Abstract
Variable (or noisy) ventilation (VV) has been demonstrated in animal models of acute lung injury to be superior to constant (or conventional) ventilation (CV), in terms of improved gas exchange and mitigation of lung injury, for reasons that are not entirely clear. We hypothesized that the efficacy of VV is related to the fact that recruitment and derecruitment of lung units are dynamic processes. To test this hypothesis, we modeled the lung computationally as a symmetrically bifurcating airway tree terminating in elastic units. Each airway was fully open or completely closed, at any point in time, according to its pressure history. The model is able to accurately mimic previous experimental measurements showing that the lungs of mice injured by acid aspiration are better recruited after 60 min of VV than CV. The model also shows that recruitment/derecruitment dynamics contribute to the relative efficacy of VV, provided lung units open more rapidly than they close once a critical opening or closing pressure threshold has been crossed. We conclude that the dynamics of recruitment and derecruitment in the lung may be important factors responsible for the benefits of VV compared with CV.
Keywords: mechanical ventilation, noisy ventilation, lung elastance, acute lung injury
conventional mechanical ventilation delivers a set tidal volume (Vt) to the lungs with exactly the same frequency for every breath. By contrast, the pattern of natural breathing varies from breath to breath, and it has been postulated that such variability may be important for normal physiological function. Accordingly, interest has recently begun to focus on the use of variable (or noisy) ventilation (VV) for the treatment of acute lung injury (ALI). Although not all studies have found VV to be advantageous (28), most have reported superior oxygenation and/or lung mechanics compared with the conventional ventilation (CV) approach (8, 9, 12, 13, 16, 19, 22, 23, 25–27, 37, 39). This raises the intriguing question as to the mechanisms underlying the relative efficacy of VV. Suki et al. (38) explained it as being due to a form of stochastic resonance, whereby the nonlinear shape of the pulmonary pressure-volume curve leads to a greater level of open lung when Vt is varied breath-to-breath. However, this is a static view of the situation, in which the amount of open lung at any point in time is determined purely by the current pressure, with no explicit dependence on time. In fact, recruitment and derecruitment of the lung can be highly dynamic (1, 11, 21), with the result that the amount of open lung is strongly determined by pressure history. Because of these dynamics, occasional large Vt values during VV have the potential not only to recruit the lung but to keep it open for significant periods of time, possibly serving to maintain a greater mean level of open lung than would be the case if Vt were kept uniform and modest.
We therefore hypothesized that the efficacy of VV compared with CV might be due in part to the dynamic nature of recruitment and derecruitment. Given that these dynamics depend significantly on the type and extent of lung injury, understanding their implications for VV could be important, for example, for eventually designing optimal Vt sequences to treat particular types of lung injury. To test this hypothesis, we performed a computational modeling study in which the effects of VV were compared with those of CV. Using a model of recruitment and derecruitment dynamics that we developed previously (20), we simulated the effects of ALI in mice (39) and examined how the application of varying Vt levels on a breath-by-breath basis affects the mean amount of open lung compared with that achieved with CV.
METHODS
An anatomically based computational model that has been described in detail previously (20) was used for this study. Briefly, the model describes a commercial small animal ventilator (flexiVent, Scireq, Montreal, PQ, Canada) connected to a mouse lung, the latter represented by a symmetrically bifurcating airway tree with identical acinar units appended to the last airway generation. The elastance of each acinar unit is equal to the total lung elastance at baseline (El0) times the number of acinar units. Each airway can exist in either of two states, open or closed, and can make a transition between these states as a function of pressure and time (21). Each airway is associated with a virtual trajectory (0 ≤ x ≤ 1) and has a critical opening and closing pressure (Po and Pc, respectively). Movement along the virtual trajectory occurs at a speed proportional to the difference between the current pressure and Po (toward increasing values of x) or Pc (toward decreasing values of x), the constants of proportionality being so and sc, respectively. If the airway is closed and x reaches 1, the airway will immediately open. The converse occurs if x reaches 0. In other words
| (1) |
To run the model with CV, mean Vt (Vtmean) was set at 0.2 ml (∼8 ml/kg in mice) and breathing frequency (f) was 240 breaths/min. For VV, the mean values of Vt and f were the same as for CV, but each varied randomly breath-by-breath, so as to keep the minute ventilation over each breath (f × Vt) constant. Vt adhered to the probability density function (39)
| (2) |
where Vmin = 0.7Vtmean, Vmax = 2.25Vtmean, and v0 is a normalization constant such that Vp = 0.9Vtmean. This probability density function (see Fig. 1 in Ref. 39) is constant between Vmin and Vp and decreases according to a power law above Vp.
