Abstract
Study Objectives:
To compare the breathing instability and upper airway collapsibility between patients with pure OSA (i.e. 100% of apneas are obstructive) and patients with predominant OSA (i.e., coexisting obstructive and central apneas).
Design:
A cross-sectional study with data scored by a fellow being blinded to the subjects' classification. The results were compared between the 2 groups with unpaired student t-test.
Setting and interventions:
Standard polysomnography technique was used to document sleep-wake state. Ventilator in pressure support mode was used to introduce hypocapnic apnea during CO2 reserve measurement. CPAP with both positive and negative pressures was used to produce obstructive apnea during upper airway collapsibility measurement.
Participants:
21 patients with OSA: 12 with coexisting central/mixed apneas and hypopneas (28% ± 6% of total), and 9 had pure OSA.
Measurements:
The upper airway collapsibility was measured by assessing the critical closing pressure (Pcrit). Breathing stability was assessed by measuring CO2 reserve (i.e., ΔPCO2 [eupnea-apnea threshold]) during NREM sleep.
Results:
There was no difference in Pcrit between the 2 groups (pure OSA vs. predominant OSA: 2.0 ± 0.4 vs. 2.7 ± 0.4 cm H2O, P = 0.27); but the CO2 reserve was significantly smaller in predominant OSA group (1.6 ± 0.7 mm Hg) than the pure OSA group (3.8 ± 0.6 mm Hg) (P = 0.02).
Conclusions:
The present data indicate that breathing stability rather than upper airway collapsibility distinguishes OSA patients with a combination of obstructive and central events from those with pure OSA.
Citation:
Xie A; Bedekar A; Skatrud JB; Teodorescu M; Gong Y; Dempsey JA. The heterogeneity of obstructive sleep apnea (predominant obstructive vs pure obstructive apnea). SLEEP 2011;34(6):745-750.
Keywords: CO2 reserve, Pcrit, OSA
INTRODUCTION
Since obstructive sleep apnea (OSA) is characterized by futile respiratory efforts against a closed upper airway, patients with OSA are believed to have anatomical/functional deficits in the upper airway (UAW). However, patients very rarely present with pure OSA in clinical practice, with most OSA patients exhibiting some proportion of central and/or mixed events. It is not clear why both types of apnea occur in the same individual during the same night, but the coexistence of two types of apnea indicates more complicated underlying disorders than increased UAW resistance alone. In fact, unstable breathing control has been implicated in the pathogenesis of both central and obstructive sleep apnea. Some patients with OSA exhibit evidence of unstable ventilatory control (i.e., increased control system “loop gain,”) that could contribute to UAW narrowing and obstruction.1,2 We have noticed that obstructive apnea (OA) begins at the nadir of respiratory drive,3 and that superimposing an unstable respiratory motor output precipitated airway obstruction during sleep in snoring subjects.4 We have also observed that increasing chemoreceptor stimulation (via increased inhalation of CO2) reduced UAW resistance in sleeping subjects.5 Hence the occurrence of OA appears to involve aspects of both upper airway collapsibility and respiratory control instability.2,6 On the other hand, when the central respiratory output drops to zero, central apnea (CA) occurs regardless of UAW structural and functional status. It is very likely that the phenotype of sleep apnea (CA vs. OA) is shaped by the interaction of respiratory drive and UAW resistance. We therefore hypothesized that for patients with equally severe OSA, those with pure OSA have a more collapsible airway than those with coexisting obstructive and central apneas (predominant OSA); while the predominant OSA group have more significant instability of the respiratory system than the pure OSA group. To test this hypothesis, we measured CO2 reserve (i.e., ΔPCO2 [eupnea- apneic threshold]) to approximate the breathing propensity for instability and UAW critical closing pressure (Pcrit) to assess the UAW collapsibility in patients with OSA, and compared these 2 indexes between the 2 subgroups (pure OSA vs. predominant OSA).
