ± SE | Significance | |
|---|---|---|---|
| A. | |||
| APAP/veh | 3 | 0.3 | *p<0.05 vs. saline |
| APAP/CYC 50 mg/kg | 2 | 0.4 | *p<0.05 vs. saline; **p<0.05 vs. APAP/veh |
| Saline | 0 | 0 | |
| B. | |||
| APAP/veh | 2 | 0.2 | *p<0.05 vs. saline |
| APAP/CYC 10 mg/kg | 2 | 0.3 | *p<0.05 vs. saline |
| Saline | 0 | 0.1 | |
Western blot assays for HIF-1α induction were performed using the nuclear extracts from the livers of the mice sacrificed at 1 h. HIF-1α induction was significantly increased above saline in the APAP/veh mice; however, HIF-1α induction in the APAP/CYC mice was similar to that of the saline treated mice (Fig. 5E).
Effect of Low Dose CYC on APAP toxicity and metabolism
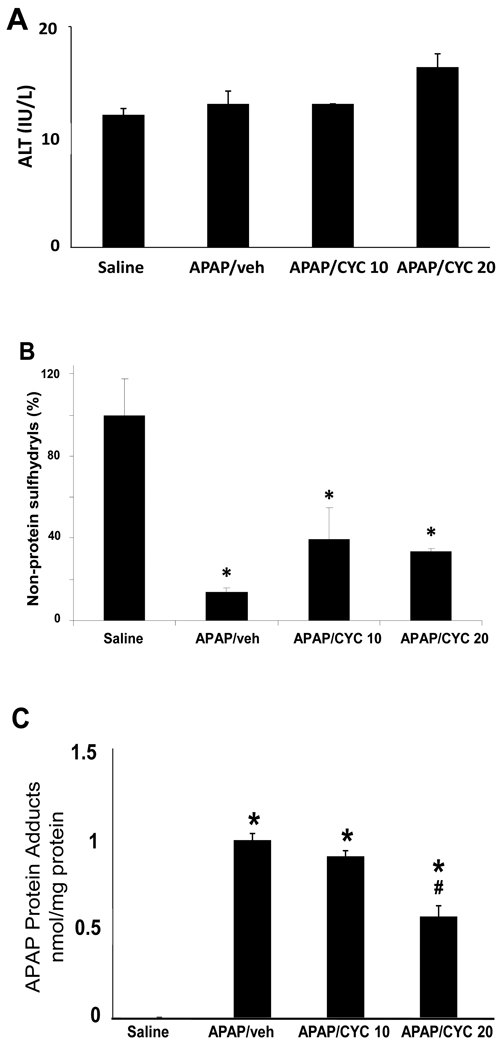
Based on the results of the 50 mg/kg CYC study described above, additional studies were designed to examine lower doses of CYC that did not inhibit the metabolism of APAP. Mice were treated with CYC at 10 or 20 mg/kg and were sacrificed at 30 minutes or 1 h. At 30 minutes, no differences were detected in ALT, hepatic non-protein sulfhydryls, hepatic protein adducts or HIF-1α induction for the mice treated with APAP/CYC 10 mg/kg compared to the mice treated with APAP/saline (data not shown). For the mice treated with APAP/CYC 20 mg/kg and sacrificed at 30 minutes, no differences were detected in ALT levels or depletion of hepatic non-protein sulfhydryls, compared to the APAP/saline mice at 30 minutes. However, APAP protein adduct levels were decreased in APAP/CYC 20 mg/kg mice at 30 minutes, compared to the APAP/saline mice at 30 minutes. The 1 hour data for these experiments (Fig. 6A) demonstrated comparable values for ALT and comparable depletion of hepatic non-protein sulfhydryls among the three groups of APAP treated mice (Fig. 6B). However, differences in the levels of hepatic APAP protein adducts were noted among the groups at 1 h. Levels of hepatic protein adducts were increased above saline in the APAP/veh, APAP/CYC 10 mg/kg and the APAP/CYC 20 mg/kg mice (Fig. 6C), but were significantly lower in the APAP/CYC 20 mg/kg mice compared to the other APAP treated groups. These data indicated that the 20 mg/kg dose of CYC also inhibited the metabolism of APAP at 30 minutes and at 1 hour.
Figure 6. Comparison of CYC 10 mg/kg and CYC 20 mg/kg in acetaminophen (APAP) treated mice at 1 h.
Mice were treated with APAP/veh, APAP/CYC (10 mg/kg), or APAP/CYC (20 mg/kg) or saline and sacrificed at 1 h. A. ALT values in the APAP and saline mice. No differences were detected between the four groups of mice at 1 h. B. Hepatic non-protein sulfhydryl levels. Hepatic non-protein sulfhydryl levels were equally depleted in all APAP groups compared to the saline mice (*p<0.05). C. Hepatic APAP protein adduct levels. Adduct levels were significantly increased above saline mice in the APAP/veh, APAP/CYC (10 mg/kg), and APAP/CYC (20 mg/kg) mice (*p<0.05). However, adducts were lower in the APAP/CYC (20 mg/kg) group (#p<0.05) compared to the APAP/CYC (10 mg/kg) group.
