Abstract
The quantity and serotypes of enteroviruses (EVs) in the influent of a local sewage treatment plant were compared to local clinical EV cases to determine if testing of sewage is adequate for an EV surveillance system. The study was carried out from August 1994 to December 2002. Monthly influent specimens were processed by organic flocculation, and dilutions of concentrate were inoculated onto a number of different cell types for virus isolation. EVs were detected in 88 of 100 monthly influent samples. Sewage EV titers were calculated by using software provided by the U.S. Environmental Protection Agency for most-probable-number determination. All 1,068 sewage EV isolates were further grouped (echovirus, coxsackievirus B, coxsackievirus A, or poliovirus) by cell culture host range analysis (growth pattern of isolates on passage to seven cell lines), and 39.0% of the 1,022 EV isolates categorized as non-poliovirus EVs were specifically serotyped. For clinical cases, primary virus isolation tests were performed on specimens submitted by local hospitals and EV isolates submitted by hospitals were serotyped. Clinical EVs were documented for 81 of the 100 months studied. In all, 694 EV isolates from clinical cases were serotyped. Annually, between 4 and 11 different serotypes of non-poliovirus EVs were identified in sewage and from 9 to 19 different non-poliovirus EV serotypes were identified from clinical specimens. Usually, the most commonly detected sewage EV serotypes were similar to the most commonly detected clinical serotypes; e.g., for 1997, echovirus 6 accounted for 53.1% of the typed sewage isolates and 39.4% of the clinical infections, while in 1998, echovirus 30 accounted for 50.0 and 46.1%, respectively. In 1999, 60.3% of the EVs from clinical cases and 79.7% of the sewage isolates were echovirus 11; in 2000, 33.3% of the EVs from clinical cases and 40.7% of the sewage isolates were coxsackievirus B5; and in 2001, 44.1% of the EVs from clinical cases and 36.2% of the sewage isolates were echovirus 13. Annual peaks of both sewage EV titers and clinical cases occurred in late summer or early fall. In some years, early spring sewage EVs portended some of the EVs that would predominate clinically during the following summer.
Because of Milwaukee's massive waterborne outbreak of cryptosporidiosis in early April 1993 (6) and the ensuing public relations disaster for the two city-operated local water treatment plants, the Milwaukee Health Department virus laboratory was directed to develop the capability to test environmental water specimens for human enteric viruses, specifically enteroviruses (EVs), in an effort to monitor the water treatment plants' source water for pathogens. Also, at that time, because the U.S. Environmental Protection Agency (EPA) was to promulgate an information collection rule (ICR) (4) requiring large water treatment plants to test source water for such viruses, it was prudent for the laboratory to develop such techniques for use in monitoring of the source water of Milwaukee's two water treatment plants. To comply with political demands, the laboratory became a U.S. EPA ICR-approved laboratory.
Assuming that Lake Michigan source water would have low titers of viruses, the personnel of the local sewage treatment plant were approached to provide sewage to be used as a positive control to test our ability to use the U.S. EPA ICR procedure for virus monitoring by organic flocculation and cell culture (16) to detect EVs. Because of our success in using the organic flocculation procedure with the U.S. EPA-mandated BGM cell culture method, this short-term project turned into a monthly screening of the sewage plant influent and effluent to monitor the plant's efficiency in the inactivation of viruses and the discharge of viruses into Lake Michigan, which is also the source water for Milwaukee's two drinking water treatment plants.
Detection of EVs in Milwaukee sewage was expected since many researchers (1, 2, 8, 12, 15) have shown great success in EV detection with similar procedures. However, our approach was different in that we undertook a long-term project for one site, grouped all sewage EVs by cell culture host range analysis, and serotyped a large portion of the sewage EV isolates, and since ours is also a clinical diagnostic virus laboratory, we could compare the EVs found in sewage to those that were causing clinical problems in the community. Two earlier studies comparing sewage EVs with reported clinical EV data, a 3-year study (1974 to 1977) in Reading, Great Britain (15), and a 1979-to-1981 Canadian study (9), demonstrated a similarity of clinical and sewage EVs. Since ours is a clinical laboratory, we also used multiple types of cell cultures to maximize clinical virus recovery and suspected that two cell lines (RD and Caco-2), which we relied on for clinical isolation of EVs, might be useful for environmental screening as an adjunct to the U.S. EPA ICR-mandated BGM cells. The 1970s British study (15) showed the usefulness of inoculating five different cell types, and the Canadian study (9) used two cell types, Vero and BSC-1. Another purpose of this study, considering the changing pattern of medical diagnostic testing, was to show that as we continue to experience a drop in the number of clinical specimens submitted for EV diagnosis, by adding routine testing of sewage to supplement the testing of clinical specimens, we could enhance our knowledge of which EVs are circulating in the community.
MATERIALS AND METHODS
Sewage collection.
