Abstract
We investigated the effects of cannabinoid receptor agonists on (1) oral cancer cell viability in vitro and (2) oral cancer pain and tumor growth in a mouse cancer model. We utilized immunohistochemistry and western blot to show that human oral cancer cells express CBr1 and CBr2. When treated with WIN55,212-2 (non-selective), ACEA (CBr1-selective) or AM1241 (CBr2-selective) agonists in vitro, oral cancer cell proliferation was significantly attenuated in a dose-dependent manner. In vivo, systemic administration (0.013M) of WIN55,212-2, ACEA, or AM1241 significantly attenuated cancer-induced mechanical allodynia. Tumor growth was also significantly attenuated with systemic AM1241 administration. Our findings suggest a direct role for cannabinoid mechanisms in oral cancer pain and proliferation. The systemic administration of cannabinoid receptor agonists may have important therapeutic implications wherein cannabinoid receptor agonists may reduce morbidity and mortality of oral cancer.
Keywords: cannabinoids, cell viability, cancer mouse model, cancer pain, CB1 receptor, CB2 receptor
Oral cancer represents 3% of all cancers and its overall survival rate of 50% places it among the worst of all cancers [18,31]. Approximately 50,000 new cases of head and neck cancer are diagnosed each year in the United States [11]. Therefore, there is a concerted effort to discover its cure. Many different agents are currently being investigated for their palliative or anti-proliferative properties on cancer. Of particular interest are cannabinoids, a group of chemicals found in Cannabis sativa Linnaeus plant and their derivatives [2,8,27]. The two widely recognized cannabinoid receptors, CBr1 and CBr2, are G-protein-coupled receptors [22]. CBr1 is expressed mainly in the central nervous system (CNS). CBr2 is mainly expressed in the immune system and peripheral tissues. Additionally CBr1 and CBr2 are also present in keratinocytes [2]. Several studies provide evidence that cannabinoids may be effective in treatment of cancer pain and/or inhibition of tumor growth in cancers such as glioma, bone and skin squamous cell carcinoma [2,4,15,25,27]. Here we demonstrate the anti-nociceptive and anti-proliferative effects of systemic administration of cannabinoid receptor agonists on human oral cancer cells.
The human oral cancer cell lines HSC3 (ATCC, Manassas, VA) and SCC9 (generous gift of Dr. Randall Kramer, UCSF) were cultivated in Dulbecco’s Modification of Eagle’s Medium with 4.5 g/L glucose, l-glutamine, and sodium pyruvate, supplemented with 10% fetal bovine serum. Primary normal oral keratinocytes (NOK) were harvested from normal gingival tissues and cultured as previously described [24]. Tissue collection was approved by the UCSF Committee on Human Research and consent was obtained from patients. NOK were cultured in Defined Keratinocyte Serum-free media (Invitrogen, Carlsbad, CA). All three cell lines were supplemented with100 μg/mL streptomycin sulfate, 100 U/mL penicillin and 25 μg/mLfungizone and cultivated at 37°C with 5% CO2.
We investigated the presence of cannabinoid receptors on human oral cancer cells using immunofluorescence. HSC3 cells were grown on cover slips overnight, then washed with PBS and fixed in cold acetone for 10 minutes. Incubation with primary goat polyclonal anti-CBr1 antibody (1:500) (Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit polyclonal anti-CBr2, (1:500) (Abcam, Cambridge, MA) was performed at 4°C overnight. The cells were incubated with the secondary anti-goat IgG-FITC (1:500) (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-rabbit Texas Red-conjugated antibody (1:500) (Abcam, Cambridge, MA) for 1 hour at room temperature. The nuclei were stained with Hoechst-33342 (Molecular Probes, Carlsbad, CA). Cover slips were mounted on in Gel-Mount (Biomeda Corp., Foster City, CA) and visualized on a Nikon Eclipse E600 microscope using epi uorescence. The images were captured and analyzed with a RT Spot Camera and RT Spot Software (Diagnostics Instruments Inc., Sterling Heights, MI). Controls included the omission of the primary antibodies for CBr1 and CBr2 during incubation.
We used western blot to confirm CBr1 and CBr2 expression. HSC3, SCC9 and NOKs were lysed in Nonidet P-40 lysis buffer. Protein concentration was determined by BCA Protein Assay Kit (Pierce, Rockford, IL). Proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Micron Separation Inc, Westborough, MA) using a semi-dry blotting apparatus (Bio-Rad, Hercules CA). The membranes were developed using ECL Chemiluminescence Kit (Amersham) and bands were detected by exposure to X-ray film. The blots were quantified and assigned rvu using an image analysis program (NIH Image, http://www.rsb.info/nih-image).
