Abstract
High-pressure CO2 treatment has been studied as a promising method for inactivating bacterial spores. In the present study, we compared this method with other sterilization techniques, including heat and pressure treatment. Spores of Bacillus coagulans, Bacillus subtilis, Bacillus cereus, Bacillus licheniformis, and Geobacillus stearothermophilus were subjected to CO2 treatment at 30 MPa and 35°C, to high-hydrostatic-pressure treatment at 200 MPa and 65°C, or to heat treatment at 0.1 MPa and 85°C. All of the bacterial spores except the G. stearothermophilus spores were easily inactivated by the heat treatment. The highly heat- and pressure-resistant spores of G. stearothermophilus were not the most resistant to CO2 treatment. We also investigated the influence of temperature on CO2 inactivation of G. stearothermophilus. Treatment with CO2 and 30 MPa of pressure at 95°C for 120 min resulted in 5-log-order spore inactivation, whereas heat treatment at 95°C for 120 min and high-hydrostatic-pressure treatment at 30 MPa and 95°C for 120 min had little effect. The activation energy required for CO2 treatment of G. stearothermophilus spores was lower than the activation energy for heat or pressure treatment. Although heat was not necessary for inactivationby CO2 treatment of G. stearothermophilus spores, CO2 treatment at 95°C was more effective than treatment at 95°C alone.
Dormant bacterial spores are highly resistant to many physical treatments and chemical agents, including heat, drying, radiation, and chemicals such as hydrogen peroxide (11), and inactivation of such spores is the main objective of food sterilization. Currently, heat, radiation, and chemical preservatives are the major food sterilization methods (29). Of these methods, moist heat treatment is most commonly used (32). Moist heat at temperatures below 100°C is defined as a disinfection process (29), which is meant to destroy disease-causing or other harmful microorganisms but not to kill bacterial spores (2). On the other hand, moist heat at 121°C or higher temperatures is defined as a sterilization process (29), which destroys all forms of life, especially microorganisms, including bacterial spores (2). The high temperature required for heat sterilization can damage the nutritive value, color, and taste of foods (17).
Hydrostatic pressure treatment can also kill dormant bacterial spores (3, 12, 34). However, at moderate temperatures, extremely high pressures (more than 600 MPa) are required to inactivate bacterial spores (19, 30). Such a pressure treatment would be difficult to apply to the food industry (19, 30).
There have been many studies of the effects of high-pressure CO2 treatment, including gaseous and supercritical states, on inactivation of bacteria at moderate temperatures (20 to 40°C) and pressures (5 to 35 MPa) (6, 15, 22). At moderate temperatures and pressures, CO2 treatment significantly inactivates bacterial vegetative cells, whereas pressure treatment alone has little effect (19, 30). There have been some previous studies of inactivation of bacterial spores by CO2 treatment (1, 4, 5, 16, 18); for example, CO2 treatment can substantially inactivate bacterial spores at temperatures above 50°C. Another study showed that Geobacillus stearothermophilus spores were poorly inactivated by CO2 treatment at 35°C and 20 MPa (18).
The G. stearothermophilus spore is one of the most heat-resistant spores of aerobic microorganisms. G. stearothermophilus usually causes flat sour spoilage of canned liquid foods, such as coffee, during storage in automatic vending machines. Because of the heat resistance of this microorganism's spores, they are often used as a biological indicator to evaluate the effectiveness of sterilization processes (7, 20, 26). In general, foods are cooked at temperatures around 100°C, and it is assumed that the foods are not damaged by heat treatment at temperatures below 100°C. For commercial applications, the sterilization period is calculated by using the 5×D (decimal reduction time) concept (21). Based on this concept, 5-log-order killing of bacterial spores (especially G. stearothermophilus spores) at temperatures below 100°C is desired to preserve food quality while providing sterilization. For this reason, in the present study we compared the effects of heat, pressure, and CO2 treatment on inactivation of the spores of five bacteria, including G. stearothermophilus. We also investigated the effect of CO2 treatment on inactivation of G. stearothermophilus spores at temperatures below 100°C.
MATERIALS AND METHODS
Bacteria.
