Abstract
Purpose of review
Dietary saturated fatty acids (SFAs) have been implicated in promoting the metabolic syndrome and atherosclerotic cardiovascular disease. Recent evidence suggests that SFAs promote the metabolic syndrome by activating Toll-like receptor 4 (TLR4). Here we examine emerging molecular evidence that SFAs directly engage pathways of innate immunity, thereby promoting inflammatory aspects of the metabolic syndrome.
Recent findings
Accumulation of SFA in the body is tightly regulated by stearoyl-CoA desaturase 1, an enzyme that converts endogenous SFA to monounsaturated fatty acids. Recent studies have demonstrated that the accumulation of SFA seen with genetic deletion or inhibition stearoyl-CoA desaturase 1 promotes inflammation, TLR4 hypersensitivity, and accelerated atherosclerosis. Therefore, stearoyl-CoA desaturase 1 may play an unexpected role in suppressing inflammation by preventing excessive accumulation of endogenous SFA-derived TLR4 agonists. In parallel, several independent laboratories have demonstrated that TLR4 is necessary for dietary SFAs to induce obesity, insulin resistance, and vascular inflammation in rodent models.
Summary
The metabolic syndrome and atherosclerotic cardiovascular disease have long been linked to dietary SFA intake and inflammation. Recent mechanistic insights into how SFAs and downstream metabolites can potentiate inflammation-driven metabolic disease are discussed here.
Keywords: atherosclerosis, insulin resistance, obesity, polyunsaturated fatty acids, saturated fatty acids, Toll-like receptor 4
Introduction
It has been estimated that nearly a quarter of American adults have the metabolic syndrome [1]. This syndrome is a collection of metabolic abnormalities, including abdominal obesity, insulin resistance, hypertriglyceridemia, hypertension, and low high-density lipoprotein cholesterol (HDLc) levels [1–3]. The metabolic syndrome is thought to also be a predictor of atherosclerotic cardiovascular disease (ASCVD) risk in humans [1–3]. The question still remains whether all aspects of the metabolic syndrome originate from a single underlying mechanism, or whether these diverse comorbidities arise from distinct origins [4,5]. Quite strikingly, all aspects of the metabolic syndrome have been closely associated with chronic inflammation [6–10], potentially implicating the innate immune system as the root cause for the syndrome as a whole. Just as inflammation has been strongly linked to metabolic syndrome comorbidities, dietary fat intake has long been proposed as another causative factor [11–13]. In this regard, not necessarily the quantity, but the quality of dietary fat consumed strongly predicts the prevalence of metabolic syndrome and atherosclerosis [11–13]. Diets enriched in saturated fatty acids (SFA) have been associated with increased risk for obesity [11], insulin resistance [12], and atherosclerosis [13], whereas diets containing long chain ω-3 polyunsaturated fatty acids (PUFA) from fish oils have been shown to protect against these same metabolic diseases [11–14]. Accordingly, the purpose of this review is to discuss recent insights regarding the direct engagement of Toll-like receptors (TLRs) by dietary fatty acids and how this impacts metabolic disease.
Role of innate immune system in metabolic syndrome and atherosclerosis
The immune system can be broadly characterized into two closely related pathways known as adaptive and innate immunity. The adaptive immune system generates responses to ‘nonself’ antigenic epitopes and promotes immunological memory. In contrast, the innate immune response is the first line of defense against invading pathogens, wherein highly conserved pathogen-associated molecular patterns (PAMPs) are recognized by cognate pattern recognition receptors (PRRs). Activation of the innate immune system controls macronutrient metabolism [15] and promotes the metabolic syndrome and atherosclerosis [7–10]. Furthermore, fatty acids themselves may ‘highjack’ the innate immune system to elicit some of their biological effects [16–22]. Even though there is a large body of evidence linking inflammation to obesity, insulin resistance, and atherosclerosis, mechanistic understanding of how the innate immune system affects these lipid-driven metabolic diseases remains elusive. Probably the best understood connection between inflammation and metabolic disease comes from decades of work linking innate immunity to atherosclerosis [23]. It has long been known that blood monocyte-derived macrophages represent a major cellular component of the atherosclerotic plaque. Furthermore, activation of macrophage PRRs accelerates ASCVD through several distinct mechanisms, including promotion of monocyte adhesion to the vascular endothelium [24], smooth muscle cell proliferation [25], cholesterol accumulation [26], cytokine/chemokine burden [24], and initiating macrophage apoptosis [27].