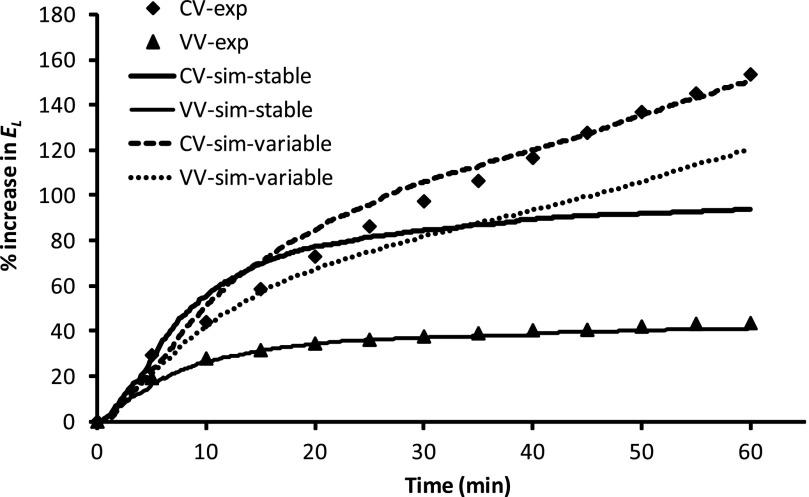
Fig. 1.
Comparison of simulated (sim) and experimentally measured (exp) mean time course of percent changes in total lung elastance (El) over 60 min of conventional ventilation (CV) or variable ventilation (VV) at 3 cmH2O positive end-expiratory pressure (PEEP). Simulated curves are averages of 400 independent simulations drawn from distributions for closing and opening pressures and speeds, and the same set of model parameters were used for CV and VV under stable (mean airway critical pressure and speeds remain constant during ventilation) or variable (mean airway critical pressure increases linearly with time during ventilation) condition. Experimental curves are averages of 8 mice for CV and VV.
Inspiration is driven by the ventilator when its piston moves forward in a sinusoidal waveform. Expiration occurs passively against the set level of positive end-expiratory pressure (PEEP) as a result of the elastic recoil pressure generated by the lungs and the combined flow resistance of the open airways.
The opening and closing speed constants are distributed among the various airways of the model according to uniform distributions, such that so ∼ unif[0,So] and sc ∼ unif[0,Sc], where So and Sc are scale factors. The critical opening and closing pressures follow a normal distribution, such that Pc ∼ N[μc,σc] and Po ∼ N[μo,σo], where μc and μo are the mean closing and opening pressures, respectively, and σc and σo are the corresponding standard deviations. We found in a previous study (21) that the difference between Po and Pc can be assumed to be the same for all airways in acid-injured mice. Together with the assumption that σc = σo, this means that, instead of having independent distributions for Pc and Po, we effectively have the same distribution for both, but one is shifted by a fixed amount (ΔP) relative to the other. To mimic the increased propensity for small airways to close more easily than large airways, the mean critical closing pressure is assumed to increase linearly with generation number from zero in the trachea to μc in the last generation, in accordance with experimental observations (17, 29). The model thus has five independent adjustable parameters (μc, σc, μo, So, and Sc) that, together, define the probability distribution functions from which the open and closing pressures and speeds for each airway are randomly drawn.
The model was initialized to have all airways open and all values of x set to 1. Simulations began with a run-in period of 5 min of ventilation followed by two deep inflations (DIs; sinusoidal, peak-to-peak value 1 ml, period 4 s). Immediately after the DIs, CV or VV was applied for 60 min, as described above. During this period, we determined the open fraction [O(t)] of the lung as a function of time, defined as the fraction of units in communication with the tracheal opening. We also determined the time course of model elastance [El(t)], in analogy with experimental practice, by fitting the conventional one-compartment linear model of the lung
to the simulated pressure [Pl(t)], ventilated lung volume [Vl(t)], and flow [V̇l(t)] signals from each breath using multiple linear regression (10), where Rl(t) is lung resistance. We assessed the efficacies of CV and VV in terms of the changes in O(t) and El(t) from their values immediately following the two DIs to their final values at the end of the 60-min ventilation period (the values used for this calculation being the averages of these quantities over 5-min periods). We performed a Monte-Carlo simulation under each set of conditions (i.e., values of μc, σc, μo, σo, So, and Sc) by performing 400–500 runs using independent values for the individual airway properties drawn from the appropriate probability distributions. The averages of O(t) and El(t) were determined from each set of simulations.
