Abstract
Purpose of review
The metabolic syndrome has become a leading health concern in developed countries. In the search for strategies to combat this growing problem, stearoyl-CoA desaturase 1 (SCD1) inhibition has been proposed as an attractive therapeutic strategy. However, recent studies warn of potentially harmful consequences of SCD1 inhibition. The purpose of this review is to discuss recent insights into the potential for SCD1 inhibitors as viable metabolic syndrome therapeutics.
Recent findings
SCD1 converts saturated fatty acids (SFAs) to monounsaturated fatty acids (MUFAs). Although SCD1 inhibition protects against diet-induced obesity, hepatic steatosis, and insulin resistance, recent studies have demonstrated that the accumulation of SCD1 substrates (SFA) can promote inflammation, atherosclerosis, steatohepatitis, and pancreatic beta cell dysfunction in preclinical rodent models. This suggests SCD1 may play a critical role in suppressing inflammatory diseases by shuttling proinflammatory SFAs into less biologically active MUFA-enriched neutral lipids. Given this, SCD1 inhibitors given in conjunction with anti-inflammatory agents may provide a useful strategy to prevent the metabolic syndrome without deleterious side-effects seen with SCD1 inhibition alone.
Summary
SCD1 inhibitors continue to hold promise as metabolic syndrome therapeutics; yet consideration must be taken to avoid the proinflammatory side-effects secondary to accumulation SCD1 substrates (SFAs).
Keywords: inflammation, metabolic syndrome, saturated fatty acids, stearoyl-CoA desaturase
Introduction
Developed countries throughout the world are now facing increased prevalence of the metabolic syndrome [1], which represents a collection of symptoms including abdominal obesity, insulin resistance, hypertriglyceridemia, hypertension, and low high-density lipoprotein cholesterol (HDL-C) levels [1–3]. Although each of these individual symptoms likely arises from distinct origins, there has been a concerted effort to identify drug targets that may comprehensively ameloriate all symptoms of the metabolic syndrome simultaneously. SCD1 inhibitors have shown promise to do just this, given that genetic deletion or pharmacologic inhibition of SCD1 improves most of the aspects of the metabolic syndrome in preclinical rodent models [4–6]. Simply by catalyzing the conversion of saturated fatty acid (SFA) to monounsaturated fatty acid (MUFA), SCD1 plays a gatekeeper role in partitioning endogenous and dietary fatty acids into metabolically active or inactive pools. As a result, inhibition of SCD1 results in striking protection against most aspects of the metabolic syndrome, yet promotes some inflammatory diseases. Given the fact that SCD1 inhibition has been reported to have both beneficial and detrimental effects, the purpose of this review is to discuss how SCD1 integrates aspects of the metabolic syndrome and inflammation, and to critically discuss the potential for SCD1 inhibition to become a viable and well tolerated therapeutic strategy in humans.
SCD1 and metabolic disease: the good news for SCD1 inhibitors
Since being originally identified in the late 1980s [7], SCD1 has been the focus of over 300 studies examining its critical role in lipid metabolism. Mice harboring a natural mutation in the SCD1 gene have impaired biosynthesis of hepatic triglycerides and cholesteryl esters, initially linking SCD1 function to both hepatic steatosis and hyperlipidemia [8]. Several subsequent studies have confirmed that SCD1 promotes both steatosis [9–12, 13••,14••] and hypertriglyceridemia [13••,14••,15,16]. Additionally, mice with targeted deletion of SCD1 are protected against both diet-induced and leptin deficiency-induced obesity [17,18]. In fact, down-regulation of SCD1 expression may be a critical component of leptin’s potent antiobesity and antisteatotic actions [18]. SCD1 deficiency also improves glucose tolerance [17–21], which may be explained by enhanced insulin sensitivity in the liver [19], adipose tissue [22], and skeletal muscle [23]. Proof of concept that SCD1 inhibitors may be useful to combat the metabolic syndrome have come from recent studies utilizing antisense oligonucleotide (ASO)-mediated inhibition of SCD1 [13••,14••,19,24]. Collectively, these studies demonstrated that targeted inhibition of SCD1 is extremely effective in preventing diet-induced obesity, hepatic steatosis, and insulin resistance [13••,14••,19,24].