Influent samples were collected monthly from the Jones Island wastewater treatment plant. The Jones Island wastewater treatment plant is located south of downtown Milwaukee and is one of two plants that treat sewage for 1.2 million people in the Milwaukee metropolitan area. Each plant services about one-half of the area residents, with the Jones Island plant servicing most of the city of Milwaukee, as well as northern and western suburbs. There is some overlap between the sections of the metropolitan area serviced by each plant. The treatment process at the Jones Island plant consists of preliminary, primary, and secondary (activated sludge) treatments, then phosphorus removal and disinfection with chlorine, followed by dechlorination with sulfur dioxide before discharge into Lake Michigan. Samples were obtained from influent on a routine basis, usually the third Tuesday of the month, collected at 4 a.m., stored at 4°C, and transported to the laboratory, and processing was started within 6 h of collection. Influent samples were 1-liter flow-proportioned grabs of the three major flows entering the plant. Influent samples were obtained monthly from August 1994 to December 2002 (except for September 1994).
Cell cultures.
Rhesus monkey kidney (RMK) primary, human rhabdomyosarcoma (RD), human embryonic lung (HEL), and Buffalo green monkey kidney (BGM) cells were purchased from BioWhittaker, Walkersville, Md. Human epidermoid carcinoma of the larynx (HEp-2) and human adenocarcinoma of the colon (Caco-2) cells were obtained from the American Type Culture Collection, Manassas, Va. Human foreskin (HFS) cells were obtained at low passage from a local hospital virus laboratory that starts its own cultures every 6 months. Culture media, Eagle's minimum essential medium with Earle's salt solution (MEM) and fetal bovine serum (FBS), were purchased from Sigma, St. Louis, Mo. All cell culture media contained HEPES buffer, l-glutamine, penicillin, streptomycin, gentamicin sulfate, and amphotericin B. Cell cultures were grown in CO2 incubators at 35.5°C and 4.5% CO2. Stock cell cultures were grown in 75- or 162-cm2 plastic flasks (Costar, Corning, N.Y.) with 5 or 10% FBS-MEM and split weekly. Twenty-four-well plates (Costar) of heteroploid cell types were grown in 5% FBS-MEM and maintained on 2% FBS-MEM. Diploid cell types were grown and maintained with 10% FBS-MEM. RMK primary cultures were grown in 24-well plates on 10% FBS-MEM and maintained with 2% FBS-MEM. Stock cell cultures and uninoculated 24-well plastic plates of cells were kept in a CO2 incubator separate from inoculated cultures. Cell cultures inoculated with sewage concentrate were kept in a different CO2 incubator than were plates inoculated with clinical specimens.
Organic flocculation procedure.
Concentration of virus in sewage specimens was accomplished by an organic flocculation procedure described in the U.S. EPA ICR virus-monitoring protocol (4, 16). A brief description of the procedure follows. At room temperature, 1 N HCl was added to a 2% beef extract V solution of wastewater to lower the pH to 3.5 ± 0.1. After continued stirring for 30 min, the solution was dispensed into four 250-ml centrifuge bottles and spun at 2,500 × g for 15 min at 4°C. The supernatant was discarded, and all four pellets were resuspended in a total of 30 ml of 0.15 M sodium phosphate buffer. After the pH was adjusted to 7.0 to 7.5, the concentrate was refrigerated at 4°C for 15 to 30 min to allow the pellet to dissolve completely. After readjustment of the pH to 9.0 to 9.5 with NaOH and centrifugation at 6,000 × g for 10 min at 4°C, the supernatant was poured off and saved. Finally, HCl was added to the saved supernatant to adjust the pH to 7.0 to 7.5, and then the sample was stored at 4°C until it was used to inoculate cell cultures.
Total culturable virus assay.
Detection of virus in concentrated sewage specimens was accomplished with a modified form of the U.S. EPA ICR water-monitoring protocol (4, 16). A brief description of our modification (14) of the procedure follows. A 1:8 dilution of the sewage concentrate in 2% FBS-MEM was filtered through a 0.2-μm-pore-size sterilizing filter pretreated with 2% beef extract. The filtered dilution of sewage was inoculated into the wells of 24-well plastic plates by addition of 0.5 ml of dilution per well, or 12 ml per 24-well plate. BGM and RD cells were used for the entire study. Caco-2 cells were used from 1997 to 2002, and HFS cells were used from 1994 to 1996. For virus adsorption, the inoculated 24-well plates were incubated for 2 h at 35.5°C. After the incubation period, the inoculum was removed and each well was washed with 0.5 ml of saline. After aspiration of the saline, 0.5 ml of 2% FBS-MEM was added to each well. The 24-well plates were then incubated at 35.5°C and 4.5% CO2 and examined microscopically for a cytopathic effect (CPE) daily. Cultures were maintained for 14 days, and wells with no CPE were not blind passaged. The EV CPE was typical and was distinct from the reovirus CPE that occurred mainly on BGM cells.
Cell culture host range analysis of sewage EV isolates.
All initial sewage EVs were grouped as echovirus, coxsackievirus A, coxsackievirus B, or poliovirus by growth pattern upon subculture to seven cell types: RMK, HEp-2, HEL, HFS, RD, BGM, and Caco-2. When a cell culture well inoculated with the filtered sewage dilution showed a significant CPE (2 to 4+), the fluid in the well was diluted 1:20 with 2% FBS-MEM and 0.05 ml was added to one well of seven different cell types (as described above). Over a period of 2 to 5 days, the newly inoculated cells were observed microscopically for the development of a CPE. Typical groupings (Table 1) were based on the experience of this laboratory with isolate typing and observation of the CPE patterns seen upon subculturing.