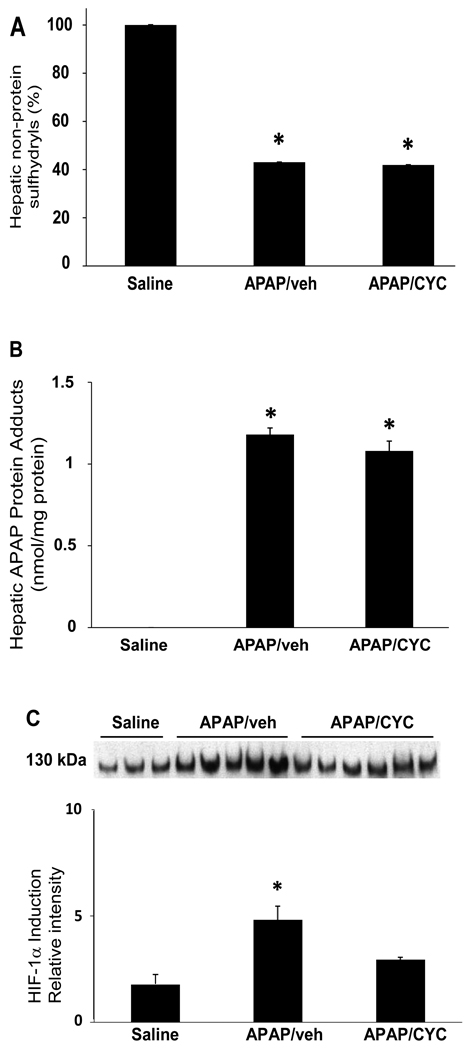
To further examine the CYC 10 mg/kg dose, a separate experiment was performed in which mice were treated with APAP/veh or APAP/CYC 10 mg/kg and sacrificed at 1 h. ALT (data not shown) values were comparable in the saline mice, the APAP/veh, and the APAP/CYC mice at 1 h and consistent with the data above (Fig 6A). Levels of hepatic non-protein sulfhydryls (Fig 7A) were reduced to comparable levels in the APAP/veh and the APAP/CYC mice. Similarly, hepatic APAP protein adduct levels (Fig. 7B) were elevated in the APAP/veh and APAP/CYC 10 mg/kg mice and consistent with the results above (Fig. 6C). HIF-1α induction was examined and indicated that HIF-1α induction was increased in the APAP/veh mice at 1 h but was reduced in the APAP/CYC 10 mg/kg mice, as demonstrated by densitometry analysis of individual mice (Fig. 7C). Thus, the CYC 10 mg/kg dose did not alter the metabolism of APAP and reduced HIF-1α induction, implicating the involvement of MPT in HIF-1α in APAP toxicity. These data clearly indicated that HIF-1α induction was a result of additional events important in the development of APAP toxicity.
Figure 7. HIF-1α induction in mice treated with APAP/CYC 10 mg/kg, sacrificed at 1 h.
Mice were treated with APAP/veh, APAP/CYC (10 mg/kg), or saline and sacrificed at 1 h. ALT values were comparable between the groups. A. Non-protein sulfhydryls. Non-protein sulfhydryl depletion was comparable in the APAP/veh and APAP/CYC mice (*p<0.05) compared to saline mice. B. APAP protein adducts. APAP protein adducts were increased above saline in both groups of APAP mice (*p<0.05). C. Densitometry analysis of western blots of HIF-1α in hepatic liver confirmed that HIF-1α induction was reduced in individual APAP/CYC mice (*p<0.05) compared to APAP/veh mice.
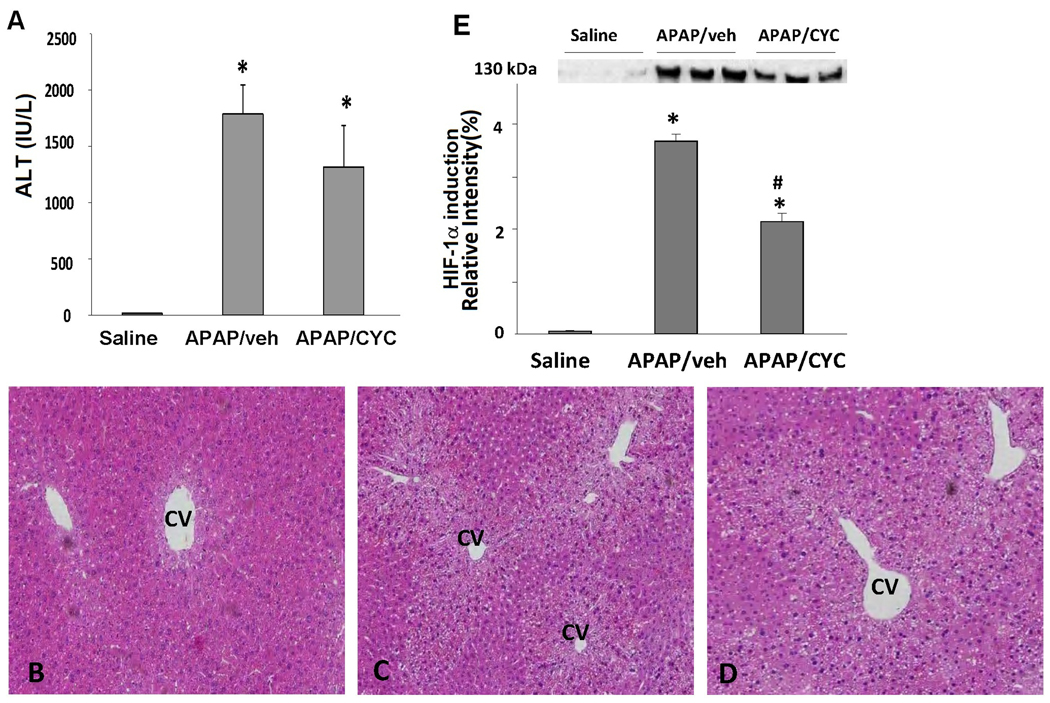
Effect of CYC 10 mg/kg on acetaminophen toxicity and HIF-1α induction at 4 h
In further experiments, the duration of effect of the MPT inhibitor CYC 10 mg/kg on HIF-1α induction in APAP toxicity was examined. In this experiment, mice were treated with APAP/veh or APAP/CYC 10 mg/kg and sacrificed at 4 h. ALT values were significantly increased in the APAP/veh and the APAP/CYC mice compared to the saline mice and there were no differences in ALT values between the APAP groups (Fig. 8A). Histologic examination of liver tissues of the mice showed similar degrees of hepatic necrosis in the two groups of APAP treated mice (Table 1B; Fig. 8B). Similar to the 1 h data (Fig. 7A, Fig. 7B), hepatic GSH and APAP protein adducts were comparable among the APAP/veh and APAP/CYC mice at 4 h (data not shown). Western blot assays for HIF-1α induction in the APAP treated mice showed that HIF-1α was strongly induced at 4 h in the APAP/veh treated mice (Fig. 8C). However, HIF-1α induction was reduced in the APAP/CYC compared to the APAP/veh mice. Thus, CYC 10 mg/kg reduced HIF-1α induction in APAP toxicity in mice at 1 h and this was a sustained effect that persisted at 4 h.
Figure 8. Effect of CYC (10 mg/kg) on APAP toxicity and HIF-1α induction at 4 h.