Although the protective effects of SCD1 inhibition on obesity, hepatic steatosis, and insulin resistance are quite striking, effects on plasma lipid levels in rodents have not been as remarkable. Indeed, SCD1 activity is positively correlated with plasma triglyceride levels in mice and in humans [15], yet SCD1 inhibition or deletion does not always result in lower plasma triglyceride [12,21,25]. The most striking plasma triglyceride lowering effects of SCD1 deficiency are seen in chow-fed asebia mice (the naturally occurring SCD1 mutant) [8,15], and in hyperlipidemic mice lacking the low-density lipoprotein receptor (LDLr−/−) [13••,14••,20]. However, chow-fed mice with targeted deletion of SCD1 only have a modest reduction in plasma triglyceride when maintained on a SV129 background [10,11, 15,16], and no difference in plasma triglyceride when maintained on a C57BL/6 [12,25] or BTBR [21] background. Hence, the background strain of experimental mice and diet must be taken into consideration when examining the effects of SCD1 inhibition of plasma triglyceride. SCD1 inhibition or deficiency also has inconsistent effects on plasma cholesterol levels in circulating low and high-density lipoproteins (LDL-C and HDL-C). As for HDL-C, chow-fed asebia mice have a substantial increase in HDL-C levels [8]; yet mice with targeted deficiency of SCD1 have either normal or diminished HDL-C levels [16,21,25]. We have likewise seen that ASO-mediated inhibition of SCD1 lowers HDL-C levels in LDLr−/−, apoB100/100 mice [13••,14••]. However, a recent study suggests that SCD1-deficient mice treated with the liver x receptor (LXR) agonist T0901317 have significantly more HDL-C than T0901317-treated wild-type controls [16]. For LDL-C, SCD1 inhibition either has no effect [13••, 14••,20] or slightly increases LDL-C levels [12,21,25, 26]. Collectively, these mixed results demonstrate that additional work is needed to determine whether SCD1 has a role in modulating the dyslipidemia associated with the metabolic syndrome.
Although the metabolic syndrome has been the target of most SCD1 inhibitor programs, recent evidence suggests that SCD1 inhibitors may also hold promise as anticancer agents [27–35,36••,37]. In support of this, SCD1 expression and activity is increased in several human cancers, chemically induced tumors, and transformed cell lines [27–32]. It is generally agreed that SCD1 expression in these hyperproliferative models is critical for driving the terminal steps of de-novo lipogenesis to provide for increased energy needs. Using an unbiased RNAi screen, SCD1 was identified as a potent regulator of human cancer cell survival [33]. Also, specific inhibition of SCD1 impairs cancer cell proliferation, in-vitro invasiveness, and tumor formation [34,35,36••,37]. In a series of elegant studies, work from the laboratory of Ariel Igal has demonstrated that SCD1 may be required for neoplastic cells to survive lipotoxic stress, given that SCD1 inhibition augments basal apoptosis and sensitizes cancer cells to the cytotoxic effects of SFA [34]. Recent mechanistic insight has shown that cancer cells require SCD1 to complete glucose-driven lipogenesis, and SCD1 inhibition results in the activation of AMP-activated protein kinase (AMPK), thereby inactivating acetyl-CoA carboxylase to functionally impair uncontrolled proliferation [35]. Collectively, these studies suggest that SCD1 inhibitors continue to hold promise as anticancer therapeutics.
SCD1 and inflammatory disease: the warning signs against SCD1 inhibition
Although SCD1 inhibition has profoundly positive effects on many aspects of the metabolic syndrome and cancer, several recent studies have uncovered some unexpected warning signs that suggest the accumulation of SCD1 substrates (SFA) may be problematic. One major concern originates from a striking skin phenotype seen in mice lacking SCD1 [37–39]. It has been well documented that gene-targeted SCD1-deficient mice [37] and asebia 2J mice [38] have an epidermal lipid barrier dysfunction that results in accelerated transepidermal water loss. In line with this, SCD1-deficient mice have impaired cold tolerance and maladapted thermoregulation [40,41]. Recently, it has been demonstrated that a portion of the metabolic abnormalities seen in SCD1-deficient mice may arise directly from alterations in skin barrier dysfunction [41,42••]. In an important study, Sampath and colleagues [42••] demonstrated that mice lacking SCD1 specifically in the skin have increased energy expenditure and are resistant to high-fat diet-induced obesity and glucose intolerance. These results illustrate an underappreciated cross-talk between skin SCD1 and peripheral tissue in maintenance of energy homeostasis.