TABLE 1.
Key for grouping of sewage EV isolates by cell culture host range analysis, using production of CPE on various cell types as an indicator
| Group | CPEa on cell type:
|
||||||
|---|---|---|---|---|---|---|---|
| MK-I | HEp-2 | HFS | HEL | BGM | RD | Caco-2 | |
| Poliovirus | + | + | + | + | + | + | + |
| Coxsackievirus A | ± | 0 | 0 | 0 | 0 | + | 0 |
| Coxsackievirus B | + | + | 0 | 0 | + | 0 | ± |
| Echovirus | + | 0 | + | + | ± | + | + |
+, extensive CPE; ±, some CPE; 0, no CPE.
A portion of the isolates demonstrating patterns that suggested a non-poliovirus EV was then selected for the LBM microneutralization test (7) with pools A to H. Selection was nonrandom. However, a representative sample of the most common host range types, plus any unusual host range types, was selected for specific serotyping. LBM serum samples were obtained from the World Health Organization EV center in Denmark. All clinical isolates were subjected to the cell culture host range analysis and then serotyped specifically by the LBM microneutralization procedure. At times, monospecific neutralizing antisera for coxsackievirus serotypes A4, A6, and A10 were used, as well as immunofluorescent monoclonal antibody tests in shell vials with reagents from Chemicon International, Temecula, Calif. Greater than 90% of the typed isolates could be identified with the LBM neutralization system.
MPN titer calculation.
The U.S. EPA, Cincinnati, Ohio, supplied a computer program for the most-probable-number (MPN) calculations for the ICR. The program uses the number of replicates inoculated, the number of positive replicates, and the inoculation volume to calculate the MPN (CPE units). This program also calculates 95% confidence intervals for MPN titers.
Clinical specimens.
Specimens for primary virus isolation were received from Milwaukee area hospitals and clinics, as well as from the Milwaukee County Medical Examiner's Office. Local hospitals, which maintain their own virus laboratories, use this laboratory as a reference laboratory for EV serotyping and send cell culture EV isolates for serotyping. All clinical specimens were processed and inoculated onto 24-well plates of the same seven cell types used for host range analysis. These cultures were kept for 12 to 14 days and observed daily for CPE. Over the period of this study, essentially all Milwaukee area clinical EV isolates were serotyped by this laboratory.
QC.
In May 1997, our laboratory was approved by the U.S. EPA to perform ICR virus testing. As part of the achievement and maintenance of approval status, we passed two on-site inspections and audits and analyzed performance evaluation and quality control (QC) samples as required. Also, for our environmental testing, each lot of beef extract used in the organic flocculation concentration procedure was screened for adequate virus recovery. For the screening, 1 liter of 2% beef extract was inoculated with 200 infection-forming units of poliovirus 3 (U.S. EPA-approved QC virus) and then processed as described above. The average recovery for eight screenings was 73%.
In addition to the above-described QC procedures, the sensitivities of our cell lines were demonstrated by (i) laboratory participation in College of American Pathologists proficiency testing for virus isolation; (ii) the laboratory's ability to isolate a variety of viruses from different types of clinical specimens, with the same cell lines used for our environmental testing; and (iii) the use of a poliovirus 3 positive control plus a negative control with BGM cells for each sewage sample tested.
RESULTS
During a 100-month study, EV was isolated from 88 of the monthly sewage samples and was detected clinically during 81 of the months. The sewage EVs were compared to the EVs that were detected clinically during the same period of time.
Seasonal appearance of EVs.
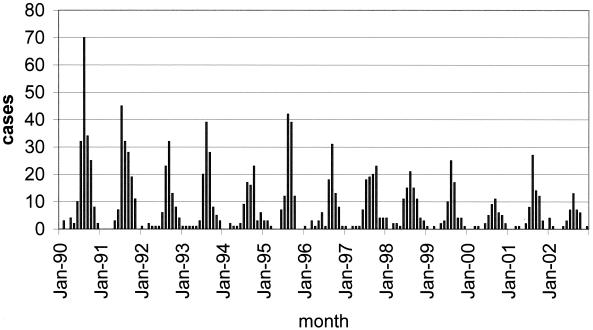
This laboratory has a long experience with the diagnosis of EV infections. Every summer from 1975 to 2002 (13, 14), we have documented, by virus isolation, that EVs have caused clinical problems in the Milwaukee area. However, the magnitude of the annual summer-fall clinical EV peaks (Fig. 1) has been decreasing since the early 1990s (from 1990 to 2002, the annual number of EV cases declined from 190 to 43). This decreased annual peak size reflects a drop in nongenital clinical virus culture specimens (from 1990 to 2002, the annual number of specimens decreased gradually from 8,715 to 1,083) submitted to the Milwaukee Health Department. Also, since 1996, a few local hospitals have begun doing their own virus culturing and since then have only used this laboratory as a reference laboratory to serotype their EV isolates. However, we do receive all local EV isolates for serotyping, indicating that less virus culture for EVs has been performed in recent years and thus giving momentum to the idea of sewage testing to better understand which EVs are circulating in the community.
FIG. 1.
Clinical EV cases confirmed each month by isolation of virus, 1990 to 2002.