Like ASCVD, activation of the innate immune system can also promote insulin resistance and obesity. It has been well over 100 years since Williamson and Lond [28] first reported that high-dose sodium salicylate treatment improved glycosuria and diabetes, a result that has been substantiated by many others [29–31]. Interestingly, salicylate’s ability to protect against insulin resistance depends entirely on its ability to inhibit inflammation driven by an inhibitor of nuclear factor kappa B kinase beta (IKKβ), and to prevent lipid-induced decreases in insulin-stimulated insulin receptor substrate 1 (IRS-1) tyrosine phosphorylation and IRS-1-associated PI-3-kinase activation [30,31]. In fact, many studies have supported the idea that cytokine signaling directly promotes insulin resistance [7,8]. A much more recent concept is that the innate immune system may be causally linked to obesity [7,8,32,33]. In 2003, two landmark papers challenged the predominant ‘adipocyte-centric’ view of obesity when they demonstrated that adipose tissue contains a substantial population of macrophages, and macrophage-driven adipose inflammation contributes significantly to the pathogenesis of obesity [32,33]. Collectively, activation of the innate immune system is strongly associated with ASCVD, insulin resistance, and obesity, and recent evidence suggests that much of this association can be traced to a unique family of PRRs known as TLRs (Fig. 1).
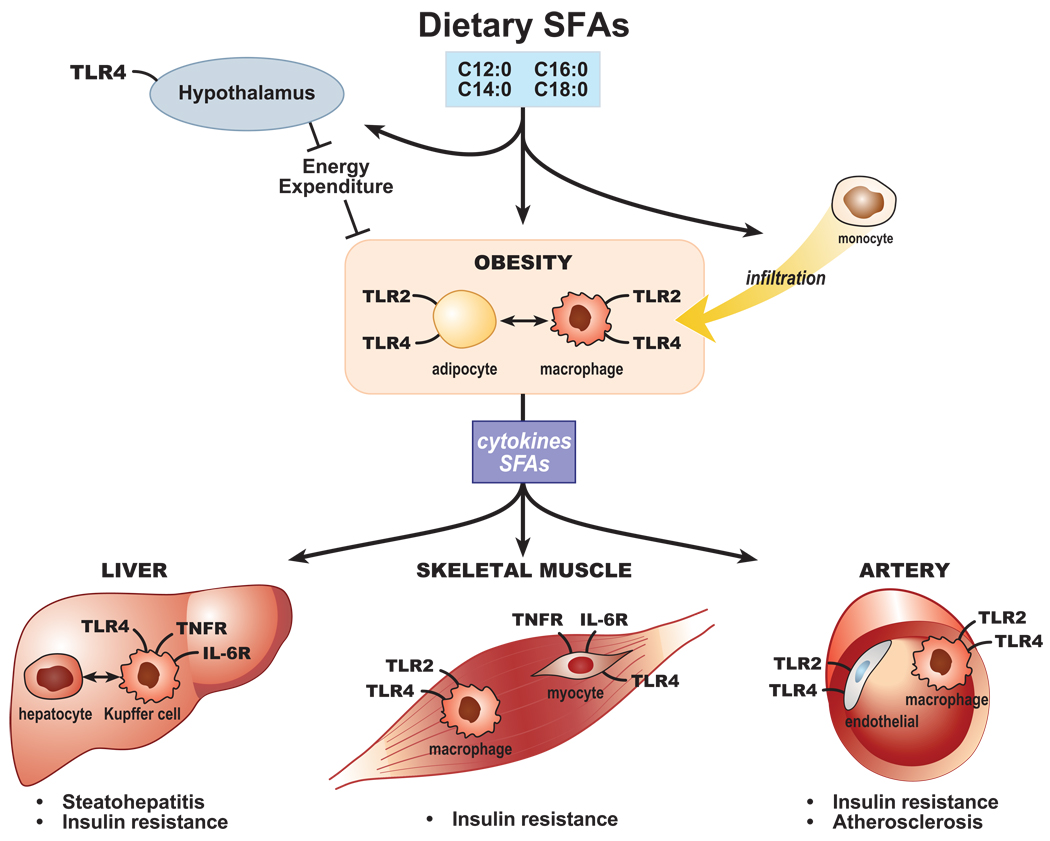
Figure 1. Scheme of saturated fatty acid activity upon Toll-like receptor 2 and Toll-like receptor 4 as a crossroads for metabolic diseases and inflammation.
