Abstract
The formation and refinement of synaptic circuits are areas of research that have fascinated neurobiologists for decades. A recurrent theme seen at many CNS synapses is that neuronal connections are at first imprecise, but refine and can be rearranged with time or with experience. Today, with the advent of new technologies to map and monitor neuronal circuits, it is worthwhile to revisit a powerful experimental model for examining the development and plasticity of synaptic circuits-the retinogeniculate synapse.
Introduction
The visual system is one of the best-understood sensory systems. It serves as an experimental model for sensory information processing as well as circuit development. Visual information encoded in the retina is transmitted via retinal ganglion cells (RGCs) to thalamic relay neurons in the dorsal lateral geniculate nucleus (LGN). Relay neurons, in turn, project to the primary visual cortex where higher order processing occurs. The retinogeniculate synapse is a powerful system for studying synaptic plasticity during development of the central nervous system (CNS). This simple circuit is accessible to both morphological and functional studies. Unlike most CNS synapses, where multiple developmental processes overlap over a short period of time, maturation of the retinogeniculate synapse extends over many weeks, facilitating the dissection of processes of axon targeting, of synapse formation, strengthening and elimination, and of experience-dependent plasticity.
The current understanding of synapse refinement at the retinogeniculate connection can be generalized to other areas in the CNS, including the cerebellum, brainstem and somatosensory system [1–3]. Development can be divided into three distinct phases: 1) axon targeting and rearrangement into proper lamina; 2) fine scale functional refinement, and 3) stabilization and maintenance of the refined circuitry (Figure 1). In this review, we draw from early studies in different species as well as recent ones from mice to review what is currently known about the mechanisms underlying the three phases and what questions remain unresolved.
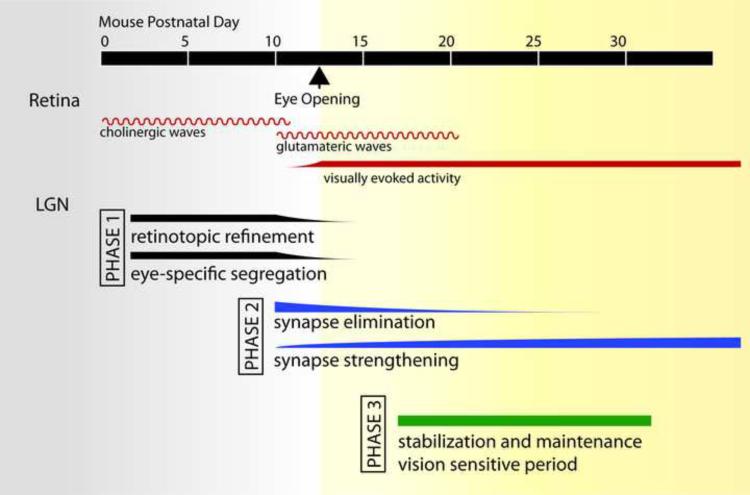
Figure 1. Developmental phases of retinogeniculate connectivity in mouse.
In the retina (red lines), spontaneous activity in the form of cholinergic waves is followed by glutamatergic waves [5]. Eye-opening in mice occurs around postnatal day 12–14 (arrowhead). Visually evoked activity begins shortly before this, when light can be first detected through closed eyelids and persists throughout adulthood. Development of retinal connections to the dorsal lateral geniculate (LGN) can be divided into 3 phases. During phase I (black), axon refinement occurs during retinopic refinement and eye-specific segregation. Throughout the second phase (blue), synaptic connections are further refined through continued elimination and strengthening of synapses. The third phase (green) involves the stabilization and maintenance of established connections. This period encompasses a period in which retinogeniculate connectivity can be influenced by visual experience.
A Simple Circuit with Precise Connectivity
A striking feature of the retinogeniculate circuit is its anatomical and functional architecture. Early in development, RGC axons that reach the LGN must choose from numerous possible target neurons to form connections. The precise anatomical organization of these connections can be seen on multiple levels: (1) retinotopic mapping, whereby relative locations of RGCs in the retina are preserved in the LGN [4,5] (2) eye-specific segregation, by which projections from each eye terminate in non-overlapping territories within the LGN [5] (3) laminar specificity, in which different RGC types project to distinct cellular layers in the LGN [6]; and (4) subcellular specificity, whereby RGCs preferentially synapse onto proximal, rather than distal dendrites of LGN relay neurons [7]. Functionally, this precise connectivity is reflected in the receptive field properties of LGN cells. In the mature CNS, in vivo physiology from cat and other mammals has demonstrated that receptive fields of relay neurons are dominated by the inputs of one or two retinal ganglion cells; a single RGC drives nearly all the action potentials of the postsynaptic relay neuron [8,9].
A prime example of precise structural and functional organization is the laminar organization of inputs to the LGN. In many mammalian species, RGCs have been morphologically classified into 15~20 different subtypes, and each is thought to have distinct functional properties such as selectively responding to onset or offset of light (ON- or OFF-RGCs, respectively), or to a specific direction [6,10]. In addition to eye-specific layers, RGC subsets send axon terminals to distinct cellular layers in many species. The laminar organization that is a hallmark of monkey, cat, and ferret LGN was previously thought to be absent in mice, where such cellular layers are not readily discernable. However, recent identification of transgenic animals that each label a different RGC subset has revealed that subtypes of RGCs restrict their axon terminals to distinct and stereotyped laminae in the mouse LGN. Four such examples of transgenic subset lines are JAMB (labels OFF direction-selective RGCs) [11], CB2 (transient OFF-α RGCs) [12]; DRD4 (ON-OFF bistratified direction selective RGCs) [13], and BD (ON-OFF bistratified direction selective RGCs) [14] mice. Each labels a unique functional subset of RGCs whose axons terminate in restricted regions of the LGN (see Figure 2). It would be interesting to determine whether these laminae are mutually exclusive or overlap as they do in the mouse superior colliculus [15]. While the former would suggest segregation of parallel functional pathways, the latter scenario would open the possibility that certain RGC types converge onto single target neurons.
