Abstract
Study Objectives:
Upper airway (UA) collapsibility is a major factor in the pathophysiology of sleep disordered breathing (SDB). We hypothesized that the negative expiratory pressure (NEP) technique could distinguish between normal children and children with SDB even during wakefulness.
Design:
During wakefulness, NEP of −5 and −10 cm H2O was applied during expiration in seated and supine positions. UA muscle activity (EMG) was measured using intra-oral electrodes.
Setting:
Sleep laboratory.
Participants:
Twenty children with snoring, 20 with obstructive sleep apnea syndrome (OSAS), and 20 controls.
Measurements and Results:
The ratio of the area under the expiratory flow-volume curve during NEP compared to tidal breathing (RatioNEP) was calculated. Similarly, EMG area under the curve during NEP as a ratio of baseline was measured (RatioEMG). There were significant differences in RatioNEP between controls and snorers and controls and OSAS, at both pressures, in both the seated and supine positions; P < 0.0001 for all (e.g., RatioNEP at −5 cm H2O, seated: 1.8 ± 0.5, 2.1 ± 0.4, and 3.0 ± 0.6 for OSAS, snorers, and controls, respectively). However, no significant differences were found between snorers and OSAS. For RatioEMG, no significant differences were found between groups.
Conclusions:
RatioNEP distinguishes between normal children and children with SDB, be it snoring or OSAS, indicating that these children have a more collapsible UA even during wakefulness. However, it does not differentiate between snorers and OSAS, highlighting the important role of UA muscle activity during sleep. NEP technique does not elicit a different UA muscle activity response between controls and children with SDB.
Citation:
Carrera HL; McDonough JM; Gallagher PR; Pinto S; Samuel J; DiFeo N; Marcus CL. Upper airway collapsibility during wakefulness in children with sleep disordered breathing, as determined by the negative expiratory pressure technique. SLEEP 2011;34(6):717-724.
Keywords: Obstructive sleep apnea, snoring, electromyogram
INTRODUCTION
The obstructive sleep apnea syndrome (OSAS) is a common disorder in children,1,2 characterized by recurrent upper airway collapse during sleep. One of the mechanisms implicated in the pathogenesis of OSAS is increased collapsibility of the upper airway. Upper airway collapse during sleep is thought to occur when the activity of upper airway dilator muscles is not enough to compensate for an anatomically small upper airway.3
The negative expiratory pressure (NEP) technique has been used to evaluate upper airway collapsibility in adults during wakefulness. The technique consists of applying negative pressure at the mouth during early expiration during tidal breathing. The obtained flow-volume curve is compared with the flow-volume curve of the preceding expiration. Studies in adults have shown that the flow-volume curve obtained during NEP is higher than the flow-volume curve of the preceding expiration in both normal subjects and subjects with sleep disordered breathing (SDB); however, this increase in expiratory flow above normal tidal breathing is smaller in snorers and patients with OSAS than in normal adults.4–6 Thus, NEP can differentiate between healthy adults and adults with a collapsible upper airway.
During wakefulness, pharyngeal dilator muscles such as the genioglossus have a higher activity in patients with OSAS than controls.7,8 At sleep onset, genioglossal activity decreases, and the smaller and more collapsible upper airway in patients with OSAS is prone to collapse.9,10 However, the increase in airflow with NEP is thought to be a passive phenomenon,11 although this has not been extensively studied.
To our knowledge, the NEP technique has not been evaluated in children with SDB. SDB in children differs from that in adults. Children have a less collapsible upper airway than adults in response to negative pressure applied continuously during sleep.12 Children also have a greater ventilatory drive than adults13 and increased upper airway neuromotor activation compared to adults.12 Thus, it is possible that they have a different upper airway response to NEP during wakefulness. Furthermore, studies have not fully evaluated the upper airway electromyographic (EMG) response to NEP in patients with OSAS.
We hypothesized that the NEP technique could distinguish between normal children and children with SDB, even during wakefulness. We also hypothesized that NEP would not elicit upper airway muscle activity, and that controls and children with SDB would manifest similar EMG responses to NEP. We therefore evaluated upper airway collapsibility during wakefulness in children with SDB (snoring and OSAS) compared to normal control children by assessing flow-volume curves and EMG upper airway muscle activity during the application of NEP at 2 levels of pressure (−5 and −10 cm H2O). In order to evaluate whether the supine position was associated with greater upper airway collapsibility, we applied NEP to subjects in both the seated and supine position.
METHODS
Children with SDB (either primary snoring or OSAS) and normal controls were studied. Subjects underwent baseline spirometry and polysomnography. They then underwent NEP testing during wakefulness. Intra-oral EMG was measured during NEP. The Institutional Review Board of the Children's Hospital of Philadelphia approved the study. Written informed consent was obtained from the parent or legal guardian. Assent was obtained from subjects aged 7 years and older.
Subjects
Children with SDB were recruited from the Sleep Center at The Children's Hospital of Philadelphia, and controls were recruited from the general population by means of advertisements. Inclusion criteria included age 6-16 years and no significant medical conditions other than SDB. The lower age limit was chosen to exclude children too young to cooperate with the experimental setup. As many children with OSAS have asthma,14 children with asthma were included if symptoms were well-controlled, spirometry was normal (forced vital capacity [FVC] and forced expiratory volume in 1 second [FEV1] ≥ 80% predicted and FEV1/FVC ≥ 80%) on the day of study, and albuterol had not been used on the day of study.
Three groups were recruited: Controls (apnea hypopnea index [AHI] < 2/h), snorers (defined as a history of snoring ≥ 3 nights/week; AHI < 2/h) and OSAS (AHI ≥ 2/h).15–17
Spirometry
Spirometry (Morgan Scientific, Inc., Haverhill, MA) was performed using standard pediatric methods18 in order to rule out pulmonary disease; particularly the presence of intrathoracic obstruction. Only children with normal spirometry, as described above, were included.