We investigated the effects of cannabinoid receptor agonists on human oral cancer cell proliferation using the MTS assay (CellTiter 96® Aqueous One Solution Cell Proliferation Assay, Promega Corporation). HSC3 cells (1×103/well) were plated on a 96-well plate. The cells were serum-starved for 24 hours to allow synchronization. Serial dilutions (0.1, 1, 2.5, 5, 10 and 50 μM) of WIN55,212-2 (non-selective), ACEA (CBr1-selevtive), and AM1241(CBr2-selective) were prepared in 0.2% DMSO/water and delivered to each group. Vehicle (0.2% DMSO) served as the control. The plates were incubated and assayed every 24 hours for a period of 4 days. At the time of assay, 20 μl of MTS reagent was added to each well. Plates were incubated for 2 hours in the dark. Absorbance was recorded using a microplate reader (Biorad, Model 680) calibrated to 490 nm.
The oral cancer mouse model was produced by inoculating HSC3 cancer cells into the hindpaw of mice as previously described [21,28]. Experiments were performed on female Foxn1nu, athymic, immunocompromised mice ranging from 4–5 weeks old and weighing 20–25g at the time of inoculation. Mice were housed in a temperature-controlled room on a 12:12 h light cycle with ad libitum access to food and water. The UCSF Committee on Animal Research approved all procedures and researchers were trained under the Animal Welfare Assurance Program.
We used Alzet-2000 constant flow rate pumps (Durect Corporations, Cupertino, CA) to administer the cannabinoid receptor agonists systemically over a period of 2 weeks. Mice were divided into four experimental groups. The pumps were filled with 50% DMSO/water as control or 0.013 M concentrations of WIN55,212–2, ACEA or AM1241 dissolved in 50% DMSO/water (65, 48 and 65 mg/kg dosages, respectively). The pumps were placed in the back of each animal 4 days after tumor inoculation, when gross tumor formation was evident. Under general anesthesia with isoflurane, a small incision was made in the skin of the back. The pump was placed subcutaneously and the incision was closed using surgical clips.
Behavioral testing for mechanical allodynia was performed as described previously [21,28]. Testing was performed by an observer blinded to the experimental groups, in the evening between 16:00–19:00 h. Mice were placed in a plastic cage with a wire mesh floor, allowing access to the paws. The tumor-bearing paw was tested using an electronic von Frey anesthesiometer (IITC Life Sciences, Woodland Hills) after thirty minutes was allowed for acclimatization. A positive response was noted if the paw was sharply withdrawn and if there was an immediate inching upon application of an increasing force with the von Frey rigid probe tip. The withdrawal threshold was de ned as the force (g) that was sufficient to elicit the above withdrawal response. The mechanical stimuli were presented at least 3 minutes apart to allow resolution of previous stimuli. Each animal was tested five times and the measurements were averaged and compared to the baseline measurements for each animal which was obtained 24 hours and on the day of inoculation prior to tumor inoculation.
Tumor growth was measured using a 520M Plethysmometer (IITC Life Sciences, Woodland Hills) to determine the paw volume. The animal’s tumor-bearing paw was inserted into a water cell, which measures the change in pressure due to immersion. Paw volume measurements were repeated 3 times and the results were averaged. The measurements for paw withdrawal and tumor growth were recorded on days 4, 7, 9, 11, 13, 15, and 18 days post-inoculation.
Data are reported as mean ± SE. Statistical analysis was performed using ANOVA and post-hoc Tukey’s test (P<0.05 considered statistically significance).
Immunohistochemistry of HSC3 cells shows that human oral cancer cells produce CBr1 and CBr2 in abundance as evidenced by the homogeneous cytoplasmic immunoreactivity (Figure 1a). The results of the western blot (Figure 1b) confirms the expression of CBr1 and CBr2 on two human oral cancer cell lines and shows that the cancer cell lines have a higher level of CBr expression compared to human NOKs.
Figure 1.