METHODS
Subjects
We studied 21 patients with OSA diagnosed via overnight polysomnography (i.e. all patients had an apnea/hypopnea index [AHI] > 10/h of sleep time). Of the apneas, ≥ 50% were classified as obstructive in nature. Among these 21 subjects, 9 presented with pure OSA (100% of apneas were obstructive) and 12 with predominant OSA (100% > obstructive apneas > 50%). Subjects were excluded if they had chronic obstructive pulmonary disease/asthma, concomitant medical illness such as renal/liver dysfunction, or other confounding sleep disorders. All subjects provided informed consent prior to participation. The experimental protocol was approved by the University of Wisconsin Center for Health Sciences Human Subjects Committee.
The experiment was completed within 2 weeks following the diagnostic study before the subjects received any treatment. All patients reported to the laboratory in the evening, having refrained from alcohol and caffeinated beverages during or after their evening meal. Zolpidem 10 mg was given prior to bed to facilitate sleep and to suppress the arousability from sleep for purpose of measuring Pcrit and apneic threshold.
Experimental Set-Up
Subjects slept with a full face mask, through which they were connected to a breathing circuit that could be directed to either a positive pressure source (ventilator) (1∼25 cm H2O) or an adjustable negative pressure source (-1∼-10 cm H2O) from wall suction. A 200 liter capacitance was placed in the inflow port to maintain the level of negative pressure applied to the mask nearly constant during the respiratory cycle. The positive pressure source and vacuum source were attached to the inspiratory circuit through T-tubing, which was easily switched from one to the other.
Polysomnographic Methods and Respiratory Monitoring
Standard polysomnography technique was used to document the sleep-wake state.7 In addition, ventilation was measured with a pneumotachograph (Model 3700, Hans Rudolph). Nasal/mask pressure was measured with a differential pressure transducer (DP 103, Validyne) from a side port on the mask. Calibrated respiratory inductive plethysmography (Inductotrace, Ambulatory Monitoring Inc.) was used to assess respiratory effort. End-tidal PCO2 (PETCO2) was measured by a gas analyzer (AMETEK, Model CD-3A), which was calibrated by known gases.
Hypocapnic Apnea Threshold and CO2 Reserve
Breathing stability during NREM sleep was assessed by measuring the CO2 reserve (i.e., the proximity between eupneic PCO2 and apneic threshold PCO2).8 First we administered sufficient positive airway pressure (CPAP) to eliminate airway obstruction and stabilize breathing. Then we administrated pressure support ventilation in a step-wise fashion by increasing the pressure by 2 cm H2O every 1 min until apnea and periodic breathing occurred. We confirmed this apneic threshold PetCO2 by abruptly increasing pressure to the previously determined level in one step.
Upper Airway Critical Closing Pressure (Pcrit)
Measurements of Pcrit were performed in a standard supine posture with the head positioned in a contoured pillow to assure a constant position. During stable NREM sleep, CPAP was increased by 2 cm H2O every 1 min until flow limitation was eliminated, and this holding pressure was then maintained throughout the study. For each trial, nasal pressure was lowered from the holding pressure by 1 cm H2O for 4-6 breaths and then returned to holding pressure for 1 min before dropping to the next level. This sequence was repeated until airflow ceased (absolute Pcrit) or arousal occurred.
Data Analysis
For the CO2 reserve assessment, central apnea was defined as absence of airflow and respiratory effort for ≥ 10 sec. The apnea threshold was determined by averaging the PETCO2 of the 3 consecutive breaths immediately preceding the apnea.8 The difference between eupneic PETCO2 during stable breathing and the apneic threshold PETCO2 was calculated as the CO2 reserve.