Polysomnography
Baseline overnight polysomnography (using Rembrandt, MedCare, Buffalo, NY) was performed and the following parameters recorded: electroencephalograms, electrooculograms, submental and tibial electromyograms, chest and abdominal wall movement by inductance plethysmography (Respitrace, Ambulatory Monitoring Inc., Ardsley, NY), ECG, airflow by nasal pressure (Pro-Tech, Mukilteo, WA) and 3-pronged thermistor (Pro-Tech, Woodinville, WA), end-tidal PCO2 (Novametrix 7000; Novametrix, Wallingford, CT), arterial oxygen saturation and pulse waveform, (Masimo, Irvine, CA or Nonin, Plymouth, MN) and digital, infrared video. Sleep architecture and respiratory parameters were analyzed using standard pediatric criteria.19
NEP Measurements
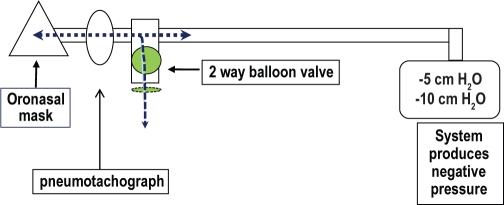
The respiratory circuit is shown in Figure 1. Flow was measured with a heated pneumotachograph (Hans-Rudolph, Inc., Shawnee, KS) with a linearity range of ± 2.6 L/s, connected to an oronasal mask (Philips Respironics, Andover, MA) and a differential pressure transducer (Validyne, Northridge, CA). Pressure was measured at the mask via a noncompliant tube connected to a differential pressure transducer (Validyne, Northridge, CA). A customized device capable of rapidly generating negative pressure (Phillips Respironics, Andover, MA) was connected to the pneumotachograph via a 2-way balloon valve (Hans-Rudolph, Inc., Shawnee, KS). The balloon valve was activated by custom-written software when inspiratory flow reached a threshold level between 0.05-0.01 L, taking into account the time delay for balloon inflation (TestPoint, SuperLogics, Inc., Natick, MA). At that point, negative (subatmospheric) pressure was delivered during early expiration (within 0.1-0.2 seconds) for 1 second. Flow and pressure transducers were calibrated prior to each study.
Figure 1.
Respiratory circuit for measuring NEP.
EMG recordings during NEP were obtained using non-invasive, intraoral mouthpieces that were custom molded for each subject.8 An impression of the sublingual fossa and lower teeth was made using a dental-grade, vinyl silicone putty (Splash', Wennigsen, Germany) and a dental mandibular tray modified to allow impression material to contact the floor of the mouth so that the material was closely opposed to the lower surface of the tongue. The mold enclosed the inferior front 4-5 teeth, and was then trimmed to remove excessive bulk. Unipolar surface electrodes made of 30-gauge Teflon-coated stainless steel wire (Sequim, WA) were sewn into the inferior surface of the mold. EMG signals were amplified, rectified, and integrated on a moving-time-average basis, with a time constant of 200 ms. The impedance was checked before each study to ensure it was < 20 Ω. Mask pressure, tidal volume, airflow, and EMG were displayed using PowerLab software (ADInstruments, Colorado Springs, CO). Volume was obtained by integration of the flow signal. Data analysis was performed using custom software (PowerBasic, Inc, Englewood, FL).
Procedure
The NEP technique involves obtaining flow-volume curves during quiet tidal breathing during wakefulness. The flow-volume curves were obtained during application of NEP of −5 and −10 cm H2O at the onset of expiration in both the supine and seated positions.
During the study, the subjects sat comfortably while wearing EMG mouthpieces and an oronasal mask, and were distracted by watching television. An oronasal mask was used, as many children with OSAS are mouth-breathers, and also to avoid interference with the EMG mouthpiece. First, in order to evaluate upper airway muscle EMG activity and distinguish voluntary movements such as swallows, subjects were asked to perform maximal genioglossal maneuvers such as protruding their tongue as forcibly as possible, and to swallow. To avoid artifact, the EMG signal was assessed at the beginning and end of each trial (each pressure level and position), and compared with the previous maneuvers and recordings. Furthermore, the EMG signal was displayed on the screen continuously during the study, allowing for the identification of artifact if the intraoral mouthpiece moved. After a period of regular quiet breathing, NEP was applied during early expiration and was maintained for 1 second. NEP was applied when inspiratory and expiratory tidal volumes were equal, using accepted guidelines for measuring tidal volume.20 Thus, it was assumed that that the application of NEP was made at the same end expiratory lung volume (EELV). NEP was repeated several times at each level of pressure of NEP. The procedure was then repeated with the child in the supine position.
Data Analysis
The flow-volume curve obtained during NEP was compared to the flow-volume curve of the preceding breath. We established a parameter termed RatioNEP as the ratio of the area under the flow-volume curve during NEP to the preceding flow-volume curve at the same time points (0.25 sec, 0.5 sec, 0.75 sec, and 1 sec). Area under the curve of the flow-volume curve was used as it has been shown that this formula seems most sensitive in identifying lung measurement abnormalities.21 Previous studies have used the area under the curve for NEP determination,6 but we used a modification whereby the flow-volume curves (normal tidal breathing and NEP) were aligned by time at 0.25, 0.5, 0.75, and 1 second, in order to account for the age-related variability in respiratory rate and tidal volume in children. The average of the best NEP maneuvers (based on lack of EMG or respiratory artifacts, similar tidal volume, regular waveforms and similar expiratory times) in each subject was used for analysis. On average, 6 breaths were analyzed for each time point, pressure level, and position. Similarly, EMG area under the curve during NEP as a ratio of baseline was measured (RatioEMG).
Statistical Analysis
Histograms and one-sample Kolmogorov-Smirnov tests were used to determine normalcy of distribution. For demographic values, descriptive statistics were computed for each group, and differences among the 3 groups were examined using analysis of variance (ANOVA) or Kruskal-Wallis tests. Post hoc pairwise tests using the Bonferroni correction factor were conducted. Paired t-tests or Wilcoxon matched-pairs signed rank tests were used to compare the paired position data and the paired pressure level data within each group separately. The association between AHI and RatioNEP was assessed using Spearman correlation. SPSS for Windows was used for descriptive statistics, group comparisons, and paired analyses (SPSS for Windows, Release 16.0.1. 2007. Chicago: SPSS Inc.).
For each of the 2 primary outcomes (RatioNEP and RatioEMG), mixed effects models, accounting for the repeated measurements of subjects in the 2 positions and the 2 pressure levels, and at the different time points (1.00, 0.75, 0.50, and 0.25 seconds), were used to examine the effects of group, position, pressure level, and time point, as well as interactions between/among these factors; gender, age, and body mass index z-score were also included in the models. Assessment of main effects was followed by post hoc pairwise comparisons of least squares means; Bonferroni correction factors were applied as needed. For pairwise comparisons based on the 3-way interaction of group-by-position-by-pressure, N = 24 comparisons were involved; we therefore required a P-value of ≤ 0.0021 (0.05/24) for statistical significance. The SAS proc mixed procedure (SAS Institute Inc. 2008. SAS/STAT 9.2 User's Guide. Cary, NC: SAS Institute Inc.) was used for mixed effects modelling.