B6C3F1 mice were treated with APAP (200 mg/kg IP) followed by CYC (10 mg/kg IP) or vehicle (veh). The mice were sacrificed at 4 h. A. Serum ALT values. ALT values were increased above saline in the APAP/veh and APAP/CYC mice (*p<0.05). B. Representative H&E stained sections in mice treated with saline, APAP/veh and APAP/CYC. The APAP/veh and APAP/CYC treated mice had increased necrosis compared to the saline mice. C. Western blot assays and densitometry of HIF-1α induction in liver homogenates. HIF-1α induction was reduced in the APAP/CYC (*p<0.05) compared to the APAP/veh mice at 4 h.
Discussion
HIF-1α is a major regulator of cellular homeostasis and is best known for its role in triggering adaptive responses secondary to hypoxia. The transcriptional upregulation of HIF-1α is associated with gene upregulation for pathways associated with angiogenesis, antioxidant defense and gluconeogenesis. The non-hypoxic induction of HIF-1α is less well understood. Studies using in vitro approaches have shown that oxidative stress may induce HIF-1α. For example, it was shown that arsenite induces oxidative stress in pre-adipocyte cells leading to the induction of HIF-1α protein levels in human ovarian cancer cells (Salazard et al., 2004). Deshmane (Deshmane et al., 2009) showed that a viral protein known as Vpr induced oxidative stress by increased hydrogen peroxide formation that in turn led to HIF-1α induction in HIV-1 infected neurons. In addition, nitrosylation reactions (i.e., posttranslational protein modifications that involve the covalent attachment of a NO group to the sulfhydryl groups on proteins) have been shown to lead to HIF-1α stabilization, leading to HIF protein induction [reviewed in (Sun et al., 2006)] and the transcription of target genes.
Oxidative stress has been well established as an early phase event in APAP mediated toxicity. Early supporting data for the role of oxidative stress in APAP toxicity include reports citing the upregulation of inducible nitric oxide synthase (Gardner et al., 2002) in APAP toxicity in rats and the colocalization of nitrotyrosine, an indicator of peroxynitrite, with necrotic hepatocytes in APAP toxicity in mice (Hinson et al., 1998). Peroxynitrite is formed by the rapid reaction of superoxide (SO) with nitric oxide. Subcellular fractionation studies have previously shown that peroxynitrite is primarily localized to the mitochondria of APAP treated animals (Cover et al., 2000). Although less is known about SO, some evidence indicates its involvement in APAP toxicity. The administration of human recombinant superoxide dismutase (SOD) to rats decreased their susceptibility to the toxic effects of APAP (Nakae et al., 1990). However a more recent report suggested that SOD 1 knockout mice were more resistant to APAP toxicity than wild type mice (Lei et al., 2006). These mice had lower ALT values and less glutatione depletion than the WT mice. However, the protection conferred by SOD1 deletion was associated with less hepatic protein nitration and reduced levels of CYP2E1. The reduced levels of CYP2E1 suggest that the mechanism was decreased metabolism of APAP to the toxic metabolite.
