Abstract
Pseudomonas mendocina KR-1 grew well on toluene, n-alkanes (C5 to C8), and 1° alcohols (C2 to C8) but not on other aromatics, gaseous n-alkanes (C1 to C4), isoalkanes (C4 to C6), 2° alcohols (C3 to C8), methyl tertiary butyl ether (MTBE), or tertiary butyl alcohol (TBA). Cells grown under carbon-limited conditions on n-alkanes in the presence of MTBE (42 μmol) oxidized up to 94% of the added MTBE to TBA. Less than 3% of the added MTBE was oxidized to TBA when cells were grown on either 1° alcohols, toluene, or dextrose in the presence of MTBE. Concentrated n-pentane-grown cells oxidized MTBE to TBA without a lag phase and without generating tertiary butyl formate (TBF) as an intermediate. Neither TBF nor TBA was consumed by n-pentane-grown cells, while formaldehyde, the expected C1 product of MTBE dealkylation, was rapidly consumed. Similar Ks values for MTBE were observed for cells grown on C5 to C8 n-alkanes (12.95 ± 2.04 mM), suggesting that the same enzyme oxidizes MTBE in cells grown on each n-alkane. All growth-supporting n-alkanes (C5 to C8) inhibited MTBE oxidation by resting n-pentane-grown cells. Propane (Ki = 53 μM) and n-butane (Ki = 16 μM) also inhibited MTBE oxidation, and both gases were also consumed by cells during growth on n-pentane. Cultures grown on C5 to C8 n-alkanes also exhibited up to twofold-higher levels of growth in the presence of propane or n-butane, whereas no growth stimulation was observed with methane, ethane, MTBE, TBA, or formaldehyde. The results are discussed in terms of their impacts on our understanding of MTBE biodegradation and cometabolism.
Methyl tertiary butyl ether (MTBE) is a branched alkyl ether that is presently widely used as an oxygenating compound in gasoline sold in the United States. The extensive use of MTBE over the last 20 years has resulted in widespread distribution of this compound in the environment. Groundwater resources have been particularly impacted by MTBE, primarily through gasoline contamination derived from leaking underground storage tanks (14, 25). The U.S. Environmental Protection Agency presently classifies MTBE as a possible human carcinogen and has issued a drinking water advisory for MTBE of 20 to 40 ppb (28).
Although MTBE can be slowly biodegraded under anaerobic conditions (1, 4, 24, 31), the fastest rates of MTBE biodegradation consistently appear to occur under aerobic conditions. A limited number of aerobic organisms that utilize MTBE as the sole source of carbon and energy for growth have been identified. These organisms include Rubrivivax strain PM-1 (10), Hydrogenophaga flava ENV 735 (12), Mycobacterium austroafricanum (5), and others (20). A variety of other organisms have also been identified that can cometabolically degrade MTBE but cannot grow with this compound as the sole source of carbon and energy. These organisms include gaseous n-alkane-utilizing bacteria (17, 23, 26) and fungi (11), as well as other hydrocarbon-utilizing bacteria grown on n-pentane (6), cyclohexane (2), and aromatics (13, 16).
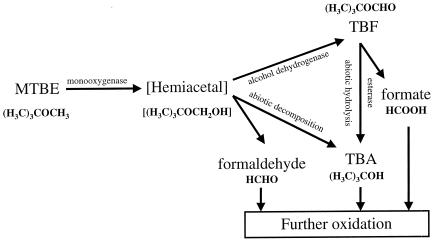
The aerobic cometabolic degradation of MTBE is thought to be initiated by a variety of monooxygenase enzymes. In propane-grown cells of Mycobacterium vaccae, JOB5 MTBE has been proposed to be initially oxidized to an unstable hemiacetal intermediate by an alkane-inducible short-chain alkane monooxygenase (23). This unstable hemiacetal has also been proposed to be further oxidized rapidly by an alcohol dehydrogenase to yield tertiary butyl formate (TBF). Biological and chemical hydrolysis of TBF generates tertiary butyl alcohol (TBA) that is itself further oxidized by the same monooxygenase responsible for initiating MTBE oxidation (23) (Fig. 1). Production of TBF has also been reported during the cometabolic degradation of MTBE by a filamentous fungus, a Graphium sp., after growth on gaseous n-alkanes (11). In contrast, TBF has not been reported as an intermediate in MTBE oxidation by n-pentane (6), n-butane (17), and other propane-oxidizing bacteria (26). In these organisms, it is likely that the hemiacetal initially generated from MTBE oxidation rapidly dismutes to form TBA and formaldehyde, the products expected from a conventional O-dealkylation reaction. Notably, all of the n-alkane-grown bacteria presently known to cometabolically oxidize MTBE, including M. vaccae JOB5, are also reported to further oxidize TBA.
FIG. 1.
Proposed pathways of bacterial MTBE oxidation to TBA. The figure summarizes the various pathways, enzyme activities, and intermediates believed to be involved in the bacterial oxidation of MTBE to TBA. The hemiacetal intermediate has not been detected.
In the present study, we investigated the potential for cometabolic degradation of MTBE by Pseudomonas mendocina KR-1. This organism is perhaps best known for its ability to degrade another important groundwater pollutant, trichloroethylene, through the activity of a toluene-induced toluene-4-monooxygenase (18, 32). A previous study has reported that toluene-grown cells of this organism do not oxidize MTBE (26). However, it has also been reported that this strain also grows on several medium-chain alkanes (18), compounds that have been previously shown to support cometabolic MTBE degradation in other organisms (6). As the majority of MTBE enters the environment as part of gasoline mixtures and P. mendocina KR-1 utilizes two major components of gasoline, toluene and n-alkanes, we were interested in more fully defining the hydrocarbon utilization pattern of this organism and in investigating the MTBE-oxidizing activity of cells grown on these substrates. Our results show that n-alkane-grown cells of this organism rapidly degrade MTBE but do not generate TBF or further oxidize TBA. Our results also show that gaseous n-alkanes that do not support the growth of this organism are also oxidized by n-alkane-grown cells and that this activity can stimulate the growth of n-alkane-grown cells.
MATERIALS AND METHODS
Materials.
Pseudomonas mendocina KR-1 was generously supplied by Amgen Inc. (Thousand Oaks, Calif.). Benzene (≥99% purity), n-butane (99% purity), 1-butanol (99% purity), 2-butanol (99.5% purity), ethylbenzene (99.8% purity), formaldehyde (37% aqueous solution), n-heptane (99% purity), 1-heptanol (98% purity), 2-heptanol (98% purity), n-hexane (≥99% purity), 1-hexanol (≥99% purity), 2-hexanol (99% purity), isobutane (99% purity), isopentane (99% purity), 2-methyl-1-propanol (99.5% purity), 2-methylpentane (99.5% purity), 2-methyl-2-propanol (TBA) (99.3% purity), MTBE (99.8% purity), n-octane (99% purity), 2-octanol (97% purity), 1-pentanol (99% purity), 2-pentanol (98% purity), 1-propanol (99.9% purity), 2-propanol (99.5% purity), toluene (99.8% purity), o-xylene (98% purity), m-xylene (≥99% purity) and p-xylene (≥99% purity) were obtained from Sigma Aldrich Chemical Co. (Milwaukee, Wis.). Acetylacetone (reagent grade), methanol (99% purity), and n-pentane (99.5% purity) were obtained from Fisher Scientific (Pittsburgh, Pa.). Absolute ethanol was obtained from Aaper Alcohol and Chemical Co. (Shelbyville, Ky.). 1-Octanol (99% purity) was obtained from Lancaster Synthesis Inc. (Windham, N.H.). Methane and ethane (CP grade) were supplied by National Specialty Products (Durham, N.C.). Propane (instrument grade) was supplied by Air Products and Chemicals Inc. (Allentown, Pa.).