The remaining model parameters were set as described in our previous study (20). Baseline (all airways open) total lung resistance (Rl0) was 2 cmH2O·s·ml−1 and total lung elastance (El0) was 22 cmH2O/ml. The ventilator tubing resistance (Req) was 0.41 cmH2O·s·ml−1 and the ventilator cylinder gas elastance (Egas) was 185 cmH2O/ml to match those of the flexiVent ventilator (21). A seven-generation airway tree was assumed in most simulations, and the size ratio between successive airway generations was 0.9. The model equations were integrated with a time step size (Δt) of 0.002 s. For the sensitivity analyses, a Δt of 0.001 s and an eight-generation airway tree were used. All simulations were performed with MATLAB (version 2007, MathWorks, Natick, MA) on a Linux cluster in the serial run mode. Each pair of Monte-Carlo simulations of CV and VV took ∼75 min on a single processor in the cluster.
RESULTS
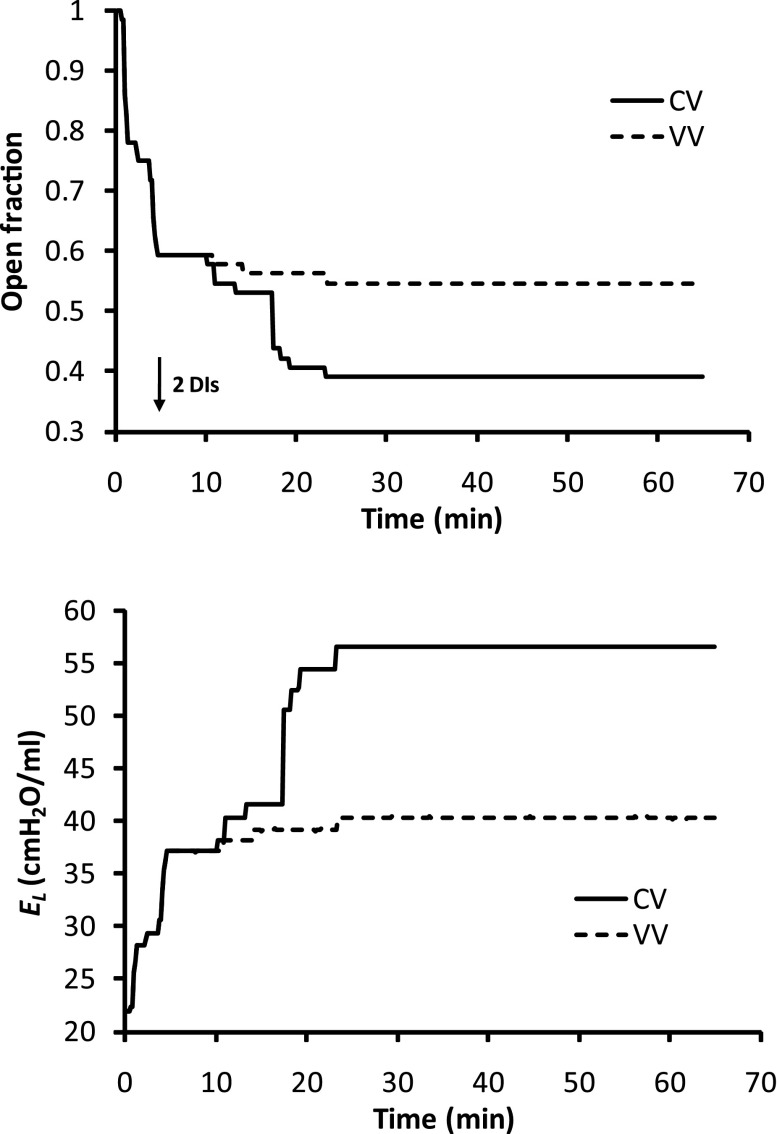
We first attempted to get the computational model to mimic the time courses of El(t) measured in a previous study comparing CV with VV in mice with ALI caused by aspiration of a small dose of HCl (39). In this prior study, a recruitment maneuver was followed by 60 min of ventilation against 3 cmH2O PEEP using the CV or VV protocols described in methods. The mean time courses for El(t) obtained experimentally are shown in Fig. 1. We found that, with use of the following trial-and-error procedure, the model could be made to accurately mimic the VV data. We began with an initial set of parameters based on previous experience (20, 21) and simulated the time course of elastance over the 60-min ventilation period. The result was compared visually with the experimental data, the model parameters were modified, and the new prediction was compared with the data again. This process was repeated 40 times until a visually good match to the data was obtained. This is shown by the thin solid line in Fig. 1, which is the mean of 400 Monte-Carlo runs of the model driven with VV using μc = 3 cmH2O, μo = 9 cmH2O, σc = σo = 3 cmH2O, So = 0.11 s−1·cmH2O−1, and Sc = 0.011 s−1·cmH2O−1. By contrast, when the model was driven with CV