Bacillus coagulans JCM2257 and Bacillus licheniformis JCM2505 were obtained from the Japan Collection of Microorganisms (Saitama, Japan). Bacillus cereus IAM1110 and Bacillus subtilis IAM1069 were obtained from the Institute of Molecular and Cellular Biosciences (Tokyo, Japan). G. stearothermophilus ATCC 12980 was obtained from the American Type Culture Collection (Manassas, Va.).
Media and culture conditions.
Overnight cultures of Bacillus and Geobacillus strains grown in nutrient broth (Difco, Detroit, Mich.) were transferred to sporulation agar plates, which consisted of nutrient agar (Difco) containing 1 μg of Mn2+/ml. The plates were incubated at 37°C (Bacillus) or 55°C (Geobacillus) for 10 days.
Preparation of spore suspensions.
Spores were collected by flooding the surface of a culture with sterile distilled water and then scraping the surface with sterile microscope slides. The spores collected were washed three times by centrifugation at 8,000 × g for 10 min, resuspended in sterile distilled water, and stored at 4°C until they were used. Suspensions were diluted to obtain approximately 106 CFU/ml.
Heat treatment.
Spore suspensions (2 ml) were transferred into glass test tubes (10 by 100 mm), which were then immersed in a water bath equilibrated at 85°C for 20 or 40 s or 1, 3, 5, 60, 120, or 180 min.
Pressure treatment.
Spore suspensions were sealed in sterile screw-cap plastic tubes (capacity, 5 ml; Greiner Labortechnik Co., Ltd., Frickenhausen, Germany) that were pressurized with a prototype pressurization apparatus (24). The time needed to achieve the treatment pressure was approximately 60 s for 200 MPa. The decompression time was approximately 10 s. The temperature of the pressure cell was regulated with a temperature-controlled water bath. Combinations of hydrostatic pressure (200 MPa) and temperature (35 and 95°C) and a total holding period of 120 min were used in this study.
CO2 treatment.
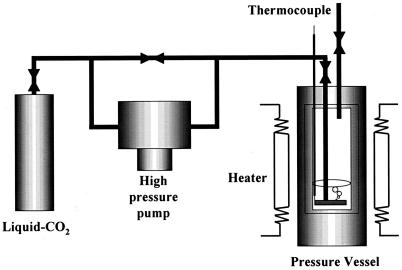
Spore suspensions (10 ml) were poured into sterile stainless test tubes (104 by 17 mm). Each test tube was placed in the vessel of a prototype pressurization apparatus (AKICO Co., Tokyo, Japan) (Fig. 1), and the tube was pressurized with CO2. Approximately 3 min was needed to achieve a treatment pressure of 30 MPa, and the decompression time was approximately 2 min. The temperature in the pressure cell was regulated with a temperature-controlled heater. Combinations of pressure (30 MPa), temperature (35, 55, 65, 75, 85, and 95°C), and total holding period (0, 30, 60, and 120 min) were used in this study.
FIG. 1.
Apparatus for high-pressure CO2 treatment.
Measurement of the number of surviving spores.
The number of surviving spores was determined by the viable plate count method by using nutrient agar (Difco). Prior to counting, the plates containing G. stearothermophilus were incubated at 55°C for 24 h, while the four Bacillus strains were incubated at 37°C for 24 h.
D and z values.
Decimal reduction times for heat treatment (heat treatment D values), decimal reduction times for hydrostatic pressure treatment (pressure treatment D values), and decimal reduction times for high-pressure carbon dioxide treatment (CO2 treatment D values) were calculated from the negative reciprocals of the slopes of the regression lines from the straight portions of the survival curves. The high-pressure carbon dioxide z value (CO2 treatment z value) was defined as the temperature required to decrease the CO2 treatment D value 10-fold in the CO2 treatment experiments. CO2 treatment z values were calculated by determining the negative reciprocals of the slopes of the log CO2 treatment D value curves (log CO2 treatment D value versus temperature).
Arrhenius plot.
Rahn suggested that microbial destruction was due to inactivation of a single critical molecule in the cell and, therefore, could be assumed to follow first-order kinetics (28). Therefore, to evaluate the effects of CO2 treatment on the rates of inactivation of bacterial spores, a simplified mathematical model based on first-order kinetics was used (17, 27): dN/dt = −kN, or, in the integrated form, ln (N/N0) = −kt, where N0 and N are the average viable spore counts measured prior to the CO2 treatment (i.e., control) and at time t (including time zero), respectively, and k is the specific death rate constant or death rate coefficient, which is a function of temperature. The slope of the linear regression analysis of ln (N/N0) versus time t is equal to −k. A survival curve for each spore type was plotted for each temperature. Regression lines were then generated within the linear portions of the survival curves.