In addition to side-effects of SCD1 inhibition seen in the skin, we have recently uncovered an unexpected problem in the artery wall [13••,14••]. The rationale for studying effects of SCD1 on atherosclerosis originated from the fact that hepatic cholesterol esterification driven by acyl-CoA : cholesterol acyltransferase 2 (ACAT2) is intimately involved in promoting the secretion of atherogenic MUFA-rich apoB-containing lipoproteins [43–45]. Since mice lacking SCD1 have severely impaired hepatic MUFA-rich cholesteryl ester biosynthesis [8], we hypothesized that SCD1 inhibition would diminish the cholesteryl ester-driven atherogenicity of LDL-C, and therefore protect against atherosclerosis. To test this idea we utilized ASO-mediated knockdown of SCD1 in a well characterized mouse model of hyperlipidemia and atherosclerosis. In agreement with previous studies [8–12,13••,14••,15–24], inhibition of SCD1 strongly protected against diet-induced obesity, hepatic steatosis, and insulin resistance. However, to our surprise, SCD1 inhibition dramatically augmented aortic atherosclerosis and macrophage inflammatory response [13••]. Similarly, mice with a genetic deletion of SCD1 also had increased systemic inflammation and atherosclerosis, despite being largely protected against symptoms of the metabolic syndrome [46•]. Collectively, three studies have warned strongly that inhibition SCD1 results in accelerated atherosclerosis in appropriate hyperlipidemic mouse models [13••,14••,46•]. Interestingly, there is one study that suggests SCD1 inhibition reduces atherosclerosis in cholesterol-fed C57BL/6J mice exposed to chronic intermittent hypoxia (CIH) [47]. However, it is important to consider that this model represents a condition when plasma cholesterol levels only reached an average of 228 mg/dl, giving rise to very modest atherosclerotic lesions in the mice [47]. Hence, it is difficult to know whether these results should be applied to the typical cholesterol-driven progression of atherosclerotic lesion formation, which is primarily associated with chronic elevation of LDL-C in rodents and humans.
In addition to problems with skin barrier dysfunction and atherosclerosis, recent evidence suggests that SCD1 inhibitors may also promote pancreatic β cell death. In fact, pancreatic β cells are exquisitely sensitive to SFA-induced lipotoxicity [47–53,54•]. There is now overwhelming evidence that SFAs compromise secretory function and promote apoptosis in multiple β cell models [47–53,54•]. In an important study, Busch and colleagues [53] identified subpopulations of β cells that were resistant to SFA-induced apoptosis, and found that these SFA-resistance subpopulations gained resistance by up-regulating SCD1. Furthermore, SCD1 inhibition rendered the previously SFA-resistant cells to once again become sensitive to SFA-induced apoptosis [53]. A recent mechanistic insight suggests SCD1 may shuttle proapoptotic SFAs into less biologically active MUFA-enriched neutral lipids, thereby protecting against β cell apoptosis [54•]. Although many cell-based studies have warned that the accumulation of SCD1 substrates (SFA) may promote pancreatic β cell apoptosis, most of these warnings have not been taken seriously since mice lacking SCD1 have overall improved insulin sensitivity and glucose tolerance [13••,14••,17–23]. However, it was recently demonstrated that the warnings seen in cultured β cell models occur in vivo. In this study by Flowers and colleagues [21], it was shown that mice lacking SCD1 in the diabetes prone BTBR leptinob/ob background had diminished glucose-stimulated insulin secretion and signs of SFA-induced lipotoxicity in β cells in vivo. Collectively, these studies demonstrate that SCD1 plays an essential role in maintaining normal pancreatic β cell function, warning against SCD1 inhibition in these critical cells.
As we move forward, it will also be important to consider other recent studies of side-effects of SCD1 inhibition seen in the liver and colon. Recently, Chen and colleagues [55] demonstrated that mice lacking SCD1 had accelerated dextran sulfate sodium (DSS) and bacterial colitis. However, a subsequent study questioned these results challenging that SCD1-deficient mice have increased fluid intake [56], which may have confounded the original study since DSS was administered via a water vehicle. Additional studies are needed to clarify whether SCD1 inhibitors affect inflammatory colitis. In the liver, it has long been thought that SCD1 inhibition would be beneficial since SCD1-deficient mice are protected against steatosis and hepatic insulin resistance [9–12, 13••,14••]. However, two recent studies demonstrate that SCD1 inhibition in the context of a very-low-fat (VLF) or methionine-choline-deficient (MCD) diet is not without consequences. When SCD1-deficient mice were fed a VLF, high-sucrose diet, a progressive phenotype of hypercholesterolemia and cholestasis was uncovered [25]. Interestingly, the hypercholesterolemia and cholestasis seen with SCD1 deficiency was corrected by supplementation with dietary unsaturated fat, but not saturated fat [25]. Results from this important study suggest that SCD1 may be conditionally essential for normal hepatocyte function when dietary unsaturated fat is limited. In another interesting study, SCD1-deficient mice were fed a MCD diet in order to study effects on steatohepatitis progression [57••]. Results from this study showed that SCD1-deficient mice fed a MCD diet had markedly increased hepatocellular apoptosis, and SCD1 inhibition sensitized hepatocytes to SFA-induced apoptotic cell death [57••]. Results from this study are strikingly similar to those discussed earlier in pancreatic β cells [47–53,54•].