The absolute Pcrit was defined as the level of mask pressure at which inspiratory airflow first ceased. We were able to obtain this in 18 of 21 subjects. In addition, in all subjects, we obtained a derived Pcrit as determined from peak inspiratory flow-UAW pressure relationships. Peak inspiratory flow of the 3rd through 5th breaths with unambiguous flow limitation during reduction in CPAP was averaged at each level of mask pressure. Pcrit was derived by the zero-flow intercept from the least-square linear regression of maximal flow vs. mask pressure as previously described.9 All data were collected only from flow-limited breaths with no associated arousal.
Unpaired t-test was used for all of comparisons between the 2 groups. All data are expressed as mean ± SE.
RESULTS
As Table 1 shows, there was no difference in age, body mass index (BMI), or AHI between the 2 groups. Subjects in both groups were above middle age and overweight. They had moderate to severe sleep apnea syndrome (mean AHI 47-49 events/h of sleep), which was distributed similarly between the 2 groups in terms of sleep stage (45.9 ± 9.3 vs. 45.7 ± 9.4 events/h in NREM sleep, P = 0.75; 57.0 ± 13.1 vs. 50.2 ± 16.3, P = 0.77 in REM sleep) and sleep position (AHI in supine position 51.4 ± 12.2 vs. 61.6 ± 16.1 events/h, P = 0.64). The only difference, by design, was the percentage of central and mixed apneas in the 2 groups, which was 0% in the pure OSA group versus 28.2% ± 6.3% in the predominant OSA group.
Table 1.
Subject description
| Pure OSA group | Predominant OSA group | P-value | |
|---|---|---|---|
| Number | 9 (2 F + 7 M) | 12 (1 F + 11 M) | N/A |
| Age, y | 50.6 ± 3.8 | 54.1 ± 2.6 | 0.40 |
| BMI, kg/m2 | 36.8 ± 2.8 | 34.3 ± 1.7 | 0.43 |
| AHI, events/h | 49.2 ± 9.4 | 47.1 ± 10.4 | 0.89 |
| AHI in NREM, events/h | 45.9 ± 9.3 | 45.7 ± 9.4 | 0.75 |
| AHI in REM, events/h | 57.0 ± 13.1 | 50.2 ± 16.3 | 0.77 |
| AHI in supine, events/h | 51.4 ± 12.2 | 61.6 ± 16.1 | 0.64 |
| Obstructive apnea (% of total AH events) | 27.7 ± 14.4 | 21.5 ± 7.0 | 0.67 |
| Central apnea (% of total AH events) | 0 ± 0 | 11.4 ± 6.3 | N/A |
| Mixed apnea (% of total AH events) | 0 ± 0 | 11.8 ± 6.0 | N/A |
| Obstructive hypopnea (% of total AH events) | 72.3 ± 14.4 | 50.6 ± 11.6 | 0.26 |
| Central hypopnea (% of total AH events) | 0 ± 0 | 4.7 ± 4.7 | N/A |
BMI, body mass index; AHI, apnea-hypopnea index.