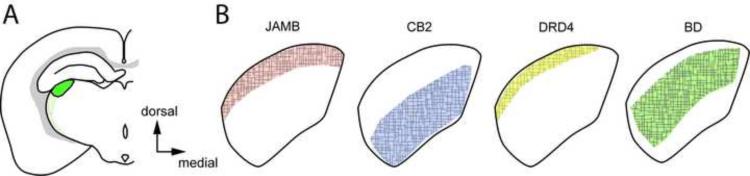
Figure 2. Laminar patterns of axon terminal projections in the mouse LGN.
A. The location of the dorsal LGN in a mouse coronal section (green). B. Schematic representation of axon terminal zones in the LGN of transgenic mouse lines that selectively label functional subsets of RGCs including: JAMB, OFF-direction selective RGCs; CB2, transient OFF-α RGCs; DRD4-RGCs, ONOFF direction selective RGCs; and BD, ON-OFF direction selective RGCs [11–14]. Colored regions represent regions of the LGN that contained labeled axons of the different RGC subsets in the mature LGN. It is still unclear how these territories change over development. Identical contours of a coronal LGN section were drawn for simplicity and may represent different rostrocaudal sections.
The remarkable specificity of the retinogeniculate synapse observed in the adult is not fully established in the young animal. How does this precise circuit develop? Recent work has harnessed the power of mouse genetics to address these questions. Despite the poor acuity of the mouse, features of its visual system have many similarities to that of primates and carnivores [16–18]. We review the molecular and activity-dependent mechanisms identified in these studies.
Distinct Phases of Development at the Retinogeniculate Synapse
Phase I: Axon Mapping and Rearrangement
Once RGC axons reach the LGN, large-scale structural changes occur, including retinotopic refinement and eye-specific segregation. Early in development, inputs from the two eyes target overlapping regions in the LGN. Gradually, inputs from the ipsi- or contra- lateral eye become segregated into separate layers through a process that involves the elimination of mistargeted synapses and the strengthening of correct ones [19,20]. In cat and monkey, eye-specific segregation is complete before birth, and in mice and ferrets, before eye-opening. Thus, the process of eye-specific segregation occurs prior to visual experience and is mediated by spontaneous activity rather than vision. Several recent reviews extensively cover mechanisms of eye-specific segregation that will not be repeated here [5,21].
Phase II: Synapse Elimination and Strengthening
During the period when axons from different eyes overlap, functional synapses are present [19,22]. With the establishment of eye-specific layers synaptic refinement was generally thought to be complete. However, electrophysiological and EM studies have revealed that at this stage, synapses are still immature and evoke weak excitatory currents [23,24]. In mice, the majority of eye-specific segregation is complete by P8–10, immediately before eye-opening (P12–14) [25]. At this point, a single LGN cell receives 10 or more RGC inputs. Over the next three weeks, synapses strengthen 20-fold while the average number of RGC inputs (also referred to as single fiber inputs) onto a given relay neuron decreases to 1–3 [23,26,27]. Thus, synaptic refinement continues well after eye specific segregation is complete. The most robust functional refinement occurs around the time of eye opening. Between P8–16, more than half of the afferent inputs are functionally eliminated while the average strength of remaining inputs increases 8-fold. This period, which we define as the second phase of synapse remodeling, is dependent on spontaneous activity, not vision [26] (Figure 1). Spontaneous retinal activity during this period encompasses both retinal waves driven by glutamatergic transmission as well as spiking of individual RGCs. The detailed features of patterned activity that is important for this stage of synaptic refinement, and whether they are the same as those that drive the first phase of synapse remodeling remain unclear.
During the second phase of synapse refinement, the number of binocularly responsive LGN cells progressively decreases until eye-opening [27]. However, synaptic refinement at this stage does not solely represent remnant traces of eye-specific segregation. Even relay neurons located in regions of the LGN that become strongly monocular soon after birth (lateral LGN) are innervated by more than 10 RGCs just before eye-opening [23]. Temporally, this period corresponds to the sharpening of LGN receptive fields that occurs after eye opening [9,28]. Refinement could result from local competition between RGCs from a single eye, either between RGCs of the same, or different functional subtype. It is unknown how the RGC subtype-specific axon laminae (Figure 2) develop relative to one another, and whether they are still being refined during the second phase. Functional classification of RGCs is ambiguous at early stages, obscuring identities of RGC subtypes that drive an LGN cell. Newly available RGC subset markers will be invaluable in future studies to determine potential competition between RGC subtypes in synaptic maturation.