Saturated fatty acids (SFAs) derived from the diet and/or adipose tissue lipolysis enter the circulation and influence several tissues through activity upon resident cells expressing Toll-like receptor (TLR) 2 and TLR4. TLR pathway activation via the tumor necrosis factor receptor (TNFR) induces proinflammatory cytokines [e.g., tumor necrosis factor (TNF), and IL-6, chemokines, and adhesion molecules, which may promote inappropriate macrophage recruitment to tissues, including the arterial subintima and adipose tissue. Intercellular communications also modify the obesity phenotype itself in part through activity upon the hypothalamus and lead to steatohepatitis, atherosclerosis, and insulin resistance.
Toll-like receptor family: master regulators of inflammatory disease
TLRs are a family of type I transmembrane receptors, currently thought to comprise at least 13 members in mammals, that specifically recognize a variety of microbial PAMPs and trigger host cellular responses. They have been the subject of several recent comprehensive reviews [34–36]. TLRs are generally composed of three domains: an extracellular domain of leucine-rich repeat motifs thought to be involved in ligand binding; a transmembrane domain that may determine receptor localization to the plasma membrane (TLR1, TLR2, TLR4, TLR5, TLR6) or to intracellular membranes (TLR3, TLR7, TLR8, TLR9); and an intracellular tail containing a conserved Toll/interleukin-1 receptor (TIR) domain common to the IL-1 and IL-18 receptors. Upon ligand binding, TLR dimerization produces a TIR–TIR complex that in turn recruits intracellular TIR-containing adaptor proteins that transduce the signal into the cytosol. Of five described TLR adaptors, three are considered as bridging or regulatory adaptors, whereas myeloid differentiation primary response protein 88 (MyD88, adaptor for all TLRs except TLR3) and TIR domain-containing adaptor inducing interferon-β (TRIF, adaptor for TLR3 and TLR4) are signaling adaptors that lead to activation of mitogen-activated protein kinases and transcription factors [e.g., nuclear factor (NF)-κB], and induction of cytokines.
TLR activation by infecting pathogens bearing TLR-specific molecular motifs [e.g., bacterial lipopeptides (BLPs) for TLR2, lipopolysaccharide (LPS) for TLR4] induces tissue inflammation and antimicrobial responses in the host. To a degree of oversimplification, TLRs can be grouped into two ligand categories, lipid (TLR1, TLR2, TLR4, TLR6) and nucleic acid (TLR3, TLR7, TLR8, TLR9). TLR2 and TLR4, which respond to bacterial cell wall lipids, are the best characterized TLRs, having been shown to play critical roles in several infectious and inflammatory disorders. Presumably reflecting structural similarity between microbial and host membrane lipids, TLR2 and TLR4 have been reported also to recognize select host lipids and to play important roles in the pathogenesis of noninfectious, inflammatory diseases of host lipid dysregulation like ASCVD, insulin resistance, and obesity.
In-vitro evidence that fatty acids activate (saturated fatty acids) or suppress (ω-3 polyunsaturated fatty acids) Toll-like receptor signaling
Interest in SFAs as potential TLR2 and TLR4 stimuli emerged several years ago [17] from the realization that the activity of microbial stimuli upon both TLR2 (i.e., BLP) and TLR4 [i.e., lipopolysaccharide (LPS)] is borne by their saturated fatty acyl moieties. Indeed, deacylated BLPs are inactive upon TLR2 [37], whereas a library of synthetic lipopeptides with SFA acyl substitutions stimulate TLR2 [38]. Lipid A, the endotoxic moiety of the LPS molecule, is hexaacylated in Salmonella and Escherichia coli with the SFAs lauric, myristic, and/or palmitic acid. Both deacylated lipid A and lipid A variants acylated with unsaturated fatty acids lose activity upon interaction with TLR4, and in fact act as antagonists [39–41], whereas a library of synthetic lipid A mimetics hexaacylated with SFAs elicit TLR4-dependent signals [42].