PETCO2 and CO2 Reserve
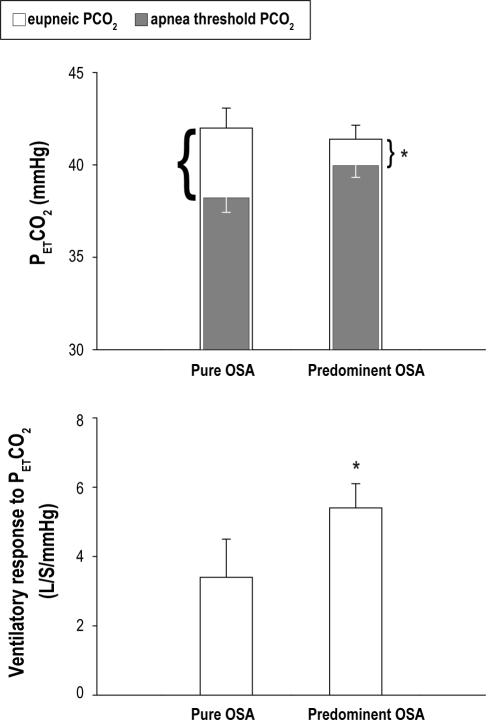
The 2 subgroups had a similar eupneic PETCO2 during both wakefulness (predominant vs. pure OSA group: 40.4 ± 1.4 vs. 39.8 ± 0.6 mm Hg, P = 0.78) and sleep (41.5 ± 0.9 vs. 42.1 ± 1.0 mm Hg, P = 0.65). Both groups underwent a slight increase in PETCO2 from wakefulness to sleep (1.2 ± 1.0 vs. 1.6 ± 1.5 mm Hg, P = 0.84). However, the CO2 reserve (i.e., the difference between the eupneic PETCO2 and the apneic threshold PETCO2) was significantly smaller in the predominant OSA group (1.6 ± 0.7 mm Hg) than the pure group (3.8 ± 0.6 mm Hg, P = 0.019; Figure 1, top). The reduced CO2 reserve in the predominant OSA group was due primarily to an increased controller gain below eupnea. The slope of ΔVE/ΔPETCO2 in the predominant group vs. pure group was 5.4 ± 0.7 vs. 3.4 ± 1.1 L/min/mm Hg, P = 0.025 (Figure 1, bottom). The severity of sleep apnea (AHI) was moderately correlated to the CO2 reserve in predominant OSA group (r2 = 0.61, P = 0.03) but not correlated to the CO2 reserve in the pure OSA group (r2 = 0.31, P = 0.42).
Figure 1.
Stability of respiratory control. (TOP) Respiratory control is less stable, as indicated by a narrower CO2 reserve in the predominant group than the pure obstructive group. The entire height of each bar represents the eupneic PETCO2; the solid portion of the bar represents the apneic threshold PETCO2; and the open portion of the bar represents the difference between the eupneic PETCO2 and the threshold PETCO2, i.e., the CO2 reserve, which is significantly smaller in the predominant OSA group than the pure OSA group. (BOTTOM) The slope of ventilatory response to hypocapnia is significantly greater in the predominant OSA group than the pure OSA group. PETCO2, end tidal PCO2. *P < 0.05 for the CO2 reserve (highlighted by the brackets next to each bar) on the TOP; while for the slope on the BOTTOM between the two groups.
Respiratory Flow Limitation and Pcrit
Most subjects showed various degrees of flow limitation during sleep at atmospheric pressure, which was often but not always associated with snoring. The minimum positive pressure required to normalize the airflow (i.e., holding pressure) was 10.0 ± 0.6 cm H2O in the pure OSA group and 11.6 ± 0.6 in the predominant OSA group; the difference was not significant (P = 0.07).
We were able to obtain absolute Pcrit on 7 of 9 patients in the pure OSA group and on 11 of 12 patients in the predominant OSA group. For each individual, the absolute Pcrit over 2-4 repeat trials varied within 2 cm H2O between the maximum and minimum values in most (16 of 18) patients. The other 3 patients aroused before we obtained zero flow. Their derived Pcrit was about −5∼-6 cm H2O.
Figure 2 shows the response of airflow to a transient drop of mask pressure in a patient with OSA. Lowering CPAP from the holding pressure progressively caused more and more severe flow limitation and reduction in inspiratory flow (Panels A to C). A further drop of CPAP from 11 cm H2O to 3 cm H2O resulted in the absence of flow despite the presence of inspiratory effort as indicated by Respitrace, which was defined as absolute Pcrit. When examining the breaths following the abrupt reduction of CPAP, we noticed that the 1st −2nd breath almost always had less flow limitation and less reduction of flow rate than the ensuing 2-4 breaths, while there was little inter-breath difference in airflow across the 3rd-6th breaths.
Figure 2.