RESULTS
Study Group
The study group is shown in Table 1. Age was similar between groups. However, SDB subjects were more likely to be male and to have a higher BMI z-score.
Table 1.
Study group
| Controls | Snorers | OSAS | |
|---|---|---|---|
| N | 20 | 20 | 20 |
| Age (yr) | 13 ± 3 | 12 ± 3 | 12 ± 3 |
| Males (N, %)a | 7 (35) | 14 (70) | 14 (70) |
| BMI z-scoreb | 0.3 ± 1.4 | 1.6 ± 0.9 | 2.2 ± 0.8 |
| AHI (N/h)c | 0.0 (0.0-0.9) | 0.7 (0.0-1.9) | 8.1 (2.2-46.5) |
Data depicted as mean ± SD for normally distributed data and median (range) for nonparametric data.
BMI, body mass index; AHI, apnea hypopnea index.
P = 0.013 for controls vs. snorers and OSAS.
P < 0.0005 for controls vs. snorers and controls vs. OSAS; no difference between snorers and OSAS (P = 0.25).
P < 0.0005 for OSAS vs. controls and OSAS vs.snorers.
RatioNEP and RatioEMG
Typical examples of the flow-volume curves and EMG signal obtained during NEP are depicted in Figure 2. Data for RatioNEP and RatioEMG at −5 cm H2O in the seated position are depicted in Table 2 and Figures 3 and 4. Data for RatioNEP and RatioEMG at −10 cm H2O and for seated vs supine positions are shown in Tables 3 and 4.
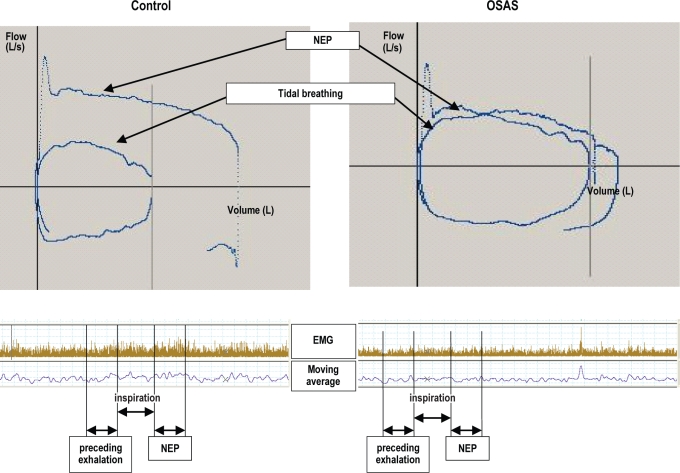
Figure 2.
The flow-volume curves and EMG signals from a control subject (left panel) and subject with OSAS (right panel) during NEP compared to tidal breathing. In the control subject, the flow-volume curve during NEP is larger than during the preceding tidal exhalation. In the OSAS subject, the difference between the two curves is much smaller. There were no differences in the EMG before and after NEP. NEP, negative expiratory pressure; OSAS, obstructive sleep apnea syndrome.
Table 2.
RatioNEP and RatioEMG at different time points at a pressure of −5 cm H2O in the seated position
| Controls | Snorers | OSAS | |
|---|---|---|---|
| RatioNEP | |||
| N | 20 | 20 | 20 |
| RatioNEP 1s | 3.0 ± 0.6* | 2.1 ± 0.4 | 1.8 ± 0.5 |
| RatioNEP 0.75s | 3.5 ± 0.8* | 2.4 ± 0.5 | 2.0 ± 0.5 |
| RatioNEP 0.5s | 4.9 ± 1.7* | 2.9 ± 0.7 | 2.5 ± 0.7 |
| RatioNEP 0.25s | 12.8 ± 8.8* | 5.5 ± 1.9 | 4.9 ± 1.9 |
| RatioEMG | |||
| N | 17 | 17 | 17 |
| RatioEMG 1s | 1.1 ± 0.4 | 1.1 ± 0.3 | 1.2 ± 0.3 |
| RatioEMG 0.75s | 1.0 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.3 |
| RatioEMG 0.5s | 1.1 ± 0.3 | 1.4 ± 0.6 | 1.2 ± 0.4 |
| RatioEMG 0.25s | 1.3 ± 0.7 | 1.8 ± 1.0 | 1.3 ± 0.5 |
Data depicted as mean ± SD.
There were significant differences in RatioNEP for all time points between controls vs. snorers and controls vs. OSAS (both P < 0.0001). There were no significant differences in RatioNEP for snorers vs. OSAS. There were no significant differences in RatioEMG between any of the groups.
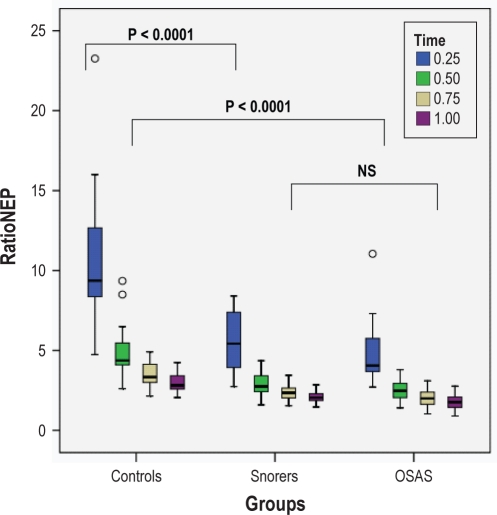
Figure 3.
Box plots of RatioNEP at different time points and at a pressure of −5 cm H2O in the seated position are shown for the 3 groups. The box represents the interquartile range which contains 50% of values. The line across the box indicates the median. The whiskers extend from the box to the highest and lowest values, excluding outliers. Outliers (o) are defined as cases with values between 1.5 and 3 box lengths from either end of the box. For ease of presentation, 2 extreme outliers were omitted; these were controls at the 0.25 time point with values of 41.54 and 27.14.