The involvement of MPT in APAP toxicity has also been addressed by multiple laboratories (Kon et al., 2004; Kon et al., 2007; Masubuchi et al., 2005; Reid et al., 2005). MPT is a common mechanism implicated in cellular injury and has been reported to occur in a number of models of cellular injury involving ischemia, anoxia, and oxidative stress. MPT may occur through increased Ca2+, a known mechanism in APAP toxicity (Corcoran et al., 1987), as well as oxidative stress. Since we previously reported that HIF-1α induction occurred very early in APAP toxicity (i.e., 1 h) (James et al., 2006), (Fig 1) and prior to evidence of hepatic injury, we postulated that HIF-1α induction was secondary to oxidative stress and that administration of a MPT inhibitor to APAP treated mice would reduce HIF-1α induction.
Hypoxia is a well described mechanism by which hepatotoxicity may occur (Fuhrmann et al., 2010; Kan et al., 2008; Schemmer et al., 1999) and from a physiologic standpoint, the centrilobular regions of the liver are the most susceptible to hypoxia (Schemmer et al; 1999). It is not believed that APAP toxicity is mediated by a hypoxic mechanism, but the evidence supporting this conclusion is indirect (Roberts et al., 1991). Significant microcirculatory alterations occur early in APAP toxicity and these alterations could lead to hypoxia (McCuskey et al., 2006; McCuskey et al., 2008). For example, experiments performed by Ito and McCuskey (Ito et al., 2004) using intravital microscopy showed that swelling to sinusoidal endothelial cells occurred as early as a half hour after APAP. In addition, extravasation of red blood cells from the sinusoids to the extra-sinusoidal regions occurred by 2 h, but was not statistically significant until 6 h (Ito et al., 2004). To test the potential role of hypoxia in APAP toxicity, studies with pimonidazole were performed to examine the temporal relationship between toxicity events and the expression of cellular markers of hypoxia. Oxygen-dependent nitroreductase activity is homogenously distributed throughout the liver (Arteel et al., 1995) and 2-nitroimidazole binding, as reflected by immunohistochemical assays for pimonidazole, occurs whenever regional oxygen concentrations are less than 14 uM (Varia et al., 1998). Initial studies confirmed that pimonidazole did not alter the metabolism of APAP (Fig. 2), nor did it alter the sensitivity of the mice to APAP. Subsequent examination of the temporal profile and regional localization of pimonidazole staining in the livers of the APAP treated mice showed no evidence of hypoxia at 1 or 2 h (Fig. 3). Throughout the 48 h time course study, the pattern of staining increased in intensity across time and was distributed initially in the midzonal regions with progression to periportal regions of the liver. Notably, the centrilobular regions were spared. Thus the time course and distribution of pimonidazole staining failed to implicate hypoxia as a relevant mechanism for either toxicity or for induction of HIF-1α in the early stages of APAP toxicity. Although the periportal regions of the liver are less susceptible to hypoxia than the centrilobular regions of the liver (Schemmer et al., 1999), previous data on covalent binding in APAP toxicity lend support to the data of the present manuscript. For example, previously published data of immunohistochemical assays for covalent binding, an oxygen dependent, P450-mediated event, showed homogenous and intense binding at early time points (eg., 1 and 2 h) in APAP treated mice (Robert et al., 1991). The pattern of intense pimonidazole staining at later time points (24 and 48 h) in the time course with higher distribution in the periportal regions may be consistent with hepatic ischemia secondary to congestion and pooling of blood in the hepatic parenchyma (Bajt et al., 2008). Peak levels of hemoglobin in liver in mice treated with APAP have been previously shown to occur at 6 hours and persist until at least 24 h (Lawson et al., 2000).
Alternatively it is possible that injury to the sinusoidal endothelium (McCuskey et al., 2006; McCuskey et al., 2008; Ito et al., 2004) in the very early stages of APAP toxicity may have limited the distribution of PIM into the centrilobular areas of the liver. We previously noted that significant elevation of plasma hyaluronic acid did not occur until 4 h in mice with APAP toxicity (Donahower et al., 2010). Since plasma hyaluronic acid is a functional marker of sinusoidal endothelial cell injury, it is likely that the microcirculation in the early stages of APAP toxicity would be sufficient to allow for distribution of PIM to the cells in the centrilobular areas. Consistent with our data, Ito et al. also noted changes in the hepatic sinusoidal diameter and the intersinusoidal distance at the 4 hr time point (Ito et al., 2003). In addition, Yin et al. (Yin et al., 2009) also recently reported staining for pimonidazole at the 5 h time point in APAP toxicity in the Balb/cJ mouse.