using the same set of parameter values, the predicted El(t) increased rapidly and initially overpredicted the experimental values. Later, El(t) flattened out, however, and underpredicted the experimental values (thick solid line, Fig. 1). Despite extensive searching of the model parameter space, it was not possible to obtain a percent change in El(t) that gave a good match to the experimental curve in both magnitude and slope of increase under CV. In particular, it was not possible to have the model reproduce the initial rapid increase in El(t) followed by the later slower increase in the CV data using only a single set of model parameter values. We therefore hypothesized that the model parameters were not constant during CV as a result of ongoing development of lung injury, in contrast to the relatively protective VV during which injury level remained stable. To simulate injury development in CV, a trial-and-error approach was again used. After 40 trials, a good reproduction of the experimental CV data in injured mice (dashed line in Fig. 1) was obtained when μc increased linearly in time from an initial value of 3 cmH2O to 4.5 cmH2O after 60 min, μo increased correspondingly from 9 to 10.5 cmH2O, and So and Sc remained fixed at 0.016 and 0.011 s−1·cmH2O−1, respectively. By contrast, when we allowed μo and μc to increase in this way with VV, we obtained a worse fit to the data (dotted line in Fig. 1) than when μo and μc remained constant. Figure 2 shows examples of O(t) and El(t) from a single run of the model using the parameters corresponding to the VV best-fit case in Fig. 1. Because of the finite size of the model airway tree (7 generations), the curves are somewhat jagged in appearance due to the macroscopic manifestations of individual derecruitment events (the average of 400 such curves gives the smooth curves shown in Fig. 1). Nevertheless, the ability of VV to prevent derecruitment compared with CV is clearly apparent.
Fig. 2.
Time history of acinar units' open fraction and El in CV or VV mode, in the VV best-fit case. Two consecutive deep inflations (DIs) were applied at 5 min (arrow); then CV (same as before DI) or VV was applied.