The response of airflow to a transient drop of Pmask in a patient with OSA. (A) Lowering CPAP from the holding pressure (11) to 8 cm H2O caused flow limitation. (B and C) Transition from Pmask of 11 cm H2O to 6 cm H2O and 4 cm H2O, respectively, showed further reduction in inspiratory flow. Panel D. Pcrit is demonstrated at a Pmask of 3 cm H2O, which resulted in the absence of flow despite the presence of inspiratory effort as indicated by a Respitrace. Note the inter-breath differences in airflow across the 6 breaths after an abrupt reduction in Pmask (arrow). EEG, electroencephalogram; Pmask, pressure at mask.
As Figure 3 shows, most patients had an absolute Pcrit between 1 and 3 cm H2O. The mean absolute Pcrit was 2.0 ± 0.4 cm H2O in the pure OSA group and 2.7 ± 0.4 cm H2O in the predominant OSA group, and there was no significant difference between the 2 groups (P = 0.27). The derived Pcrit was −0.36 ± 1.0 cm H2O in the pure OSA group and 0.89 ± 0.77 cm H2O in the predominant OSA group, and the difference was also not significant (P = 0.33).
Figure 3.
The passive collapsibility (either derived or observed) of the upper airway did not differ between the 2 groups. The majority of patients have Pcrit > 0 (atmospheric pressure). The horizontal line represents the group mean Pcrit. There were 2 missing data points in the pure group and 1 in the predominant group for the absolute Pcrit measurement because those 3 patients aroused before we were able to obtain the absolute Pcrit, zero airflow. Pcrit, critical closing pressure.
DISCUSSION
This study demonstrated that in clinically diagnosed OSA patients, those with a combination of central, mixed, and obstructive apneas had a steeper slope of ventilatory response to ΔPETCO2 below eupnea and a narrower CO2 reserve relative to the pure OSA patients. On the other hand, passive collapsibility of the upper airway did not differ between the two groups, as they both showed a similar elevation of Pcrit above atmosphere pressure. These findings indicate that (1) increased upper airway collapsibility is commonly seen in most patients in both phenotypes of OSA and (2) neurochemical control instability more likely contributes to the sleep disordered breathing in patients with a combination of central and obstructive apneas.
Methodological Concerns
We quantified the propensity for respiratory control instability and sleep apnea by measuring CO2 reserve (i.e., the difference in PCO2 between eupnea and the apneic threshold). With the withdrawal of wakeful influences breathing becomes critically dependent on CO2, as shown by the unmasking of a highly sensitive hypocapnic-induced apneic threshold in NREM sleep.10,11 Unstable and often periodic breathing patterns during sleep have been shown to occur when the CO2 reserve is narrowed.8,12,13 In turn, a reduced CO2 reserve occurs when controller gain (slope of the CO2 ventilatory response below and/or above eupnea) and/or plant gain (change in V. a required to reach the apneic threshold)14–16 are increased, as demonstrated in patients with heart failure17 or experimentally in hypoxic environments.8,12 Therefore, the CO2 reserve presents an index of breathing control stability, that is, the greater the slope of the CO2 ventilatory response, the closer the proximity of eupneic PETCO2 to apnea threshold PETCO2, and the less stable the control system.15
To examine the propensity for upper airway collapsibility, Pcrit was measured after the method of Schwartz et al.18 Following the reduction in CPAP from holding pressure, we and others9 observed the greatest increase in flow resistance over the subsequent third to fifth breath. Accordingly, we used these breaths to determine the derived Pcrit. We used the Pcrit obtained by this method as an index of a patient's inherent airway collapsibility.19,20 However, we could not rule out the influence on Pcrit from variations in lung inflation and/or airway tonicity.21 Finally, we did observe that the “derived” Pcrit (from pressure-flow regression) was less than the “absolute” Pcrit (at observed zero flow); however, both measurements of Pcrit were nearly identical in the pure OSA group and combined central/obstructive patient group.