Phase III: Maintenance of Refined Circuitry and Vision-dependent Plasticity
Synaptic refinement continues between P16–32 in mice, albeit to a lesser extent than during the second developmental period. Single fiber strength increases an additional 2–3 fold and the average number of inputs innervating a relay neuron decreases by another 1–2 (Figure 3) [26]. The final stage of circuit refinement was thought to be independent of visual activity, as dark rearing animals from birth (chronic dark rearing) had no significant effect on receptive field refinement [29], pruning, or strengthening of synapses [26]. Surprisingly, dark rearing after a period of visual experience (late dark rearing) resulted in an increase in the number of inputs and weakening of RGC input strength (Figure 3). These findings revealed a previously unrecognized period of refinement that is sensitive to vision. Re-exposure to light after 7 days of deprivation resulted in rapid pruning and strengthening again, indicating that the changes in circuitry are reversible at this stage. Further investigation revealed that this plasticity is triggered by prior visual experience and occurs only during a discrete period of time, thus defining this phase as a critical period for the retinogeniculate synapse [30].
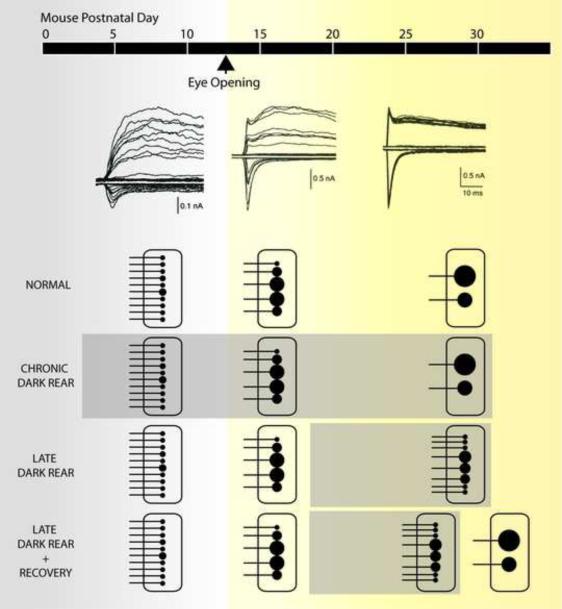
Figure 3.
Functional development of the mouse retinogeniculate synapse. (Top) Representative excitatory synaptic responses to incremental increases in optic nerve stimulation from P11, 17 and 28 mice [23]. Synaptic currents are recorded while alternating the holding potential between −70 mV (inward currents) and +40 mV (outward currents) to assess both AMPAR and NMDAR currents, respectively. (Bottom) Schematic representation of synaptic refinement is drawn to symbolize the experimental data for number of inputs and synaptic strength at different points in development [26,30]. Under normal conditions, synaptic strength increases by 20-fold and afferent inputs decrease from more than 10 down to just 1–3. An RGC input is symbolized by a horizontal line connected to a black circle; the size of the circle represents the relative strength of the input. Each black circle represents an axon making numerous contacts (release sites) with the postsynaptic neuron. Sensory deprivation by dark rearing animals from birth (chronic dark rear) shows no significant difference to normally reared mice. However, dark rearing after a period of visual experience (late dark rear), results in an increase in number of RGC inputs and weakening of established connections. During this period, these changes can be reversed when the animal is re-exposed to light (late dark rear + recovery).
How can we reconcile the effects of late dark rearing with the fact that retinogeniculate synapses of chronically dark reared mice appear to refine normally? We propose a model in which spontaneous activity drives the majority of refinement of eye-specific segregation, retinotopy, and synaptic connections during the first two phases of development. By P20, a coarse approximation of the mature network is established and a single RGC makes connections to only a few of many potential postsynaptic partners. During the late phase of synapse remodeling, which is triggered by vision, the circuit can be further sculpted by visual experience. Based on visual experience during this time, an RGC can strengthen, weaken, or eliminate existing connections, or make new ones. Deprivation during this late phase of plasticity is an extreme and sudden change that strongly destabilizes the partially refined circuit, resulting in a robust change in synaptic strength and connectivity. In the case of mice dark reared from birth, this late phase of plasticity is never triggered, and thus the circuit remains in its default configuration.
Mechanisms Underlying Vision-Independent Plasticity
The mouse retinogeniculate connection has become a useful assay system for identifying mechanisms of synapse refinement. Several candidate molecules that play a role in eye-specific segregation have been identified from the screening of mutant mice for abnormalities. Mutations that disrupt spontaneous retinal activity, such as the nicotinic acetylcholine receptor β2 subunit (β2) null mice and nob mice show abnormalities in eye-specific segregation [31–33]. Disruption of axon guidance cues such as Wnt/ryk [34] and Eph/ephrin gradients also results in abnormal retinotopic map refinement in addition to disrupted segregation [4,35].
An unexpected theme that has emerged from the identification of a number of these molecules is their relationship to the immune system [36,37]. Recently, class I major histocompatibility complex (MHCI) molecules were found at a number of synapses in the CNS during development and adulthood, including in the retinogeniculate synapse [38–40]. Mutant mice with disrupted expression of MHC I molecules demonstrate defects in eye specific layer formation [38,41]. Electrophysiological studies of the role of MHCI show altered synaptic function although the specific defects differ depending on the particular synapse [41–44].
Another component of the immune system, the complement cascade, which is involved in marking foreign material for phagocytosis, have also been implicated in synapse elimination. Mice deficient in proteins in this cascade, C1q or C3, exhibit pronounced defects in eye-specific segregation [45]. Recordings from the retinogeniculate synapse at P30 show that, in addition to one strong input, multiple small weak inputs innervate an average relay neuron. These results suggest a working model in which complement proteins play a role in the proper removal of synapses that are destined for elimination [45].