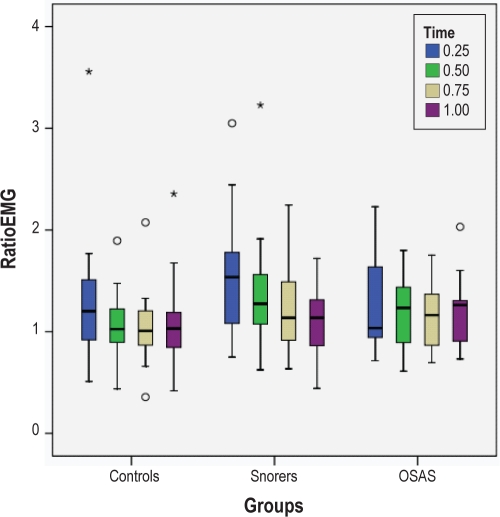
Figure 4.
Box plots of RatioEMG at different time points and at a pressure of −5 cm H2O in the seated position are shown for the 3 groups. The box represents the interquartile range which contains 50% of values. The line across the box indicates the median. The whiskers extend from the box to the highest and lowest values, excluding outliers. Outliers (o) are defined as cases with values between 1.5 and 3 box lengths from either end of the box. Extreme outliers (*) are cases with values > 3 box lengths from either end of the box. There were no significant differences between groups. For ease of presentation, one extreme outlier was omitted; this was a snorer at the 0.25 time point with a value of 5.26.
Table 3.
RatioNEP and RatioEMG at different time points for −5 vs. −10 cm H2O
| −5 cm versus −10 cm H2O seated | −5 cm versus −10 cm H2O supine | ||||
|---|---|---|---|---|---|
| RatioNEP | |||||
| All | RatioNEP | 1s | 2.3 ± 0.7 vs 3.3 ± 1.3 | 1.9 ± 0.7 vs 2.4 ± 1.2 | |
| 0.75s | 2.6 ± 0.9 vs 3.9 ± 1.7 | 2.2 ± 0.9 vs 3.0 ± 1.6 | |||
| 0.5s | 3.5 ± 1.5 vs 5.4 ± 2.9 | 2.9 ± 1.4 vs 4.2 ± 3.0 | |||
| 0.25s | 7.8 ± 6.4 vs 12.7 ± 9.7 | 6.0 ± 5.0 vs 9.6 ± 10.4 | |||
| Control | RatioNEP | 1s | 2.9 ± 0.6 vs 4.6 ± 1.1 | 2.5 ± 0.6 vs 3.5 ± 1.3 | |
| 0.75s | 3.5 ± 0.8 vs 5.5 ± 1.5 | 3.0 ± 0.8 vs 4.4 ± 1.8 | |||
| 0.5s | 4.9 ± 1.7 vs 8.1 ± 3.2 | 4.2 ± 1.6 vs 6.7 ± 3.8 | |||
| 0.25s | 12.8 ± 8.8 vs 20.6 ± 12.5 | 9.7 ± 7.0 vs 17.6 ± 14.9 | |||
| Snorers | RatioNEP | 1s | 2.1 ± 0.4 vs 2.9 ± 0.8 | 1.8 ± 0.6 vs 2.1 ± 0.6 | |
| 0.75s | 2.4 ± 0.5 vs 3.4 ± 0.9 | 1.9 ± 0.6 vs 2.5 ± 0.8 | |||
| 0.5s | 2.9 ± 0.7 vs 4.5 ± 1.6 | 2.4 ± 0.7 vs 3.3 ± 1.0 | |||
| 0.25s | 5.7 ± 1.8 vs 10.3 ± 4.1 | 4.4 ± 1.2 vs 6.3 ± 1.9 | |||
| OSAS | RatioNEP | 1s | 1.8 ± 0.5 vs 2.4 ± 0.8 | 1.5 ± 0.5 vs 1.7 ± 0.7 | |
| 0.75s | 2.0 ± 0.5 vs 2.7 ± 0.8 | 1.7 ± 0.5 vs 1.9 ± 0.9 | |||
| 0.5s | 2.5 ± 0.7 vs 3.5 ± 1.0 | 2.1 ± 0.7 vs 2.5 ± 1.1 | |||
| 0.25s | 4.9 ± 1.9 vs 7.1 ± 2.8 | 3.8 ± 1.8 vs 4.8 ± 2.0 | |||
| RatioEMG | |||||
| All | RatioEMG | 1s | 1.1 ± 0.4 vs 1.4 ± 0.8 | 1.2 ± 0.5 vs 1.6 ± 1.0 | |
| 0.75s | 1.1 ± 0.4 vs 1.5 ± 1.1 | 1.3 ± 0.6 vs 1.9 ± 1.5 | |||
| 0.5s | 1.2 ± 0.5 vs 1.8 ± 1.2 | 1.5 ± 0.8 vs 2.0 ± 1.7 | |||
| 0.25s | 1.4 ± 0.8 vs 2.3 ± 2.2 | 1.8 ± 1.3 vs 2.3 ± 2.0 | |||
| Control | RatioEMG | 1s | 1.1 ± 0.4 vs 1.4 ± 1.1 | 1.1 ± 0.4 vs 1.5 ± 0.9 | |
| 0.75s | 1.0 ± 0.4 vs 1.5 ± 1.5 | 1.3 ± 0.8 vs 1.9 ± 2.0 | |||
| 0.5s | 1.1 ± 0.3 vs 1.6 ± 1.2 | 1.3 ± 0.7 vs 2.2 ± 2.3 | |||
| 0.25s | 1.3 ± 0.7 vs 1.9 ± 1.6 | 1.5 ± 1.0 vs 2.2 ± 2.5 | |||
| Snorers | RatioEMG | 1s | 1.1 ± 0.4 vs 1.4 ± 0.6 | 1.3 ± 0.7 vs 1.7 ± 1.6 | |
| 0.75s | 1.2 ± 0.4 vs 1.5 ± 0.8 | 1.3 ± 0.6 vs 1.7 ± 1.2 | |||
| 0.5s | 1.4 ± 0.6 vs 2.1 ± 1.5 | 1.5 ± 0.9 vs 1.8 ± 1.2 | |||
| 0.25s | 1.8 ± 1.0 vs 2.8 ± 3.0 | 2.0 ± 1.5 vs 2.1 ± 1.6 | |||
| OSAS | RatioEMG | 1s | 1.2 ± 0.3 vs 1.5 ± 0.8 | 1.4 ± 0.5 vs 1.6 ± 0.5 | |
| 0.75s | 1.2 ± 0.3 vs 1.5 ± 0.8 | 1.5 ± 0.6 vs 2.0 ± 1.3 | |||
| 0.5s | 1.2 ± 0.4 vs 1.7 ± 1.0 | 1.6 ± 0.8 vs 2.1 ± 1.4 | |||
| 0.25s | 1.3 ± 0.5 vs 2.1 ± 1.9 | 1.9 ± 1.4 vs 2.7 ± 2.0 |
Data shown as mean ± SD
Table 4.