Typically, since 1990 (Fig. 1), the local EV season began in June and peak activity generally occurred in August or September, with the last outbreak cases being detected in November. However, sporadic clinical EV cases occurred throughout the winter. Each season, many clinical EV serotypes were detected, with three or four serotypes predominating. The most common EV serotypes varied from year to year. No matter which serotypes predominated, the age distribution for confirmed infections in each season was similar, with approximately 60% of the infections documented for those less than 12 months of age. The reported clinical picture for confirmed cases also did not vary much from year to year, with approximately 40% of the confirmed cases identified as aseptic meningitis cases. Typical age distributions and clinical data for Milwaukee Health Department confirmed cases have been reported previously (10).
During the years of comparative clinical and sewage testing, 9 to 19 (average, 13.7) different clinical EV serotypes were detected each year (Table 2) but only between 4 and 11 (average, 7.4) serotypes were detected in sewage, always fewer serotypes than were detected for clinical specimens. From 1994 to 2002, peak EV titers in sewage occurred during the same months when seasonal clinical EV activity was evident (Fig. 2), with 556 (80.1%) of the clinical cases and 747 (69.9%) of the sewage isolates detected during July, August, September, and October. Maximum seasonal sewage MPN-per-liter titers varied greatly, with the highest maximum, 3,347 MPN/liter (95% confidence interval, 2,028 to 4,842 MPN/liter), occurring in July 1997 and the lowest maximum, 237 MPN/liter (95% confidence interval, 113 to 385 MPN/liter), occurring in September 1996. Each year, the peak in diagnosed clinical infections and the peak in sewage EV MPN-per-liter titers occurred in either the summer or the early fall. However, some years with a relatively large number of clinical cases (1995, 119 clinical cases; 1998, 89 clinical cases) had relatively low maximum sewage titers (913 and 631 MPN/liter, respectively). Also, the converse was seen in 2002, when a high maximum sewage EV titer (3,218 MPN/liter, 95% confidence interval of 1,778 to 5,727 MPN/liter) was observed in July but only 43 EV infections were confirmed for the year. Thus, EV disease and EVs in sewage peak during the same time frame each year (comparison of the percentage of total sewage isolates and the percentage of total clinical cases by month: Wilcoxon signed-rank test, W+ = 36, W− = 42; n = 12; P = <0.8501) but a linear quantitative relationship does not exist.
TABLE 2.
Number of different non-poliovirus EV serotypes
| Yr | No. of clinical serotype | No. of sewage serotype |
|---|---|---|
| 1994 | 19 | 6 |
| 1995 | 17 | 8 |
| 1996 | 13 | 5 |
| 1997 | 13 | 7 |
| 1998 | 12 | 7 |
| 1999 | 9 | 4 |
| 2000 | 14 | 11 |
| 2001 | 11 | 10 |
| 2002 | 15 | 9 |
FIG. 2.
Comparison of monthly clinical EV cases with monthly EV sewage titers, August 1994 to December 2002.
Sewage EVs by group.
Over the 100 months of the study, 1,068 EVs were isolated from sewage and grouped by cell culture host range analysis (Table 3). These analyses indicated that 1,022 of the isolates were non-poliovirus EVs and 39.0% of these isolates were further identified as to serotype. Echovirus was the group with the most detections (859 viruses), and coxsackievirus type A was that with the least (12 viruses). Only 46 polioviruses were isolated from sewage, with no poliovirus being detected in 2000, 2001, or 2002. With the recent change in the pediatric vaccination policy, which advocates the exclusive use of inactivated poliovirus vaccine after January 2000 (3), isolation of poliovirus from environmental samples may become rare.
TABLE 3.
Number of sewage EV isolates grouped by cell culture host range analysis
| Yr | No. of isolates
|
Total | No. of non-poliovirus isolates | No. of non-poliovirus isolates typed | % of non-poliovirus isolates typed | |||
|---|---|---|---|---|---|---|---|---|
| Poliovirus | Coxsackievirus A | Coxsackievirus B | Echovirus | |||||
| 1994 | 10 | 0 | 19 | 29 | 58 | 48 | 13 | 27.1 |
| 1995 | 10 | 5 | 54 | 37 | 106 | 96 | 37 | 38.5 |
| 1996 | 9 | 3 | 15 | 35 | 62 | 53 | 24 | 45.3 |
| 1997 | 3 | 0 | 0 | 212 | 215 | 212 | 49 | 13.1 |
| 1998 | 8 | 0 | 2 | 86 | 96 | 88 | 40 | 45.5 |
| 1999 | 6 | 0 | 13 | 121 | 140 | 134 | 69 | 51.5 |
| 2000 | 0 | 1 | 32 | 72 | 105 | 105 | 59 | 56.2 |
| 2001 | 0 | 0 | 14 | 103 | 117 | 117 | 47 | 40.2 |
| 2002 | 0 | 3 | 2 | 164 | 169 | 169 | 61 | 36.1 |
| Total | 46 | 12 | 151 | 859 | 1,068 | 1,022 | 399 | 39.0 |
Cell sensitivity for isolation of various EV groups.