We used a mild sedative medication, 10 mg of zolpidem, during the Pcrit and apnea threshold measurement to facilitate sleep and suppress the arousability from sleep. The combination of solid sleep, mild sedation, and high level of nasal CPAP should create a relatively hypotonic state in the UAW muscles. Since both groups of patients used the same dosage of zolpidem, its effect, if any, on the Pcrit and CO2 reserve should not affect our conclusion. All data were collected during stage 2-3 NREM sleep in the supine position to avoid any influence of sleep states or body position on the Pcrit measurements.
Causes/Implications of the Elevated Pcrit in OSA Patients
Both pure OSA patients and the combined central/obstructive patient groups showed a positive Pcrit (2-2.3 cm H2O) and required higher CPAP holding pressures (10-11 cm H2O) to ensure airway patency compared to normal individuals.22–24 These data suggest a highly collapsible UAW in all OSA patients. Our study did not examine the causes of increased passive collapsibility of the airway in each individual. However, our patients did have a significantly increased body mass, which may partially account for the elevation of Pcrit because fat deposition in the airway wall and fatty infiltration of the tongue would increase the soft tissue mass.25 Moreover, obesity reduces resting lung volume, resulting in loss of caudal traction on upper airway structures and parallel increases in pharyngeal collapsibility.26 The positive Pcrit predisposes them to UAW occlusion during sleep even with the normal decline in pharyngeal dilator muscle activity that occurs upon withdrawal of the wakefulness stimulus.27
Reduced CO2 Reserve in the Combined Central/Obstructive Group
Our combined central/obstructive apnea patients differed from the pure OSA patient group primarily in showing a steeper ventilatory response slope to CO2 below eupnea, thereby causing a narrower reserve of PaCO2 between eupnea and the apneic threshold, i.e., less breathing control stability. Unstable ventilation and increased control system loop gain have been linked to OSA in several clinical and experimental studies.1–6
Why and how does respiratory instability predispose to obstructive events? It is known that maintenance of pharyngeal patency requires sufficient neuromotor activation of the UAW dilator muscles to offset the negative intraluminal pressure during inspiration and/or to prevent passive airway collapsibility at end-expiration. The UAW muscles are modulated by the respiratory control system,28,29 so that the upper airway resistance is inversely correlated with the intensity of the respiratory drive.5,30 The high chemosensitivity in the predominant OSA group patients means that the respiratory control system will over-respond to a given set of chemoreceptor stimuli (likely to an excess ventilatory response at apnea termination and/or apnea in response to relatively small reductions in PaCO2). When the apneic threshold is very close to the spontaneously breathing PCO2, as seen in the predominant OSA group, hyperpneas very easily drive PaCO2 below apneic threshold, resulting in a temporary cessation of the respiratory drive. Airway imaging studies revealed that airway narrowing or closure often occurs early after the onset of a “central” apnea and even in the absence of inspiratory effort.3 Furthermore, transient hypocapnia may decrease hypoglossal nerve activity more than phrenic nerve activity, causing an imbalance in the activity of pump vs. upper airway muscle activities.31 In addition, since OSA patients have their pharyngeal patency more sensitive to withdrawal of ventilatory drive than normal individuals,30 they are expected to be more vulnerable to breathing instability, and any respiratory fluctuations readily predisposes their upper airways to occlusion. This tendency is especially true in individuals with Pcrit close to atmospheric pressure,32 as seen in most of our subjects.
We emphasize that our study has only examined two factors, i.e., chemosensitivity and UAW collapsibility, as significant contributors to OSA. However, many other factors such as arousability and recruitability of upper airway dilator muscle are also important in determining the breathing pattern and type of apneas.33–35 This is further complicated by the fact that chemosensitivity will also play an important role in determining both arousability and muscle recruitment ability.