RatioNEP and RatioEMG at different time points for the seated vs. supine positions
| Seated versus supine −5 cm H2O | Seated versus supine −10 cm H2O | |||
|---|---|---|---|---|
| RatioNEP | ||||
| All | RatioNEP | 1s | 2.3 ± 0.7 vs 1.9 ± 0.7 | 3.2 ± 1.2 vs 2.4 ± 1.2 |
| 0.75s | 2.6 ± 0.9 vs 2.2 ± 0.9 | 3.9 ± 1.7 vs 3.0 ± 1.7 | ||
| 0.5s | 3.5 ± 1.6 vs 2.9 ± 1.4 | 5.4 ± 3.0 vs 4.2 ± 3.0 | ||
| 0.25s | 7.8 ± 6.4 vs 6.0 ± 5.0 | 12.8 ± 9.7 vs 9.7 ± 10.5 | ||
| Control | RatioNEP | 1s | 3.0 ± 0.5 vs 2.6 ± 0.6 | 4.5 ± 1.0 vs 3.5 ± 1.3 |
| 0.75s | 3.5 ± 0.8 vs 3.0 ± 0.8 | 5.5 ± 1.5 vs 4.4 ± 1.8 | ||
| 0.5s | 4.9 ± 1.7 vs 4.2 ± 1.6 | 8.1 ± 3.2 vs 6.7 ± 3.8 | ||
| 0.25s | 12.8 ± 8.8 vs 9.7 ± 7.0 | 20.6 ± 12.5 vs 17.6 ± 14.9 | ||
| Snorers | RatioNEP | 1s | 2.1 ± 0.4 vs 1.7 ± 0.6 | 2.8 ± 0.6 vs 2.1 ± 0.6 |
| 0.75s | 2.4 ± 0.5 vs 1.9 ± 0.6 | 3.5 ± 0.9 vs 2.5 ± 0.9 | ||
| 0.5s | 2.9 ± 0.8 vs 2.4 ± 0.7 | 4.5 ± 1.6 vs 3.3 ± 1.1 | ||
| 0.25s | 5.5 ± 1.9 vs 4.4 ± 1.2 | 10.3 ± 4.2 vs 6.3 ± 1.9 | ||
| OSAS | RatioNEP | 1s | 1.8 ± 0.5 vs 1.5 ± 0.5 | 2.4 ± 0.8 vs 1.6 ± 0.7 |
| 0.75s | 2.0 ± 0.5 vs 1.7 ± 0.5 | 2.7 ± 0.8 vs 1.9 ± 0.9 | ||
| 0.5s | 2.5 ± 0.7 vs 2.1 ± 0.7 | 3.5 ± 1.0 vs 2.5 ± 1.1 | ||
| 0.25s | 4.9 ± 1.9 vs 3.8 ± 1.8 | 7.1 ± 2.8 vs 4.8 ± 2.0 | ||
| RatioEMG | ||||
| All | RatioEMG | 1s | 1.1 ± 0.3 vs 1.2 ± 0.5 | 1.3 ± 0.6 vs 1.6 ± 1.0 |
| 0.75s | 1.1 ± 0.4 vs 1.3 ± 0.6 | 1.5 ± 1.1 vs 1.8 ± 1.5 | ||
| 0.5s | 1.2 ± 0.4 vs 1.5 ± 0.8 | 1.8 ± 1.3 vs 2.0 ± 1.7 | ||
| 0.25s | 1.4 ± 0.8 vs 1.8 ± 1.3 | 2.2 ± 2.2 vs 2.3 ± 2.0 | ||
| Control | RatioEMG | 1s | 1.0 ± 0.3 vs 1.1 ± 0.3 | 1.1 ± 0.3 vs 1.5 ± 0.8 |
| 0.75s | 1.0 ± 0.4 vs 1.3 ± 0.8 | 1.5 ± 1.5 vs 1.9 ± 2.0 | ||
| 0.5s | 1.1 ± 0.3 vs 1.3 ± 0.7 | 1.6 ± 1.2 vs 2.2 ± 2.3 | ||
| 0.25s | 1.3 ± 0.7 vs 1.5 ± 1.0 | 1.9 ± 1.6 vs 2.2 ± 2.5 | ||
| Snorers | RatioEMG | 1s | 1.0 ± 0.4 vs 1.1 ± 0.4 | 1.2 ± 0.5 vs 1.2 ± 0.3 |
| 0.75s | 1.2 ± 0.4 vs 1.2 ± 0.5 | 1.5 ± 0.8 vs 1.6 ± 1.2 | ||
| 0.5s | 1.3 ± 0.6 vs 1.5 ± 0.9 | 2.0 ± 1.5 vs 1.6 ± 1.1 | ||
| 0.25s | 1.7 ± 1.0 vs 2.0 ± 1.5 | 2.7 ± 3.1 vs 1.9 ± 1.3 | ||
| OSAS | RatioEMG | 1s | 1.2 ± 0.3 vs 1.4 ± 0.5 | 1.5 ± 0.8 vs 1.9 ± 1.2 |
| 0.75s | 1.2 ± 0.3 vs 1.5 ± 0.6 | 1.5 ± 0.8 vs 2.0 ± 1.3 | ||
| 0.5s | 1.2 ± 0.4 vs 1.6 ± 0.8 | 1.7 ± 1.0 vs 2.1 ± 1.4 | ||
| 0.25s | 1.3 ± 0.5 vs 1.9 ± 1.4 | 2.1 ± 1.9 vs 2.7 ± 2.0 |
Data shown as mean ± SD
RatioNEP was significantly greater in controls vs. snorers and controls vs. OSAS in the seated position at both pressure levels (both P < 0.0001). Similar differences were observed between groups in the supine position at both pressure levels (both P < 0.0001). However, there were no differences between snorers and OSAS. For RatioEMG, no significant differences were found between groups at either pressure level or in either position.