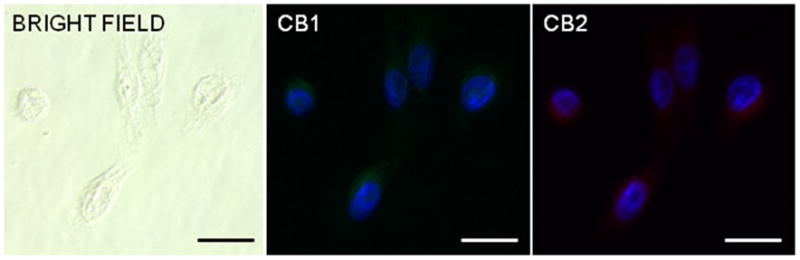
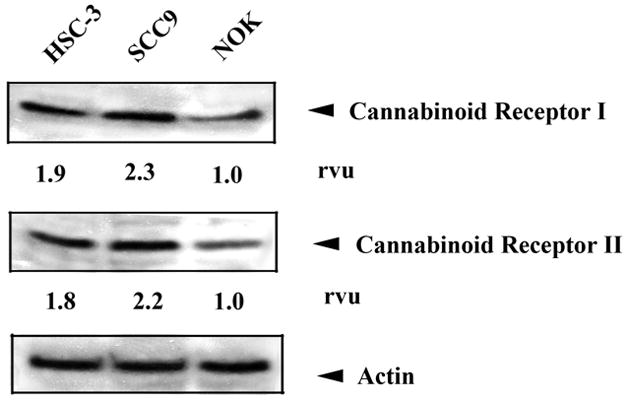
CBr1 and CBr2 are present in human head and neck cancer cells (a). Immunofluorescence of human squamous cell carcinoma (SCC) cells revealed homogeneous cytoplasmic and membrane staining for CBr1 and CBr2. Representative bright field (left panel) and corresponding immunofluorescence images for CBr1 (green, middle) and CBr2 (red, right panel). Scale bar=20μm. Western blot analysis of cannabinoid receptor expression in NOK, HCS3 and SCC9 cells revealed that all three cell lines express CBr1 and CBr2 (b). The expression of Actin was also demonstrated as control. Image analysis of the results of western blot indicates that the cancer cell lines express a higher level of cannabinoid receptors compared to NOK.
WIN55-212-2, ACEA, and AM1241 reduced cell viability significantly in a dose-dependant manner after four days (Figure 2). The lowest dose that showed a significant decrease in viability on day 4 with each agonist was 2.5 μM (Tukey’s test). At this concentration, WIN55,212-2, decreased proliferation to 36% (p<0.05) and ACEA decreased proliferation to 74% (p<0.05). The same concentration of AM1241 initially increased proliferation to 125% (p<0.05) after one day of treatment but ongoing treatment reduced proliferation to 84% after 4 days (p<0.05).
Figure 2.
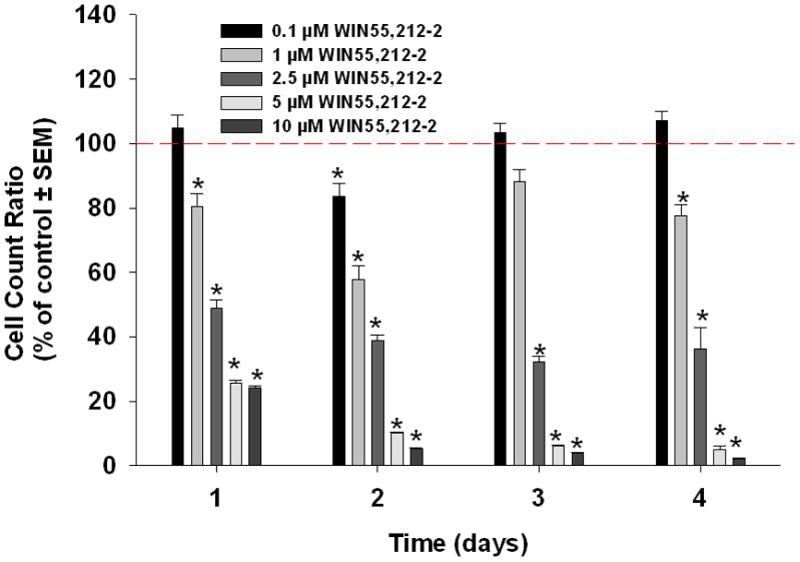