The temperature dependence of the rate constant k can generally be expressed with the Arrhenius equation (31). The natural logarithm of the absolute value of the specific death rate (ln k) as a function of the reciprocal absolute temperature (T) is plotted as follows: ln k = ln A − (Ea/R)(1/T), where A is the frequency factor, R is the gas constant (8.314 J/mol • K), and Ea is the activation energy of the CO2 treatment inactivation process (in joules per mole), which also provides a measure of the temperature sensitivity of the bacterial spores under various conditions.
Statistical analysis.
All experiments were repeated three times. In each experiment, one sample was used. The data presented below are the means of three replicate experiments.
RESULTS
Evaluation of the heat resistance of the spores of five species of bacteria.
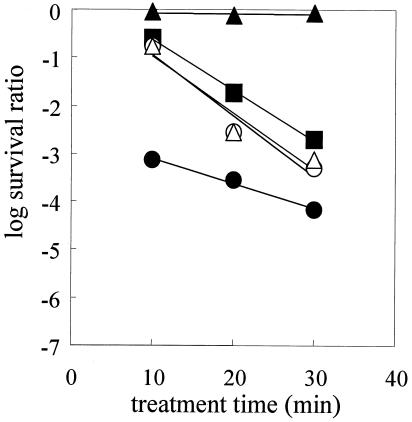
Figure 2 shows the data for inactivation of spores of five species of bacteria by heat treatment at 85°C. The survival curves exhibited tailing, and heat treatment D values for five species were calculated from the linear portions of the survival curves (Table 1). According to the heat treatment D values, the G. stearothermophilus spores were the most heat-resistant spores among the five types of spores (significant inactivation was not observed in 180 min), while the B. licheniformis spores were the most heat sensitive.
FIG. 2.
Survival curves for G. stearothermophilus (▴), B. coagulans (▪), B. licheniformis (○), B. cereus (▵), and B. subtilis (•) spores after heat treatment at 85°C.
TABLE 1.
Effect of heat treatment at 85°C, pressure treatment at 65°C and 200 MPa, and CO2 treatment at 35°C and 30 MPa on the resistance of bacterial spores
| Organism | Treatment | D value (min) |
|---|---|---|
| G. stearothermophilus | Heat | ∞ |
| Pressure | 75.2 | |
| CO2 | 385 | |
| B. subtilis | Heat | 19.0 |
| Pressure | 9.3 | |
| CO2 | 1,667 | |
| B. coagulans | Heat | 9.5 |
| Pressure | 6.9 | |
| CO2 | 164 | |
| B. cereus | Heat | 8.5 |
| Pressure | 6.9 | |
| CO2 | 133 | |
| B. licheniformis | Heat | 7.9 |
| Pressure | 8.5 | |
| CO2 | 182 |
Evaluation of the resistance of the spores of five species of bacteria to hydrostatic pressure treatment.
Figure 3 shows the data for survival of the spores of five species of bacteria after treatment at 65°C and 200 MPa. The survival curves exhibited tailing, and pressure treatment D values for the five species were calculated from the linear portions of the survival curves. The pressure treatment D values calculated are shown in Table 1. Based on the pressure treatment D values, the G. stearothermophilus spores were the most resistant spores among the spores of the five strains, while the B. cereus spores were the most sensitive. The pressure treatment D values for the G. stearothermophilus spores were approximately 11 times higher than those for B. cereus spores. Based on the results obtained, except for G. stearothermophilus, there was no clear relationship between heat resistance and pressure resistance in the bacterial spores.
FIG. 3.
Survival curves for G. stearothermophilus (▴), B. coagulans (▪), B. licheniformis (○), B. cereus (▵), and B. subtilis (•) spores after pressure treatment at 65°C and 200 MPa.