Is there a unifying mechanism underlying the side-effects of SCD1 inhibition?
It is reasonable to assume that many of the side-effects seen with SCD1 inhibition may stem from the abnormal accumulation of SCD1 substrates (SFA) in multiple tissues. In fact, there is a large body of evidence that SFAs are potent proinflammatory molecules, linking these SCD1 substrates to the promotion of a number of inflammatory diseases including atherosclerosis [58], β cell dysfunction [47–53,54•], steatohepatitis [59,60], and colitis [61]. In fact, recent evidence suggests that SFAs can activate multiple toll-like receptors (TLRs), which play a key role in innate immunity [62–67]. Therefore, one of the key roles of SCD1 may be to suppress inflammation by preventing excessive accumulation of SFA-derived TLR4 ligands. Interestingly, long-chain ω-3 polyunsaturated fatty acids (ω-3 PUFA) have been shown to counteract SFA-induced TLR4 activation in cultured macrophage cell systems [63,65–67]. Therefore, we reasoned that dietary supplementation with fish oil-derived ω-3 PUFAs may prevent the SFA-driven TLR4 hypersensitivity and accelerated atherosclerosis previously seen with SCD1 inhibition in mice-fed SFA or MUFA-enriched diets [13••]. Indeed, the accelerated atherosclerosis seen with SCD1 inhibition can be completely prevented by moderate dietary fish oil supplementation [14••]. Importantly, the proinflammatory effects of SCD1 ASO treatment can be overcome by dietary ω-3 PUFA supplementation, and the dual therapy of SCD1 ASO and dietary ω-3 PUFA provides dramatic protection against atherogenesis [14••]. Therefore, this synergistic dual therapy of SCD1 inhibition in the presence of dietary fish oil may provide a novel therapeutic approach for the metabolic syndrome and atherosclerosis. Given this outcome, it is tempting to speculate that SCD1 inhibitors given in conjunction with other anti-inflammatory agents could provide useful strategies to prevent the metabolic syndrome, while avoiding deleterious side-effects stemming from the accumulation of SCD1 substrates (SFA). Additional work to formally test this possibility is required (Fig. 1).
Figure 1. Proposed mechanism underlying the side-effects seen with SCD1 inhibition.
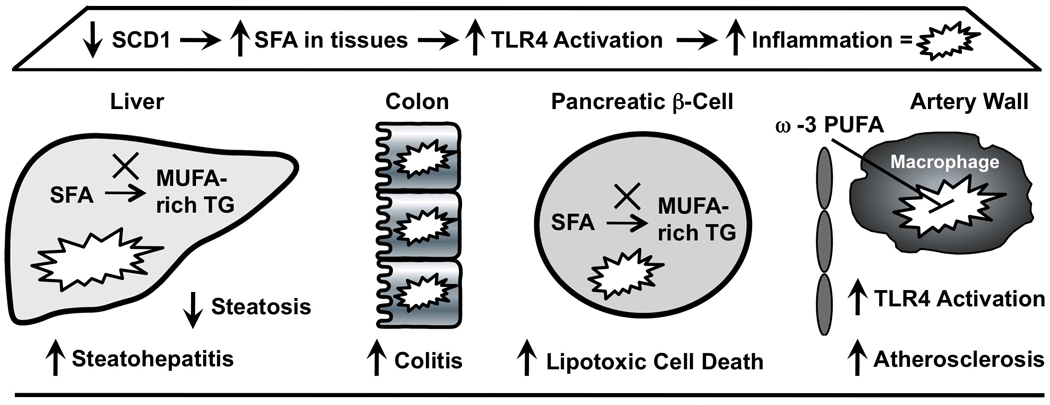
Under normal conditions, SCD1 catalyzes the conversion of saturated fatty acids (SFAs) to monounsaturated fatty acids (MUFAs), which are preferred substrates for esterification into cellular triglyceride (TG). It is reasonable to assume that many of the side-effects seen with SCD1 inhibition may stem from the abnormal accumulation of SCD1 substrates (SFA) in multiple tissues. SFAs are known to promote the activation of toll-like receptor 4 (TLR4) and drive inflammation. In the liver the accumulation of SFA promotes steatohepatitis in response to a methionine-choline-deficient diet. In the colon, the accumulation of SFA promotes DSS and bacterial-driven inflammatory colitis. In the pancreatic β-cell, the accumulation of SFA results in lipotoxicity and cell death. In the artery wall, the accumulation of SFA promotes TLR4 activation in macrophages and augments atherosclerosis. Importantly, dietary ω-3 polyunsaturated fatty acid (ω-3 PUFA) supplementation can prevent the accelerated atherosclerosis seen with SCD1 inhibition in part by dampening inflammatory signaling in macrophages.