Cell growth.
For most of the experiments described in this study, we used P. mendocina KR-1 cells grown in batch culture in glass serum vials (125 ml) sealed with Teflon-lined Mininert valves (Alltech Associates Inc., Deerfield, Ill.). The vials contained a mineral salt medium (25 ml) (33) and were inoculated (at an initial optical density at 600 nm [OD600] of ∼ 0.001) with a suspension of cells obtained from axenic cultures of P. mendocina KR-1 previously grown on agar plates containing mineral salt medium with lactate (20 mM) as the sole carbon and energy source. Unless otherwise stated, all potential liquid growth substrates were added to the sealed vials as the pure compound (5 μl; 0.02%, vol/vol) by using sterile glass microsyringes. Gaseous n-alkanes (methane, ethane, propane, n-butane, and isobutane) were added to the culture vials as required by using disposable sterile plastic syringes fitted with Acrodisc disposable filters (0.1 μm) (Gelman Laboratory, Ann Arbor, Mich.). The culture vials were incubated at 30°C in the dark for 5 days in an Innova 4900 (New Brunswick Scientific Co., Inc., Edison, N.J.) environmental shaker operated at 150 rpm. Culture growth was determined by measuring OD600 by using a Shimadzu 1601 UV/Vis spectrophotometer (Kyoto, Japan). In every experiment, a sample (50 μl) of each cell culture was streaked on mineral salt-lactate plates to subsequently confirm the purity of the culture. In some experiments (see Tables 1 and 2), cells were also grown as described above on dextrose-containing Difco Plate Count medium (Becton Dickinson and Company, Sparks, Md.). This medium contained (in grams per liter) dextrose (1.0), pancreatic digest of casein (5.0), and yeast extract (2.5).
TABLE 1.
Growth substrate range for P. mendocina KR-1
| Potential growth substratea | OD600b after 5 days |
|---|---|
| n-Alkanes | |
| Methanec | ≤0.01 |
| Ethane | ≤0.01 |
| Propane | 0.02 (0.01) |
| n-Butane | 0.02 (≤0.01) |
| n-Pentane | 0.78 (0.01) |
| n-Hexane | 0.76 (0.02) |
| n-Heptane | 0.74 (≤0.01) |
| n-Octane | 0.78 (≤0.01) |
| Branched alkanes | |
| Isobutane | 0.01 (≤0.01) |
| Isopentane | 0.01 (≤0.01) |
| 2-Methylpentane | 0.01 (≤0.01) |
| Aromatics | |
| Benzene | 0.01 (≤0.01) |
| Toluene | 0.43 (≤0.01) |
| Ethylbenzene | ≤0.01 |
| o-Xylene | 0.01 (≤0.01) |
| m-Xylene | ≤0.01 |
| p-Xylene | 0.01 (≤0.01) |
| 1° Alcohols | |
| Methanol | 0.01 (≤0.01) |
| Ethanol | 0.44 (0.01) |
| 1-Propanol | 0.54 (0.01) |
| 1-Butanol | 0.66 (0.01) |
| 1-Pentanol | 0.59 (0.02) |
| 1-Hexanol | 0.65 (0.02) |
| 1-Heptanol | 0.53 (0.01) |
| 1-Octanol | 0.56 (0.02) |
| 2-Methyl-1-propanol | 0.64 (0.02) |
| 2° Alcohols | |
| 2-Propanol | 0.02 (≤0.01) |
| 2-Butanol | 0.02 (≤0.01) |
| 2-Pentanol | 0.02 (≤0.01) |
| 2-Hexanol | 0.02 (≤0.01) |
| 2-Heptanol | 0.02 (≤0.01) |
| 2-Octanol | 0.01 (≤0.01) |
| Oxygenates and ethers | |
| MTBE | 0.01 (≤0.01) |
| TBA | 0.02 (≤0.01) |
Duplicate cultures of P. mendocina KR-1 were grown for 5 days in the presence of each substrate at an initial substrate concentration of 0.05% vol/vol.
All optical densities reported are the means of duplicate cultures. The values in parentheses indicate the range of optical densities around the means.
All cultures using gaseous alkanes (methane, ethane, propane, n-butane, and isobutane) contained 50 ml of alkane added to the sealed culture vials and were incubated for 15 days.
TABLE 2.
Oxidation of MTBE during growth of P. mendocina KR-1 on diverse growth substrates
| Growth substratea | OD600 after 5 daysb
|
MTBE remaining (μmol)c | TBA detected (μmol)d | Molar balance (%) (MTBE + TBA)e | |
|---|---|---|---|---|---|
| Minus MTBE | Plus MTBE | ||||
| Toluene | 0.33 (0.02) | 0.31 (≤0.01) | 39.7, 40.7 | 1.6, 1.2 | 99 |
| n-Octane | 0.76 (0.01) | 0.76 (0.03) | 1.8, 3.0 | 37.3, 34.0 | 91 |
| n-Heptane | 0.72 (≤0.01) | 0.73 (≤0.01) | 4.1, 4.6 | 35.3, 33.3 | 92 |
| n-Hexane | 0.64 (0.02) | 0.63 (≤0.01) | 12.7, 12.2 | 27.5, 26.0 | 93 |
| n-Pentane | 0.67 (<0.01) | 0.65 (≤0.01) | 15.7, 13.4 | 25.8, 27.9 | 99 |
| n-Butane | 0.04 (≤0.01) | 0.02 (≤0.01) | 43.4, 42.9 | 0.2, 0.2 | 103 |
| n-Propane | 0.01 (≤0.01) | 0.01 (≤0.01) | 41.1, 42.6 | 0.2, 0.2 | 100 |
| Ethane | 0.02 (≤0.01) | 0.01 (≤0.01) | 39.5, 42.7 | 0.2, 0.2 | 98 |
| 1-Octanol | 0.56 (≤0.01) | 0.45 (0.02) | 42.4, 40.2 | 0.5, 0.3 | 99 |
| 1-Heptanol | 0.51 (≤0.01) | 0.51 (≤0.01) | 42.8, 42.8 | 0.1, 0.2 | 102 |
| 1-Hexanol | 0.55 (≤0.01) | 0.47 (≤0.01) | 40.7, 42.0 | 1.2, 1.0 | 101 |
| 1-Pentanol | 0.58 (≤0.01) | 0.47 (0.01) | 39.1, 39.5 | 1.0, 0.9 | 96 |
| 1-Butanol | 0.59 (≤0.01) | 0.53 (0.01) | 43.4, 43.8 | 0.3, 0.3 | 105 |
| 1-Propanol | 0.52 (≤0.01) | 0.48 (≤0.01) | 40.6, 42.3 | 0.1, 0.3 | 99 |
| Ethanol | 0.51 (≤0.01) | 0.47 (≤0.01) | 41.6, 42.3 | 0.2, 0.1 | 100 |
| MTBE | NAf | 0.01 (≤0.01) | 42.5, 43.4 | 0.2, 0.2 | 103 |
| Dextrose | 1.51 (0.02) | 1.52 (0.04) | 45.8, 44.4 | 0.0, 0.0 | 107 |
All potential growth substrates other than dextrose (full-strength Plate Count media) were initially present at concentrations equivalent to 500 μmol of carbon.
Duplicate cultures of P. mendocina KR-1 were grown for 5 days in the presence of each potential growth substrate in the absence (minus MTBE) and presence (plus MTBE) of MTBE (42 μmol). The values reported are the means of the optical densities of the two cultures after 5 days. The values in parentheses represent the ranges of values around the means.