Free SFAs have indeed been demonstrated to elicit TLR4-dependent and TLR2-dependent responses in several cell types. Using transfected RAW 264.7 macrophages, Lee et al. [17,20] reported that multiple SFAs, but most prominently lauric acid (C12 : 0), signal via TLR4-MyD88 to activate NF-κB, and via TLR4-TRIF, to activate interferon-stimulated regulatory element; by contrast, several ω-3 and ω-6 PUFAs attenuate C12 : 0 and LPS responses through effects at the level of TLR4 [17,21,22]. The ω-3 PUFA docosahexanoic acid (DHA) also more globally inhibits cellular responses to TLR2, TLR3, TLR5, and TLR9 agonists [20,22]. Like LPS, C12 : 0-elicited responses in transfected 293T cells require the TLR4 accessory molecule MD2 and are potentiated by CD14 [22]. Moreover, C12 : 0 also signals via TLR2 in cooperation with TLR1 or TLR6, but not via TLR3, TLR5, or TLR9. Additional cell types in which SFAs have been shown to elicit TLR4-dependent signals include dendritic cells [19], primary and human acute monocytic leukemia cell line (THP-1) macrophages [16,43], adipocytes [16], endothelial and smooth muscle cells [44,45], and osteoclasts [46••]. TLR2-dependent responses are reported in adipocytes [47] and myotubes [48]. Endogenous SFAs released from adipocytes activate cocultured macrophages via TLR4 [18], indicating the potential for cellular crosstalk in adipose tissue. Collectively, there is a growing body of evidence that SFAs promote, whereas long chain PUFA antagonize, TLR4-dependent and TLR2-dependent signaling in multiple cell models [16–22,42–45,46••,47,48].
In-vivo evidence that Toll-like receptor 4 is necessary for saturated fatty acids to induce metabolic syndrome and atherosclerotic cardiovascular disease
Although SFAs can clearly modulate TLR signaling in cell models [16–22,42–45,46••,47,48], the specific involvement of TLRs in the pathogenesis of obesity, insulin resistance, and ASCVD has only very recently come to light. In an elegant study, Shi et al. [16] demonstrated that SFAs activate TLR4-dependent signaling in both macrophages and adipocytes, and mice lacking TLR4 are protected against insulin resistance driven by intravenous lipid infusion [16]. In addition to effects in macrophages and adipocytes, SFAs can activate TLR4 in the hypothalamus, which triggers a central inflammatory response that results in resistance to anorexigenic signals [49••]. Several additional studies [50–52] have shown that mice lacking TLR4 are protected against high-fat diet-induced obesity and insulin resistance, clearly linking TLR4 to the progression of the metabolic syndrome.
In addition to TLR4’s role in promoting obesity and insulin resistance, TLR signaling plays an important role in ASCVD progression (Fig. 1). It has been clearly demonstrated that TLR4 is abundantly expressed in macrophages contained within atherosclerotic plaques [53], and that mice lacking either TLR4 itself or its downstream adapter protein MyD88 are protected against atherosclerosis [54,55]. In agreement, humans with mutations in TLR4 are less susceptible to atherosclerosis [56,57]. This is likely explained, in part, by TLR4’s role in vascular endothelial cell activation [45], cytokine/chemokine generation [56,57,45], outward vascular remodeling [58], and macrophage apoptosis [59]. Interestingly, bone-marrow transplantation studies have shown that macrophage TLR4 plays a very minor role in SFA-driven atherosclerosis [60•]. In addition to TLR4, TLR2 has been implicated in promoting ASCVD [61], yet whether SFA-driven ASCVD depends on TLR2 has not been examined.
The stearoyl-CoA desaturase 1 story: accumulation of endogenous saturated fatty acids accelerates atherosclerosis and inflammatory colitis
Although there is clear evidence that dietary SFAs promote the metabolic syndrome and ASCVD [11–13], it is still debated whether endogenous SFAs produced from de novo synthesis can act in a similar fashion. SFA levels in the body are tightly regulated by stearoyl-CoA desaturase 1 (SCD1), an enzyme that converts endogenous SFA to monounsaturated fatty acids (MUFAs). Mice lacking SCD1 accumulate large amounts of SFAs in multiple tissues, yet are protected against diet-induced obesity, hepatic steatosis, and insulin resistance [62]. Based on data generated in SCD1 null mice, SCD1 inhibition has been proposed as an attractive strategy for preventing the metabolic syndrome [62]. However, these early studies on SCD1 null mice failed to examine the consequences of endogenous SFA accumulation on innate immunity.
In early 2008, we learned the first lesson that endogenous SFAs can indeed promote innate immunity and inflammatory disease [63••]. In this study, it was shown that mice lacking SCD1 had enhanced dextran sulfate sodium (DSS)-induced and bacterial-induced inflammatory gene expression and exaggerated colitis. The authors suggested that SCD1 may serve a protective function against proinflammatory signaling. In support of this concept, our group recently demonstrated that antisense oligonucleotide (ASO)-mediated inhibition of SCD1 resulted in SFA enrichment of macrophage membranes, TLR4 hypersensitivity, and striking enhancement of atherosclerosis [64••]. Likewise, mice genetically lacking SCD1 also have increased inflammation and accelerated atherosclerosis [65•]. Interestingly, we now know that moderate dietary supplementation with ω-3 PUFA from fish oil can completely prevent the accelerated atherosclerosis seen with SCD1 ASO treatment (unpublished observations; J.M.B. and L.L.R.). This finding strongly supports the work of Hwang and coworkers [19–22] demonstrating that ω-3 PUFAs can effectively counteract SFA-induced TLR4 activation in cultured macrophages and dendritic cells. Mechanistic understanding of how this reciprocal relationship occurs will likely lead to novel therapeutic strategies for the metabolic syndrome and ASCVD.