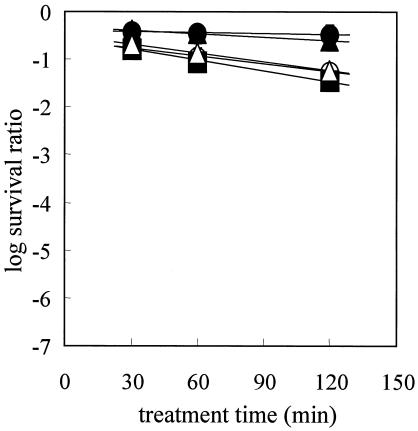
Evaluation of the resistance of the spores of five species of bacteria to high-pressure CO2 treatment.
Figure 4 shows the data for survival of the bacterial spores after treatment with CO2 at 35°C and 30 MPa. The survival curves exhibited tailing, and CO2 treatment D values were calculated from the linear portions of the survival curves. The calculated CO2 treatment D values are shown in Table 1. Based on the CO2 treatment D values, the B. subtilis spores were the most resistant spores among the spores of the five strains, while the B. cereus spores were the most sensitive. The G. stearothermophilus spores were more sensitive than other spores to CO2 treatment. The CO2 treatment D values for B. subtilis spores were approximately 13 times higher than those for B. coagulans spores, indicating that resistance of the spores to CO2 treatment was not correlated with resistance to heat and pressure.
FIG. 4.
Survival curves for G. stearothermophilus (▴), B. coagulans (▪), B. licheniformis (○), B. cereus (▵), and B. subtilis (•) spores after CO2 treatment at 35°C and 30 MPa.
Effect of CO2 treatment on inactivation of G. stearothermophilus spores at temperatures below 100°C.
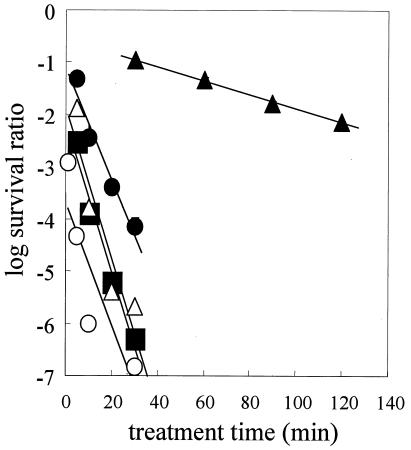
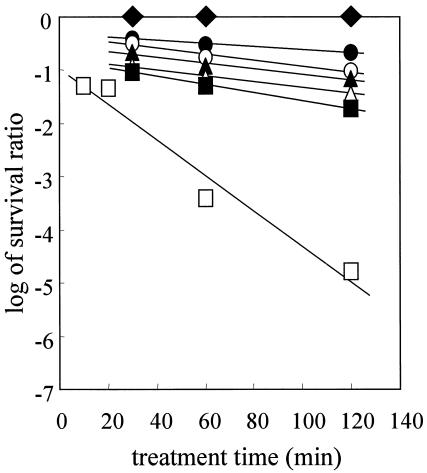
Previous studies showed that it is difficult to completely inactivate G. stearothermophilus spores by CO2 treatment at 35°C (18). However, we speculated that CO2 treatment would inactivate G. stearothermophilus spores at higher temperatures. Therefore, we examined the effect of temperature on inactivation of G. stearothermophilus spores by CO2 treatment at 30 MPa. We found that the inactivation rate increased proportionally with the treatment temperature (Fig. 5). The calculated CO2 treatment D values are shown in Table 2. Although temperatures from 35 to 85°C did not inactivate the G. stearothermophilus spores, the spores were inactivated by CO2 treatment for 120 min at 95°C and 30 MPa.
FIG. 5.
Effect of temperature on survival of G. stearothermophilus spores at 30 MPa and 35°C (•), 55°C (○), 65°C (▴), 75°C (▵), 85°C (▪), and 95°C (▪). ♦, survival curve for G. stearothermophilus spores after heat treatment at 95°C.
TABLE 2.
D values for G. stearothermophilus spores
| Treatment | Temp (°C) | D value (min) |
|---|---|---|
| CO2 (30 MPa) | 35 | 345 |
| 55 | 182 | |
| 65 | 196 | |
| 75 | 179 | |
| 85 | 130 | |
| 95 | 29.9 | |
| Heat | 95 | 2,450 |
CO2 treatment z values for inactivation of G. stearothermophilus spores.