Amount of MTBE remaining in each of the MTBE-containing (plus MTBE) cultures after 5 days, as determined by GC.
Amount of TBA detected in the medium of each of the MTBE-containing cultures (plus MTBE) after 5 days, as determined by GC.
Sum of the mean values reported for MTBE remaining and TBA accumulated after 5 days, expressed as a percentage of the value of the initial amount of MTBE added (42 μmol). The reported values have been rounded to the nearest integer.
NA, not applicable.
In experiments requiring concentrated suspensions of n-alkane-grown cells, the cells were grown as described above except that they were harvested after 2-days growth. The cells were harvested from the culture medium by centrifugation (10,000 × g for 10 min), and the resulting cell pellet was resuspended in buffer (10 ml of 50 mM sodium phosphate, pH 7). The washed cells were sedimented again by centrifugation (as above), and the resulting cell pellet was finally resuspended with buffer (1.0 ml, as above) to a final protein concentration of 3 to 15 mg of total cell protein ml−1. The cells were stored at 4°C and were used within 4 h.
Reaction conditions.
Reactions following the degradation of MTBE, TBA, TBF, and 1° alcohols and the effects of n-alkanes on MTBE degradation were all conducted in glass serum vials (10 ml). The reaction vials were prepared by adding buffer (∼900 μl of 50 mM sodium phosphate, pH 7), after which the vials were sealed with butyl rubber stoppers and aluminum crimp seals (Wheaton Scientific, Millville, N.J.). Liquid substrates (MTBE, TBA, TBF, 1° alcohols, and liquid n-alkanes) were added to the sealed vials from aqueous stock solutions by using microsyringes. Gaseous n-alkanes (methane, ethane, propane, and n-butane) were added directly to the sealed vials, and the excess pressure in the vials was then released by briefly inserting a syringe needle (2.5 cm, 22 gauge) into the stopper. The final concentration of gaseous n-alkane in the reaction vial was then determined by analysis of the gas phase by gas chromatography (GC) (see below). The aqueous-phase concentration of each alkane was then calculated by using solubility data for each gas, assuming a total gas (air plus n-alkane) pressure of 1 atm. In all experiments, the reaction vials were prepared immediately before use and were then incubated for 5 min in a shaking water bath (30°C and 150 rpm) to allow equilibration of substrates between the gas and liquid phases. The reactions were then initiated by the addition of an aliquot (100 μl) of a concentrated cell suspension to give a final reaction volume of 1 ml and a total cell protein content of ∼0.3 to 1.5 mg. The reaction vials were then returned to the shaking water bath and were sampled for analysis by GC according to the demands of each experiment.
Experiments investigating the production and consumption of formaldehyde were conducted under the same conditions as described above. To quantify changes in formaldehyde concentration, the reaction mixture was removed from the reaction vial and was centrifuged in an Eppendorf microfuge (14,000 rpm for 2 min) to sediment the cells. Three samples (100 μl each) were taken from the supernatant and were diluted to a final volume of 1 ml with water. The sample was mixed with the test reagent (20) and incubated in the dark at 37°C for 1 h. The concentration of formaldehyde was determined spectrophotometrically (412 nm) by using calibration plots developed by using standard solutions of formaldehyde (0 to 2 mM). Neither MTBE nor TBA interfered with this assay at concentrations of up to 1 mM.
Determination of kinetic constants.
Kinetic constants (Vmax and Ks) were determined for MTBE oxidation by using the small-scale reactions described above. In these experiments, concentrated suspensions of cells grown on C5 to C8 n-alkanes were used. The reactions were conducted by using a range of initial MTBE concentrations (0 to ∼50 mM dissolved MTBE). Initial time course experiments with cells grown on each n-alkane indicated that the rate of TBA production was constant over a 30-min period. The incubations were therefore conducted for a total of 25 min, and the final concentration of TBA generated after this time was determined by GC (see below). The rate of MTBE oxidation was derived from this final TBA concentration with the assumption that no further oxidation of TBA occurred during the reaction period (see Results). The kinetic constants were derived by computer-fitting the data by nonlinear regression to a single substrate-binding model [y = Vmax · x/(Ks + x)] by using GraphPad Prism version 3.0a for Macintosh (GraphPad Software, San Diego, Calif.).
Analytical methods.
For all experiments, the concentrations of MTBE, TBA, TBF, n-alkanes, and alcohols were determined by GC. For the analysis of MTBE, TBA, TBF, and 1° alcohols, aqueous samples (2 μl) were taken directly from the culture or reaction vials and were injected into a Shimadzu GC-8A gas chromatograph (Kyoto, Japan) fitted with a flame ionization detector and a stainless steel column (0.3 × 183 cm) filled with Porapak Q (60 to 80 mesh) (Waters Associates, Framingham, Mass.). The analysis was conducted by using a column temperature of 160°C, an injection port temperature of 200°C, and a detector temperature of 220°C. Nitrogen was used as the carrier gas at a flow rate of 15 ml/min. In experiments that followed the time course of n-alkane and MTBE consumption during cell growth (see Fig. 5), gas-phase samples (25 μl) were removed by using gas-tight syringes with dry heat-treated needles (45 s at 350° C). The samples were directly injected into a Shimadzu GC-14A gas chromatograph fitted with a flame ionization detector and a DB-MTBE capillary column (30 m × 0.45 mm (internal diameter), 2.55-μm film; J & W Scientific, Folsom, Calif.). The analysis was conducted by using a column temperature of 35°C, an injection port temperature of 200°C, and a detector temperature of 220°C. Nitrogen was used as the carrier gas at a flow rate of 5 ml/min. Both gas chromatographs were interfaced to Hewlett-Packard HP3395 (Palo Alto, Calif.) integrators for data collection.
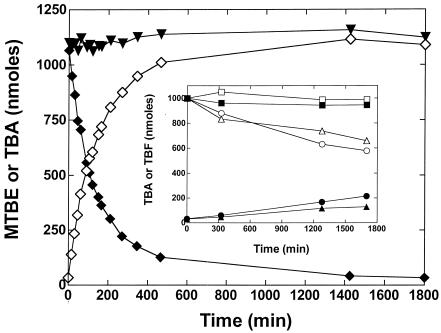
FIG. 5.
Consumption of MTBE and gaseous n-alkanes during cell growth on n-pentane. A series of cultures of P. mendocina KR-1 were grown in sealed glass serum vials (125 ml) containing mineral salt medium (25 ml) with either n-pentane alone (87 μmol) or with n-pentane (87 μmol) and either MTBE (16.8 μmol), methane, ethane, propane, or n-butane (44.6 μmol of each gas). Each of the cultures was conducted in duplicate. Gas phase samples (25 μl) were removed at the indicated times and were analyzed by GC as described in Materials and Methods. The figure shows the time course of n-pentane consumption (open symbols) and supplementary substrate consumption (solid symbols) for the cultures grown with n-pentane plus MTBE (□, ▪); n-pentane plus methane (▵, ▴); n-pentane plus ethane (▿, ▾); n-pentane plus propane (⋄, ♦); n-pentane plus n-butane (○, •); and n-pentane alone (*). The symbols represent the mean value of each data set for the duplicate cultures, while the error bars show the range of values for the data for the duplicate cultures.
Formaldehyde was quantified by using the colorimetric method described by Nash (21). Cell protein concentrations were determined by using the Biuret assay (7) after cell material was solubilized for 30 min at 65°C in 3 N NaOH and insoluble material was sedimented by centrifugation in an Eppendorf microfuge (14,000 rpm for 5 min). Bovine serum albumin was used as the standard. The concentration of MTBE in saturated aqueous solution at room temperature (23°C) was taken as 0.544 M (11). The dimensionless Henry's constant for MTBE at 30°C was taken as 0.0255 (19). The aqueous solubilities of methane, ethane, propane, and n-butane at 1 atm at 30°C were taken as 1.41, 1.81, 1.44, and 1.01 mM, respectively (30).