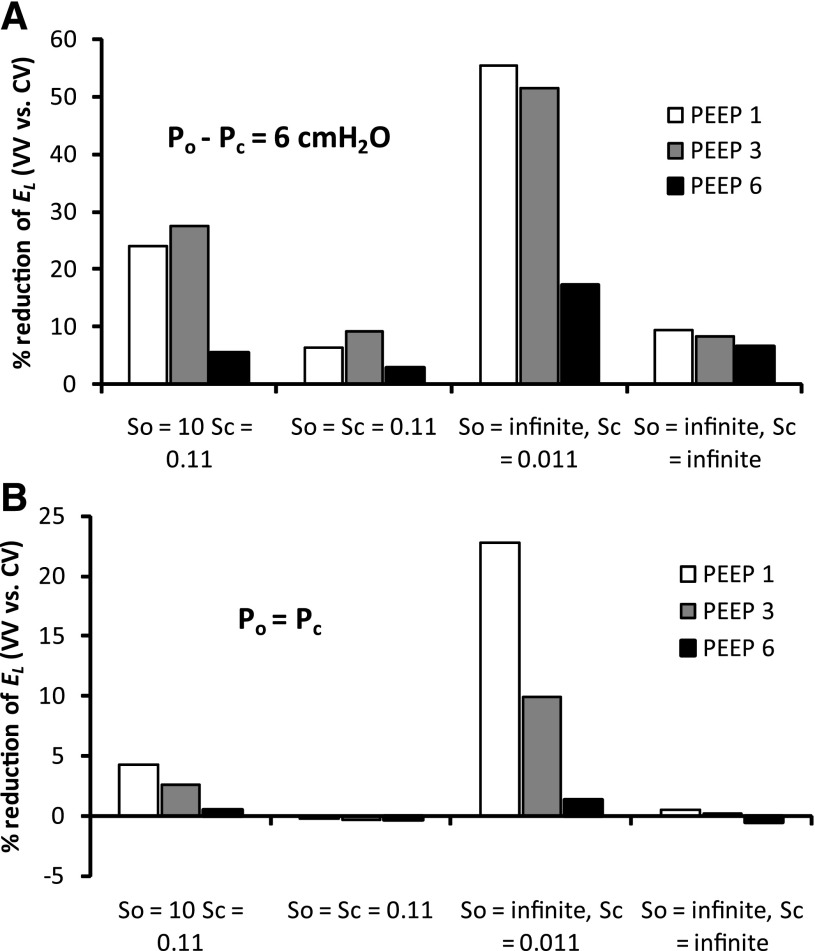
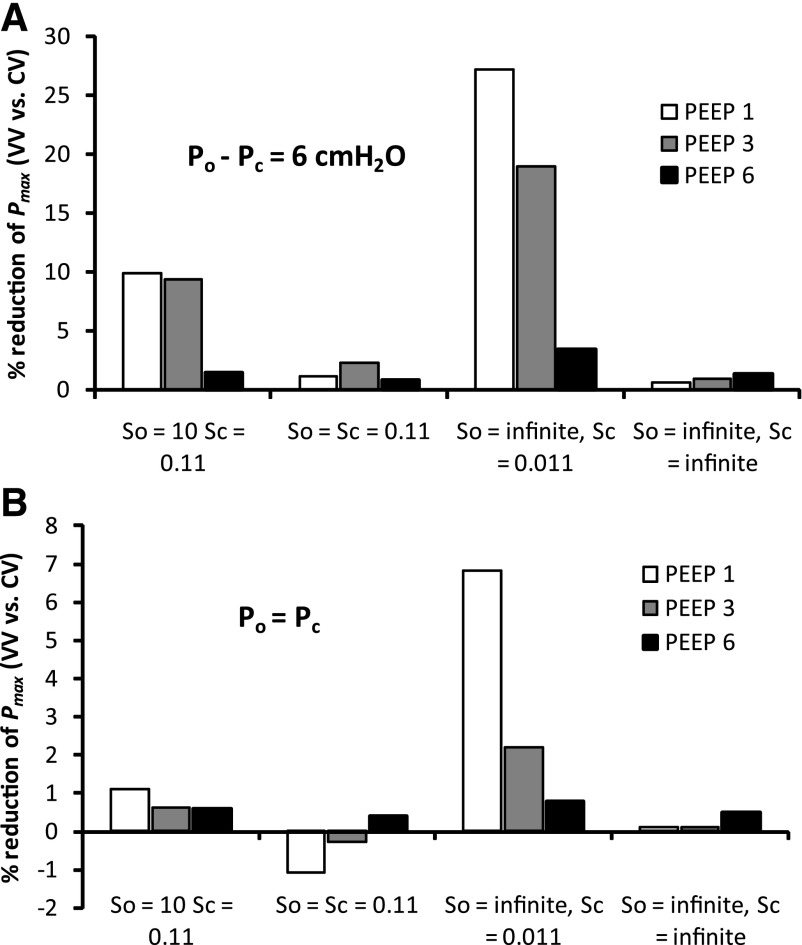
We next investigated how dependent the improved performance of VV was, relative to CV, on PEEP and the various parameters of the model. Figure 3 shows the percent reduction in El at the end of the 60-min ventilation period obtained with VV relative to CV (a positive value indicating that VV is better at maintaining the lung open). Figure 3A shows that when the critical opening and closing pressures differ, as in the previous simulations (μo − μc = 6 cmH2O), VV improves on CV at 1–3 cmH2O PEEP, whether the speeds of opening and closing are the same or not or, even, whether opening and closing events occur instantaneously once their respective pressure thresholds have been crossed. This is not the case, however, when there is no difference between the opening and closing pressures (μo = μc) and the opening and closing speeds (So = Sc) are the same (Fig. 3B). A somewhat similar picture applies to the peak airway pressures generated at the end of the 60-min ventilation periods (averaged over the final 5 min; Fig. 4), although in this case the conditions μo = μc and So = Sc lead to VV actually being more injurious (i.e., producing higher pressures) than CV. We found that these conclusions were not altered by using a smaller time step (0.001 vs. 0.002 s) or a larger airway tree (8 vs. 7 generations).