Effect of Different Pressure Levels
Pressure level affected both RatioNEP and Ratio EMG (Table 3). For RatioNEP, higher values were found at a pressure of −10 cm H2O than −5 cm H2O for controls and snorers in both positions (seated: P < 0.0001 for both groups; supine: P < 0.0001 for controls and P = 0.001 for snorers). A similar trend that did not reach statistical significance was seen in the OSAS group (P = 0.006 for −5 vs. −10 cm H2O in the seated position and P = 0.16 in the supine position). At both −5 and −10 cm H2O, there continued to be differences in RatioNEP between the control and snoring groups and between the control and OSAS groups (P < 0.0001).
RatioEMG was greater at −10 cm H2O than at −5 cm H2O (P < 0.0001). However, no differences were found between groups.
Effect of Seated vs Supine Positions
RatioNEP was generally lower (i.e., demonstrating a more collapsible upper airway) in the supine compared to the seated position (Table 4). At −5 cm H2O, significant positional differences were found only in the control group (P = 0.0005). However, at −10 cm H2O, differences between seated vs. supine were found in all groups (P < 0.0001, P = 0.0012, and P = 0.0011 for controls, snorers, and OSAS, respectively). In both positions, RatioNEP differed between controls and snorers and controls and OSAS (P < 0.0001), but did not differ significantly between snorers and OSAS.
RatioEMG was higher in the supine position than in the seated position (P = 0.004), but there was no significant difference between groups when the 2 positions were compared (Table 4).
Effect of Time
Significant differences in RatioNEP between controls and snorers and between controls and OSAS were observed at all time points (P < 0.0001 for all), while no differences were observed between snorers and OSAS at any of the time points. There was no difference in RatioEMG between groups at any time point.
Effects of Gender, Age, and Body Mass Index
Gender, age and BMI z-score had a statistically significant effect on RatioNEP (P = 0.039, 0.0005, and 0.0007, respectively). For gender, males tended to have slightly higher RatioNEP values (least squares mean ± SE for males: 4.77 ± 0.24; for females: 4.66 ± 0.24). For age, the coefficient ± SE was 0.036 ± 0.001, indicating that with increasing age, RatioNEP also increased. For BMI z-score, the coefficient ± SE was −0.012 ± 0.003, indicating that as BMI z-score increased, RatioNEP decreased.
Neither gender nor age nor BMI had a significant effect on RatioEMG (P = 0.88, 0.66, and 0.34, respectively).
Effect of Severity of SDB on RatioNEP
The correlation between AHI and RatioNEP was assessed in the 3 groups at each pressure level and position. In general, there was no correlation between AHI and RatioNEP in control subjects; only one of 16 correlation coefficients reached statistical significance, namely the correlation between AHI and RatioNEP at 1 second (Table 5). In snorers, there was no statistically significant correlation between AHI and RatioNEP. However, significant inverse correlations were found between AHI and RatioNEP in the children with OSAS. Thus, in children with OSAS, 10 of 16 correlation coefficients examining the relationship between AHI and RatioNEP at each level of pressure, position, and time were statistically significant, with correlation coefficients ranging from r = −0.447 (P = 0.048) to r = −0.560 (P = 0.010).
Table 5.
Spearman correlation of apnea hypopnea index with RatioNEP for each group at each level of pressure and each position
| Seated |
Supine |
|||||
|---|---|---|---|---|---|---|
| −5 cm H2O | −10 cm H2O | −5 cm H2O | −10 cm H2O | |||
| Controls | RatioNEP | 1s | −0.115 | 0.236 | 0.093 | −0.516* |
| 0.75s | −0.156 | −0.023 | < 0.0005 | −0.427 | ||
| 0.5s | −0.133 | −0.104 | −0.085 | −0.436 | ||
| 0.25s | −0.083 | −0.035 | −0.334 | −0.350 | ||
| Snorers | RatioNEP | 1s | 0.066 | 0.055 | 0.099 | 0.188 |
| 0.75s | −0.037 | −0.109 | 0.047 | 0.220 | ||
| 0.5s | −0.132 | −0.289 | −0.041 | 0.336 | ||
| 0.25s | −0.303 | −0.362 | −0.275 | 0.056 | ||
| OSAS | RatioNEP | 1s | −0.530* | −0.292 | −0.523* | −0.517* |
| 0.75s | −0.508* | −0.329 | −0.560* | −0.557* | ||
| 0.5s | −0.447* | −0.440 | −0.465* | −0.538* | ||
| 0.25s | −0.416 | −0.357 | −0.395 | −0.460* | ||
P < 0.05
DISCUSSION
The main finding of this study is that children with SDB have a more collapsible upper airway than normal children, even during wakefulness. Thus, RatioNEP could differentiate controls from children with SDB at all time points, in both positions, and at both levels of pressure. However, RatioNEP could not differentiate between snorers and OSAS. This study demonstrates that children with SDB, including children with snoring but no obstructive apnea, have a more collapsible UA than controls, even during wakefulness. Furthermore, this study has shown that the NEP technique is not associated with differing patterns of upper airway muscle activation during wakefulness in normal children compared to children with snoring or OSAS.
We evaluated whether different pressure levels of NEP might evoke different upper airway responses in children with SDB. At pressure of −10 cm H2O, RatioNEP values were higher in controls and snorers, with a similar trend in the OSAS group; this was associated with an increased EMG response.
We also investigated whether the supine position increased UA collapsibility in comparison to the seated position, and whether the response to position differed within groups. We found lowest values for RatioNEP in the supine position in all groups despite increased EMG activity, reflecting increased upper airway collapsibility in this position. However, the positional difference in NEP only became apparent in the SDB group at the higher driving pressure of −10 cm H2O. Studies in adults with OSAS have shown increases in upper airway collapsibility in the seated position even at −5 cm H2O.4,6 Thus, in children the supine position may have less influence on upper airway collapsibility, consistent with clinical findings that the supine position is less important in the etiology of OSAS in the pediatric population.22
We were not able to differentiate between snorers and OSAS using NEP. This finding is in accordance with other studies showing that both anatomic factors (augmented mechanical load) which increase UA collapsibility and a blunted neuromotor response during sleep are required for the development of OSAS.23 As the NEP technique is primarily assessing passive UA mechanical properties (UA collapsibility in the absence of a compensatory neuromuscular response), only one side of the equation is being evaluated. Moreover, the categorization of children with SDB as snorers or OSAS is somewhat artificial as there is clearly a spectrum of disease, ranging from primary snoring to upper airway resistance syndrome to obstructive hypoventilation to OSAS, and thus any attempts to divide patients into categories will be somewhat arbitrary. Furthermore, children may shift from one category to another, e.g., by gaining weight.