RESULTS
Growth substrate range.
Our initial experiments aimed to define the range of major gasoline hydrocarbons and some of their potential initial metabolites used by P. mendocina KR-1 as growth-supporting substrates. The organism grew well on toluene but did not grow on any of the other aromatic compounds (benzene, ethylbenzene, or the three xylene isomers) under the conditions tested (Table 1). The organism also grew well on all of the liquid n-alkanes (C5 to C8) but not on any of the gaseous n-alkanes (C1 to C4) or simple branched alkanes tested. The results reported in Table 1 for all gaseous alkane substrates are for cultures incubated with ∼25-fold-higher molar concentrations than those for the C5 to C8 n-alkanes, and the cultures were incubated for 15 days rather than 5 days. To address possible toxic effects associated with the higher solubility of gaseous alkanes compared to that of liquid n-alkanes, cultures were also prepared with the same molar concentrations of these gases as the liquid n-alkanes. This change did not result in cell growth on the gaseous n-alkanes (data not shown). Together, these results suggest that the n-alkane growth substrate range of this organism is effectively limited to n-pentane and longer-chain n-alkanes. In contrast to the results with n-alkanes, a wider range of 1° alcohols (C2 to C8) supported strong cell growth (Table 1), while no growth was observed with any 2° alcohols (C3 to C8) or with either MTBE or TBA.
We subsequently examined the potential for MTBE oxidation by P. mendocina KR-1 cells cultivated on the growth substrates identified in Table 1. Cells were grown for 5 days under carbon-limited conditions (500 μmol of carbon from each substrate) in the presence or absence of MTBE (42 μmol) (Table 2). After growth was completed, as determined by both the complete consumption of the growth substrate and the lack of further change in culture density (OD600), the concentrations of MTBE, TBA, and TBF in each culture medium were determined by GC. There was a slight inhibitory effect on the final culture density observed for cells grown on 1° alcohols in the presence of MTBE compared to that for cells grown on the same substrates without MTBE (Table 2). Despite this effect, minimal MTBE consumption or TBA production was observed in the cultures grown on 1° alcohols (Table 2). There was no consistent effect of MTBE on the final culture density for cells grown on dextrose-containing medium, toluene, and n-alkanes (Table 2). A low level of TBA accumulation (1.2 μmol) was observed with toluene-grown cells, and this accumulation was accompanied by a corresponding slight decrease in the residual amount of MTBE (Table 2). In the case of n-alkanes, much higher levels of TBA (≤35 μmol) were detected and were matched by correspondingly higher levels of MTBE consumption (Table 2). No TBA production or MTBE consumption was observed with dextrose-grown cells. As expected, no MTBE oxidation or TBA production was observed with cultures incubated with non-growth-supporting substrates such as methane, ethane, propane, n-butane, or MTBE itself. The molar balances for MTBE consumption and TBA accumulation in all cultures were consistently high (≥90%). No TBF production (minimum detection limit, 20 nmol ml−1) was observed in the culture medium of any of the incubations included in Table 2.
Products of MTBE degradation.
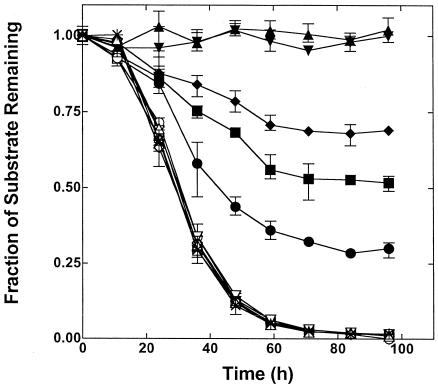
Resting (nonproliferating) n-pentane-grown cells immediately and rapidly consumed MTBE and TBA accumulated in the reaction medium in stoichiometric amounts relative to the amount of MTBE consumed (Fig. 2). Again, TBF was not observed as a transient intermediate during this reaction. The apparent lack of TBA consumption observed during the time course experiment described in Fig. 2 could reflect a potent competitive inhibition of TBA oxidation by the low concentrations of residual MTBE in the reaction medium. Likewise, the apparent lack of TBF production might have been caused by a rapid rate of biological TBF hydrolysis that masked the accumulation of this compound during MTBE oxidation. Both of these possibilities were tested by incubating n-pentane-grown cells with either TBA or TBF alone (1 mM). No consumption of TBA was observed over an ∼30-h time period (Fig. 2, inset). In the case of TBF, a slow rate of substrate consumption and TBA production was observed in the presence of n-pentane-grown cells. However, these rates were the same as the abiotic hydrolysis rate observed when TBF was incubated in the absence of cells.
FIG. 2.
Time course of MTBE consumption and TBA production by n-pentane-grown cells. n-Pentane-grown cells (total protein, 1.33 mg) were incubated with MTBE (1.1 μmol) in a reaction vial (10 ml), and the changes in reactant concentrations were determined over time by GC, as described in Materials and Methods. The figure shows the time course for MTBE consumption (⧫), TBA production (◊), and the molar balance (MTBE + TBA) (▾) for the reaction. The inset shows the time course of TBA consumption and TBA production from TBF hydrolysis. n-Pentane-grown cells (total protein, 0.3 mg) were incubated with either TBA (1 μmol) or TBF (1 μmol) in a reaction vial (10 ml) as described in Materials and Methods. The changes in reactant and product concentrations were determined over time by GC. The figure shows the time course for TBA consumption for a reaction containing n-pentane-grown cells (▪) and a reaction conducted without cells (buffer alone) (□). The figure also shows the time course for TBF consumption for a reaction conducted with (○) and without (▵) n-pentane-grown cells and the corresponding production of TBA in the presence (•) and absence (▴) of cells.
The results presented in Fig. 2 suggest that the oxidation of MTBE by n-alkane-grown cells of P. mendocina KR-1 involves an O-dealkylation reaction that is expected to yield both formaldehyde and TBA as reaction products. Formaldehyde production was not detected by our GC analysis during the reaction described in Fig. 2. In a separate experiment, a more sensitive colorimetric analysis (21) was used to explore the possibility of formaldehyde production and consumption during MTBE oxidation. n-Pentane-grown cells were incubated with MTBE (1 μmol), and samples were analyzed at 30-min intervals to determine accumulation of TBA and formaldehyde, as described in Materials and Methods. The cells generated 0, 62, 134, 186, and 220 nmol of TBA after 0, 30, 60, 90, and 120 min, respectively. Much lower amounts of formaldehyde (0, 14.5, 4.9, 3.5, and 0 nmol, respectively) were detected at the same time points. We also examined the consumption of formaldehyde by these cells in the absence of MTBE. The cells consumed >98% of the added formaldehyde (1 mM) in 2 h. In contrast, ≥99% of the added formaldehyde was recovered after 2 h in reactions conducted with either the same amount of boiled cells (95°C for 10 min) or no cells.
Kinetics of MTBE degradation.