Fig. 3.
Percent reduction of El in VV mode compared with CV mode at the end of ventilation when critical opening and closing pressures (Po and Pc, respectively) are equal (Po = Pc) or different (Po − Pc = 6 cmH2O) under 4 different combinations of closing and opening speeds: So = 0.11 and Sc = 0.011, So = Sc = 0.11, So = ∞ and Sc = 0.011, or So = ∞ and Sc = ∞.
Fig. 4.
Percent reduction of mean peak airway opening pressure in VV mode compared with CV mode at the end of ventilation for parameter combinations described in Fig. 3 legend.
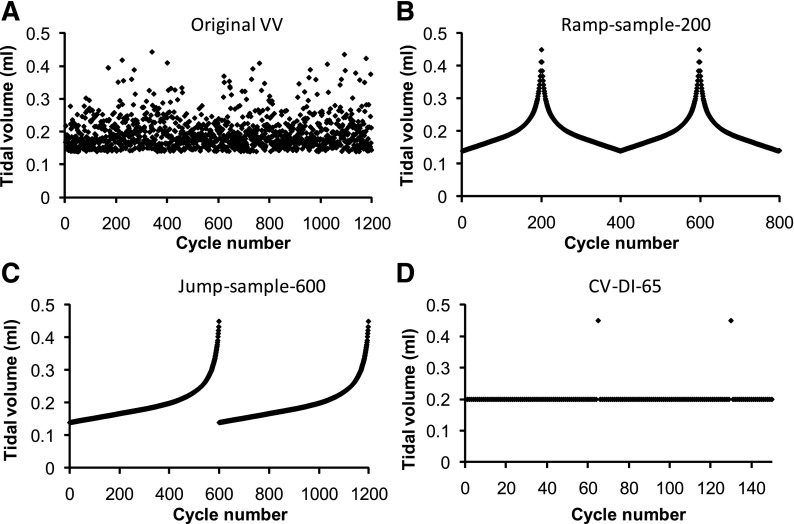
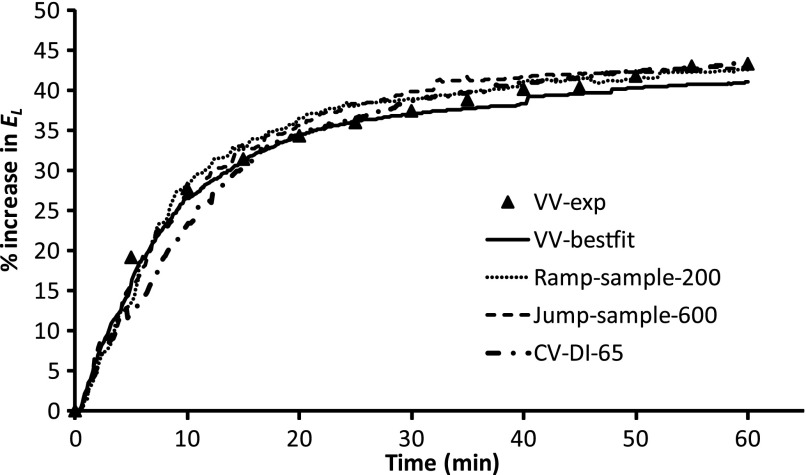
Finally, the original VV series of Vt values was modified in various ways to investigate how efficacy depends on the randomness of the signal and its probability density distribution function. Figure 5A shows the original VV series of Vt values. This series of values was sorted into ascending order and then sampled uniformly from beginning to end to create the series of Vt values shown in Fig. 5, B and C. Figure 5B shows a pair of identical symmetrical peaks using 200 samples. Figure 5C shows a pair of identical monotonically increasing curves using 600 samples. Thus, while the signals in Fig. 5, B and C, have the same probability distribution functions as the original random signals, by ordering the data points according to amplitude, we eliminated the stochastic nature of the original signals. Finally, Fig. 5D shows a sequence of constant Vt values at the mean of the previous sequences (0.2 ml) with single DI (Vt = Vmax) applied every 65 cycles. This is similar to the CV complemented with periodic DIs in the original experimental study (39). The time courses of percent increase in El produced when each of these Vt sequences was applied to the model were very similar and are shown in Fig. 6. Note that when we repeated the model simulations using increased time durations between the appearances of large Vt values, the predicted increases in El were greater than those seen experimentally, and vice versa.