In our laboratory, Caco-2 cells (601 of 859 echoviruses) were more sensitive than BGM cells (17 of 859 echoviruses) for the isolation of echoviruses (Table 4) (chi square test = 432.93; P ≤ 0.001). However, echoviruses also frequently produced a CPE on RD cells (219 of 859 echoviruses). BGM cells were more sensitive for type B coxsackieviruses (126 of 151 isolates) than were Caco-2 cells (15 of 151 isolates) (chi-square test = 63.56; P ≤ 0.001). RD cells detected the most polioviruses (22 of 46 poliovirus isolates). No matter the type of EV or the cell system used, more than 90% of the isolate CPE was detected in 7 days or less (14). It is noteworthy that reoviruses were mainly detected on BGM cells, with a CPE only becoming apparent at 7 or more days postinoculation (data not shown).
TABLE 4.
Number of sewage EVs isolated on various cell types in 1994 to 2002
| Cell typea | No. of isolates of EVb group
|
No. of Total isolates | |||
|---|---|---|---|---|---|
| Poliovirus | Coxsackievirus A | Coxsackievirus B | Echovirus | ||
| BGM | 16 | 0 | 126 | 17 | 159 |
| RD | 22 | 12 | 1 | 219 | 254 |
| Caco-2 | 6 | 0 | 15 | 601 | 622 |
| HEp-2 | 0 | 0 | 9 | 8 | 17 |
| HFS | 2 | 0 | 0 | 14 | 16 |
| Total | 46 | 12 | 151 | 859 | 1,068 |
BGM and RD cells were used in all years. HFS cells were used in 1994, 1995, and 1996. Caco-2 cells were used in 1997 to 2002. HEp-2 cells were used in 1999 to 2002.
EVs were grouped by cell culture host range analysis and specific typing.
Yearly comparison of sewage and clinical EVs.
Table 5 compares the EVs isolated from sewage with the EVs isolated from clinical cases for each year of the study. For many years, the most commonly detected clinical EV serotype was also the most commonly detected sewage serotype. For example, in 1997, echovirus 6 accounted for 39.4% of the clinical infections and 53.1% of the typed sewage isolates, while in 1998, echovirus 30 accounted for 46.1 and 50.0%, respectively. In 1999, 60.3% of the EVs from clinical cases and 79.9% of the typed sewage isolates were echovirus 11, and for 2000, 33.3% of the EVs from clinical cases and 40.7% of the sewage isolates were coxsackievirus B5. Also, in 2001, 44.1% of the clinical EVs and 36.2% of the sewage isolates were echovirus 13. But the most common clinical EV for a specific year is not always the most common EV in sewage, as is the case for 1994, 1995, and 2002. In 1994, echovirus 18 was the most frequently detected clinical EV (15.0% cases) but was not detected in sewage, and in 1995, coxsackievirus B4 was the most frequently isolated clinical EV (21.0% of the cases) but was only the second most commonly typed sewage EV (21.6% of the isolates), behind echovirus 11, at 35.1% of the sewage isolates typed. In 2002, coxsackievirus B1 (20.9% of the cases) was the most common clinical EV but only accounted for 3.3% of the typed sewage isolates.
TABLE 5.
EV comparisons: clinical cases versus sewage isolates
| Virus | % of clinical cases/% of typed sewage isolates in:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | |
| N/N | 80/13 | 119/37 | 86/24 | 99/49 | 89/36 | 68/69 | 42/39 | 68/47 | 43/61 |
| echo-2 | 1.3/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| echo-3 | 0/0 | 0.8/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/3.4 | 0/6.4 | 18.6/34.4 |
| echo-4 | 0/0 | 4.2/0 | 3.5/4.2 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| echo-5 | 5.0/0 | 1.7/0 | 0/0 | 0/0 | 0/0 | 1.5/0 | 0/0 | 0/0 | 0/0 |
| echo-6 | 7.5/46.2 | 0.8/2.7 | 4.7/16.7 | 39.4/53.1 | 0/11.1 | 0/6 | 2/11.9 | 0/8.5 | 0/3.3 |
| echo-7 | 6.3/0 | 0/0 | 16.3/37.5 | 4.0/24.5 | 0/5.6 | 0/0 | 0/0 | 0/0 | 13.9/26.2 |
| echo-9 | 0/0 | 9.2/5.4 | 0/0 | 0/0 | 0/0 | 1.5/0 | 4.8/3.4 | 1.5/8.5 | 2.3/0 |
| echo-11 | 0/15.4 | 15.1/35.1 | 7.0/0 | 2.0/8.2 | 1.1/16.7 | 60.3/79.7 | 4.8/16.9 | 0/8.5 | 2.3/0 |
| echo-12 | 0/0 | 0/0 | 0/0 | 0/4.1 | 0/0 | 0/0 | 0/0 | 0/0 | 0/1.6 |
| echo-13 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/1.7 | 44.1/36.2 | 0/0 |
| echo-14 | 1.3/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/1.7 | 0/0 | 0/0 |
| echo-15 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/1.7 | 0/0 | 0/0 |