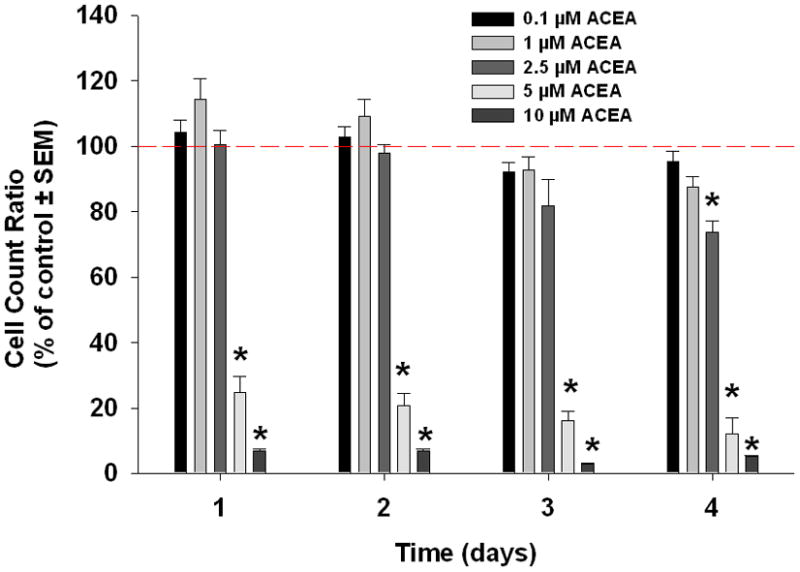
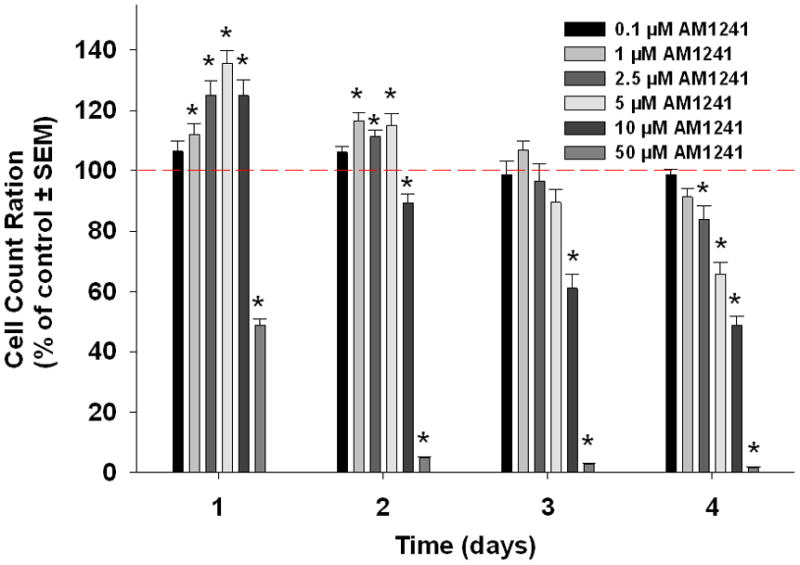
Cannabinoid agonists decrease oral cancer cell viability in vitro. Mean percent change in cell viability relative to control in oral cancer cells treated with WIN55,212-2(a), ACEA (b), or AM1241(c), as measured by MTS assay. WIN55,212-2, ACEA and AM1241 caused a dose-dependant decrease in HSC3 viability (n=7/group, * p<0.05 ANOVA).
The systemic administration of cannabinoid receptor agonists significantly attenuated cancer pain. WIN55,212-2 treatment significantly increased mean paw withdrawal threshold on days 7 (19%, p<0.05), 15 (21%, p<0.01) and 18 (34%, p<0.01) compared to control. ACEA treatment significantly increased paw withdrawal threshold on day 18 (24%, p<0.05) and AM1241 treatment resulted in a significant increase on days 15 (27%, p<0.05) and 18 (26%, p<0.05) (Tukey’s test).
Hindpaw tumors in the AM1241 group were significantly smaller than the control group on days 7 (26%, p<0.05), 9 (39%, p<0.001), 11 (21%, p<0.05) and 18 (40%, p<0.01) (Tukey’s test). The tumors in the WIN55,212-2 treated mice were significantly smaller than control on day 9 (21%, p<0.01). After day 9, there was a trend of tumor volume reduction, but the difference was not statistically significant. The ACEA treated group also showed a trend of tumor volume reduction, however, the difference was not statistically significant.