The NEP technique was initially developed to detect intrathoracic expiratory flow limitation during spontaneous tidal breathing,24 and was subsequently used to examine upper airway collapsibility. Studies in adults with SDB have found similar results to the current study, with overlapped values in snorers and OSAS which did not allow for the identification of a cut-off value for each group.4,6 In children, NEP has been used to evaluate asthma and cystic fibrosis.25,26 To our knowledge no prior studies of NEP have been conducted in children with OSAS.
The application of a negative pressure during expiration increases airflow compared to the preceding expiration because of the increased driving pressure between the alveoli and the oronasal airway. This increase is smaller in the presence of upper or lower airway collapse. Using the interrupter technique for measuring upper airway resistance, it has been observed that NEP does not increase expiratory airway resistance per se in awake normal controls; however, an increased resistance was found in awake adults snorers.5 This reflects upper airway collapse.
The application of negative pressure during inspiration results in activation of dilator pharyngeal muscles as a compensatory reflex to preserve upper airway patency.27 It has been assumed that the application of negative pressure during expiration does not induce upper airway muscle activity. However, to our knowledge, only one study has evaluated this. Tantucci et al. measured EMG in response to NEP in a relatively small sample of normal adults, using intra-oral EMG in 2 subjects and external surface EMG in 8 subjects.11 In our study of a relatively large group of children, using intra-oral EMG measurements, we did see a small increase in RatioEMG during NEP, although this increase was proportionately much smaller than the increase in RatioNEP. Thus, upper airway muscle activation may contribute in part to the NEP response. EMG activity during NEP increased at more negative pressure levels (i.e., for −10 cm H2O vs. −5 cm H2O) and in the supine compared to the seated position, indicating that some degree of upper airway muscle activation was elicited. Of note, however, changes in EMG were similar between groups. Therefore, the differences in upper airway collapsibility between controls and children with sleep disordered breathing (evaluated with RatioNEP) were not consequent to differences in upper airway muscle activation. Thus, overall, when NEP is applied to assess upper airway collapsibility, the passive mechanics of the upper airway are being evaluated. Consistent with this, NEP has been shown to correlate with the hypotonic critical closing pressure (Pcrit).28
This study has several limitations. There was a difference in gender between controls and the SDB groups, with a preponderance of females in the control group. However, the mixed effects model was used to control for this in data analyses. In young children, OSAS occurs equally among both genders,14 whereas in adults, OSAS occurs more commonly in males; the gender balance in adolescents has not been evaluated in population-based studies. Further studies are needed to evaluate the effect of gender on NEP at different developmental stages.
Children in both of the SDB groups were also more obese than the controls. This is not surprising, as obesity is a risk factor for SDB.14 Obesity can result in smaller lung volumes, and therefore less tracheal traction and increased UA collapsibility, as UA length correlates with UA collapsibility.29 Low EELV constitutes a contributing factor for OSAS.31 Pulmonary function has been evaluated in obese children, although studies have used different methods and different populations. One study found that the functional residual capacity (FRC) was < 80% predicted in 43% of severely obese children (body mass index z-score > 3 SD).32 Li et al. also demonstrated an FRC < 80% predicted in 46% of obese children with a mean body mass index of 30 kg/m2,33 and Marcus et al. found a mean FRC of 82% ± 21% in obese children with a mean body mass index of 33 kg/m2.34 It is not known whether the obese children in the current study had a lower EELV compared to the controls, especially in the supine position. Therefore, whether the NEP values were the result of low EELV affecting UA collapsibility cannot be elucidated with this study.
In summary, the NEP technique is a useful tool for studying upper airway mechanics in children with habitual snoring and OSAS, with abnormalities being evident even during wakefulness. As NEP is similar between children with OSAS and snorers, it suggests that these children have similar degrees of passive upper airway collapsibility during wakefulness. The reason for the difference in upper airway patency during sleep in children with OSAS versus snorers may be related to differences in compensatory upper airway muscle activation during sleep. It can be speculated that both children with habitual snoring and those with OSAS have intrinsically increased upper airway collapsibility, but those with snoring are able to mount a compensatory upper airway neuromotor response during sleep, thereby preventing upper airway collapse. The NEP technique primarily evaluates the passive upper airway properties, and does not take into accounts the effect of upper airway dilatory muscles. As it is possible to use NEP to obtain a cohort of children with the same degree of intrinsic upper airway collapsibility, this technique may be useful in future studies evaluating the effects of upper airway neuromotor activity during sleep.
DISCLOSURE STATEMENT
Phillips Respironics, Inc., provided modified CPAP equipment to provide the subatmospheric pressure; otherwise this was not an industry supported study. Dr. Marcus has received research support and equipment from Phillips Respironics unrelated to this study. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the children and their families for their enthusiastic participation in this study. The authors also thank the respiratory therapists in the pulmonary function laboratory and the sleep technologists for their assistance.
Dr. Larramona Carrera was supported by BA08/90101 Instituto de Salud Carlos III, Ministry Science and Education of Spain, Fundacio Parc Tauli and Sociedad Española de Neumologia Pediatrica. Dr. Marcus was supported by NIH grants RO1 HL58585 and UL1 RR-024134. Phillips Respironics, Inc, provided modified CPAP equipment to provide the subatmospheric pressure.