CYC has been previously utilized as an inhibitor of MPT in both in vitro and in vivo studies of APAP toxicity. CYC is reported to inhibit MPT by binding to cyclophilin D, one of the three proteins comprising the MPT pore (Halestrap et al., 1997). Kon et al. (Kon et al., 2004) utilized CYC to block MPT in an in vitro model of cultured hepatocytes exposed to APAP. He demonstrated that CYC blocked both MPT and toxicity but these effects were short-lived (Kon et al., 2004). MPT was noted to be sensitive to CYC at early time points (3–6 h after APAP), but insensitive to CYC at later time points (9–16 h after APAP). Similar short term effects on MPT were observed using another MPT inhibitor, NIM811, a non-immunosuppressive analogue of CYC (Kon et al., 2004). CYC was also utilized in experiments involving freshly isolated hepatocytes treated with APAP and was shown to block MPT (Reid et al., 2005). Because freshly isolated hepatocytes do not lose cytochrome P450 activity, they may represent a better in vitro model for studies of APAP toxicity. In this model, (Reid et al., 2005) the addition of CYC to hepatocyte media at 2 h, after the metabolic phase of toxicity, was associated with protection from toxicity, a reduction in oxidative stress, and a reduction in the loss of mitochondrial membrane potential.
In the present study, high dose CYC (50 mg/kg) protected from toxicity but interfered with the metabolism of APAP (Fig. 5D) and subsequently lowered HIF-1α induction. In previous work using an in vivo model of APAP toxicity, Masubuchi et al. (Masubuchi et al., 2005) reported that CYC (50 mg/kg) attenuated APAP toxicity and noted that the observed swelling in the mitochondria of mice treated with APAP was eliminated with the treatment of the mice with CYC. In addition, the decrease in mitochondrial membrane potential elicited by APAP was reversed by treatment with CYC (Masubuchi et al., 2005). However, the data of the present study suggests that the 50 mg/kg dose of CYC inhibited the toxicity of APAP via inhibition of metabolism as demonstrated by reduced levels of hepatic APAP protein adducts (Fig. 5D). Previous in vitro studies utilizing human hepatic microsomes found that CYC had inhibitory effects on CYP P450 metabolism (Hopkins et al., 2010), including CYP 1A2, an important CYP P450 isoform for APAP metabolism (reviewed in Bessems et al., 2001). To our knowledge, no data have examined the inhibitory potential of CYC on mouse CYP 1A2, 2E1 or 3A4. Our data demonstrate that lack of hepatic GSH depletion per se (Fig. 5C) did not adequately measure the potential effects of CYC on the metabolism of APAP. However, the finding that 50 mg/kg of CYC led to lower APAP covalent binding (Fig. 5D) and reduced HIF-1α induction (Fig. 5E) clearly shows the importance of APAP metabolism leading to HIF-1α induction.
In contrast to the 20 mg/kg dose of CYC, treatment with low dose CYC (10 mg/kg) did not alter the metabolism of APAP as demonstrated by comparable values for hepatic GSH and hepatic APAP protein adducts (Fig. 6B, Fig. 6C). Notably, low dose treatment with CYC lowered HIF-1α induction at 1 h (Fig. 7C) and this effect was sustained at 4 h (Fig. 8E). However, the mice treated with low dose CYC (10 mg/kg) had similar amounts of necrosis as the APAP/veh mice (Table 1; Fig 8D).
The induction of HIF-1α in mice treated with low doses of APAP (15 mg/kg, 30 mg/kg, and 100 mg/kg; Fig. 1B) was unexpected as these doses do not cause hepatic necrosis. Moreover, 15 mg/kg of APAP is a therapeutic dose. Since the induction of HIF-1α was inhibited by the MPT inhibitor CYC (Figure 5E, Figure 7D), the data suggest that low doses of APAP produce some level of mitochondrial toxicity (ie., MPT) without overt necrosis. Assuming that oxidative stress is the key event occurring as a result of MPT and leading to both HIF-1α induction and toxicity, there may be either an APAP dose-dependent difference in the oxidative stress species leading to HIF-1α induction and toxicity, or there may be a threshold for oxidant mediated necrosis.
In summary, the data of the present study implicate a mechanism involving oxidative stress and MPT in the induction of HIF-1α in APAP toxicity in the mouse. The data suggest that hypoxia is not a relevant mechanism for HIF-1α induction in the early stages of APAP toxicity. These findings have application for understanding the endogenous protective mechanisms of the liver in response to toxin mediated injury. For example, HIF-1α upregulates VEGF, which we previously found to be important in hepatocyte regeneration in APAP toxicity (Donahower et al., 2006; Donahower et al., 2010). Understanding the cellular responses that trigger the upregulation of pathways that facilitate repair following hepatic injury may ultimately have relevance for future therapies designed to treat APAP toxicity in the clinical setting.
Acknowledgements
This work was supported by NIDDK (DK-075936 – LPJ; R01 DK079008 – JAH) and the University of Arkansas for Medical Sciences College of Medicine Children’s University Medical Group Fund Grant Program and the Arkansas Biosciences Institute through Arkansas Tobacco Settlement Funds.
ABBREVIATIONS
- APAP
acetaminophen
- ALT
alanine aminotransferase
- HIF-1α
hypoxia inducible factor-1α
- CYC
cyclosporine
- MPT
mitochondrial permeability transition
- PIM
pimonidazole
- SO
superoxide
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement. Drs. James and Hinson have filed a patent (0263839) with the U.S. patent office for the development of a point of care test for the diagnosis of acetaminophen toxicity. The other authors have no conflict of interest to disclose.
Contributor Information
Shubhra Chaudhuri, Email: schaudhuri@uams.edu.
Sandra S. McCullough, Email: mcculloughsandras@uams.edu.