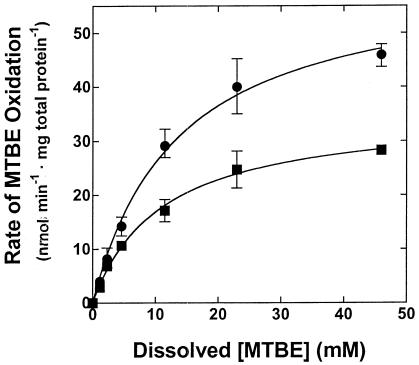
The apparent lack of TBA consumption during MTBE oxidation by n-pentane-grown cells indicated that the kinetics of MTBE oxidation could be investigated directly by determining the rates of accumulation of TBA. Values for Ks and Vmax for MTBE oxidation were determined for cells grown on each of the n-alkane growth substrates identified in Table 1. The cells grown on each of the n-alkanes had similar Ks values (12.95 mM; standard deviation [SD], 2.04; r2 ≥ 0.973) and, with the exception of n-pentane-grown cells (Vmax = 35.3 nmol min−1 mg of total protein−1; SD, 2.28), similar Vmax values for MTBE oxidation (average Vmax = 61.1 nmol min−1 mg of total protein−1; SD, 2. 66) (Fig. 3).
FIG. 3.
Kinetics of MTBE oxidation by P. mendocina KR-1. The figure shows a plot of the specific rate of TBA production from MTBE versus that from a dissolved MTBE concentration for cells of P. mendocina KR-1 grown on C5 to C8 n-alkanes. The reactions were conducted as described in Materials and Methods. The figure shows the average rate of TBA production for cells grown on n-pentane (two different cultures) (▪) and for cells grown on n-hexane, n-heptane, and n-octane (one culture for each n-alkane) (•). The error bars show the range of values around the mean. The plotted curve represents the computer fit to the single-substrate-binding model described in Materials and Methods.
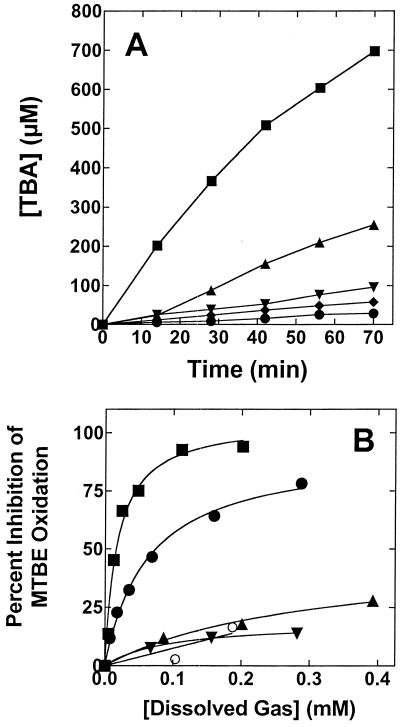
The similar Ks values for MTBE oxidation suggested that the same MTBE-oxidizing enzyme was expressed by this organism during growth on all of the n-alkanes tested. Further support for this model was obtained from the results of an experiment that examined possible inhibitory effects of n-alkanes on MTBE oxidation. All of the n-alkane growth substrates identified in this study (Table 1) were potent inhibitors of MTBE oxidation by n-pentane-grown cells (Fig. 4A). Surprisingly, both n-butane and propane also strongly inhibited MTBE oxidation, whereas ethane and methane were no more inhibitory than nitrogen (Fig. 4B). The levels of inhibition caused by various concentrations of propane and n-butane were fitted to a single substrate-binding model, as described in Materials and Methods. Good fits (r2 ≥ 0.98) were obtained in both cases. Apparent Ki values (Kiapp) of 63 and 18 μM were obtained from this analysis for propane and n-butane, respectively.
FIG. 4.
Effects of n-alkanes on MTBE oxidation by n-pentane-grown cells. The figure shows the effects of growth-supporting (A) and non-growth-supporting n-alkanes (B) on the time course of TBA production from MTBE by n-pentane-grown cells. (A) n-Pentane-grown cells (total protein, 0.47 mg) were incubated with MTBE (42 μmol) in a reaction vial (10-ml) as described in Materials and Methods. The figure shows the time course of TBA production from MTBE for cells incubated with MTBE alone (▪) or with MTBE and each of the following n-alkanes (30 μmol): n-pentane (♦), n-hexane (•), n-heptane (▾), and n-octane (▴). (B) The figure shows a plot of the effects of gaseous n-alkanes on TBA production from MTBE by n-pentane-grown cells. Cells (total protein, 1.8 mg) were incubated with MTBE (2.8 μmol) in reaction vials (10 ml) with various concentrations of gaseous n-alkanes as described in Materials and Methods. After being incubated for 25 min, the reaction medium was analyzed by GC to quantify the production of TBA. The figure shows the effects of increasing dissolved concentrations of nitrogen (○), methane (▾), ethane (▴), propane (•), and n-butane (▪) on TBA production, relative to a control reaction conducted without gaseous n-alkanes. The plotted curve represents the computer fit to the single-substrate-binding model described in Materials and Methods.
Oxidation of gaseous alkanes.
One possible explanation for the inhibitory effect of propane and n-butane on MTBE oxidation by n-pentane-grown cells is that these gases act as substrates for the alkane-oxidizing enzyme system and thereby inhibit MTBE oxidation through competitive interactions at the active site of the enzyme. This possibility also suggested to us that the non-growth-supporting gaseous n-alkanes might also be consumed by cells grown on C5 to C8 n-alkanes. This possibility was examined by following the time course of substrate consumption during the growth of cells on either n-pentane alone or on n-pentane plus either methane, ethane, propane, n-butane, or MTBE. The time course of n-pentane consumption was essentially indistinguishable for each of the cultures (Fig. 5). Approximately 48% of the added MTBE was consumed during the incubation period, and the time course of MTBE consumption closely followed that of n-pentane consumption, albeit at a slower rate. Notably, consumption of MTBE ceased after the n-pentane had been fully consumed. In the case of the gaseous n-alkanes, no detectable consumption (≤3%) of either methane or ethane occurred throughout the entire incubation. In contrast, ∼30% of the added propane and ∼70% of the added n-butane were consumed during the incubation period. As with MTBE, the consumption of both gaseous n-alkanes occurred concurrently with n-pentane consumption and there was no evidence of continued consumption of these compounds after the n-pentane had been fully consumed. Control experiments without cells showed minimal losses (≤3%) of each of the substrates used in this experiment over a 5-day period (data not shown). A gas chromatographic analysis of the reaction media after completion of the experiment described in Fig. 5 did not reveal the accumulation of likely initial products of propane or n-butane oxidation, such as 1° alcohols. This finding suggested that the products of propane and n-butane oxidation were most likely consumed by n-pentane-grown cells. In a separate experiment, we examined this possibility by incubating n-pentane-grown cells with each 1° alcohol (C2 to C4) (5 mM) in small-scale reactions (1 ml). The rate of consumption of each alcohol was determined by GC over 45 min with four alcohol concentration determinations obtained for each reaction. In each case, the rate of alcohol consumption was close to constant (r2 ≥ 0.93) and the specific rates for ethanol, 1-propanol, 1-butanol, and 1-pentanol consumption were estimated as 21, 79, 155, and 74 nmol min−1 total mg of protein−1, respectively.
Effects of cosubstrates on cell growth.