SUMMARY
The major finding of this study is that quantitative differences in CO2 reserve, reflecting differences in the propensity for respiratory control stability, distinguishes combined central/obstructive OSA from pure OSA. The present study also emphasizes the equally elevated Pcrit in the two groups, indicating that upper airway collapsibility is a universal problem for all types of OSA patients. Since the majority of OSA patients have some degree of coexisting central events, our observations suggest that central respiratory instability and upper airway collapsibility act synergistically in contributing to OSA, at least in most OSA patients. These observations deepened our understanding of the importance of heteropathy for each phenotype of OSA and may offer new therapeutic options for the treatment of refractory OSA. We would predict, for example, that stabilization of breathing pattern would reduce AHI in certain types of OSA patients. Further work is required to determine how these variables interact, and whether treatment of central system stability in some OSA patients may also relieve a significant number of obstructive events.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEGMENTS
We would like to thanks Dominic S. Puleo for technique assistance. Research work performed at Pulmonary Physiology Laboratory, VA Hospital, Madison, WI. This work was supported by the VA Medical Research Service; American Lung Association of Wisconsin; and NIH-NHLB1 (Grant 1RC1HL099724-01).
REFERENCES
- 1.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:181–90. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 2.Jordan AS, Wellman A, Edwards JK, et al. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea. J Appl Physiol. 2005;99:2020–7. doi: 10.1152/japplphysiol.00410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol. 1995;78:1806–15. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
- 4.Warner G, Skatrud JB, Dempsey JA. Effect of hypoxia-induced periodic breathing on upper airway obstruction during sleep. J Appl Physiol. 1987;62:2201–11. doi: 10.1152/jappl.1987.62.6.2201. [DOI] [PubMed] [Google Scholar]
- 5.Badr MS, Skatrud JB, Simon PM, Dempsey JA. Effect of hypercapnia on total pulmonary resistance during wakefulness and during NREM sleep. Rev Respir Dis. 1991;144:406–14. doi: 10.1164/ajrccm/144.2.406. [DOI] [PubMed] [Google Scholar]
- 6.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–58. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 7.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 8.Xie A, Skatrud JB, Barczi SR, et al. Influence of cerebral blood flow on breathing stability. J Appl Physiol. 2009;106:850–6. doi: 10.1152/japplphysiol.90914.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patil SP, Punjabi NM, Schneider H, O'Donnell CP, Smith PL, Schwartz AR. A simplified method for measuring critical pressures during sleep in the clinical setting. Am J Respir Crit Care Med. 2004;170:86–93. doi: 10.1164/rccm.200309-1239OC. [DOI] [PubMed] [Google Scholar]
- 10.Skatrud JB, Dempsey JA. Interaction of sleep state and chemical stimuli in sustaining rhythmic ventilation. J Appl Physiol. 1983;55:813–22. doi: 10.1152/jappl.1983.55.3.813. [DOI] [PubMed] [Google Scholar]
- 11.Xie A, Wong B, Phillipson EA, Slutsky AS, Bradley TD. Interaction of hyperventilation and arousal in the pathogenesis of idiopathic central sleep apnea. Am J Respir Crit Care Med. 1994;150:489–95. doi: 10.1164/ajrccm.150.2.8049835. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Effect of ventilatory drive on carbon dioxide sensitivity below eupnea during sleep. Am J Respir Crit Care Med. 2002;165:1251–60. doi: 10.1164/rccm.2110041. [DOI] [PubMed] [Google Scholar]
- 13.Xie A, Skatrud JB, Puleo DS, Dempsey JA. Influence of arterial O2 on the susceptibility to posthyperventilation apnea during sleep. J Appl Physiol. 2006;100:171–7. doi: 10.1152/japplphysiol.00440.2005. [DOI] [PubMed] [Google Scholar]