Conclusion
The metabolic syndromehas become a major global health concern, and SCD1 inhibition shows promise as an attractive strategy to treat this complex syndrome. However, recent evidence suggests that the accumulation of SCD1 substrates (SFA), namely the saturated fatty acid products of endogenous lipogenesis, is not without side-effects. Recent evidence suggests SCD1 may play a critical role in suppressing multiple inflammatory diseases by shuttling proinflammatory SFAs into less biologically active MUFA-enriched neutral lipids. Hence, SCD1 inhibitors given in conjunction with anti-inflammatory agents such as fish oil may provide a useful strategy to prevent the metabolic syndrome while avoiding the deleterious side-effects stemming from the accumulation of SCD1 substrates (SFA). SCD1 inhibitors continue to hold promise as metabolic syndrome therapeutics, yet additional work is needed to ensure these compounds are well tolerated in the context of inflammatory disease.
Acknowledgements
The authors are supported by grants from the National Institutes of Health (NIH-1K99HL096166–01 to J.M.B. and NIH-P01-HL49373 to L.L.R), and the National Center for Complimentary and Alternative Medicine (NCCAM-P50AT002782 to L.L.R.).
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 266–267).
- 1.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 4.Cohen P, Ntambi JM, Friedman JM. Stearoyl-CoA desaturase-1 and the metabolic syndrome. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:271–280. doi: 10.2174/1568008033340117. [DOI] [PubMed] [Google Scholar]
- 5.Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase as a new drug target for obesity treatment. Obes Rev. 2005;6:169–174. doi: 10.1111/j.1467-789X.2005.00177.x. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki M, Ntambi JM. Role of stearoyl-coenzyme A desaturase in lipid metabolism. Prostaglandins Leukot Essent Fatty Acids. 2003;68:113–121. doi: 10.1016/s0952-3278(02)00261-2. [DOI] [PubMed] [Google Scholar]
- 7.Christy RJ, Yang VW, Ntambi JM, et al. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev. 1989;3:1323–1335. doi: 10.1101/gad.3.9.1323. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki M, Kim YC, Gray-Keller MP, et al. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem. 2000;275:30132–30138. doi: 10.1074/jbc.M005488200. [DOI] [PubMed] [Google Scholar]
- 9.Asilmaz E, Cohen P, Miyazaki M, et al. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest. 2004;113:414–424. doi: 10.1172/JCI19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyazaki M, Dobrzyn A, Man WC, et al. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and independent mechanisms. J Biol Chem. 2004;279:25164–25171. doi: 10.1074/jbc.M402781200. [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki M, Dobrzyn A, Sampath H, et al. Reduced adiposity and liver steatosis by stearoyl-CoA desaturase deficiency are independent of peroxisome proliferators-activated receptor-alpha. J Biol Chem. 2004;279:35017–35024. doi: 10.1074/jbc.M405327200. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki M, Flowers MT, Sampath H, et al. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007;6:484–496. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 13. Brown JM, Chung S, Sawyer JK, et al. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation. 2008;118:1467–1475. doi: 10.1161/CIRCULATIONAHA.108.793182. First study to demonstrate that inhibition of SCD1 with antisense oligonucleotides promotes severe atherosclerosis in mice fed saturated and monounsaturated-enriched diets.