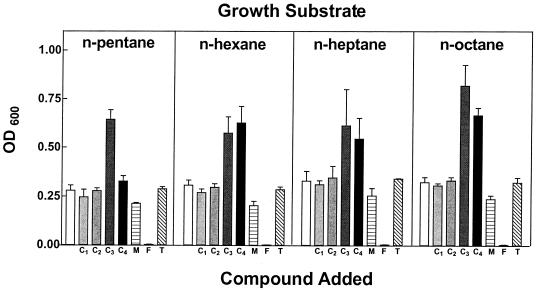
The consumption of both MTBE and the selected gaseous n-alkanes shown in Fig. 4 led us to examine the effects of higher concentrations of these compounds during the growth of cells on liquid n-alkanes (C5 to C8). The effects of these gaseous n-alkanes (450 μmol/reaction) were compared with the effects of equivalent amounts of MTBE, TBA, and formaldehyde. Several consistent trends were observed in the results of this experiment (Fig. 6). First, the presence of MTBE had a slight (∼15%) but consistent inhibitory effect on growth. The amount of TBA generated in these incubations averaged 60 μmol (2.4 mM in solution) for each alkane growth substrate. Second, the presence of TBA (18 mM in solution) had no detected effect on cell growth, while an equivalent concentration of formaldehyde completely inhibited cell growth. Third, there was no discernible effect of either methane or ethane on culture growth. Fourth, the presence of either propane or n-butane consistently led to an increase in culture growth, compared to that for cultures grown with C5 to C8 n-alkanes alone. The lowest level of growth stimulation (∼18%) by gaseous n-alkanes was observed with cells grown on n-pentane in the presence of n-butane. The highest level of stimulation was observed with cells grown on n-octane in the presence of propane (∼156%). The average level of growth stimulation for all C5 to C8 n-alkanes combined was ∼113% in the presence of propane and ∼74% in the presence of n-butane. To determine whether the differences in growth (measured at OD600) were directly related to changes in biomass production, we examined the relationship between culture density (OD600) and protein content. The combined data were fitted to a straight line by linear regression with a final r2 value of 0.98 (data not shown).
FIG. 6.
Effects of cosubstrates on growth yield of cells grown on liquid n-alkanes. The figure shows the effects of gaseous n-alkanes, MTBE, and its metabolites on the final-culture density of P. mendocina KR-1 cells grown under carbon-limited conditions on C5 to C8 n-alkanes. A series of cultures of P. mendocina KR-1 were prepared in glass serum vials (125 ml) as described in Materials and Methods. The cultures contained either an n-alkane growth substrate alone (0.02%, vol/vol) (white bars) or (in sequence from left to right) each n-alkane growth substrate (0.02%, vol/vol) with either methane (C1), ethane (C2), propane (C3), n-butane (C4), MTBE (M), formaldehyde (F), or TBA (T) added individually (450 μmol of each compound). The cultures were grown for 5 days, and each culture condition was replicated at a minimum of three times. The figure shows the average final optical density (OD600) for all of the cultures. The error bars show the range of values of the final optical densities.
DISCUSSION
The results of this study have characterized two different but related features of the cometabolic activities of n-alkane-grown cells of P. mendocina KR-1. The main focus has been on characterizing the degradation of MTBE. A secondary focus has been on the effects of non-growth-supporting n-alkanes on both the MTBE-oxidizing activity and the n-alkane-dependent growth of this organism. The similarities and differences between these two classes of substrates and their impact on our understanding of MTBE degradation and cometabolism are discussed in the following sections.
Cometabolism of MTBE.
Our results have provided strong evidence supporting the conclusion that MTBE oxidation by alkane-grown cells of P. mendocina KR-1 is a cometabolic process that involves the activity of an inducible n-alkane-oxidizing monooxygenase enzyme system. Our evidence supporting this conclusion is that the organism was unable to grow on MTBE as the sole source of carbon and energy under the conditions tested (Table 1). However, MTBE was rapidly oxidized to a monooxygenase-compatible product, TBA, by cells after growth on n-pentane (Fig. 5) and the other n-alkanes (Table 2) but not after growth on the corresponding 1° alcohol products of these n-alkanes (Table 2). While these conclusions are similar to those made in several previous studies of MTBE oxidation by n-alkane-oxidizing bacteria (6, 23, 26), our present results offer several new insights into the diversity of MTBE-oxidizing systems. First, this study extends the range of n-alkanes known to support cometabolic MTBE-oxidizing activity to include n-alkanes up to n-octane. This finding is significant, as most MTBE enters the environment as part of a gasoline and C5 to C8 n-alkanes are important components of these hydrocarbon mixtures. The presence of high concentrations of n-alkanes in gasoline raises the possibility that cometabolic degradation of MTBE might occur in gasoline-impacted environments if appropriate environmental conditions were met. In subsequent studies with P. mendocina KR-1, we have also observed MTBE-oxidizing activity after growth of cells on n-nonane and n-decane but not on n-undecane or n-dodecane (C. A. Smith and M. R. Hyman, unpublished results), an observation that extends this range of substrates even further.
A second notable feature of the MTBE-oxidizing activity of P. mendocina KR-1 is that, unlike that for M. vaccae JOB5 (23), this process does not appear to involve the generation of TBF. Our present hypothesis is that TBF production by M. vaccae JOB5 is due to the activity of an alcohol dehydrogenase that can oxidize the transient hemiacetal intermediate that is presumed to be generated by all monooxygenase enzymes acting on the methoxy group of MTBE. If this is correct, the lack of TBF production observed in this study most likely reflects differences in the catalytic capabilities of the alcohol dehydrogenases in P. mendocina KR-1 and TBF-generating organisms such as M. vaccae JOB5 (23) rather than differences in the mechanism of MTBE oxidation by the alkane monooxygenases in these organisms.
The third and most novel feature of MTBE oxidation by P. mendocina KR-1 is the apparent inability of this organism to further oxidize low concentrations of TBA (Fig. 2). In all cases so far reported, n-alkane-oxidizing bacteria have been shown to oxidize both MTBE and TBA, typically through the activity of the same monooxygenase enzyme. However, TBA often accumulates to various degrees during MTBE oxidation, and this accumulation can generally be attributed to the kinetic features (Ks or Vmax) of TBA as a monooxygenase substrate that are less favorable than those of MTBE. Note that the Ks value we have determined for MTBE for n-alkane-grown cells in this study (12.95 mM) is unusually high and exceeds most values reported for other MTBE-cometabolizing bacteria (6, 17, 23) by at least an order of magnitude. If the same kinetic trends for MTBE and TBA seen with other organisms also apply to P. mendocina KR-1, it may be that n-alkane-grown cells of P. mendocina KR-1 can oxidize TBA. However, this reaction may be significant or detectable only at unusually high TBA concentrations.
Cometabolism of non-growth-supporting n-alkanes.
We have provided several lines of evidence that, in addition to oxidizing MTBE, cells of P. mendocina grown on C5 to C8 n-alkanes cometabolically oxidize at least two non-growth-supporting gaseous n-alkanes, propane and n-butane. For example, propane and n-butane were consumed during growth of cells on n-pentane (Fig. 5) and both compounds were also shown to be potent inhibitors of MTBE oxidation by n-pentane-grown cells (Fig. 4B). Both of these observations are compatible with these compounds acting as substrates for an alkane-induced alkane-oxidizing enzyme system. We also demonstrated that higher concentrations of propane and n-butane led to consistent and substantial increases in biomass production during growth on all of the truen-alkane growth substrates (C5 to C8). The simplest interpretation of this result is that the metabolites generated from propane and n-butane oxidation are further metabolized productively by alkane-grown cells. In support of this model, we have confirmed that n-pentane-grown cells can oxidize C2 to C5 1° alcohols and have shown that the 1° alcohol growth substrate range of this organism (C2 to C8) is broader than the corresponding n-alkane substrate range (Table 1). Taking these points together, we conclude that the increased biomass observed during cell growth in the presence of propane and n-butane (Fig. 6) is most likely due to further metabolism of the products of propane and n-butane oxidation. Based on our growth studies (Table 1), these products are expected to be 1o rather than 2o alcohols.