This is the first study to show the presence of CBr1 and CBr2 on human oral cancer cells. Application of synthetic cannabinoid receptor agonists dose-dependently attenuated oral cancer cell viability in vitro. We also demonstrated that systemic administration of synthetic cannabinoids attenuated chronic cancer pain and proliferation in a mouse cancer model. The three agonists used in this study are highly selective for their target receptors, indicating the likelihood that our findings are due to the activation of the targeted cannabinoid receptors. WIN55,212-2 is highly specific (>90%) with a high affinity (1.9 nM) for functional receptors in rat cerebellar membranes [14]. This agonist has been shown to bind both CBr1 and CBr2 with Ki values of 62.3 and 3.30 nM respectively [3]. ACEA has been shown to bind to CBr1 with Ki value of 1.4 nM with a 2000-fold selectivity for CBr1 over CBr2 [10]. In contrast, AM1241 has high affinity for the human CBr2 with a Ki value of 7 nM and its affinity for the human CBr2 is more than 80-fold stronger than CBr1 [32]. These agonists have proven efficacy and receptor selectivity in many studies on cancer pain and proliferation [2,15,23,25].
Our current results agree with those shown previously by us as well as others. Local administration of WIN55,212-2 or AM1241 can attenuate mechanical allodynia in head and neck cancer [7] and systemic administration of cannabinoid receptor agonists reduce pain in other cancer models such as fibrosarcoma and bone cancer [13,15]. Here we showed that the systemic route of administration of cannabinoid receptor agonists is also effective in decreasing oral cancer pain. The anti-nociceptive effects of cannabinoids can manifest through multiple routes. The two subtypes of cannabinoid receptors are expressed in different tissues. CBr1 is mostly expressed in the CNS while the CBr2 is mostly expressed in the immune system and peripheral tissues [2,8]. CBr2 is also present in some areas of the CNS [17,30] such as spinal cord and dorsal root ganglia [19]. Furthermore, CBr1 and CBr2 are both expressed in keratinocytes [2] and oral cancer cells. In this study, since the agonists were administered systemically, the analgesic effect may have been through the activation of cannabinoid receptors in the local tissues and/or the CNS [15]. Cannabinoids can induce anti-nociception through CBr1 of the CNS [20]. WIN55,212-2 can penetrate the blood brain barrier (BBB) although the penetration is low (0.0060%) [20]. BBB penetration for ACEA and AM1241 is not quantified. Although present, the functional role of CBr2 in the CNS remains unclear and requires further investigation [15]. In local tissue, activation of CBr2 on keratinocytes results in the release of endogenous opioids that may contribute to the local anti-nociceptive effects of CBr2 receptor agonists [12,23]. Based on the presence of CBr1 and CBr2 on head and neck cancer cells and our previous finding that cannabinoids locally reduce cancer pain, it is possible that the activation of these receptors on cancer cells may result in a similar mechanism of endogenous opioid release.
Cannabinoids have been shown to have anti-proliferative effects in different cancers including skin cancer [2,27]. Casanova et al. showed that local administration of WIN55,212-2 or JWH-133 (CBr2-selective) inhibited skin tumor growth in mice [2]. In our study, in vitro administration of WIN55,212-2, ACEA, or AM1241was effective in reducing human cancer cell viability in a dose-dependant manner. We unexpectedly found that AM1241 treatment resulted in an increase in cell counts after 24 hours. There are few reports suggesting that cannabinoids may have pro-proliferative effects in cancer [6,9,29]. This pro-proliferative effect is mediated through cleavage of growth factor precursors by metalloproteinases, which leads to trans-activation of the epidermal growth factor receptor and is not due to CBr activation [4,9]. These effects were seen at 1/10 of the pro-apoptotic concentration that may occur during intermittent treatment with a drug [4,9]. In our study, this proliferative effect was observed with AM1241 24 hours following drug treatment. However, this agonist decreased proliferation significantly over the 4-day course of the in vitro experiment. Furthermore, AM1241 also decreased proliferation significantly in vivo.
In vitro, WIN55,212-2 decreased cell viability at a lower concentration relative to AM1241 or ACEA. This finding did not translate to the in vivo studies where AM1241 was more effective in inhibiting tumor growth over the course of 18 days. This finding might be due to the differences between in vitro and in vivo experiments. In the in vitro study, the compound was delivered directly to the cells in a single dose whereas in the in vivo study, the compound was delivered systemically, at a constant rate and over a period of 2 weeks. In this systemic route of delivery, some of the compound may have been deposited in other tissues. Another explanation may be the effects on the tumor microenvironment on the cancer cells. It is possible that the tumor micro-environment affects the expression levels and/or the mechanism of action of the two cannabinoid receptors, which may lead to CBr2 agonist being more effective in inhibiting tumor growth.