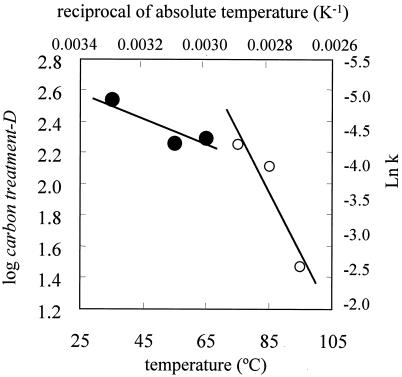
Next, we examined the kinetics of CO2 inactivation of G. stearothermophilus spores at various temperatures. Figure 6 shows the effect of temperature on the log CO2 treatment D values. There were two straight portions in the regression lines in the log CO2 treatment D value graphs, and CO2 treatment z values were calculated from the slopes of the straight portions of the regression lines. CO2 treatment z values are shown in the Table 3. The CO2 treatment z values were 135°C (35 to 85°C) and 25.7°C (75 to 95°C), respectively. These results indicate that an increase in the inactivation rate depends on the increase in temperature more for the high temperature range than for a lower temperature range (Table 3).
FIG. 6.
Effect of temperature on log CO2 treatment D values and Arrhenius plots of the logarithm of the rate constant for inactivation of G. stearothermophilus spores at 30 MPa.
TABLE 3.
Effect of temperature on the CO2 treatment z value and activation energy of G. stearothermophilus spores subjected to CO2 treatment at 30 MPa
| Temp (°C) | CO2 treatment-z value (°C) | Activation energy (kJ/mol) |
|---|---|---|
| 35-85 | 135 | 18.0 |
| 75-95 | 25.7 | 94.5 |
Determination of the activation energy for inactivation of G. stearothermophilus spores by CO2 treatment.
Figure 6 also shows the Arrhenius plots of the logarithm of the rate constant for inactivation of G. stearothermophilus spores by CO2 treatment. There were two linear portions in the regression lines, which were used to calculate the activation energies. The activation energies were 18.0 kJ/mol (35 to 65°C) and 94.5 kJ/mol (75 to 95°C).
DISCUSSION
Previous studies have shown that G. stearothermophilus spores are highly resistant to heat (7, 20, 26). Similarly, we found that at 85°C the heat treatment D value for G. stearothermophilus spores was remarkably higher than the values for four other species. Other studies have shown that bacterial spores cannot be effectively inactivated by pressure treatment at room temperature (8, 10, 23, 25, 30). Because we were interested in the combined effects of pressure and heat, we examined the ability of pressure to sterilize spores at 65°C. We found that the spores used in this study were not inactivated by heat treatment at 70°C for up to 540 min (data not shown). We also found that the pressure treatment D value for G. stearothermophilus spores was approximately 11 times higher than the value for B. cereus spores. Except for G. stearothermophilus, there was no clear relationship between the heat resistance and the pressure resistance of bacterial spores. This result partially agreed with the results of a previous study (23). However, in the previous study, spores were subjected to a pressure treatment at 5 to 10°C and 588 MPa, and the spores of only two of the six Bacillus species used were significantly inactivated (23). Therefore, it is difficult to compare the results of this study and the results of the previous study.
The effect of CO2 treatment on inactivation of bacterial spores was investigated at 35°C and 30 MPa. Based on our results, B. subtilis spores were the most resistant spores among the spores of the five species tested, while B. coagulans spores were the most sensitive. The G. stearothermophilus spores were not the most resistant spores. Specifically, the CO2 treatment D value for B. subtilis spores was approximately 13 times higher than that for B. coagulans spores and was approximately 4.3 times higher than that for G. stearothermophilus spores. The CO2 treatment resistance did not correlate with heat resistance and pressure resistance. These results suggest that there are different mechanisms for inactivation of bacterial spores by CO2, heat, and pressure.
G. stearothermophilus spores were inactivated approximately 5 orders of magnitude by CO2 treatment for 120 min at 95°C and 30 MPa. In contrast, G. stearothermophilus spores were inactivated only approximately 50% by pressure treatment for 120 min at 95°C and 30 MPa (data not shown). Therefore, CO2 treatment was substantially more effective than pressure treatment alone for inactivating G. stearothermophilus spores.