Possible mechanisms by which saturated fatty acids and ω-3 polyunsaturated fatty acids may regulate Toll-like receptor 2 and Toll-like receptor 4 signaling
The molecular mechanisms by which fatty acids impact TLR2-dependent and TLR4-dependent signaling remain incompletely resolved. Given that the acyl chains of lipid A directly bind the crystal structure of the MD2-TLR4 complex [66••], the potential exists for free SFAs to act as direct ligands to MD2-TLR4. This said, it is unclear how likely it would be for free SFAs presented on a carrier protein to recapitulate the precise presentation of acyl chains in lipid A, and one group was unable to detect direct binding of stearic acid (C18 : 0) to MD2-TLR4 [67•]. Alternatively, it is possible that SFAs may be converted to TLR4-active metabolites. One group has proposed that palmitate (C16 : 0) may require conversion to ceramide for activity, as palmitate induction of cytokines is attenuated by inhibiting either conversion to palmitoyl-CoA or de novo ceramide synthesis, and is mimicked by cell treatment with ceramide [43]. It is notable in this regard that ceramide has structural similarity to lipid A [68], itself elicits TLR4-dependent cellular responses [69], and, though controversial, may act as an intermediate in TLR4-dependent responses elicited by LPS and other stimuli [68,70,71]. Incorporation of SFAs into lysophosphatidylcholine (LPC) also yields proinflammatory LPC species [63••], though it is not clear that their activity involves TLRs. On the other hand, PUFAs may be metabolized into anti-inflammatory resolvins [72], or attenuate LPS-induced responses indirectly through activating peroxisome proliferator-activated receptors [73].
SFAs and PUFAs may also possibly impact TLR signaling through effects upon the lipid and protein composition of the raft membrane microdomains to which TLR4 is thought to be translocated upon activation [74]. SFAs pack well into membrane rafts; indeed, the majority of raft proteins are thought to be palmitoylated and/or myristoylated [75], and many, including TRIF-related adaptor molecule (TRAM), a coadaptor for TRIF in the TLR4 pathway, require acylation for proper membrane localization and function [76,77]. By contrast, PUFAs appear to disrupt raft order and lipid composition in the plasma membrane [78,79], displacing signaling proteins from rafts [80,81] and reducing TLR4 cell surface expression [82]. Given our incomplete mechanistic understanding, it has become quite clear that this area is a fertile ground for future research.
Conclusion
The metabolic syndrome has become a major global health concern, and dietary SFA intake plays a significant role in the development of this complex syndrome. Recent evidence suggests that both dietary and endogenous SFA can promote the metabolic syndrome and ASCVD by activating TLRs. Although the connection between SFAs and TLR activation has been strongly supported, the molecular details of how free fatty acids activate TLR-dependent signaling are still largely unknown. Further mechanistic understanding of how SFAs promote and long chain PUFAs prevent TLR activation will likely lead to novel therapeutic strategies for the metabolic syndrome and ASCVD.