For many years cannabinoids have been used for medicinal and recreational purposes. Recently, studies have focused on the therapeutic effects of cannabinoids on different cancers. The current study was the first to investigate the therapeutic effects of synthetic cannabinoids on oral cancer. Our results suggest that systemic administration of cannabinoids decease oral cancer pain. We have previously demonstrated the effects of morphine, which is the first line of treatment for pain in cancer patients, on paw withdrawal using the cancer pain mouse model [28]. Morphine reversed cancer-induced reductions in paw withdrawal threshold by 40–50%. In comparison, cannabinoid receptor agonists reversed cancer-induced pain (40% of control) with similar efficacy without the sedating/tolerance side effects of opioids. The present findings suggest that cannabinoid treatment may be a promising alternative therapy for oral cancer pain management. Furthermore, CBr2 agonism is not only palliative, but it may also be effective in inhibiting oral cancer growth, making the agonist a particularly desirable therapeutic agent. CBr1 activation has been linked to catalepsy and behavior change. Although no behavior change was observed between groups by the blinded researcher, these behavioral effects may be of concern to some researchers. Systemic CBr2 administration does not cause the psychoactive effects demonstrated by activation of CB1 receptors or opiates [12,15]. Based on the results of our study, CBr2 may be effective in the treatment of head and neck cancer by decreasing the morbidity as well as the morality of this cancer with out affecting the patient’s behavior or catalepsy.
Research Highlights.
CBr agonists decrease oral SCC proliferation in vitro
Systemic administration of CBr agonists decreases cancer pain in mice
Systemic administration of CBr2 agonist decreases oral SCC proliferation in mice
Figure 3.
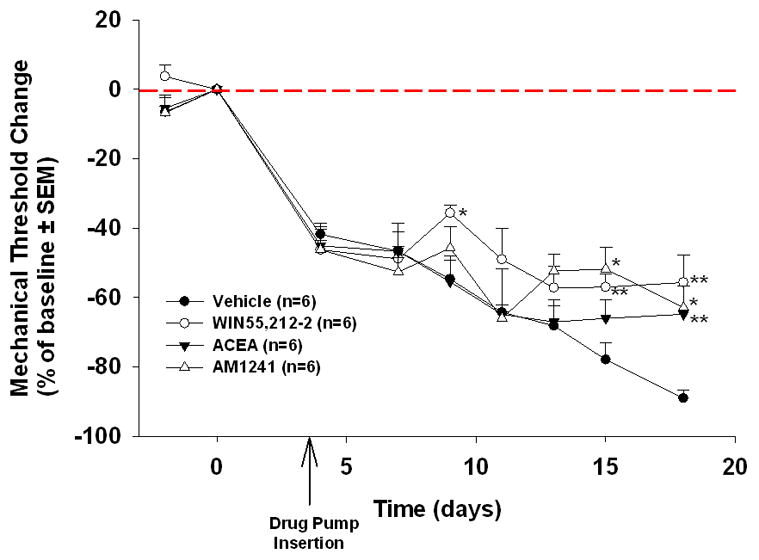
Cannabinoid agonists attenuate oral carcinoma-induced pain. WIN55,212-2, significantly increased mean paw withdrawal threshold on days 7, 15 and 18 relative to control. ACEA, showed a significant increase in paw withdrawal threshold on day 18. AM1241, showed significantly increased mean paw withdrawal threshold on day 15 and 18. (n=6/group, *p<0.05, ** p<0.01 ANOVA).
Figure 4.
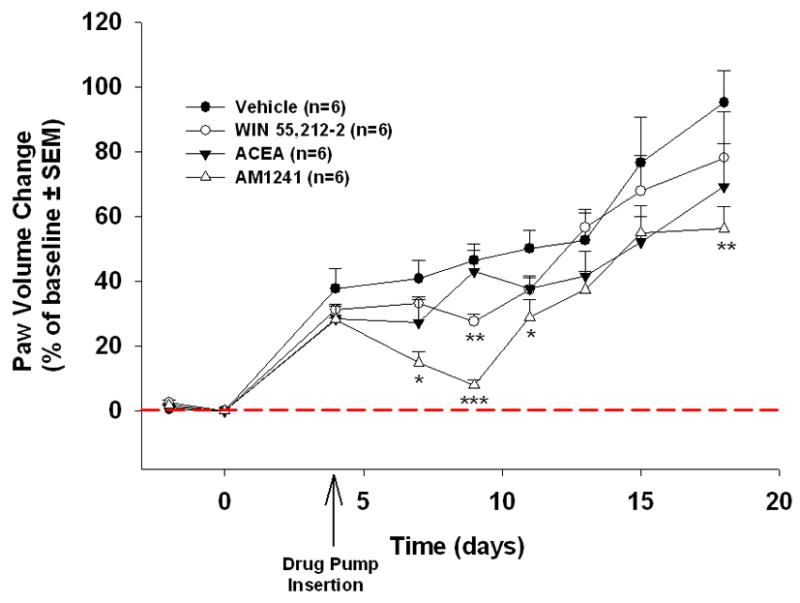
Cannabinoid agonists attenuate oral cancer growth. WIN55,212-2, significantly reduced tumor volume compared to control on day 9 (ANOVA). AM1241, significantly reduced tumor volume compared to control on days 7, 9, 11 and 18. (n=6/group, *p<0.05, **p<0.01, ***p<0.001 ANOVA). There was a trend for ACEA to reduce tumor volume.