Leah Hennings, Email: henningsleah@uams.edu.
Pippa M. Simpson, Email: psimpson@mcw.edu.
Jack A. Hinson, Email: hinsonjacka@uams.edu.
Laura P. James, Email: lameslaurap@uams.edu.
References
- Arteel GE, Thurman RG, Yates JM, Raleigh JA. Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion in rat liver. Br. J. Cancer. 1995;72:889–895. doi: 10.1038/bjc.1995.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albana E, Polli G, Chiarpotto E, Biasi F, Dianzani MU. Paracetamol-stimulated lipid peroxidation in isolated rat and mouse hepatocytes. Chem. Biol. Interact. 1983;47:249–263. doi: 10.1016/0009-2797(83)90161-8. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Yan HM, Farhood A, Jaeschke H. Plasminogen activator inhibitor-1 limits liver injury and facilitates regeneration after acetaminophen overdose. Toxicol. Sci. 2008;104:419–427. doi: 10.1093/toxsci/kfn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg AC, Janssen H, Sprong D, Hofland I, Blommestijn G, Raleigh JA, Varia M, Balm A, Van Velthuyzen L, Delaere P, Sciot R, Haustermans KMG. Hypoxia and perfusion measurements in human tumors - initial experience with pimonidazole and IUdR. Acta. Oncol. 2001;40:924. doi: 10.1080/02841860152708198. [DOI] [PubMed] [Google Scholar]
- Bessems JGM, Vermeulen NPE. Paracetamol (Acetaminophen)- induced toxicity: molecular and biochemical mechanisms, Analogues and Protective Approaches. Crit. Rev. Toxicol. 2001;31(1):55–138. doi: 10.1080/20014091111677. [DOI] [PubMed] [Google Scholar]
- Bonello S, Zahringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Gorlach A. Reactive oxygen species activate the HIF 1 alpha promoter via a functional NFҚβ site. Arterioscler. Thromb. Vasc. Biol. 2007;27:755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic. Biol. Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Casteilla L, Rigoulet M, Penicaud L. Mitochondrial ROS metabolism: modulation by uncoupling proteins. IUBMB Life. 2001;52:181–188. doi: 10.1080/15216540152845984. [DOI] [PubMed] [Google Scholar]
- Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia inducible factor 1 alpha during hypoxia: a mechanism of O2 sensing. J. Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- Copple BL, Rondelli CM, Maddox JF, Hoglen NC, Ganey PE, Roth RA. Modes of cell death in rat liver after monocrotaline exposure. Tox. Sci. 2003;77:172–182. doi: 10.1093/toxsci/kfh011. [DOI] [PubMed] [Google Scholar]
- Corcoran GB, Wong BK, Neese BL. Early sustained rise in total liver calcium during acetaminophen hepatotoxicity in mice. Res. Commun. Chem. Pathol. Pharmacol. 1987;58(3):291–305. [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Baji ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol. Exp. Ther. 2000;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- Deshmane SL, Mukherjee R, Fan S, Valle LD, Michels C, Sweet T, Rom I, Khalili K, Rappaport J, Amini S, Sawaya BE. Activation of the oxidative stress pathway by HIV-1 Vpr leads to induction of hypoxia-inducible factor 1α expression. J. Biol. Chem. 2009;284:11364–11373. doi: 10.1074/jbc.M809266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahower BC, McCullough SS, Simpson PM, Stowe CD, Hennings L, Kurten R, Hinson JA, James LP. Human recombinant vascular endothelial growth factor (hrVEGF) reduces necrosis and enhances hepatocyte regeneration in a mouse model of acetaminophen toxicity. J. Pharmocol. Exp. Ther. 2010;334:33–43. doi: 10.1124/jpet.109.163840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahower B, McCullough SS, Kurten RC, Lamps LW, Simpson P, Hinson JA, James LP. Vascular endothelial growth factor and hepatocyte regeneration in acetaminophen toxicity. Am J. Physiol. Gastrointest. Liver. Physiol. 2006;291:G102–G109. doi: 10.1152/ajpgi.00575.2005. [DOI] [PubMed] [Google Scholar]
- Duyndam MCA, Hulscher TM, Fontijn D, Pinedo HM, Boven E. Induction of vascular endothelial growth factor expression and hypoxia inducible factor 1 alpha protein by the oxidative stressor arsenite. J. Biol. Chem. 2001;276:48066–48076. doi: 10.1074/jbc.M106282200. [DOI] [PubMed] [Google Scholar]
- Fuhrmann V, Jager B, Zubkova A, Drolz A. Hypoxic hepatitis: epidemiology, pathophysiology and clinical management. Wien. Klin. Wochenschr. 2010;122(5–6):129–139. doi: 10.1007/s00508-010-1357-6. [DOI] [PubMed] [Google Scholar]
- Gardner CR, Laskin JD, Dambach DM, Sacco M, Durham SK, Bruno MK, Cohen SD, Gordan MK, Gerecke DR, Zhou P, Laskin DL. Reduced hepatotoxicity of acetaminophen in mice lacking inducible nitric oxide synthase: potential role of tumor necrosis factor- α and interleukin 10. Toxicol. Appl. Pharmacol. 2002;184:27–36. [PubMed] [Google Scholar]