- 14.Dempsey JA, Smith CA, Przybylowski T, et al. The ventilatory responsiveness to CO2 below eupnoea as a determinant of ventilatory stability in sleep. J Physiol. 2004;560:1–11. doi: 10.1113/jphysiol.2004.072371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp Physiol. 2005;90:13–24. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- 16.Khoo MC. Determinants of ventilatory instability and variability. Respir Physiol. 2000;122:167–82. doi: 10.1016/s0034-5687(00)00157-2. [DOI] [PubMed] [Google Scholar]
- 17.Xie A, Skatrud JB, Puleo DS, Rahko PS, Dempsey JA. Apnea-hypopnea threshold for CO2 in patients with congestive heart failure. Am J Respir Crit Care Med. 2002;165:1245–50. doi: 10.1164/rccm.200110-022OC. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol. 1988;64:535–42. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- 19.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–26. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz AR, O'Donnell CP, Baron J, et al. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med. 1998;157:1051–7. doi: 10.1164/ajrccm.157.4.9706067. [DOI] [PubMed] [Google Scholar]
- 21.Series F, Marc I. Effects of continuous negative airway pressure-related lung deflation on upper airway collapsibility. J Appl Physiol. 1993;75:1222–5. doi: 10.1152/jappl.1993.75.3.1222. [DOI] [PubMed] [Google Scholar]
- 22.Gold AR, Marcus CL, Dipalo F, Gold MS. Upper airway collapsibility during sleep in upper airway resistance syndrome. Chest. 2002;121:1531–40. doi: 10.1378/chest.121.5.1531. [DOI] [PubMed] [Google Scholar]
- 23.Meurice JC, Marc I, Carrier G, Series F. Effects of mouth opening on upper airway collapsibility in normal sleeping subjects. Am J Respir Crit Care Med. 1996;153:255–9. doi: 10.1164/ajrccm.153.1.8542125. [DOI] [PubMed] [Google Scholar]
- 24.Henke KG, Dempsey JA, Badr MS, Kowitz JM, Skatrud JB. Effect of sleep-induced increases in upper airway resistance on respiratory muscle activity. J Appl Physiol. 1991;70:158–68. doi: 10.1152/jappl.1991.70.1.158. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz AR, Gold AR, Schubert N, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144:494–8. doi: 10.1164/ajrccm/144.3_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz AR, Patil SP, Squier S, Schneider H, Kirkness JP, Smith PL. Obesity and upper airway control during sleep. J Appl Physiol. 2010;108:430–5. doi: 10.1152/japplphysiol.00919.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fogel RB, White DP, Pierce RJ, et al. Control of upper airway muscle activity in younger versus older men during sleep onset. J Physiol. 2003;553:533–44. doi: 10.1113/jphysiol.2003.045708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gauda EB, Carroll TP, Schwartz AR, Smith PL, Fitzgerald RS. Mechano- and chemoreceptor modulation of respiratory muscles in response to upper airway negative pressure. J Appl Physiol. 1994;76:2656–62. doi: 10.1152/jappl.1994.76.6.2656. [DOI] [PubMed] [Google Scholar]
- 29.Shea SA, Akahoshi T, Edwards JK, White DP. Influence of chemoreceptor stimuli on genioglossal response to negative pressure in humans. Am J Respir Crit Care Med. 2000;162:559–65. doi: 10.1164/ajrccm.162.2.9908111. [DOI] [PubMed] [Google Scholar]
- 30.Series F, Cormier Y, Desmeules M, La Forge J. Effects of respiratory drive on upper airways in sleep apnea patients and normal subjects. J Appl Physiol. 1989;67:973–9. doi: 10.1152/jappl.1989.67.3.973. [DOI] [PubMed] [Google Scholar]
- 31.Haxhiu MA, Mitra J, van Lunteren E, Prabhakar N, Bruce EN, Cherniack NS. Responses of hypoglossal and phrenic nerves to decreased respiratory drive in cats. Respiration. 1986;50:130–8. doi: 10.1159/000194919. [DOI] [PubMed] [Google Scholar]
- 32.Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–32. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–33. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 34.Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol. 2007;103:1929–41. doi: 10.1152/japplphysiol.00561.2007. [DOI] [PubMed] [Google Scholar]
- 35.Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol. 2008;105:1389–405. doi: 10.1152/japplphysiol.90408.2008. [DOI] [PubMed] [Google Scholar]