Acknowledgments
This work was supported by PACCTR summer fellowship, NIH/NCRR/OD UCSF-CTSI Grant Number TL1 RR024129 and NIH/NIDCR R21 DE018561. We thank S.H. Achdjian and D. Dang for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexander A, Smith PF, Rosengren RJ. Cannabinoids in the treatment of cancer. Cancer Let. 2009;285:6–12. doi: 10.1016/j.canlet.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Casanova ML, Blázquez C, Martínez-Palacio J, Villanueva V, Fernández-Aceñero MJ, Huffman JW, Jorcano JL, Guzmán M. Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J Clin Invest. 2003;111:43–50. doi: 10.1172/JCI16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. Comparison of the Pharmacology and Signal Transduction of the Human Cannabinoid CB1 and CB2 Receptors. Molecular Pharmacology. 1995;48:443–450. [PubMed] [Google Scholar]
- 4.Flygare J, Sander B. The endocannabinoid system in cancer Potential therapeutic target? Seminars in Cancer Bio. 2008:1–14. doi: 10.1016/j.semcancer.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Galve-Roperh I, Sánchez C, Cortés ML, Gómez del Pulgar T, Izquierdo M, Guzmán M. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nature Med. 2000;6:313–319. doi: 10.1038/73171. [DOI] [PubMed] [Google Scholar]
- 6.Gardner B, Zhu LX, Sharma S, Tashkin DP, Dubinett SM. Methanadamide increases COX-2 expression and tumor growth in murine lung cancer. FASEBJ. 2003;17:2157–9. doi: 10.1096/fj.03-0254fje. [DOI] [PubMed] [Google Scholar]
- 7.Guerrero AV, Quang P, Dekker N, Jordan RC, Schmidt BL. Peripheral cannabinoids attenuate carcinoma-induced nociception in mice. Neuroscience Letters. 2008;433:77–81. doi: 10.1016/j.neulet.2007.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzmán M. Cannabinoids: Potential Anticancer Agents. Nature Reviews. 2003;3:745–755. doi: 10.1038/nrc1188. [DOI] [PubMed] [Google Scholar]
- 9.Hart S, Fischer OM, Ullrich A. Cannabinoids induce cancer cell proliferation via tumor necrosis factor alpha-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Cancer Res. 2004;64:1943–5. doi: 10.1158/0008-5472.can-03-3720. [DOI] [PubMed] [Google Scholar]
- 10.Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, Murphy V, Pertwee RG, Campbell WB. Synthesis and Characterization of Potent and Selective Agonists of the Neuronal Cannabinoid Receptor (CB1) Journal of Pharmacology and Experimental Therapeutics. 1999;289:1427–1433. [PubMed] [Google Scholar]
- 11.Holmes JD, Martin RA, Gutta R. Characteristics of head and neck cancer patients referred to an oral and maxillofacial surgeon in the United States for Management. Journal of Oral and Maxillofacial Surgery. 2010;68:555–561. doi: 10.1016/j.joms.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Dever G, Makriyannis A, Vanderah TW, Mata HP, Malan TP., Jr CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci. 2005;102:3093–8. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kehl LJ, Hamamoto DT, Wacnik PW, Croft DL, Norsted BD, Wilcox GL, Simone DA. A cannabinoid agonist differentially attenuates deep tissue hyperalgesia in animal models of cancer and inflammatory muscle pain. Pain. 2003;103:175–86. doi: 10.1016/s0304-3959(02)00450-5. [DOI] [PubMed] [Google Scholar]
- 14.Kuster JE, Stevenson JI, Ward SJ, D’Ambra’ TE, Haycock DA. Aminoalkylindole Binding in Rat Cerebellum: Selective Displacement by Natural and Synthetic Cannabinoids. The Journal of Pharmacology and experimental Therapeutics. 1995;264:352–365. [PubMed] [Google Scholar]
- 15.Lozano-Ondoua AN, Wright C, Vardanyan A, King T, Largent-Milnes TM, Nelson M, Jimenez-Andrade JM, Mantyh PW, Vanderah TW. A cannabinoid 2 receptor agonist attenuates bone cancer-induced pain and bone loss. Life Sciences. 2010;86:646–65. doi: 10.1016/j.lfs.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKallip RJ, Lombard C, Fisher M, Martin BR, Ryu S, Grant S, Nagarkatti PS, Nagarkatti M. Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood. 2002;100:627–634. doi: 10.1182/blood-2002-01-0098. [DOI] [PubMed] [Google Scholar]