- Halestrap AP, Connern CP, Griffiths EJ, Kerr PM. Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol. Cell. Biochem. 1997;174:167–172. [PubMed] [Google Scholar]
- Hinson JA, Pike SL, Pumford NR, Mayeux PR. Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem. Res. Toxicol. 1998;11:604–607. doi: 10.1021/tx9800349. [DOI] [PubMed] [Google Scholar]
- Hinson JA, Bucci TJ, Irwin LK, Michael SL, Mayeux PR, Hinson JA. Effect of inhibitors of nitric oxide synthase on acetaminophen-induced hepatotoxicity in mice. Nitric Oxide. 2002;6:160–167. doi: 10.1006/niox.2001.0404. [DOI] [PubMed] [Google Scholar]
- Hinson JA, Roberts DR, James LP. Mechanisms of acetaminophen-induced hepatic necrosis. In: Uetrecht J, editor. Handbook of Experimental Pharmacology 196: Adverse Drug Reactions. Springer: New York; 2009. pp. 370–395. [Google Scholar]
- Hopkins S, Scorneaux B, Huang Z, Murray GM, Wring S, Smitley C, Harris R, Erdmann F, Fischer G, Ribeill Y. SCY-635, a novel nonimmunosuppressive analog of cyclosporine that exhibits potent inhibition of hepatitis C virus RNA replication in vitro. Antimicrob. Agents Chemo. 2010;54(2):660–672. doi: 10.1128/AAC.00660-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Bethea NW, Abril ER, McCuskey RS. Early hepatic microvascular injury in response to acetaminophen toxicity, 2003. Microcirculation. 2003;10:391–400. doi: 10.1038/sj.mn.7800204. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulphide formation and oxidant stress during acetaminophen induced hepatotoxicity in mice in vivo: the protective effects of allopurinol. J. Pharmocol. Exp. Ther. 1990;255:935–941. [PubMed] [Google Scholar]
- James LP, Donahower B, Burke AS, McCullough SS, Hinson JA. Induction of the nuclear factor HIF1-alpha in acetaminophen toxicity: evidence for oxidative stress. Biochem. Biophys. Res. Commun. 2006;343:171–176. doi: 10.1016/j.bbrc.2006.02.143. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J. Pharmacol. Exp. Ther. 1973;187:195–202. [PubMed] [Google Scholar]
- Kan W, Hsieh C, Schwacha MG, Choudhry MA, Raju R, Bland KI, Chaudry IH. Flutamide protects against trauma-hemorrhage-induced liver injury via attenuation of the inflammatory response, oxidative stress, and apoptosis. J. Appl. Physiol. 2008;105:595–602. doi: 10.1152/japplphysiol.00012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Tox. Sci. 2001;62:212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- Kon K, Ikejema K, Okumura K, Aoyama T, Arai K, Takei Y, Lemasters JJ, Sato N. Role of apoptosis in acetaminophen hepatotoxicity. J. Gastroenterol. Hepatol. 2007;22:S49–S52. doi: 10.1111/j.1440-1746.2007.04962.x. [DOI] [PubMed] [Google Scholar]
- Kon K, Jae-Sung K, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatol. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Koop DR. Oxidative and reductive metabolism by cytochrome P450 2E1. FASEB J. 1992;6:724–730. doi: 10.1096/fasebj.6.2.1537462. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Tox. Sci. 2000;54:509–516. doi: 10.1093/toxsci/54.2.509. [DOI] [PubMed] [Google Scholar]
- LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- Lei XG, Zhu JH, McClung JP, Aregullin M, Roneker CA. Mice deficient in Cu, Zn-superoxide dismutase are resistant to acetaminophen toxicity. Biochem. J. 2006;399:455–461. doi: 10.1042/BJ20060784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters JJ, Ji S, Thurman RG. Centrilobular injury following low-flow hypoxia in isolated, perfused rat liver. Science. 1981;213:661–663. doi: 10.1126/science.7256265. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, Nieminen A, Qian T, Trost LC, Herman B. The mitochondrial permeability transition in toxic, hypoxic and reperfusion injury. Mol. Cell Biochem. 1997;174:159–165. [PubMed] [Google Scholar]
- Lemasters JJ. Mechanisms of Hepatic Toxicity V. Necrapoptosis and the mitochondrial permeability transition: shared pathways to necrosis and apoptosis. Am. J. Physio. Gastrointest. Liver Physiol. 1999;276:1–6. doi: 10.1152/ajpgi.1999.276.1.G1. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen induced liver injury in mice. J. Hepatol. 2005;42:110–116. doi: 10.1016/j.jhep.2004.09.015. [DOI] [PubMed] [Google Scholar]
- McCuskey R. Sinusoidal endothelial cells are an early target for hepatic toxicants. Clin. Hemorheol. and Microcirc. 2006;34(1–2):5–10. [PubMed] [Google Scholar]