Pentraxins are another family of molecules in the immune system that mark cells for phagocytosis and are known to bind C1q. Two pentraxin homologues found in the nervous system (NP1 and 2) are expressed in RGC axons during the period of synapse remodeling [46]. The NP1/2 knockout mouse exhibits delayed eye-specific segregation such that patterning of axonal projections is abnormal at P10, but not P30. Retinogeniculate synaptic recordings reveal substantially reduced AMPAR EPSCs, consistent with a defect in AMPAR recruitment into the synapse during the first phase of development (P6–9). However, by P17–20, single fiber AMPAR strength is normal in mutants, although relay neurons are innervated by an abnormally high number of RGC inputs [47]. It is not clear whether the disruption in innervation is corrected by P30, or whether eye specific segregation is still abnormal at P17–20. These results suggest that the role of pentraxins in synaptic strengthening changes between the first and later phases of synapse remodeling.
Are the mechanisms that regulate the segregation of eye specific layers during the first phase of retinogeniculate synapse remodeling the same as those that drive synaptic refinement during the second phase of remodeling? Some mutants, such as C1q KO mice, exhibit disruption in both anatomy and function, indicating that the two phases share common mechanisms. However, not all mutants that exhibit disruption of eye-specific segregation have correlated abnormalities in retinogeniculate function. In the β2 null mice that exhibit defects in eye specific segregation [31,32], and in ephrin triple knockout A2/A5/β2 null mice that have severe disruption of retinotopy and segregation (personal communication, D. Feldheim), in vivo recordings show remarkably little disruption in receptive field size and structure in the LGN [31,48]. Thus anatomical refinement can be dissociated from functional refinement, suggesting that regulatory mechanisms can differ between the two phases.
Synapse Strengthening and Elimination
Retinogeniculate recordings of NP1/2 and C1q KO mice show that disruption of synapse elimination can occur without affecting synapse strengthening. Over development, retinogeniculate strengthening occurs simultaneously with functional elimination of weaker afferent inputs. Hebbian mechanisms have been proposed to drive these changes [49]. In mice, bursts of presynaptic action potentials designed to mimic synchronous retinal wave activity can elicit a relatively small increase or decrease in synaptic strength, depending on whether they coincide with relay neuron bursting [50]. Studies in ferret, using paired stimuli, suggest that up to 2-fold potentiation in synaptic strength can be elicited by presynaptic activity [51]. This potentiation can account for the two-fold increase in quantal size that is found over development. However, an increase in quantal size accounts for just a fraction of the 20-fold increase in single fiber strength between P8 and P32. Since the probability of release does not significantly change over this developmental window, the number of release sites, or contacts between a single RGC and relay neuron, must increase dramatically [23].
Mechanisms Underlying Sensory Dependent Plasticity
Compared to the first two phases of synapse remodeling, little is known about the mechanisms underlying the recently identified visual experience-dependent retinogeniculate remodeling. Evidence for how sensory information can change synaptic connectivity was presented in a recent study that combined in vitro electrophysiology, EM immunolabeling and viral transfection of tagged AMPAR subunits [52]. Under basal visual activity, the GluR1 subunit is preferentially trafficked into postsynaptic densities (PSD) of retinogeniculate synapses but not corticothalamic synapses. GluR1 insertion into the PSD is regulated by calcium/calmodulin-dependent protein kinase II and small GTPase Ras activity, while removal of GluR1 is controlled by Rap kinase activity. Eye closure blocks GluR1 trafficking into the retinogeniculate PSD, thus reducing synaptic strength. It is not clear whether specific GluR1 trafficking occurs throughout development, or only during a specific period of time. However, it is likely that turnover of AMPAR subunits at retinogeniculate PSD must occur in order to remodel synaptic connections during the thalamic critical period.
Molecular mechanisms mediating the experience-dependent period of synapse remodeling should also be distinguishable from other developmental phases. MeCP2, a transcriptional regulator that has been associated with the neurodevelopmental disorder Rett Syndrome, is one such example. Analysis of retinogeniculate development in a Mecp2 null mouse showed that synapse formation, strengthening and elimination during the first and second phases are not significantly different from WT littermates. However, synapses fail to strengthen further, and the number of afferent inputs increases during the thalamic critical period, consistent with a role for MeCP2 in the modification of this circuit by sensory information [53].
Morphological Correlates to Synapse Remodeling
Much of our current understanding of structural synapse remodeling has been gained from studies in the peripheral nervous system. The neuromuscular junction (NMJ) is relatively large, accessible, and simple, facilitating in vivo imaging studies that have provided invaluable insight into the process of developmental synapse remodeling. Early in development, a single muscle fiber is transiently innervated by multiple motor neurons that are eventually eliminated until one input remains and strengthens. Retraction of axon branches is the structural correlate for this synapse elimination [54,55]. It is generally believed that a similar process occurs in the CNS, although detailed morphological analysis has been more challenging due to the significantly smaller size of the synapses, complex circuitry, and lack of accessibility to deep structures for in vivo imaging.
Similar to synapse remodeling at the NMJ, RGC projections are at first diffuse and undergo large-scale retraction to a focal region during retinotopic refinement in the mouse superior colliculus (SC) [56]. Recent studies indicate that a similar process occurs during laminar restriction of some RGCs in the SC. OFF-α RGC axon terminals initially span the full depth of the outer SC layer before becoming restricted to the deep retinorecipient layer [57]. Interestingly, the precise mechanisms of morphological development appear to be cell-type dependent [14].