| echo-16 | 0/0 | 0.8/0 | 0/0 | 0/0 | 0/0 | 0/0 | 2.4/0 | 0/0 | 0/0 |
| echo-17 | 0/0 | 0/0 | 0/0 | 18.2/0 | 1.1/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| echo-18 | 15.0/0 | 5.9/5.4 | 0/0 | 0/0 | 12.4/5.6 | 0/0 | 0/0 | 32.4/2.1 | 2.3/0 |
| echo-19 | 0/0 | 0/0 | 0/4.2 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| echo-20 | 1.3/0 | 0/2.7 | 0/0 | 0/0 | 1.1/0 | 0/0 | 2.4/0 | 1.5/0 | 0/0 |
| echo-21 | 1.3/0 | 6.7/0.8 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| echo-22 | 1.3/0 | 3.4/0 | 8.1/0 | 9.1/2.0 | 14.6/0 | 3/0 | 9.5/0 | 0/2.1 | 2.3/1.6 |
| echo-25 | 6.3/0 | 4.2/0 | 0/0 | 1.0/6.1 | 5.6/8.3 | 1.5/0 | 2.4/15.3 | 0/2.1 | 2.3/1.6 |
| echo-30 | 3.8/0 | 1.7/0 | 1.2/0 | 3.0/2.0 | 46.1/50.0 | 0/0 | 0/0 | 4.4/0 | 11.6/0 |
| echo-33 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/1.7 | 0/0 | 0/0 |
| cox-B1 | 1.3/0 | 0/0 | 0/0 | 0/0 | 2.2/2.8 | 0/0 | 0/0 | 1.5/0 | 20.9/3.3 |
| cox-B2 | 11.2/7.7 | 4.2/0 | 2.3/0 | 10.1/0 | 9.0/0 | 0/0 | 2.4/0 | 2.9/14.9 | 2.3/0 |
| cox-B3 | 0/0 | 0/0 | 0/0 | 1.0/0 | 0/0 | 0/1.5 | 0/0 | 5.9/10.6 | 4.7/0 |
| cox-B4 | 12.5/15.4 | 21.0/21.6 | 2.3/0 | 0/0 | 0/0 | 5.9/13.0 | 9.5/1.7 | 1.5/0 | 0/0 |
| cox-B5 | 5.0/0 | 14.3/16.2 | 7.0/37.5 | 0/0 | 0/0 | 0/0 | 33.3/40.7 | 1.5/0 | 7.0/0 |
| cox-B6 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 2.3/0 |
| cox-A4 | 0/0 | 5.0/0 | 0/0 | 9.1/0 | 0/0 | 0/0 | 14.3/0 | 0/0 | 0/0 |
| cox-A6 | 3.8/0 | 0/0 | 2.3/0 | 0/0 | 1.1/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| cox-A7 | 0/0 | 1.7/0 | 0/0 | 1.0/0 | 4.5/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| cox-A9 | 0/0 | 0/0 | 26.7/0 | 1.0/0 | 0/0 | 20.6/0 | 4.8/0 | 0/0 | 2.3/0 |
| cox-A10 | 2.5/0 | 0/0 | 16.3/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| cox-A16 | 3.8/0 | 0/0 | 2.3/0 | 1.0/0 | 1.1/0 | 0/0 | 0/0 | 0/0 | 4.7/1.6 |
| cox-A? | 10.0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 3/0 | 2.4/0 | 0/0 | 0/3.3 |
| ev-71 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 4.8/0 | 2.9/0 | 0/0 |
However, the comparative sewage and clinical data for each year (Table 5) must be looked at individually to understand the full scope of the complexity of EV surveillance.
1994.
The most common clinical EV, echovirus 18, was not detected in sewage in 1994. However, other common clinical EVs, coxsackieviruses B4 and B2 and echovirus 6, were detected in sewage. A high percentage of typed sewage isolates (46.2%) were echovirus 6, which accounted for only 7.5% of the clinical infections. Echovirus 11 was detected in sewage but not seen clinically.
1995.
The four most common clinical EVs (coxsackievirus B4, echovirus 11, coxsackievirus B5, and echovirus 9) in 1995 were all detected in sewage. Eleven different clinical EV serotypes were not detected in influent samples. Echovirus type 20 was detected in sewage but not seen in clinical cases.
1996.
Coxsackieviruses A9 and A10 were the most commonly detected clinical EVs in 1996 (26.7 and 16.3% of the cases, respectively), but neither was detected in sewage. Echovirus 7 was detected both clinically (16.3% of cases) and in sewage (37.5% of typed isolates), as were coxsackievirus B5 (7.0% of the clinical cases and 37.5% of the typed sewage isolates), echoviruses 6 (4.7% of the clinical cases and 16.7% of the typed sewage isolates), and echovirus 4 (3.5% of the clinical cases and 4.2% of the typed sewage isolates).
1997.
The most common clinical EV, echovirus 6 (39.4% of the cases), was also the most frequently detected sewage EV (53.1% of the typed isolates) in 1997. Echovirus 17 and coxsackievirus B2, both seen frequently in clinical cases (18.2 and 10.1%, respectively), were not detected in sewage. Echovirus 12 was detected in sewage but not in clinical cases.
1998.
Echovirus 30 was the predominant clinical EV (46.1% of the cases) and also the most commonly detected sewage EV (50.0% of the typed isolates) in 1998. One echovirus 11 infection was confirmed clinically, and echovirus 11 was also detected in sewage. However, both echovirus types 6 and 7 were detected in sewage but no clinical infections were confirmed.