- 14. Brown JM, Chung S, Sawyer JK, et al. Combined therapy of dietary fish oil and stearoyl-CoA desaturase 1 inhibition prevents the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. doi: 10.1161/ATVBAHA.109.198036. [Epub ahead of print] First study to demonstrate that dual therapy of SCD1 inhibition in the presence of dietary fish oil may provide a novel therapeutic approach for the metabolic syndrome without deleterious effects on atherosclerosis.
- 15.Attie AD, Krauss RM, Gray-Keller MP, et al. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyeridemia. J Lipid Res. 2002;43:1899–1907. doi: 10.1194/jlr.m200189-jlr200. [DOI] [PubMed] [Google Scholar]
- 16.Chu K, Miyazaki M, Man WC, Ntambi JM. Stearoyl-coenzyme A desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol Cell Biol. 2006;26:6786–6798. doi: 10.1128/MCB.00077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ntambi JM, Miyazaki M, Stoehr JP, et al. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen P, Miyazaki M, Socci ND, et al. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez-Juarez R, Pocai A, Mulas C, et al. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest. 2006;116:1686–1695. doi: 10.1172/JCI26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macdonald ML, Singaraja RR, Bissada N, et al. Absence of stearoyl-CoA desaturase-1 ameloriates features of the metabolic syndrome in LDLR-deficient mice. J Lipid Res. 2008;49:217–229. doi: 10.1194/jlr.M700478-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flowers JB, Rabaglia ME, Schueler KL, et al. Loss of stearoyl-CoA desaturase-1 improves insulin sensitivity in lean mice but worsens diabetes in leptin-deficient obese mice. Diabetes. 2007;56:1228–1239. doi: 10.2337/db06-1142. [DOI] [PubMed] [Google Scholar]
- 22.Rahman SM, Dobrzyn A, Lee SH, et al. Stearoyl-CoA desaturase 1 deficiency increases insulin signaling and glycogen accumulation in brown adipose tissue. Am J Physiol Endocrinol Metab. 2005;288:E381–E387. doi: 10.1152/ajpendo.00314.2004. [DOI] [PubMed] [Google Scholar]
- 23.Rahman SM, Dobrzyn A, Dobrzyn P, et al. Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphate 1B in muscle. Proc Natl Acad Sci USA. 2003;100:11110–11115. doi: 10.1073/pnas.1934571100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang G, Li Z, Liu F, et al. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. J Clin Invest. 2005;115:1030–1038. doi: 10.1172/JCI23962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flowers MT, Groen AK, Oler AT, et al. Cholestasis and hypercholesterolemia in SCD1-deficient mice fed a low-fat, high-carbohydrate diet. J Lipid Res. 2006;47:2668–2680. doi: 10.1194/jlr.M600203-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Wilcox D, Nguyen P, et al. Hepatic knockdown of stearoyl-CoA desaturase 1 via RNA interference in obese mice decreases lipid content and changes fatty acid composition. Front Biosci. 2007;12:3781–3794. doi: 10.2741/2352. [DOI] [PubMed] [Google Scholar]
- 27.Yahagi N, Shimano H, Hasegawa K, et al. Coordinate activation of lipogenic enzymes in hepatocellular carcinoma. Eur J Cancer. 2005;41:1316–1322. doi: 10.1016/j.ejca.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 28.Scaglia N, Caviglia JM, Igal RA. High stearoyl-CoA desaturase protein and activity levels in simian virus 40 transformed-human lung fibroblasts. Biochem Biophys Acta. 1687:141–151. doi: 10.1016/j.bbalip.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Ding SF, Habib NA, et al. Partial characterization of a cDNA for human stearoyl-CoA desaturase and changes in its mRNA expression in some normal and malignant tissues. Int J Cancer. 1994;57:348–352. doi: 10.1002/ijc.2910570310. [DOI] [PubMed] [Google Scholar]
- 30.Lu J, Pei H, Kaeck M, et al. Gene expression changes associated with chemically induced rat mammary carcinogenesis. Mol Carcinog. 1997;20:204–215. doi: 10.1002/(sici)1098-2744(199710)20:2<204::aid-mc7>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 31.Thai SF, Allen JW, DeAngelo AB, et al. Detection of early gene expression changes by differential display in the livers of mice exposed to dichloroacetic acid. Carcinogenesis. 2001;22:1317–1322. doi: 10.1093/carcin/22.8.1317. [DOI] [PubMed] [Google Scholar]