- 17.Morgan NH, Stanford IM, Woodhall GL. Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology. 2009;57:356–68. doi: 10.1016/j.neuropharm.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33–64. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 19.Pertwee RG. Cannabinoid receptors and pain. Progress in Neurobiology. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- 20.Petitet F, Jeantaud B, Bertrand P, Imperato A. Cannabinoid penetration into mouse brain as determined by ex vivo binding. Eur J Pharmacol. 1999;374(3):417–21. doi: 10.1016/s0014-2999(99)00189-2. [DOI] [PubMed] [Google Scholar]
- 21.Pickering V, Jay Gupta R, Quang P, Jordan RC, Schmidt BL. Effect of peripheral endothelin-1 concentration on carcinoma-induced pain in mice. Eur J Pain. 2008;12:293–300. doi: 10.1016/j.ejpain.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pisanti S, Malfitano AM, Grimaldi C, Santoro A, Gazzerro P, Laezza C, Bifulco M. Use of cannabinoid receptor agonists in cancer therapy as palliative and curative agents. Best Pract Res Clin Endocrinol Metab. 2009;23:117–31. doi: 10.1016/j.beem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Potenzieri C, Harding-Rose C, Simone DA. The cannabinoid receptor agonist, WIN55, 212-2, attenuates tumor-evoked hyperalgesia through peripheral mechanisms. Brain Res. 2008;1215:69–75. doi: 10.1016/j.brainres.2008.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quang PN, Schmidt BL. Peripheral endothelin B receptor agonist-induced antinociception involves endogenous opioids in mice. The Journal of Pain. 2010;11(10):917–1026. doi: 10.1016/j.pain.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sánchez C, de Ceballos ML, Gómez del Pulgar T, Rueda D, Corbacho C, Velasco G, Galve-Roperh I, Huffman JW, Ramón S, Ramón C, Guzmán M. Inhibition of glioma growth in vivo by selective activation of the CB(2) cannabinoid receptor. Cancer Res. 2001;61:5784–9. [PubMed] [Google Scholar]
- 26.Sanchez C, Galve-Roperh I, Canova C, Brachet P, Guzman M. Tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS Lett. 1998;436:6–10. doi: 10.1016/s0014-5793(98)01085-0. [DOI] [PubMed] [Google Scholar]
- 27.Sarfaraz S, Adhami VM, Syed DN, Afaq F, Mukhtar H. Cannabinoids for Cancer Treatment: Progress and Promise. Cancer Res. 2008;68:339–42. doi: 10.1158/0008-5472.CAN-07-2785. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt BL, Pickering V, Liu S, Quang P, Dolan J, Connelly ST, Jordan RC. Peripheral endothelin A receptor antagonism attenuates carcinoma-induced pain. Eur J Pain. 2007;11:406–414. doi: 10.1016/j.ejpain.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Valk P, Verbakel S, Vankan Y, Hol S, Mancham S, Ploemacher R. Anandamide, a natural ligand for the peripheral cannabinoid receptor is a novel synergistic growth factor for hematopoietic cells. Blood. 1997;90:1448–57. [PubMed] [Google Scholar]
- 30.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Stella N, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–32. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 31.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184–94. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 32.Yao BB, Mukherjee S, Fan Y, Garrison TR, Daza AV, Grayson GK, Hooker BA, Dart MJ, Sullivan JP, Meyer MD. In vitro pharmacological characterization of AM1241: a protean agonist at the cannabinoid CB2 receptor? British Journal of Pharmacology. 2006;149:145–154. doi: 10.1038/sj.bjp.0706838. [DOI] [PMC free article] [PubMed] [Google Scholar]