Acknowledgements
The present work was supported by grants from the National Institutes of Health (NIH-1K99HL096166-01 to J.M.B. and NIH-P01-HL49373 to L.L.R), the National Center for Complimentary and Alternative Medicine (NCCAM-P50AT002782 to L.L.R.), and by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (M.B.F.). The authors thank Rosanne Crooke, Mark Graham, and Richard Lee (ISIS Pharmaceuticals, Inc. Carlsbad, CA USA) for providing the SCD1 antisense oligonucleotides described here. We also thank Lois Wyrick for assistance with figure production.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 4.Kahn R. Metabolic syndrome: is it a syndrome? Does it matter? Circulation. 2007;115:1806–1810. doi: 10.1161/CIRCULATIONAHA.106.658336. [DOI] [PubMed] [Google Scholar]
- 5.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 6.Devaraj S, Singh U, Jialal I. Human C-reactive protein and the metabolic syndrome. Curr Opin Lipidol. 2009;20:182–189. doi: 10.1097/MOL.0b013e32832ac03e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Odegard JI, Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab. 2008;4:619–626. doi: 10.1038/ncpendmet0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21:1443–1455. doi: 10.1101/gad.1550907. [DOI] [PubMed] [Google Scholar]
- 9.Tobias P, Curtiss LK. Thematic review series: the immune system and atherogenesis. Paying the price for pathogen protection: toll receptors in atherogenesis. J Lipid Res. 2005;46:404–411. doi: 10.1194/jlr.R400015-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Harrison DG, Guzik TJ, Goronzy J, Weyand C. Is hypertension an immunological disease? Curr Cardiol Rep. 2008;10:464–469. doi: 10.1007/s11886-008-0073-6. [DOI] [PubMed] [Google Scholar]
- 11.Moussavi N, Gavino V, Receveur O. Could the quality of dietary fat, and not just its quantity, be related to risk of obesity? Obesity. 2008;16:7–15. doi: 10.1038/oby.2007.14. [DOI] [PubMed] [Google Scholar]
- 12.Rivellese AA, De Natale C, Lilli S. Type of dietary fat and insulin resistance. Ann N Y Acad Sci. 2002;967:329–335. doi: 10.1111/j.1749-6632.2002.tb04288.x. [DOI] [PubMed] [Google Scholar]
- 13.Brousseau ME, Schaefer EJ. Diet and coronary heart disease: clinical trials. Curr Atheroscler Rep. 2000;2:487–493. doi: 10.1007/s11883-000-0048-6. [DOI] [PubMed] [Google Scholar]
- 14.Fedor D, Kelley DS. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2009;12:138–146. doi: 10.1097/MCO.0b013e3283218299. [DOI] [PubMed] [Google Scholar]
- 15.Khovidhunkit W, Kim MS, Memon RA, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 18.Suganami T, Tanimoto-Koyama K, Nishida J, et al. Role of the Toll-like receptor4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 19.Weatherhill AR, Lee JY, Zhao L, et al. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol. 2005;174:5390–5397. doi: 10.4049/jimmunol.174.9.5390. [DOI] [PubMed] [Google Scholar]
- 20.Lee JY, Zhao L, Youn HS, et al. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 21.Lee JY, Ye J, Gao Z, et al. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 22.Lee JY, Plakidas A, Lee WH, et al. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 24.Gerszten RE, Garcia-Zepeda EA, Lim YC, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 25.Weinstein R, Stemerman MB, Maciag T. Hormonal requirements for growth of arterial smooth muscle cells in vitro: and endocrine approach to atherosclerosis. Science. 1981;212:818–820. doi: 10.1126/science.7013068. [DOI] [PubMed] [Google Scholar]
- 26.Linton MF, Atkinson JB, Fazio S. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science. 1995;267:1034–1037. doi: 10.1126/science.7863332. [DOI] [PubMed] [Google Scholar]
- 27.Feng B, Yao PM, Li Y, et al. The endoplasmic reticulum is the site of cholestero-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 28.Williamson RT, Lond MD. On the treatment of glycosuria and diabetes mellitus with sodium salicylate. Br Med J. 1901;1:760–762. doi: 10.1136/bmj.1.2100.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reid J, Macdougall AI, Andrews MM. Aspirin and diabetes mellitus. Br Med J. 1957;2:1071–1074. doi: 10.1136/bmj.2.5053.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin M-J, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IkB kinase-β. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 31.Kim JK, Kim YJ, Fillmore JJ, et al. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brikos C, O’Neill LA. Signalling of Toll-like receptors. Handb Exp Pharmacol. 2008;183:21–50. doi: 10.1007/978-3-540-72167-3_2. [DOI] [PubMed] [Google Scholar]
- 35.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 36.O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev. 2008;226:10–18. doi: 10.1111/j.1600-065X.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- 37.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 38.Muller SD, Muller MR, Huber M, et al. Triacyl-lipopentapeptide adjuvants: TLR2-dependent activation of macrophages and modulation of receptor-mediated cell activation by altering acyl-moieties. Int Immunopharmacol. 2004;4:1287–1300. doi: 10.1016/j.intimp.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Krauss JH, Seydel U, Weckesser J, Mayer H. Structural analysis of the nontoxic lipid A of Rhodobacter capsulatus 37b4. Eur J Biochem. 1989;180:519–526. doi: 10.1111/j.1432-1033.1989.tb14677.x. [DOI] [PubMed] [Google Scholar]