- 32.Kumar-Sinha C, Ignato KW, Lippman ME, et al. Transcriptome analysis of HER2 reveals a molecular connection to fatty acid synthesis. Cancer Res. 2003;63:132–139. [PubMed] [Google Scholar]
- 33.Morgan-Lappe SE, Tucker LA, Huang X, et al. Identification of Ras-related nuclear protein, targeting protein for xenopus kinesin-like protein 2, and stearoyl-CoA desaturase 1 as promising cancer targets from an RNAi-based screen. Cancer Res. 2007;67:4390–4398. doi: 10.1158/0008-5472.CAN-06-4132. [DOI] [PubMed] [Google Scholar]
- 34.Scaglia N, Igal RA. Stearoyl-CoA desaturase is involved in the control of proliferation, anchorage-independent growth, and survival in human transformed cells. J Biol Chem. 2005:25339–25349. doi: 10.1074/jbc.M501159200. [DOI] [PubMed] [Google Scholar]
- 35.Scaglia N, Igal RA. Inhibition of stearoyl-CoA desaturase 1 expression in human lung adenocarcinoma cells impairs tumorigenesis. Int J Oncol. 2008;33:839–850. [PubMed] [Google Scholar]
- 36. Scaglia N, Chisholm J, Igal RA. Inhibition of stearoyl-CoA desaturase-1 inactivates acetyl-CoA carboxylase and impairs proliferation in cancer cells: role of AMPK. PLoS One. 2009;4:e6812. doi: 10.1371/journal.pone.0006812. First study to demonstrate a requirement for SCD1 in regulating de-novo lipogenesis and the signaling pathways (AMPK/ACC) that co-ordinately regulate cancer cell hyperproliferation.
- 37.Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene inmice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001;131:2260–2268. doi: 10.1093/jn/131.9.2260. [DOI] [PubMed] [Google Scholar]
- 38.Sundberg JP, Boggess D, Sundberg BA, et al. Asebia-2J (Scd1(ab2J)): a new allele and a model for scarring alopecia. Am J Path. 2000;156:2067–2075. doi: 10.1016/S0002-9440(10)65078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng Y, Eilertsen KJ, Ge L, et al. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268–270. doi: 10.1038/15446. [DOI] [PubMed] [Google Scholar]
- 40.Lee SH, Dobrzyn A, Dobrzyn P, et al. Lack of stearoyl-CoA desaturase 1 upregulates basal thermogenesis but causes hypothermia in a cold environment. J Lipid Res. 2004;45:1674–1682. doi: 10.1194/jlr.M400039-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Binczek E, Jenke B, Holz B, et al. Obesity resistance of the stearoyl-CoA deficient (scd1 −/−) mouse results from disruption of the epidermal lipid barrier and adaptive thermoregulation. Biol Chem. 2007;388:405–418. doi: 10.1515/BC.2007.046. [DOI] [PubMed] [Google Scholar]
- 42. Sampath H, Flowers MT, Liu X, et al. Skin-specific deletion of stearoyl-CoA desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J Biol Chem. 2009;284:19961–19973. doi: 10.1074/jbc.M109.014225. First study to demonstrate that skin-specific deletion of SCD1 results in increased energy expenditure and resistance to high-fat diet-induced obesity and glucose intolerance, illustrating an underappreciated cross-talk between skin SCD1 and peripheral tissue in maintenance of energy homeostasis.
- 43.Rudel LL, Haines J, Sawyer JK, et al. Hepatic origin of cholesteryl oleate in coronary artery atherosclerosis in African green monkeys. Enrichment by dietary monounsaturated fat. J Clin Invest. 1997;100:74–83. doi: 10.1172/JCI119524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willner E, Tow B, Buhman KK, et al. Deficiency of acyl-CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci USA. 2003;100:1262–1267. doi: 10.1073/pnas.0336398100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee RG, Kelley KK, Sawyer JK, et al. Plasma cholesterol esters provided by lecithin:cholesterol acyltransferase and acyl-CoA:cholesterol acyltransferase have opposite atherosclerostic potential. Circ Res. 2004;95:998–1004. doi: 10.1161/01.RES.0000147558.15554.67. [DOI] [PubMed] [Google Scholar]
- 46. MacDonald ML, van Eck M, Hildebrand RB, et al. Despite antiatherogenic metabolic characteristics, SCD1-deficient mice have increased inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:341–347. doi: 10.1161/ATVBAHA.108.181099. Study shows that -eficient mice on the LDLr−/− background have increased inflammation and atherosclerosis.