REFERENCES
- 1.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32:731–6. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunetti L, Rana S, Lospalluti ML, et al. Prevalence of obstructive sleep apnea syndrome in a cohort of 1,207 children of southern Italy. Chest. 2001;120:1930–5. doi: 10.1378/chest.120.6.1930. [DOI] [PubMed] [Google Scholar]
- 3.Jordan AS, White DP. Pharyngeal motor control and the pathogenesis of obstructive sleep apnea. Respir Physiol Neurobiol. 2008;160:1–7. doi: 10.1016/j.resp.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferretti A, Giampiccolo P, Redolfi S, et al. Upper airway dynamics during negative expiratory pressure in apneic and non-apneic awake snorers. Respir Res. 2006;7:54. doi: 10.1186/1465-9921-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tantucci C, Duguet A, Ferretti A, et al. Effect of negative expiratory pressure on respiratory system flow resistance in awake snorers and nonsnorers. J Appl Physiol. 1999;87:969–76. doi: 10.1152/jappl.1999.87.3.969. [DOI] [PubMed] [Google Scholar]
- 6.Tamisier R, Wuyam B, Nicolle I, et al. Awake flow limitation with negative expiratory pressure in sleep disordered breathing. Sleep Med. 2005;6:205–13. doi: 10.1016/j.sleep.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–9. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz ES, White DP. Genioglossus activity in children with obstructive sleep apnea during wakefulness and sleep onset. Am J Respir Crit Care Med. 2003;168:664–70. doi: 10.1164/rccm.200301-092OC. [DOI] [PubMed] [Google Scholar]
- 9.Fogel RB, White DP, Pierce RJ, et al. Control of upper airway muscle activity in younger versus older men during sleep onset. J Physiol. 2003;553:533–44. doi: 10.1113/jphysiol.2003.045708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz ES, White DP. Genioglossus activity during sleep in normal control subjects and children with obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:553–60. doi: 10.1164/rccm.200403-262OC. [DOI] [PubMed] [Google Scholar]
- 11.Tantucci C, Mehiri S, Duguet A, et al. Application of negative expiratory pressure during expiration and activity of genioglossus in humans. J Appl Physiol. 1998;84:1076–82. doi: 10.1152/jappl.1998.84.3.1076. [DOI] [PubMed] [Google Scholar]
- 12.Marcus CL, Fernandes Do Prado LB, Lutz J, et al. Developmental changes in upper airway dynamics. J Appl Physiol. 2004;97:98–108. doi: 10.1152/japplphysiol.00462.2003. [DOI] [PubMed] [Google Scholar]
- 13.Marcus CL, Glomb WB, Basinski DJ, Davidson SL, Keens TG. Developmental pattern of hypercapnic and hypoxic ventilatory responses from childhood to adulthood. J Appl Physiol. 1994;76:314–20. doi: 10.1152/jappl.1994.76.1.314. [DOI] [PubMed] [Google Scholar]
- 14.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 15.Witmans MB, Keens TG, Davidson Ward SL, Marcus CL. Obstructive hypopneas in children and adolescents: normal values. Am J Respir Crit Care Med. 2003;168:1540. doi: 10.1164/ajrccm.168.12.954. [DOI] [PubMed] [Google Scholar]
- 16.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872–8. doi: 10.1378/chest.125.3.872. [DOI] [PubMed] [Google Scholar]
- 17.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2-9 years old: additional data and review of the literature. Pediatr Pulmonol. 2005;40:22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 19.Rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the scoring of sleep and associated events. [Google Scholar]
- 20.Bates JH, Schmalisch G, Filbrun D, Stocks J. Tidal breath analysis for infant pulmonary function testing. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/ American Thoracic Society. Eur Respir J. 2000;16:1180–92. doi: 10.1034/j.1399-3003.2000.16f26.x. [DOI] [PubMed] [Google Scholar]
- 21.Zapletal A, Hladikova M, Chalupova J, Svobodova T, Vavrova V. Area under the maximum expiratory flow-volume curve--a sensitive parameter in the evaluation of airway patency. Respiration. 2008;75:40–7. doi: 10.1159/000099615. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes Do Prado LB, Li X, Thompson R, Marcus CL. Body position and obstructive sleep apnea in children. Sleep. 2002;25:66–71. doi: 10.1093/sleep/25.1.66. [DOI] [PubMed] [Google Scholar]
- 23.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol. 2007;102:547–56. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 24.Koulouris NG, Valta P, Lavoie A, et al. A simple method to detect expiratory flow limitation during spontaneous breathing. Eur Respir J. 1995;8:306–13. doi: 10.1183/09031936.95.08020306. [DOI] [PubMed] [Google Scholar]
- 25.Tauber E, Fazekas T, Eichler I, et al. Negative expiratory pressure: a new tool for evaluating lung function in children? Pediatr Pulmonol. 2003;35:162–8. doi: 10.1002/ppul.10233. [DOI] [PubMed] [Google Scholar]
- 26.Goetghebeur D, Sarni D, Grossi Y, et al. Tidal expiratory flow limitation and chronic dyspnoea in patients with cystic fibrosis. Eur Respir J. 2002;19:492–8. doi: 10.1183/09031936.02.00220702. [DOI] [PubMed] [Google Scholar]
- 27.Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol. 1991;436:15–29. doi: 10.1113/jphysiol.1991.sp018536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montemurro LT, Bettinzoli M, Corda L, Braghini A, Tantucci C. Relationship between critical pressure and volume exhaled during negative pressure in awake subjects with sleep-disordered breathing. Chest. 2010;137:1304–9. doi: 10.1378/chest.09-2109. [DOI] [PubMed] [Google Scholar]
- 29.Malhotra A, Huang Y, Fogel RB, et al. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med. 2002;166:1388–95. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 30.Ronen O, Malhotra A, Pillar G. Influence of gender and age on upper-airway length during development. Pediatrics. 2007;120:e1028–e1034. doi: 10.1542/peds.2006-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jordan AS, White DP, Owens RL, et al. The effect of increased genioglossus activity and end-expiratory lung volume on pharyngeal collapse. J Appl Physiol. 2010;109:469–75. doi: 10.1152/japplphysiol.00373.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dubern B, Tounian P, Medjadhi N, Maingot L, Girardet JP, Boule M. Pulmonary function and sleep-related breathing disorders in severely obese children. Clin Nutr. 2006;25:803–9. doi: 10.1016/j.clnu.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Li AM, Chan D, Wong E, Yin J, Nelson EA, Fok TF. The effects of obesity on pulmonary function. Arch Dis Child. 2003;88:361–3. doi: 10.1136/adc.88.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcus CL, Curtis S, Koerner CB, Joffe A, Serwint JR, Loughlin GM. Evaluation of pulmonary function and polysomnography in obese children and adolescents. Pediatr Pulmonol. 1996;21:176–83. doi: 10.1002/(SICI)1099-0496(199603)21:3<176::AID-PPUL5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]