The CO2 treatment D value for G. stearothermophilus spores at 95°C was 29.9 min, and the atmospheric treatment D value at 95°C was 2,450 min. Therefore, the increase in the temperature during CO2 treatment decreased the CO2 treatment D value for G. stearothermophilus approximately 80-fold at 95°C. In contrast to G. stearothermophilus spores, the spores of the four Bacillus strains were easily inactivated by heat treatment at 95°C. Thus, we predicted that the four Bacillus strains would be inactivated easily by CO2 treatment at 95°C and 30 MPa. This treatment is also effective in inactivating G. stearothermophilus spores.
G. stearothermophilus spores are effectively inactivated by pressure treatment at higher temperatures (8, 13, 14). During pressure treatment, dormant bacterial spores germinated under hydrostatic pressure, and the germinated spores were inactivated (3, 12, 34). Similar to the results obtained with the pressure treatment, the bacterial spores germinated during CO2 treatment (9). Clearly, the spores that germinated during CO2 treatment at a higher temperature were heat inactivated at 95°C. In addition, reduction of the pH by CO2 might contribute to the inactivation of the bacterial spores.
The CO2 treatment z values for G. stearothermophilus spores were 135°C (35 to 85°C) and 25.7°C (75 to 95°C). The inactivation rates at the higher temperature range were more dependent on the increase in temperature than were those at the lower temperature range. In general, during heat treatment, the regression plots of log heat treatment D values versus temperatures were linear (17). However, for CO2 treatments, there were two distinct linear portions of the log CO2 treatment D value plots, and there were two corresponding linear portions in the Arrhenius plots (Fig. 6). The activation energies were 18.0 kJ/mol (35 to 65°C) and 94.5 kJ/mol (75 to 95°C) (Table 3). The Arrhenius plots for heat treatments also were linear (27). Based on the CO2 treatment z values and activation energies, the inactivation mechanisms appear to be different at 85 to 95°C. In addition, the mechanism of bacterial spore inactivation by CO2 treatment appears to be different from the mechanism of bacterial spore inactivation by heat treatment.
Similar to the results of the CO2 treatments, there were two straight portions in the regression lines of the Arrhenius plots for pressure treatment at 100 MPa (25). Similarly, both CO2 treatment and pressure treatment initiate bacterial spore germination (9). Thus, inactivation of bacterial spores occurs in two steps. We therefore expected that the G. stearothermophilus spores that germinated during CO2 treatment would be more effectively inactivated at 85 to 95°C than at a lower temperature. Microbial destruction by heat treatment has been shown to be due to inactivation of a single critical molecule in the cells and spores and therefore could be assumed to follow first-order kinetics (28). In other words, inactivation of microorganisms by heat treatment occurs in a single step, while inactivation by CO2 treatment occurs in two steps.
In addition to this evidence indicating that there is a difference in the mechanisms of inactivation, we found differences in the activation energies for bacterial spore inactivation by heat and CO2 treatments. The activation energy for inactivation by heat treatment ranged from 221 to 347 kJ/mol (21), and the activation energy for G. stearothermophilus spores was 351 kJ/mol (33) (Table 4). In contrast, the activation energy for inactivation by CO2 treatment was 94.5 kJ/mol at 75 to 95°C. Therefore, from the perspective of energy consumption, the spores were more effectively inactivated by CO2 treatment than by heat treatment.
TABLE 4.
Activation energies for inactivating bacterial spores by heat, pressure, and CO2 treatments
| Treatment | Pressure (MPa) | Activation energy (kJ/mol) | Reference(s) |
|---|---|---|---|
| Heat | 0.1 | 221-351a | 21, 33 |
| Pressure | 100 | 143-247 | 24 |
| 200 | 198-270 | 24 | |
| CO2 | 30 | 94.5a | This study |
The data include data for G. stearothermophilus spores.
For pressure treatments at 90 to 110°C, the activation energies for inactivation of bacterial spores ranged from 143 to 247 kJ/mol at 100 MPa and from 198 to 270 kJ/mol at 200 MPa (25). These data, however, did not include the data for G. stearothermophilus, but we predicted that the activation energy for inactivating G. stearothermophilus spores is higher than the activation energies for inactivating Bacillus spores. Therefore, CO2 treatment is more effective than both heat treatment and pressure treatment for inactivating bacterial spores.