- 47.Lupi R, Dotta F, Marselli L, et al. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on humans pancreatic islets: evidence that β-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl2 regulated. Diabetes. 2002;51:1437–1442. doi: 10.2337/diabetes.51.5.1437. [DOI] [PubMed] [Google Scholar]
- 48.Shimabukuro M, Zhou YT, Levi M, et al. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maedler K, Oberholzer J, Bucher P, et al. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic β-cell turnover and function. Diabetes. 2003;52:726–733. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- 50.Piro S, Anello M, Di Pietro C, et al. Chronic exposure to free fatty acids or high glucose induces apoptosis in rat pancreatic islets: possible role of oxidative stress. Metabolism. 2002;51:1340–1347. doi: 10.1053/meta.2002.35200. [DOI] [PubMed] [Google Scholar]
- 51.Maestre I, Jordan J, Calvo S, et al. Mitochondrial dysfunction is involved in apoptosis induced by serum withdrawal and fatty acids in the beta-cell line INS-1. Endocrinology. 2003;144:335–345. doi: 10.1210/en.2001-211282. [DOI] [PubMed] [Google Scholar]
- 52.El-Assaad W, Buteau J, Peyot ML, et al. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144:4154–4163. doi: 10.1210/en.2003-0410. [DOI] [PubMed] [Google Scholar]
- 53.Busch AK, Gurisik E, Cordery DV, et al. Increased fatty acid desaturation and enhanced expression of stearoyl coenzyme A desaturase protects pancreatic beta cells from lipoapoptosis. Diabetes. 2005;54:2917–2924. doi: 10.2337/diabetes.54.10.2917. [DOI] [PubMed] [Google Scholar]
- 54. Hellemans KH, Hanaert JC, Denys B, et al. Susceptibility of pancreatic beta cells to fatty acids is regulated by LXR/PPARalpha-dependent stearoyl-coenzyme A desaturase. PLoS One. 2009;4:e7226. doi: 10.1371/journal.pone.0007266. Study suggests the presence of an LXR/PPARalpha-dependent transcriptional mechanism of up-regulating SCD1, leading to the shuttling of proapoptotic SFAs into less biologically active MUFA-enriched neutral lipids, thereby protecting against β-cell apoptosis.
- 55.Chen C, Shah YM, Morimura K, et al. Metabolomics reveals that hepatic stearoyl-CoA desaturase 1 downregulation exacerbates inflammation and acute colitis. Cell Metab. 2008;7:135–147. doi: 10.1016/j.cmet.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacDonald ML, Bissada N, Vallance BA, et al. Stearoyl-CoA desaturase-1 does not promote DSS-induced acute colitis. Biochem Biophys Acta. 2009;1791:1166–1172. doi: 10.1016/j.bbalip.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li ZZ, Berk M, McIntyre TM, et al. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease. J Biol Chem. 2009;284:5637–5644. doi: 10.1074/jbc.M807616200. First study to show that despite being protected against hepatic triglyceride accumulation, SCD1-deficient mice are more susceptible to methionine-choline-deficient diet-induced steatohepatitis.
- 58.Temel RE, Rudel LL. Diet effects on atherosclerosis in mice. Curr Drug Targets. 2007;8:1150–1160. doi: 10.2174/138945007782403847. [DOI] [PubMed] [Google Scholar]
- 59.Gentile CL, Pagliassotti MJ. The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J Nutr Biochem. 2008;19:567–576. doi: 10.1016/j.jnutbio.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pickens MK, Yan JS, Ng RK, et al. Dietary sucrose is essential to the development of liver injury in the MCD model of steatohepatitis. J Lipid Res. 2009 doi: 10.1194/jlr.M900022-JLR200. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Esteve M, Navarro E, Klaassen J, et al. Plasma and mucosal fatty acid pattern in colectomized ulcerative colitis patients. Dig Dis Sci. 1998;43:1071–1078. doi: 10.1023/a:1018895121350. [DOI] [PubMed] [Google Scholar]
- 62.Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 64.Suganami T, Tanimoto-Koyama K, Nishida J, et al. Role of the Toll-like receptor4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 65.Lee JY, Zhao L, Youn HS, et al. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 66.Lee JY, Ye J, Gao Z, et al. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 67.Lee JY, Plakidas A, Lee WH, et al. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]