On the postsynaptic side, morphological changes early in development involve the extension and retraction of dendritic branches of neurons that stabilize as synapses mature [58,59]. A recent study in the tadpole tectum, which is amenable to in-vivo time lapse imaging, showed that retinotectal synapses formed on stable dendrites are more mature and less dense than those on dynamic branches [60]. NMDAR blockade or visual deprivation delayed this synaptic maturation, consistent with activity-dependent mechanisms mediating synaptic refinement.
Large-scale structural changes are also believed to underlie the first phase of mammalian retinogeniculate synapse remodeling. However, classic studies of single axon morphology do not support a scenerio in which a diffuse, complex axon arbor retracts to a focal target area during eye-specific segregation. Rather, at the earliest stages when axons between the two eyes are intermingled, individual axons hardly branch at all – axons exist as single twig-like structures with few short side branches [20]. The few side branches present in embryonic cat are even less evident in monkey [61]. As axons segregate into eye-specific regions, axon branches are removed, concurrent with robust elaboration of the axon arbor in the correct target areas (Figure 4). While much focus has been on the elimination of axon branches during eye-specific segregation, defects in this process may also be mediated by the excessive elaboration of mistargeted axons.
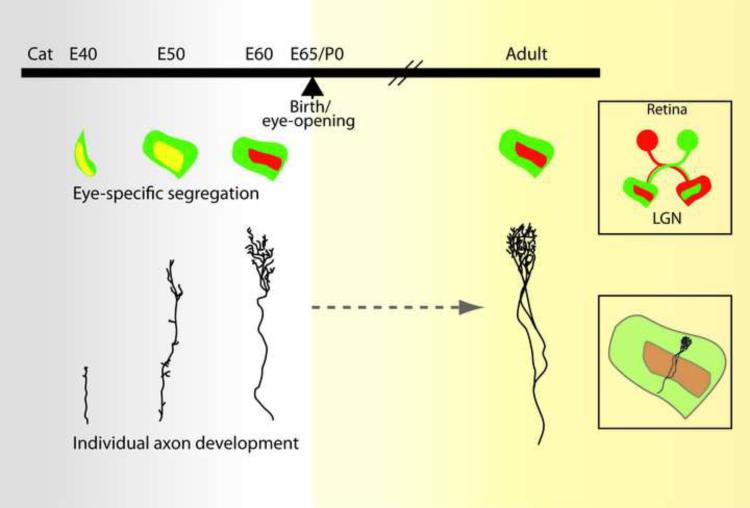
Figure 4.
Structural development of retinal axon terminals in the LGN of the cat. We revisit these classic single-RGC morphology studies to compare the anatomical changes seen over development with the changes in retinogeniculate synaptic strength and innervation observed from mice. Similar single axon studies in mice are currently not available, thus comparisons are made relative to the time of completion of eye specific segregation and eye opening. (Top) Schematic representation of the progression of eye-specific segregation in the cat. Projections from one eye colored in red, the other in green (see top right box). Yellow region represents region of overlap between the two eyes (Bottom) Illustration of morphological changes of single RGC axon arbors described from classic studies in the cat. Bottom right box shows the location of the arbor within the LGN, Images of arbors adapted from [20] (embryonic (E) time points) and [62] (adult).
The anatomical basis of synapse remodeling during the later phases of plasticity remains unknown. Electrophysiological evidence indicates that during the second phase, synapses continue to be eliminated as receptive fields sharpen. However, compared to the time of eye opening, single RGC arbors in cat show further elaboration a few weeks later (Figure 4) [62]. There appears to be a discordance between the structural and functional changes that occur. However, it is important to note that “synapse elimination” in this context does not require a reduction in the number of synapses, but rather involves a decrease in the number of cells innervating a single postsynaptic cell. Synaptic strengthening is also occurring at this time, mediated by the addition of release sites. Thus, morphological changes underlying input elimination may be mediated by fine-scale axon remodeling, postsynaptic structural changes reflected in dendritic morphology, or locally by biochemical or molecular changes at the release sites. Future studies are needed to shed light on these possibilities.
Conclusions
Much has been learned about the development of synaptic circuits in the mammalian CNS, yet many questions remain unanswered. While some CNS circuits form precisely without developmental refinement, others undergo large-scale changes over time. Is the act of refinement somehow advantageous to the mature circuit? Is the recruitment of afferent RGC inputs seen during late dark rearing due to reactivation of previously eliminated synapses, or formation of new pre- and post-synaptic partners? What are the morphological correlates to different phases of synapse remodeling? Can plasticity at the retinogeniculate synapse be reactivated? Answering these questions will provide further insight into how circuits form and how we can harness this plasticity to rewire circuits in the mature or diseased CNS. The recent development of circuit mapping tools such as trans-synaptic tracers, and high-throughput serial electron microscopy [63–65] will provide new approaches for answering these questions at the retinogeniculate synapse.