- 40.Munford RS, Hall CL. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science. 1986;234:203–205. doi: 10.1126/science.3529396. [DOI] [PubMed] [Google Scholar]
- 41.Qureshi N, Takayama K, Kurtz R. Diphosphoryl lipid A obtained from the nontoxic lipopolysaccharide of Rhodopseudomonas sphaeroides is an endotoxin antagonist in mice. Infect Immun. 1991;59:441–444. doi: 10.1128/iai.59.1.441-444.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stover AG, Da Silva Correia J, Evans JT, et al. Structure-activity relationship of synthetic Toll-like receptor 4 agonists. J Biol Chem. 2004;279:4440–4449. doi: 10.1074/jbc.M310760200. [DOI] [PubMed] [Google Scholar]
- 43.Haversen L, Danielsson KN, Fogelstrand L, Wiklund O. Induction of proinflammatory cytokines by long-chain saturated fatty acids in human macrophages. Atherosclerosis. 2009;202:382–393. doi: 10.1016/j.atherosclerosis.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 44.Ishikado A, Nishio Y, Yamane K, et al. Soy phosphatidylcholine inhibited TLR4-mediated MCP-1 expression in vascular cells. Atherosclerosis. 2009 doi: 10.1016/j.atherosclerosis.2009.01.010. [Epub ahead of print]. doi: 10.1016. [DOI] [PubMed] [Google Scholar]
- 45.Kim F, Pham M, Luttrell I, et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 46. Oh SR, Sul OJ, Kim YY, et al. Saturated fatty acids enhance osteoclast survival. J Lipid Res. 2009 doi: 10.1194/jlr.M800626. [Epub ahead of print]. doi: 10.1194. Demonstration that SFA accumulation seen with obesity can regulate bone density through a TLR4-dependent enhancement of osteoclastogenesis.
- 47.Murakami K, Bujo H, Unoki H, Saito Y. High fat intake induces a population of adipocytes to co-express TLR2 and TNFalpha in mice with insulin resistance. Biochem Biophys Res Commun. 2007;354:727–734. doi: 10.1016/j.bbrc.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 48.Senn JJ. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem. 2006;281:26865–26875. doi: 10.1074/jbc.M513304200. [DOI] [PubMed] [Google Scholar]
- 49. Milanski M, Degasperi G, Coope A, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. First demonstration that SFAs can regulate obesity by acting centrally in the hypothalamus, where they initiate a TLR4-dependent inflammatory response that results in resistance to anorexigenic signals.
- 50.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 51.Poggi M, Bastelica D, Gual P, et al. C3H/HeJ mice carrying a Toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia. 2007;50:1267–1276. doi: 10.1007/s00125-007-0654-8. [DOI] [PubMed] [Google Scholar]
- 52.Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fats. Obesity. 2008;16:1248–1255. doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- 53.Xu XH, Shah PK, Faure E, et al. Toll-like receptor 4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 54.Michelsen KS, Wong MH, Shah PK, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bjorkbacka H, Kunjathoor VV, Moore KJ, et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 56.Arbour NC, Lorenz E, Schutte BC, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 57.Kiechl S, Lorenz E, Reindl M, et al. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 58.Hollestelle SC, De Vries MR, Van Keulen JK, et al. Toll-like receptor 4 is involved in outward arterial remodeling. Circulation. 2004;109:393–398. doi: 10.1161/01.CIR.0000109140.51366.72. [DOI] [PubMed] [Google Scholar]
- 59.Seimon TA, Obstfeld A, Moore KJ, et al. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc Natl Acad Sci U S A. 2006;103:19794–19799. doi: 10.1073/pnas.0609671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Coenen KR, Gruen ML, Lee-Young RS, et al. Impact of macrophage Toll-like receptor 4 deficiency on macrophage infiltration into adipose tissue and the artery wall in mice. Diabetologia. 2009;52:318–328. doi: 10.1007/s00125-008-1221-7. Bone-marrow transplantation studies demonstrate that macrophage expression of TLR4 is not critical for high-fat diet-induced recruitment of macrophages into adipose tissue or the artery wall.
- 61.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flowers MT, Ntambi JM. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr Opin Lipidol. 2008;19:248–256. doi: 10.1097/MOL.0b013e3282f9b54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen C, Shah YM, Morimura K, et al. Metabolomics reveals that hepatic stearoyl-CoA desaturase 1 downregulation exacerbates inflammation and acute colitis. Cell Metab. 2008;7:135–147. doi: 10.1016/j.cmet.2007.12.003. First study to demonstrate that SCD1-knockout mice have increased inflammation in response to a bacterial challenge and greatly exacerbated inflammatory colitis.