- McCuskey R. The hepatic microvascular system in health and its response to toxicants. Anat. Rec. 2008;291:661–671. doi: 10.1002/ar.20663. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol. Exp. Ther. 1973a;187:185–194. [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis II. Role of covalent binding in vivo. J. Pharmacol. Exp. Ther. 1973b;187:195–202. [PubMed] [Google Scholar]
- Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high- performance liquid chromatography with electrochemical detection. Drug Metab. Dispos. 2002;30:446–451. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- Nakae D, Yamamoto K, Yoshiji H, Kinugasa T, Maruyana H, Farber JL, Konishi Y. Liposome encapsulated superoxide dismutase prevents liver necrosis induced by acetaminophen. Am. J. Pathol. 1990;136:787–795. [PMC free article] [PubMed] [Google Scholar]
- Papstefanou VP, Bozas E, Mykoniatis MG, Grypioti A, Garyfallidis S, Bartsocas CS, Nicolopoulou-Stamati P. VEGF isoforms and receptor expression throughout acute acetaminophen-induced liver injury and regeneration. Arch. Toxicol. 2007;81(10):729–741. doi: 10.1007/s00204-007-0201-x. [DOI] [PubMed] [Google Scholar]
- Pialoux V, Mounier R, Brown AD, Steinback CD, Rawling JM, Poulin MJ. Relationship between oxidative stress and HIF-1 alpha mRNA during sustained hypoxia in humans. Free Rad. Biol. Med. 2009;46:321–326. doi: 10.1016/j.freeradbiomed.2008.10.047. [DOI] [PubMed] [Google Scholar]
- Potter DW, Hinson JA. Mechanisms of acetaminophen oxidation to N-acetyl-P-benzoquinone imine by horseradish peroxidase and cytochrome P-450. J. Biol. Chem. 1987;262:966–973. [PubMed] [Google Scholar]
- Raleigh JA, Calkins-Adams DP, Rinker LH, Ballenger CA, Weissler MC, Fowler WC, Novotny DB, Varia MA. Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinomas using pimonidazole as hypoxia marker. Cancer Res. 1998;58:3765–3768. [PubMed] [Google Scholar]
- Reid AB, Kurten RC, McCullough SS, Brock RW, Hinson JA. Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J. Pharmacol. Exp. Ther. 2005;312:509–516. doi: 10.1124/jpet.104.075945. [DOI] [PubMed] [Google Scholar]
- Roberts DW, Bucci TJ, Benson RW, Warbritton AR, McRae TA, Pumford NR, Hinson JA. Immunohistochemical localization and quantification of the 3-(cystein-S-yl)-acetaminophen protein adduct in acetaminophen hepatotoxicity. Am. J. Pathol. 1991;138(2):359–371. [PMC free article] [PubMed] [Google Scholar]
- Salazard B, Bellon L, Jean S, Maraninchi M, El-Yazidi C, Orsiere T, Margotat A, Botta A, Berge-Lefranc JL. Low-level arsenite activates the transcription of genes involved in adipose differentiation. Cell Biol. Toxicol. 2004;20:375–385. doi: 10.1007/s10565-004-1471-1. [DOI] [PubMed] [Google Scholar]
- Schemmer P, Connor HD, Arteel GE, Raleigh JA, Bunzendahl H, Mason RP, Thurman RG. Reperfusion injury in livers due to gentle in situ organ manipulation during harvest involves hypoxia and free radicals. J. Pharmacol. Exp. Ther. 290:235–240. [PubMed] [Google Scholar]
- Semenza G. Signal transduction to hypoxia inducible factor 1. Biochem. Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- Sies H, de Groot H. Role of reactive oxygen species in cell toxicity. Toxicol. Lett. 1992;64–65:547–551. doi: 10.1016/0378-4274(92)90230-h. [DOI] [PubMed] [Google Scholar]
- Sun J, Steenbergen C, Murphy E. S-Nitrosylation: NO-related redox signaling to protect against oxidative stress. Antioxid. Redox. Signal. 2006;8(9–10):1693–1705. doi: 10.1089/ars.2006.8.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi E, Sakisaka S, Matsuo K, Tanikawa K, Sata M. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J. Histochem. Cytochem. 2001;49:121–130. doi: 10.1177/002215540104900112. [DOI] [PubMed] [Google Scholar]
- Tsurui Y, Sho M, Kuzumoto Y, Hamada K, Akashi S, Kashizuka H, Ikeda N, Nomi T, Mizuno T, Kanehiro H, Nakajima Y. Dual role of vascular endothelial growth factor in hepatic ischemia-reperfusion injury. Transplantation. 2005;79:1110–1115. doi: 10.1097/01.tp.0000161627.84481.5e. [DOI] [PubMed] [Google Scholar]
- Varia MA, Calkins-Adams DP, Rinker LH, Kennedy AS, Novotny DB, Fowler WC, Raleigh JA. Pimonidazole: a novel hypoxia marker for complementary study of tumor hypoxia and cell proliferation in cervical carcinoma. Gynecol. Oncol. 1998;71:270–277. doi: 10.1006/gyno.1998.5163. [DOI] [PubMed] [Google Scholar]
- Yin H, Cheng L, Holt M, Hail N, MacLaren R, Ju C. Lactoferrin protects against acetaminophen induced liver injury in mice. Hepatol. 2009;51:1007–1016. doi: 10.1002/hep.23476. [DOI] [PMC free article] [PubMed] [Google Scholar]