Acknowledgments
This work was supported by NIH RO1 EY013613 and the Children's Hospital Boston Mental Retardation and Developmental Disabilities Research Center NIH PO1 HD18655. We thank J. Hauser, B.M. Hooks, J. Morales, and A.Thompson for helpful discussion and comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lu T, Trussell LO. Development and elimination of endbulb synapses in the chick cochlear nucleus. J Neurosci. 2007;27:808–817. doi: 10.1523/JNEUROSCI.4871-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H, Zhang ZW. A critical window for experience-dependent plasticity at whisker sensory relay synapse in the thalamus. J Neurosci. 2008;28:13621–13628. doi: 10.1523/JNEUROSCI.4785-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kano M, Hashimoto K. Synapse elimination in the central nervous system. Curr Opin Neurobiol. 2009;19:154–161. doi: 10.1016/j.conb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Pfeiffenberger C, Yamada J, Feldheim DA. Ephrin-As and patterned retinal activity act together in the development of topographic maps in the primary visual system. J Neurosci. 2006;26:12873–12884. doi: 10.1523/JNEUROSCI.3595-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nassi JJ, Callaway EM. Parallel processing strategies of the primate visual system. Nat Rev Neurosci. 2009;10:360–372. doi: 10.1038/nrn2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson JR, Friedlander MJ, Sherman SM. Fine structural morphology of identified X- and Y-cells in the cat's lateral geniculate nucleus. Proceedings of the Royal Society of London - Series B: Biological Sciences. 1984;221:411–436. doi: 10.1098/rspb.1984.0042. [DOI] [PubMed] [Google Scholar]
- **8.Sincich LC, Adams DL, Economides JR, Horton JC. Transmission of spike trains at the retinogeniculate synapse. J Neurosci. 2007;27:2683–2692. doi: 10.1523/JNEUROSCI.5077-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; A recent study in the macaque showing that >95% of visually driven spike trains in LGN cell were associated with EPSPs of a single spiking RGC. LGN responses were driven by the summation of retinal EPSPs that occurred within 40ms.
- 9.Alonso J-M, Yeh C-I, Weng C, Stoelzel C. Retinogeniculate connections: A balancing act between connection specificity and receptive field diversity. Prog Brain Res. 2006;154:3–13. doi: 10.1016/S0079-6123(06)54001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- **11.Kim IJ, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–482. doi: 10.1038/nature06739. [DOI] [PubMed] [Google Scholar]; Using a transgenic labeling approach, the study identified a previously unrecognized subset of OFF-RGCs whose dendrites were uniformly asymmetric, and direction selective for upward movement. The direction of sensitivity correlated with the direction asymmetry of the dendritic arbors, suggesting that the structure and location of the dendrites could shape the function of RGCs.
- *12.Huberman AD, Manu M, Koch SM, Susman MW, Lutz AB, Ullian EM, Baccus SA, Barres BA. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron. 2008;59:425–438. doi: 10.1016/j.neuron.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors identified a transgenic mouse line (CB2-GFP) that expresses GFP exclusively in transient OFF-α RGCs. Axon terminals were restricted to the deep layers of the superior colliculus, and the formation of the laminar restriction was found to occur normally in β2 knockout mice, suggesting that cholinergic retinal waves are not necessary for laminar restriction of RGC axons.
- **13.Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. Genetic Identification of an On-Off Direction- Selective Retinal Ganglion Cell Subtype Reveals a Layer-Specific Subcortical Map of Posterior Motion. Neuron. 2009;62:327–334. doi: 10.1016/j.neuron.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified a transgenic subset line (DRD4-EGFP) that labels ON-OFF RGCs that are direction selective to posterior direction. The laminar pattern of RGC projections in LGN and SC are compared to those observed in another RGC subset line, CB2-GFP, identified in their previous study [12], and show that each RGC type has a different pattern of lamination in the LGN and SC
- *14.Kim I-J, Zhang Y, Meister M, Sanes JR. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J Neurosci. 2010;30:1452–1462. doi: 10.1523/JNEUROSCI.4779-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]; Four transgenic lines were generated that label RGC subsets that were each distinct in function and morphology. Each of these subsets was found to restrict their axon terminals in restricted laminae within the SC and LGN. The laminar development of the dendrites within the retina as well as axons in the SC differed between subtypes, suggesting differential developmental mechanisms may be subtype-specific.
- 15.Hong YK, Kim I-J, Sanes JR. Stereotyped Axonal Arbors of Retinal Ganglion Cell Subsets in the Mouse Superior Colliculus. Journal of Comparative Neurology. 2011 doi: 10.1002/cne.22595. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohki K, Reid RC. Specificity and randomness in the visual cortex. Curr Opin Neurobiol. 2007;17:401–407. doi: 10.1016/j.conb.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *17.Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J Neurosci. 2008;28:7520–7536. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study examined the receptive field properties in the mouse visual cortex, and showed that many parameters, such as orientation selectivity, spatial frequency, simple and complex receptive field organization of cortical neurons were similar to those of previous studies in other species.