- 64. Brown JM, Chung S, Sawyer JK, et al. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation. 2008;118:1467–1475. doi: 10.1161/CIRCULATIONAHA.108.793182. First study to show that pharmacologic inhibition of SCD1 function in hyperlipidemic mice results in TLR4 hypersensitivity in macrophages and markedly accelerated atherosclerosis.
- 65. Macdonald ML, van Eck M, Hildebrand RB, et al. Despite antiatherogenic metabolic characteristics, SCD1-deficient mice have increased inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:341–347. doi: 10.1161/ATVBAHA.108.181099. Study demonstrates that hyperlipidemic mice genetically lacking SCD1 have increased systemic inflammation and accelerated atherosclerosis.
- 66. Park BS, Song DH, Kim HM, et al. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. Crystal structure of the TLR4-MD-2-LPS complex was solved. Five of the six acyl chains of LPS are buried deep inside the pocket and the remaining chain is exposed to the surface of myeloid differentiation factor 2 (MD-2), forming a hydrophobic interaction with the conserved phenylalanines of TLR4.
- 67. Schaeffler A, Gross P, Buettner R, et al. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology. 2009;126:233–245. doi: 10.1111/j.1365-2567.2008.02892.x. Study shows that free SFAs do not bind directly to TLR-4/MD-2, likely ruling out the possibility that nonesterified SFAs can directly act as TLR4 ligands.
- 68.Joseph CK, Wright SD, Bornmann WG, et al. Bacterial lipopolysaccharide has structural similarity to ceramide and stimulates ceramide-activated protein kinase in myeloid cells. J Biol Chem. 1994;269:17606–17610. [PubMed] [Google Scholar]
- 69.Barber SA, Perera PY, Vogel SN. Defective ceramide response in C3H/HeJ (Lpsd) macrophages. J Immunol. 1995;155:2303–2305. [PubMed] [Google Scholar]
- 70.Cuschieri J, Bulger E, Billgrin J, et al. Acid sphingomyelinase is required for lipid Raft TLR4 complex formation. Surg Infect (Larchmt) 2007;8:91–106. doi: 10.1089/sur.2006.050. [DOI] [PubMed] [Google Scholar]
- 71.Fischer H, Ellstrom P, Ekstrom K, et al. Ceramide as a TLR4 agonist; a putative signalling intermediate between sphingolipid receptors for microbial ligands and TLR4. Cell Microbiol. 2007;9:1239–1251. doi: 10.1111/j.1462-5822.2006.00867.x. [DOI] [PubMed] [Google Scholar]
- 72.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li H, Ruan XZ, Powis SH, et al. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005;67:867–874. doi: 10.1111/j.1523-1755.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 74.Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- 75.Melkonian KA, Ostermeyer AG, Chen JZ, et al. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J Biol Chem. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- 76.Resh MD. Membrane targeting of lipid modified signal transduction proteins. Subcell Biochem. 2004;37:217–232. doi: 10.1007/978-1-4757-5806-1_6. [DOI] [PubMed] [Google Scholar]
- 77.Rowe DC, McGettrick AF, Latz E, et al. The myristoylation of TRIF-related adaptor molecule is essential for Toll-like receptor 4 signal transduction. Proc Natl Acad Sci U S A. 2006;103:6299–6304. doi: 10.1073/pnas.0510041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chapkin RS, Wang N, Fan YY, et al. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim Biophys Acta. 2008;1778:466–471. doi: 10.1016/j.bbamem.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Q, Wang M, Tan L, et al. Docosahexaenoic acid changes lipid composition and interleukin-2 receptor signaling in membrane rafts. J Lipid Res. 2005;46:1904–1913. doi: 10.1194/jlr.M500033-JLR200. [DOI] [PubMed] [Google Scholar]
- 80.Stulnig TM, Huber J, Leitinger N, et al. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J Biol Chem. 2001;276:37335–37340. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- 81.Zeyda M, Staffler G, Horejsi V, et al. LAT displacement from lipid rafts as a molecular mechanism for the inhibition of T cell signaling by polyunsaturated fatty acids. J Biol Chem. 2002;277:28418–28423. doi: 10.1074/jbc.M203343200. [DOI] [PubMed] [Google Scholar]
- 82.De Smedt-Peyrusse V, Sargueil F, Moranis A, et al. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J Neurochem. 2008;105:296–307. doi: 10.1111/j.1471-4159.2007.05129.x. [DOI] [PubMed] [Google Scholar]