Many foods are cooked at approximately 100°C. For this reason, it is desirable to kill bacterial spores by heat treatment at temperatures less than 100°C. Our results show that CO2 treatment at temperatures below 100°C can effectively inactivate bacterial spores, including those of G. stearothermophilus. For commercial food sterilization, the sterilization period is calculated based on the 5×D concept (21). Based on this calculation, treatment for 120 min at 95°C and 30 MPa is sufficient for sterilization by CO2 treatment. CO2 treatment can therefore decrease the processing temperature and pressure needed to inactivate bacterial spores, including spores of G. stearothermophilus. Decreasing the processing temperature should decrease the amount of heat damage and the required processing pressure and thereby reduce the cost of sterilization equipment.
We investigated the effect of a 120-min treatment with CO2 at 95°C and 30 MPa on the quality of commercially available milk, orange juice, coffee, and soup. Except for milk, which was coagulated, we found no noticeable reduction in quality.
REFERENCES
- 1.Ballestra, P., and J. Cue. 1998. Influence of pressurized carbon dioxide on the thermal inactivation of bacterial and fungal spores. Lebensm. Wiss. Technol. 31:84-88. [Google Scholar]
- 2.Block, S. S. 2001. Definition of terms, p. 19-28. In S. S. Block (ed.), Disinfection, sterilization, and preservation. Lea & Febiger, London, United Kingdom.
- 3.Clouston, J. G., and P. A. Wills. 1969. Initiation of germination and inactivation of Bacillus pumilus spores by hydrostatic pressure. J. Bacteriol. 97:684-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dillow, A. K., F. Dehghani, J. S. Hrkach, N. R. Foster, and R. Langer. 1999. Bacterial inactivation by using near- and supercritical carbon dioxide. Proc. Natl. Acad. Sci. 96:10344-10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enomoto, A., K. Nakamura, K. Nagai, T. Hashimoto, and M. Hakoda. 1997. Inactivation of food microorganisms by high-pressure carbon dioxide treatment with or without explosive decompression. Biosci. Bio/Technol. Biochem. 61:1133-1137. [DOI] [PubMed] [Google Scholar]
- 6.Erkmen, O. 2001. Effect of high-pressure carbon dioxide on Escherichia coli in nutrient broth and milk. Int. J. Food Microbiol. 65:131-135. [DOI] [PubMed] [Google Scholar]
- 7.Feeherry, F. E., D. T. Munsey, and D. B. Rowley. 1987. Thermal inactivation and injury of Bacillus stearothermophilus spores. Appl. Environ. Microbiol. 53:365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furukawa, S., and I. Hayakawa. 2000. Investigation of desirable hydrostatic pressure required to sterilize Bacillus stearothermophilus IFO 12550 spores and its sterilization properties in glucose, sodium chloride and ethanol solutions. Food Res. Int. 33:901-905. [Google Scholar]
- 9.Furukawa, S., T. Watanabe, T. Tai, J. Hirata, N. Narisawa, T. Kawarai, H. Ogihara, and M. Yamasaki. Effect of high pressure gaseous carbon dioxide on the germination of bacterial spores. Int. J. Food Microbiol., in press. [DOI] [PubMed]
- 10.Gould, G. W. 1973. Inactivation of spores in food by combined heat and hydrostatic pressure. Acta Aliment. 2:377-383.
- 11.Gould, G. W. 1983. Mechanisms of resistance and dormancy, p. 173-209. In A. Hurst and G. W. Gould (ed.), The bacterial spore, vol. 2. Academic Press Inc., New York, N.Y.
- 12.Gould, G. W., and J. H. Sale. 1970. Initiation of germination of bacterial spores by hydrostatic pressure. J. Gen. Microbiol. 60:335-346. [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa, I., T. Kanno, M. Tomita, and Y. Fujio. 1994. Application of high pressure for inactivation and protein denaturation. J. Food Sci. 59:159-163. [Google Scholar]
- 14.Hayakawa, I., T. Kanno, K. Yoshiyama, and Y. Fujio. 1994. Oscillatory compared with continuous high pressure sterilization on Bacillus stearothermophilus spores. J. Food Sci. 59:164-167. [Google Scholar]
- 15.Ishikawa, H., M. Shimoda, H. Shiratsuchi, and Y. Osajima. 1995. Sterilization of microorganisms by the supercritical carbon dioxide micro-bubble method. Biosci. Bio/Technol. Biochem. 59:1949-1950. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa, H., M. Shimoda, K. Tamaya, A. Yonekura, T. Kawano, and Y. Osajima. 1997. Inactivation of Bacillus spores by the supercritical carbon dioxide micro-bubble method. Biosci. Bio/Technol. Biochem. 61:1022-1023. [DOI] [PubMed] [Google Scholar]
- 17.Joslyn, L. J. 2001. Sterilization by heat, p. 695-728. In S. S. Block (ed.), Disinfection, sterilization, and preservation. Lea & Febiger, London, United Kingdom.