1999.
Echovirus 6 was the predominant clinical and sewage isolate in 1999, with 60.3% of the clinical cases and 79.5% of the typed sewage isolates, respectively. Coxsackievirus A9 was a common cause of clinical disease (20.6% of the cases) but was not detected in sewage. Coxsackievirus B4 was detected in both clinical specimens (5.9% of the cases) and sewage specimens (13.0% of the typed isolates), while echovirus 6 and coxsackievirus B3 were identified in sewage but not detected in clinical specimens.
2000.
Coxsackievirus B5 was the predominant EV in both sewage and clinical samples in 2000, with 40.7 and 33.3% of the typed isolates, respectively. Coxsackievirus B4 accounted for 9.5% of the clinical cases and 1.7% of the sewage isolates. Coxsackievirus A4 and echovirus 22 were detected clinically but not recovered from sewage, and conversely, echovirus types 13, 14, 15, and 33 were detected in sewage but not in clinical specimens.
2001.
Echovirus 13, an EV that until 2001 had been detected in the United States only rarely since 1970 (5), was the most frequently diagnosed EV for clinical infections (44.1% of cases) and also the EV most commonly detected in sewage (36.2% of typed isolates) in 2001. The other two most common clinical EVs, echovirus 18 (32.4% of the cases) and coxsackievirus B3 (5.9% of the cases), were also detected in sewage. However, a number of EVs detected in sewage, echovirus types 6, 11, 22, and 25, were not seen clinically.
2002.
Coxsackievirus B1 was the most frequently detected clinical EV (20.9% of the cases) and was also identified in sewage (3.3% of the typed isolates) in 2002. Echovirus 3 (18.6% of the clinical cases) and echovirus 7 (13.9% of the clinical cases) were both detected in sewage (3.3 and 34.4% of the typed isolates, respectively). Echovirus 6, echovirus 12, and coxsackievirus A (unidentified) were detected in sewage but were not diagnosed in clinical infections.
DISCUSSION
In Milwaukee, Wis., as in most of North America, the EV seasons are complex. In each season, many EV serotypes are present, with three to five serotypes predominating. Diagnosis of EV infections requiring hospital visits provides some insight into the types that are circulating in the community. However, because many infections are asymptomatic or subclinical, EV serotypes not detected clinically could still be shed into local sewage. This study shows that if sewage is sampled monthly and sewage isolates are compared to clinical cases, to some degree, qualitatively, a similar picture of EV activity is evident for the community. Shorter-term comparative clinical and sewage studies in Great Britain (15) and Canada (9) have also demonstrated a similarity of echoviruses and type B coxsackieviruses during the summer months.
In Milwaukee, peak detection of both clinical and sewage EVs occurred during the summer or early fall and many of the clinically relevant EVs were also detectable in sewage. However, the clinical-sewage correlation was not 100% because a greater number of EV serotypes was detected clinically than in environmental samples and some sewage serotypes were never detected in clinical infections. Also, the type A coxsackieviruses, even when present clinically, were difficult to culture from sewage even when RD cells were used. Thus, if one cannot obtain clinical specimens, a reasonable method by which to determine EV activity would be to try to isolate EVs from local sewage. The reliability of the sewage culture results is improved if more than one cell type is used for EV isolation. While BGM cells are good for isolation of type B coxsackieviruses, many of the echoviruses would have been missed if RD or Caco-2 cells had not been used. However, it is of utmost importance, when using sewage for an EV surveillance system, to assume that the clinical population in question is the population shedding EVs into the sewage. Because the influent sewage samples tested in this study were obtained from a treatment plant that services approximately half of the Milwaukee area population and this laboratory identifies essentially all of the clinical EVs from the Milwaukee metropolitan area, it is probably reasonable to assume that our testing of sewage is a reflection of local EV activity. However, our system is more qualitative then quantitative because many of the important EV parameters, such as the percentage of the population infected by each EV serotype each season, the proportion of those infected with overt clinical disease, the quantity of each EV serotype shed in a stool specimen, the EV serotype-specific inactivation rates during transit to the treatment plant, and the efficiency of recovery of specific EV serotypes by the organic flocculation procedure, are unknown. However, the system used in Milwaukee indicates that many different EV serotypes can be detected in sewage by using the organic flocculation procedure and multiple cell types for isolation of EVs. The usefulness of community sewage testing to monitor the presence of polioviruses in the face of the circulation of wild-type poliovirus in the community has been demonstrated in The Netherlands (17) and Finland (11), and similar testing of sewage may be useful for monitoring of echoviruses and coxsackieviruses.