- 18.Chalupa LM, Williams RW. In: Eye, Retina, and Visual System of the Mouse. Chalupa LM, W WR, editors. MIT Press; 2008. pp. 353–362. [Google Scholar]
- 19.Campbell G, Shatz CJ. Synapses formed by identified retinogeniculate axons during the segregation of eye input. Journal of Neuroscience. 1992;12:1847–1858. doi: 10.1523/JNEUROSCI.12-05-01847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sretavan DW, Shatz CJ. Prenatal development of retinal ganglion cell axons: segregation into eye-specific layers within the cat's lateral geniculate nucleus. Journal of Neuroscience. 1986;6:234–251. doi: 10.1523/JNEUROSCI.06-01-00234.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol. 2005;76:213–235. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Lo FS, Ziburkus J, Guido W. Synaptic mechanisms regulating the activation of a Ca(2+)-mediated plateau potential in developing relay cells of the LGN. J Neurophysiol. 2002;87:1175–1185. doi: 10.1152/jn.00715.1999. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28:955–966. doi: 10.1016/s0896-6273(00)00166-5. [DOI] [PubMed] [Google Scholar]
- 24.Bickford ME, Slusarczyk A, Dilger EK, Krahe TE, Kucuk C, Guido W. Synaptic development of the mouse dorsal lateral geniculate nucleus. J Comp Neurol. 2010;518:622–635. doi: 10.1002/cne.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaubert-Miazza L, Green E, Lo FS, Bui K, Mills J, Guido W. Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis Neurosci. 2005;22:661–676. doi: 10.1017/S0952523805225154. [DOI] [PubMed] [Google Scholar]
- 26.Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52:281–291. doi: 10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Ziburkus J, Guido W. Loss of binocular responses and reduced retinal convergence during the period of retinogeniculate axon segregation. J Neurophysiol. 2006;96:2775–2784. doi: 10.1152/jn.01321.2004. [DOI] [PubMed] [Google Scholar]
- 28.Tavazoie SF, Reid RC. Diverse receptive fields in the lateral geniculate nucleus during thlamocortical development. Nature Neuroscience. 2000;3:608–616. doi: 10.1038/75786. [DOI] [PubMed] [Google Scholar]
- 29.Sherman SM, Spear PD. Organization of visual pathways in normal and visually deprived cats. Physiol Rev. 1982;62:738–855. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- **30.Hooks BM, Chen C. Vision triggers an experience-dependent sensitive period at the retinogeniculate synapse. J Neurosci. 2008;28:4807–4817. doi: 10.1523/JNEUROSCI.4667-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study examines the critical time period in which mouse retinogeniculate connections can be altered by visual activity as described in a previous study [26]. Furthermore, this process was reversible when animals were re-exposed to light.
- 31.Grubb MS, Rossi FM, Changeux JP, Thompson ID. Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the beta 2 subunit of the nicotinic acetylcholine receptor. Neuron. 2003;40:1161–1172. doi: 10.1016/s0896-6273(03)00789-x. [DOI] [PubMed] [Google Scholar]
- 32.Muir-Robinson G, Hwang BJ, Feller MB. Retinogeniculate axons undergo eye-specific segregation in the absence of eye-specific layers. J Neurosci. 2002;22:5259–5264. doi: 10.1523/JNEUROSCI.22-13-05259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demas J, Sagdullaev BT, Green E, Jaubert-Miazza L, McCall MA, Gregg RG, Wong RO, Guido W. Failure to maintain eye-specific segregation in nob, a mutant with abnormally patterned retinal activity. Neuron. 2006;50:247–259. doi: 10.1016/j.neuron.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 34.Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, Zou Y. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- 35.Pfeiffenberger C, Cutforth T, Woods G, Yamada J, Renteria RC, Copenhagen DR, Flanagan JG, Feldheim DA. Ephrin-As and neural activity are required for eye-specific patterning during retinogeniculate mapping. Nat Neurosci. 2005;8:1022–1027. doi: 10.1038/nn1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Shatz CJ. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Datwani A, Mcconnell MJ, Kanold PO, Micheva KD, Busse B, Shamloo M, Smith SJ, Shatz CJ. Classical MHCI Molecules Regulate Retinogeniculate Refinement and Limit Ocular Dominance Plasticity. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Needleman LA, Liu XB, El-Sabeawy F, Jones EG, McAllister AK. MHC class I molecules are present both pre- and postsynaptically in the visual cortex during postnatal development and in adulthood. Proc Natl Acad Sci U S A. 2010;107:16999–17004. doi: 10.1073/pnas.1006087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu HP, Chen H, Ding Q, Xie ZH, Chen L, Diao L, Wang P, Gan L, Crair MC, Tian N. The immune protein CD3zeta is required for normal development of neural circuits in the retina. Neuron. 2010;65:503–515. doi: 10.1016/j.neuron.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fourgeaud L, Davenport CM, Tyler CM, Cheng TT, Spencer MB, Boulanger LM. MHC class I modulates NMDA receptor function and AMPA receptor trafficking. Proc Natl Acad Sci U S A. 2010;107:22278–22283. doi: 10.1073/pnas.0914064107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci U S A. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Letellier M, Willson ML, Gautheron V, Mariani J, Lohof AM. Normal adult climbing fiber monoinnervation of cerebellar Purkinje cells in mice lacking MHC class I molecules. Dev Neurobiol. 2008;68:997–1006. doi: 10.1002/dneu.20639. [DOI] [PubMed] [Google Scholar]
- 45.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 46.Bjartmar L, Huberman AD, Ullian EM, Renteria RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, et al. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26:6269–6281. doi: 10.1523/JNEUROSCI.4212-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **47.Koch SM, Ullian EM. Neuronal pentraxins mediate silent synapse conversion in the developing visual system. J Neurosci. 2010;30:5404–5414. doi: 10.1523/JNEUROSCI.4893-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study indicates that neuronal pentraxins (NP1/2) mediate the development of AMPAR-mediated transmission at the retinogeniculate synapse. NP1/2 null mice have normal presynaptic release and quantal size early in development, but drastically reduced AMPAR-mediated transmission at the retinogeniculate synapse. Later in development, single fiber input strength recovers, but defects in afferent innervation persist.