If propane and n-butane are fully metabolized during growth on other n-alkanes, it is interesting to consider why neither compound appears to support the growth of this organism when supplied as the sole source of carbon and energy (Table 1). One possibility is that these gaseous n-alkanes do not act as effective inducers of the enzymes required for alkane metabolism. The only evidence we have in support of this model is that the Vmax for MTBE oxidation in n-pentane-grown cells was consistently lower than that determined for cells grown on other n-alkanes (Fig. 3). If the level of induction is accurately reflected in the Vmax values, the approximately twofold-lower Vmax values we determined for MTBE oxidation by n-pentane-grown cells compared to those for cells grown on higher n-alkanes might suggest that the level of induction by the next-smaller compound in this homologous series, n-butane, would be less than that seen with n-pentane. Notably, a very strong effect of carbon chain length has been observed for the inductive effects of non-growth-supporting n-alkanes (C6 to C11) on a chromosomally located alkM::lacZ fusion in Acinetobacter ADP1, a strain that grows on C12 to C18 n-alkanes (22).
Another possibility is that these gaseous alkanes may induce alkane-oxidizing activity, but as discussed earlier for TBA, there may be kinetic constraint on the oxidation of these compounds that does not allow them to be oxidized at sufficient rates to support growth. For example, despite being present at a 25%-lower initial concentration (n-butane, ∼10 μM; propane, ∼14 μM), n-butane was more rapidly consumed than propane during growth of cells on n-pentane (Fig. 5). In contrast, neither ethane (initial dissolved concentration, ∼18 μM) nor methane (initial dissolved concentration, ∼14 μM) was consumed at all during this reaction. Our analysis of the inhibitory effects of gaseous n-alkanes as inhibitors of MTBE oxidation (Fig. 4B) also confirmed this trend and demonstrated that n-butane was a substantially more effective inhibitor than propane. In contrast, ethane and methane were shown to be no more effective than nitrogen as inhibitors.
The apparent Ki (Kiapp) values we determined for propane and n-butane as inhibitors of MTBE oxidation require modification to account for the presence of MTBE in the reactions (initial dissolved concentration, 2.3 mM) and the Ks for MTBE (12.95 mM; see above), as shown by the following equation:
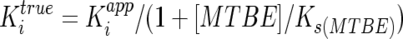 |
Note that if these compounds inhibit MTBE oxidation by acting as competitive alternative substrates, the true Ki values obtained from this equation for n-butane (Ki = 16 μM) and propane (Ki = 53 μM) are equivalent to the Ks values for these compounds as substrates (3). Although we have not determined the Ks value for the growth-supporting n-alkanes identified in this study, a progressive increase in Ks values through the homologous series of n-alkanes (C5 to C2) could account for the results we have obtained for gaseous n-alkanes (C1 to C4) in this study. A final interesting observation made in this study was that neither propane nor n-butane continued to be oxidized once n-pentane had been fully consumed (Fig. 5). This observation suggests that there may be other unforeseen physiological or enzymatic constraints that prevent this organism from using these gaseous n-alkanes as sole sources of carbon and energy for growth.
Significance of cometabolism.
According to a widely used definition of cometabolism (27), the oxidation of MTBE, propane, and n-butane we have characterized in this study are all cometabolic processes, as none of these substrates support the growth of the organism when supplied as sole carbon and energy sources. However, as discussed above, there is clear evidence that the oxidation of two gaseous n-alkanes leads to an increase in cell biomass, a feature not addressed by most models of cometabolism. The growth-stimulating effect of propane and n-butane cometabolism is an unusual observation but one that shows considerable similarities to the growth-stimulating effect recently reported for cometabolic chloromethane oxidation by methanotrophic bacteria (8). In this case, chloromethane is oxidatively dehalogenated by a constitutive methane monooxygenase activity to yield formaldehyde. Formaldehyde is then thought to be assimilated through the existing pathways required for growth on methane.
In contrast to the cometabolism of gaseous n-alkanes, there appear to be only minor effects of either TBA or MTBE on cell growth. Although there was no detectable effect of MTBE on growth of cells on n-alkanes with low amounts of MTBE (42 μmol) (Table 2), a slight but consistent decrease in cell yield was observed when cells were grown with n-alkanes and ∼10-fold-higher amounts of MTBE (Fig. 6). This finding might reflect the cumulative effect of sustained reductant depletion caused by concurrent oxidation of MTBE by a monooxygenase enzyme system. Alternatively, it could reflect a toxic effect of formaldehyde. In this study, we have shown that formaldehyde is rapidly consumed at low concentrations (1 mM), whereas high initial concentrations (18 mM) completely inhibited n-alkane-dependent growth (Fig. 6). The possibility of toxic effects of formaldehyde generation during MTBE cometabolism has not been explored to any great extent, although some effects of formaldehyde have been investigated in MTBE-metabolizing organisms (12). Note that unlike P. mendocina KR-1, organisms like M. vaccae JOB5 that generate TBF during cometabolic MTBE oxidation are likely to be spared any potential formaldehyde-dependent inhibitory effects, as the hydrolysis of TBF yields TBA and formate, a much less reactive C1 compound. It should also be recognized that further oxidation of either of the C1 products of MTBE oxidation might also help offset the reductant demands associated with a monooxygenase-catalyzed oxidation of MTBE. Again, this issue has not received significant attention to date and warrants further investigation.
A final issue raised by this study is the nature of the enzyme responsible for MTBE and n-alkane oxidation in P. mendocina KR-1. Many pseudomonads have n-alkane growth substrate ranges similar to those described in this study. For example, the well-characterized alkane hydroxylase system in P. putida GPo1 is expressed by this organism after growth on C6 to C12 n-alkanes. While n-octane-grown cells of this organism have been reported not to oxidize MTBE (26), this alkane hydroxylase is known to O-dealkylate a variety of methoxylated alkane derivatives (15). Note also that a P. aeruginosa strain that is believed to express alkane hydroxylase activity after growth on C5 to C8 n-alkanes has also been reported to oxidize n-butane, even though this compound does not support cell growth (29). Butane-oxidizing activity has also recently been reported for an alkane hydroxylase system in a Nocardioides strain grown on a range of n-alkane substrates similar to that examined in the present study (9). However, unlike the present study, neither of the studies referred to above has shown that gaseous n-alkane oxidation can promote the growth of the organism. Our ongoing research is directed at further characterizing the enzyme responsible for alkane and MTBE oxidation in P. mendocina KR-1 and investigating the similarity between this enzyme and alkane hydroxylases from diverse organisms.
Acknowledgments
This work was supported by funding to M.R.H. from the American Petroleum Institute.
The opinions expressed are those of the authors and not necessarily those of the funding agency.
REFERENCES
- 1.Bradley, P. M., F. H. Chapelle, and J. E. Landmeyer. 2001. Methyl t-butyl ether mineralization in surface-water sediment microcosms under denitrifying conditions. Appl. Environ. Microbiol. 67:1975-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corcho, D., R. J. Watkinson, and D. N. Lerner. 2000. Cometabolic degradation of MTBE by a cyclohexane-oxidizing bacteria, p. 183-189. In G. B. Wickramanayake, A. R. Gavaskar, B. C. Alleman, and V. S. Magar (ed.), Bioremediation and phytoremediation of chlorinated and recalcitrant compounds. Battelle Press, Columbus, Ohio.
- 3.Cornish-Bowden, A. 1979. Fundamentals of enzyme kinetics. Butterworths, London, United Kingdom.