EVs that are detected in late winter or early spring can at times be predictors of some of the EV types that will be predominant clinically during the following summer. For example, in 1995, echovirus 11 was detected in sewage in March and coxsackievirus B4 was detected in sewage in January, February, and March and these two viruses were the most commonly detected EVs during the following EV season, with the first clinical echovirus 11 case detected in July and the first clinical coxsackievirus B4 case detected in June. However, confounding the interpretation, coxsackievirus B4 was also detected clinically and in sewage in 1994. In the summer of 1996, echovirus 7 was one of the predominant clinical EVs (16.3% of the clinical cases), with the first clinical case in August, but this virus was detected in sewage in February 1996 and was not detected clinically or in sewage in 1995. During 1997, the predominant EV, echovirus 6 (39.4% of the clinical cases), was detected in sewage in April, with the first clinical case reported in June. In 1998, echovirus 30 (46.1% of the clinical cases) predominated and was first detected clinically in June but was detected in sewage in both January and March. Especially noteworthy in 2001 was the appearance of echovirus 13 both clinically (44.1% of the clinical cases) and in sewage (36.2% of the typed isolates). Echovirus 13 was rarely detected in the United States from 1970 until 2001. However, echovirus 13 was detected in sewage in December 2000, long before the first clinical case was detected in July 2001. Echovirus 13 was also isolated from sewage in June 2001. However, each year so many EV serotypes are detected both clinically and in sewage that it is difficult to assign any predictive value to one specific serotype that is detected early in any given year. There are years such as 1998, when echovirus 7 was detected in sewage in January and April but caused no confirmed clinical infections that year. However, echovirus 7 was detected both in sewage and clinically in 1996 and 1997, so the early 1998 sewage detections may have been carryover from community activity in the preceding 2 years. Thus, the prognostic value of early year EV sewage isolates is limited.
REFERENCES
- 1.Berg, G. 1987. Methods for recovering viruses from the environment. CRC Press, Inc., Boca Raton, Fla.
- 2.Berg, G., H. Bodily, E. Lennette, J. Melnick, and T. G. Metcalf. 1976. Viruses in water. American Public Health Association, Inc., Washington, D.C.
- 3.Centers for Disease Control and Prevention. 2000. Poliomyelitis prevention in the United States: updated recommendations of the Advisory Committee on Immunization Practices. Morbid. Mortal. Wkly. Rep. 49(RR05):1-22. [PubMed] [Google Scholar]
- 4.Federal Register. 1996. Information collection rule (ICR). Fed. Regist. 61:24345-24388. [Google Scholar]
- 5.Krishna, N., M. Little, and R. Ratard. 2001. Echovirus type 13—United States, 2001. Morbid. Mortal. Wkly. Rep. 50:777-780. [PubMed] [Google Scholar]
- 6.MacKenzie, W. R., N. Hoxie, M. Proctor, S. Gradus, K. Blair, D. Peterson, J. Kazmierzak, K. Fox, D. Addis, J. Rose, and J. Davis. 1994. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 331:161-167. [DOI] [PubMed] [Google Scholar]
- 7.Melnick, J. L., V. Rennick, B. Hampil, N. J. Schmidt, and H. H. Ho. 1973. Lyophilized combination pools of enterovirus equine antisera: preparation and test procedure for identification of field strains of 42 enteroviruses. Bull. W. H. O. 48:263-268. [PMC free article] [PubMed] [Google Scholar]
- 8.Metcalf, T. G., J. L. Melnick, and M. K. Estes. 1995. Environmental virology: from detection of viruses in sewage and water by isolation to identification by molecular biology—a trip of over 50 years. Annu. Rev. Microbiol. 49:461-487. [DOI] [PubMed] [Google Scholar]
- 9.Payment, P., R. Ayache, and M. Trudel. 1993. A survey of enteric viruses in domestic sewage. Can. J. Microbiol. 1:111-119. [DOI] [PubMed] [Google Scholar]
- 10.Piraino, F., G. Sedmak, and K. Raab. 1982. Echovirus 11 infections of newborns with mortality during the 1979 enterovirus season in Milwaukee, Wis. Public Health Rep. 97:346-353. [PMC free article] [PubMed] [Google Scholar]
- 11.Poyry, T., M. Stenvik, and T. Hovi. 1988. Viruses in sewage waters during and after a poliomyelitis outbreak and subsequent nationwide oral poliovirus vaccination campaign in Finland. Appl. Environ. Microbiol. 54:371-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao, V. C., and J. L. Melnick. 1986. Aspects of microbiology 13: environmental virology. American Society for Microbiology, Washington, D.C.
- 13.Sedmak, G., C. Abel, B. Voight, and H. Wisniewski. 1981. Seasonal occurrence of viruses in the Milwaukee area. Wis. Med. J. 80:31-35. [PubMed] [Google Scholar]
- 14.Sedmak, G., D. Bina, and J. MacDonald. 1999. Milwaukee enterovirus surveillance system: comparison of sewage isolates to clinical isolates, session 1, p. 1-21. In Proceedings of the International Symposium on Waterborne Pathogens. American Water Works Association, Denver, Colo.
- 15.Sellwood, J., J. Dadswell, and J. Slade. 1981. Viruses in sewage as an indicator of their presence in the community. J. Hyg. (London) 86:217-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Environmental Protection Agency. 1995. Virus monitoring protocol for the information collection requirements rule: ICR-EPA manual—EPA/814-B-95-002. U.S. Environmental Protection Agency, Washington, D.C.
- 17.van der Avoort, H., J. Reimerink, A. Ras, M. Muldres, and A. van Loon. 1995. Isolation of epidemic poliovirus from sewage during the 1992-3 type 3 outbreak in The Netherlands. Epidemiol. Infect. 114:481-491. [DOI] [PMC free article] [PubMed] [Google Scholar]