- 48.Cang J, Niell CM, Liu X, Pfeiffenberger C, Feldheim DA, Stryker MP. Selective disruption of one Cartesian axis of cortical maps and receptive fields by deficiency in ephrin-As and structured activity. Neuron. 2008;57:511–523. doi: 10.1016/j.neuron.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 50.Butts DA, Kanold PO, Shatz CJ. A Burst-Based “Hebbian” Learning Rule at Retinogeniculate Synapses Links Retinal Waves to Activity-Dependent Refinement. PLoS Biol. 2007;5:e61. doi: 10.1371/journal.pbio.0050061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mooney R, Madison DV, Shatz CJ. Enhancement of transmission at the developing retinogeniculate synapse. Neuron. 1993;10:815–825. doi: 10.1016/0896-6273(93)90198-z. [DOI] [PubMed] [Google Scholar]
- **52.Kielland A, Bochorishvili G, Corson J, Zhang L, Rosin DL, Heggelund P, Zhu JJ. Activity patterns govern synapse-specific AMPA receptor trafficking between deliverable and synaptic pools. Neuron. 2009;62:84–101. doi: 10.1016/j.neuron.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study monitors AMPAR subunit GluR1 trafficking at the retinogeniculate and corticothalmic synapses onto thalamic relay neurons in the LGN. Using in vitro electrophysiology and electron microscopy, these authors found that GluR1 containing AMPAR was present in intracellular pools near the PSDs of both retinogeniculate and corticogeniculate synapses. However, vision-dependent activity selectively drove trafficking of GluR1 containing AMPAR into retinogeniculate but not corticothalamic PSDs.
- **53.Noutel J, Hong YK, Leu B, Kang E, Chen C. Experience-Dependent Retinogeniculate Synapse Remodeling is Abnormal in MeCP2 Deficient Mice. Neuron. doi: 10.1016/j.neuron.2011.03.001. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]; Retinogeniculate refinement in Mecp2-null mice occurs normally until P21, but subsequently becomes abnormal resulting in disruption of connectivity and strength of the retinogeniculate circuit. These mutants also fail to exhibit vision-triggered plasticity in response to late dark rearing, suggesting that distinct regulatory mechanisms underlie the developmental phases at the retinogeniculate synapse and that Mecp2 is important for the later experience-dependent synapse remodeling.
- 54.Kasthuri N, Lichtman JW. The role of neuronal identity in synaptic competition. Nature. 2003;424:426–430. doi: 10.1038/nature01836. [DOI] [PubMed] [Google Scholar]
- 55.Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- 56.McLaughlin T, Torborg C, Feller M, O'Leary D. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- *57.Cheng TW, Liu XB, Faulkner RL, Stephan AH, Barres BA, Huberman AD, Cheng HJ. Emergence of lamina-specific retinal ganglion cell connectivity by axon arbor retraction and synapse elimination. J Neurosci. 2010;30:16376–16382. doi: 10.1523/JNEUROSCI.3455-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uses electron microscopy and the RGC subset line CB2-GFP described in [12] to examine the structural development of axon terminals in the SC. Early in development, OFF-α RGCs span the full depth of the retinorecipient layers of the SC. Axon branches are removed from the superficial layers, while those in the deep layers are stabilized, resulting in laminar restriction of axon terminals.
- 58.Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- 59.Luo L, O'Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- **60.Li J, Erisir A, Cline H. In vivo time-lapse imaging and serial section electron microscopy reveal developmental synaptic rearrangements. Neuron. 2011;69:273–286. doi: 10.1016/j.neuron.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using two photon in vivo imaging and serial electron microscopy of Xenopus tectal neurons, authors compare synapses made onto stable versus unstable dendritic branches in the Xenopus tectum. Synapses made onto retracting or extending branches were immature and dense, while those on stable branches were more mature and sparser, suggesting that synapse elimination occurs by the removal of numerous immature inputs.
- 61.Snider CJ, Dehay C, Berland M, Kennedy H, Chalupa LM. Prenatal development of retinogeniculate axons in the macaque monkey during segregation of binocular inputs. J Neurosci. 1999;19:220–228. doi: 10.1523/JNEUROSCI.19-01-00220.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sur M, Esguerra M, Garraghty PE, Kritzer MF, Sherman SM. Morphology of physiologically identified retinogeniculate X- and Y-axons in the cat. J Neurophysiol. 1987;58:1–32. doi: 10.1152/jn.1987.58.1.1. [DOI] [PubMed] [Google Scholar]
- 63.Lichtman JW, Sanes JR. Ome sweet ome: what can the genome tell us about the connectome? Curr Opin Neurobiol. 2008;18:346–353. doi: 10.1016/j.conb.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **65.Bock DD, Lee WA, Kerlin AM, Andermann ML, Hood G, Wetzel AW, Yurgenson S, Soucy ER, Kim HS, Reid RC. Network anatomy and in vivo physiology of visual cortical neurons. Nature. 2011 doi: 10.1038/nature09802. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uses two-photon in vivo imaging to determine the functional orientation tuning of neurons in the mouse visual cortex, combined with large-scale serial electron microscopy to identify local connections to interneurons. Inhibitory neurons were found to receive dense convergent inputs from excitatory cortical neurons with broad range of orientation selectivity. The continued development of large-scale serial EM technology such as those described here are will be invaluable for constructing complete wiring diagrams of neuronal circuits.