- 18.Kamihira, M., M. Taniguchi, and T. Nobayashi. 1987. Sterilization of microorganisms with supercritical carbon dioxide. Agric. Biol. Chem. 51:407-412. [Google Scholar]
- 19.Knorr, D., and V. Heinz. 2001. Development of nonthermal methods for microbial control, p. 853-877. In S. S. Block (ed.), Disinfection, sterilization, and preservation. Lea & Febiger, London, United Kingdom.
- 20.Lopez, M., I. Gonzarez, M. Mazas, J. Gonzarez, R. Martin, and A. Bernardo. 1997. Influence of recovery conditions on apparent resistance of Bacillus stearothermophilus spores. Int. J. Food Sci. Technol. 32:305-311. [Google Scholar]
- 21.Lund, D. B. 1977. Design of thermal processes for maximizing nutrient retention. Food Technol. 31:71-78. [Google Scholar]
- 22.Nakamura, K., A. Enomoto, H. Fukuhisa, K. Nagai, and M. Hakoda. 1994. Disruption of microbial cells by the flash discharge of high-pressure carbon dioxide. Biosci. Bio/Technol. Biochem. 58:1297-1301. [Google Scholar]
- 23.Nakayama, A., Y. Yano, S. Kobayashi, M. Ishikawa, and K. Sakai. 1996. Comparison of pressure resistances of spores of six Bacillus strains with their heat resistances. Appl. Environ. Microbiol. 62:3897-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogihara, H., T. Hitomi, and N. Yano. 1998. Inactivation of some foodborne pathogens and indicator bacteria by hydrostatic pressure. J. Food Hyg. Soc. Jpn. 39:436-439. (In Japanese.) [Google Scholar]
- 25.Okazaki, T., T. Yoneda, and K. Suzuki. 1994. Combined effects of temperature and pressure on sterilization of Bacillus subtilis spores. J. Jpn. Soc. Food Sci. Technol. 41:536-541. (In Japanese.) [Google Scholar]
- 26.Periago, P. M., F. P. S., M. J. Ocio, and A. Martinez. 1998. Apparent thermal resistance of Bacillus stearothermophilus spores recovered under anaerobic conditions. Z. Lebensm. Unters. Forsch. 206:63-67. [Google Scholar]
- 27.Pflug, I. J., R. G. Holcomb, and M. M. Gomez. 2001. Principles of the thermal destruction of microorganisms, p. 79-129. In S. S. Block (ed.), Disinfection, sterilization, and preservation. Lea & Febiger, London, United Kingdom.
- 28.Rahn, O. 1945. Injury and death of bacteria. MO: Biodynamica, Normandy, France.
- 29.Russell, A. D. 2001. Principles of antimicrobial activity, p. 31-55. In S. S. Block (ed.), Disinfection, sterilization, and preservation. Lea & Febiger, London, United Kingdom.
- 30.Sonoike, K. 1997. Subject for the application to food. J. Jpn. Soc. Food Sci. Technol. 44:522-530. (In Japanese.) [Google Scholar]
- 31.Torok, T., and A. D. J. King. 1966. Thermal inactivation kinetics of food-borne yeasts. J. Food Sci. 56:6-9. [Google Scholar]
- 32.Walker, H. W., and W. S. LaGrange. 1991. Sanitation in food manufacturing operations, p. 791-801. In S. S. Block (ed.), Disinfection, sterilization, and preservation. Lea & Febiger, London, United Kingdom.
- 33.Wang, D. I., J. Scharer, and A. E. Humphrey. 1964. Kinetics of death of bacterial spore at elevated temperatures. Appl. Microbiol. 12:451-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wuytack, E., S. Boven, and C. Michiels. 1998. Comparative study of pressure-induced germination of Bacillus subtilis spores at low and high pressures. Appl. Environ. Microbiol. 64:3220-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]