- 4.Finneran, K. T., and D. R. Lovley. 2001. Anaerobic degradation of methyl-tert-butyl ether (MTBE) and tert-butyl ether (TBA). Environ. Sci. Technol. 35:1785-1790. [DOI] [PubMed] [Google Scholar]
- 5.François, A., H. Mathis, D. Godeefroy, P. Pivateau, F. Fayolle, and F. Monot. 2002. Biodegradation of methyl tert-butyl ether and other fuel oxygenates by a new strain, Mycobacterium austroafricanum IFP 2102. Appl. Environ. Microbiol. 68:2754-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garnier, P., R. Auria, C. Auger, and S. Revah. 1999. Cometabolic biodegradation of methyl t-butyl ether by Pseudomonas aeruginosa grown on pentane. Appl. Microbiol. Biotechnol. 51:498-503. [DOI] [PubMed] [Google Scholar]
- 7.Gornall., A. G., C. J. Bardawill, and M. M. David. 1949. Determination of serum proteins by means of the Biuret reaction. J. Biol. Chem. 177:751-766. [PubMed] [Google Scholar]
- 8.Han, J.-I., and J. D. Semaru. 2000. Chloromethane stimulates growth of Methylomicrobium album BG8 on methanol. FEMS Microbiol. Lett. 187:77-81. [DOI] [PubMed] [Google Scholar]
- 9.Hanamura, N., C. M. Yeager, and D. J. Arp. 2001. Two distinct monoooxygenases for alkane oxidation in Nocardioides sp. strain CF8. Appl. Environ. Microbiol. 67:4992-4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson, J. R., C. E. Ackerman, and K. M. Scow. 1999. Biodegradation of methyl tert-butyl ether by a bacterial pure culture. Appl. Environ. Microbiol. 65:4788-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardison, L. K., S. S. Curry, L. M. Ciuffetti, and M. R. Hyman. 1997. Metabolism of diethyl ether and cometabolism of methyl tert-butyl ether by a filamentous fungus, a Graphium sp. Appl. Environ. Microbiol. 63:3059-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatzinger, P. B., K. McClay, S. Vainberg, M. Tugusheva, C. W. Condee, and R. J. Steffan. 2001. Biodegradation of methyl tert-butyl ether by a pure bacterial culture. Appl. Environ. Microbiol. 67:5601-5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyman, M., C. Smith, and K. O'Reilly. 2001. Cometabolism of MTBE by an aromatic hydrocarbon-oxidizing bacterium, p. 145-152. In V. S. Magar, J. T. Gibbs, K. T. O'Reilly, M. R. Hyman, and A. Leeson (ed.), Bioremediation of MTBE, alcohols, and ethers. Battelle Press, Columbus, Ohio.
- 14.Johnson, R., J. Pankow, D. Bender, C. Price, and J. Zigorski. 2000. MTBE: to what extent will past releases contaminate community water supply wells? Environ. Sci. Technol. 34:210A-217A. [DOI] [PubMed] [Google Scholar]
- 15.Katapodis, A. G., H. A. Smith, and S. W. May. 1988. New oxyfunctionalization capabilities for ω-hydroxylases: asymmetric aliphatic sulfoxidation and branched ether demethylation. J. Am. Chem. Soc. 110:897-899. [Google Scholar]
- 16.Koenigsberg, S., C. Sandefur, W. Mahaffey, M. Deshusses, and N. Fortin. 1999. Peroxygen mediated bioremediation of MTBE, p. 13-18. In B. C. Alleman and A. Leeson (ed.), Proceedings of the Fifth International in Situ and On-Site Bioremediation Symposium, vol. 3. Battelle Press, Columbus, Ohio.
- 17.Liu, C. Y., G. E. Speitel, Jr., and G. Georgiou. 2001. Kinetics of methyl t-butyl ether cometabolism at low concentrations by pure cultures of butane-degrading bacteria. Appl. Environ. Microbiol. 67:2197-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClay, K., S. Streger, and R. J. Steffan. 1995. Induction of toluene oxidation activity in Pseudomonas mendocina KR-1 and Pseudomonas sp. strain ENVPC5 by chlorinated solvents and alkanes. Appl. Environ. Microbiol. 61:3479-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller, M. E., and J. D. Stuart. 2000. Measurement of aqueous Henry's law constants for oxygenates and aromatics found in gasolines by static headspace method. Anal. Chem. 72:622-625. [DOI] [PubMed] [Google Scholar]
- 20.Mo, K., C. O. Lora, A. E. Wanken, M. Javarnmardian, X. Yang, and C. F. Kulpa. 1997. Biodegradation of methyl-t-butyl ether by pure bacterial cultures. Appl. Microbiol. Biotechnol. 47:69-72. [DOI] [PubMed] [Google Scholar]
- 21.Nash, T. 1953. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem. J. 55:416-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratajczak, A., W. Geiβdörfer, and W. Hillen. 1998. Expression of alkane hydroxylase from Acinetobacter sp. strain ADP1 is induced by a broad range of n-alkanes and requires the transcriptional activator AlkR. J. Bacteriol. 180:5822-5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith, C. A., K. T. O'Reilly, and M. R. Hyman. 2003. Characterization of the initial reactions during the cometabolic degradation of methyl tert-butyl ether (MTBE) by propane-grown Mycobacterium vaccae JOB5. Appl. Environ. Microbiol. 69:796-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somsamak, P., R. M. Cowan, and M. M. Haggblom. 2001. Anaerobic biotransformation of fuel oxygenates under sulfate-reducing conditions. FEMS Microbiol. Ecol. 37:259-264. [Google Scholar]
- 25.Squillace, P. J., J. S. Zogorski, W. G. Wilbur, and C. V. Price. 1996. Primary assessment of the occurrence and possible sources of MTBE in groundwater in the United States, 1993-1994. Environ. Sci. Technol. 30:1721-1730. [Google Scholar]
- 26.Steffan, R. J., K. McClay, S. Vainberg, C. W. Condee, and D. Zhang. 1997. Biodegradation of the gasoline oxygenates methyl tert-butyl ether, ethyl tert-butyl ether, and tert-amyl methyl ether by propane-oxidizing bacteria. Appl. Environ. Microbiol. 63:4216-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stirling, D. I., and H. Dalton. 1979. The fortuitous oxidation and cometabolism of various carbon compounds by whole-cell suspensions of Methylococcus capsulatus (Bath). FEMS Microbiol. Lett. 5:315-318. [Google Scholar]
- 28.U.S. Environmental Protection Agency. 1997. Drinking water advisory: consumer acceptability advice and health effects analysis on methyl tertiary-butyl ether (MtBE). U.S. Environmental Protection Agency publication no. (EPA) 822-F-97-008. Office of Water, U.S. Environmental Protection Agency, Washington, D.C.
- 29.van Eyk, J., and T. J. Bartles. 1968. Paraffin oxidation in Pseudomonas aeruginosa. 1. Induction of paraffin oxidation. J. Bacteriol. 96:707-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilhelm, E., R. Battino, and R. J. Wilcock. 1977. Low-pressure solubility of gases in liquid water. Chem. Rev. 77:219-262. [Google Scholar]
- 31.Wilson, J. T., J. S. Cho, B. H. Wilson, and J. A. Vardy. 2000. Natural attenuation of MTBE in the subsurface under methanogenic conditions. U.S. Environmental Protection Agency publication no. (EPA) 600-R-00-006. U.S. Environmental Protection Agency, Washington, D.C.
- 32.Winter, R. B., K.-M. Yen, and B. D. Ensley. 1989. Efficient degradation of trichloroethylene by a recombinant Escherichia coli. Bio/Technology 7:282-285. [Google Scholar]
- 33.Yeager, C. M., P. J. Bottomley, D. J. Arp, and M. R. Hyman. 1999. Inactivation of toluene 2-monooxygenase in Burkholderia cepacia G4 by alkynes. Appl. Environ. Microbiol. 65:632-639. [DOI] [PMC free article] [PubMed] [Google Scholar]