Fig. 5.
Comparison of various tidal volume (Vt) signals during ventilation. A: original VV signal. B: ramp, with 200 sample points from the original VV signal. C: jump, with 600 sample points from the original VV signal. D: CV, with DIs every 65 cycles.
Fig. 6.
Comparison of mean time course of percent changes in El over 60 min of ventilation under various modifications of the original VV signal. VV-exp, experimental data using original VV signal in Fig. 5A; VV-best fit, simulation data using original VV signal in Fig. 5A; Ramp-sample-200, simulation data using Vt in Fig. 5B; Jump-sample-600, simulation data using Vt in Fig. 5C; CV-DI-65, simulation data using Vt in Fig. 5D.
DISCUSSION
Mechanical ventilation is a key therapeutic intervention in critically ill patients, particularly those with acute respiratory distress syndrome (ARDS), but it also has the capacity to greatly exacerbate existing injury through a process known as ventilator-induced lung injury (35, 40). Clinical practice mandates the use of relatively small Vt (ideally 6 ml/kg) in ARDS on the basis of a well-publicized clinical trial (40). Even though other ventilatory strategies have yet to pass the rigors of a randomized clinical trial, a number of them seem physiologically compelling for the treatment of ALI/ARDS and have shown some promise in preclinical investigations. One such example, and the focus of the present study, is the use of a randomly varying breathing pattern. VV was originally proposed by Lefevre et al. (19) and then by Mutch and colleagues (22–27). The original rationale for VV was that it ought to vary the location where recruitment occurs within the lungs during mechanical ventilation, so that it is not always the same airways that receive the stress of atelectrauma with every breath. More recently, Thammanomai et al. (39) investigated the physiological consequences of VV in a mouse model of ALI and found it to be superior to CV in terms of lung mechanical function and biomarkers of injury. VV has also been found to be superior to CV in terms of gas exchange and respiratory mechanics in pigs during severe bronchospasm (22) and prolonged anesthesia (23) and has been observed to lead to improved gas exchange and lung mechanics compared with CV in a sheep saline lavage lung injury model (12).
VV thus appears to have promise as a ventilatory mode appropriate for ALI/ARDS, which immediately raises the question of the mechanism underlying its apparent efficacy. One possibility relates to improved surfactant function. Arold et al. found that VV increases endogenous surfactant release in normal guinea pigs (9) and that a variable stretch pattern enhances surfactant secretion in alveolar type II cells in culture (7). Reduced surface tension would be expected to improve the recruitability of the lung and to reduce tissue stress during mechanical ventilation. Suki et al. (38) proposed that the nonlinear pressure-volume curve of the lung may be critical to the benefits accruing from VV through a phenomenon known as stochastic resonance (41). Here, the concavity of the lung's pressure-volume relationship leads to an increasing mean lung volume with increasing variations in pressure, even though mean pressure remains the same (14). VV has also been reported to enhance respiratory sinus arrhythmia (24), which is known to improve ventilation-perfusion matching in the lung.
None of the explanations for VV postulated so far, however, take into account the fact that recruitment and derecruitment in the lung are dynamic processes, depending on time as well as pressure. The temporal aspects of recruitment and derecruitment are limited in the normal lung but can become very pronounced in the injured lung, as evidenced by the extended transients in lung elastance that typically occur following a recruitment maneuver (2–4). Since the occasional large Vt that occurs in VV is reminiscent of recruitment maneuvers, we hypothesized that recruitment/derecruitment dynamics might be important in defining how VV fares compared with CV. Our model simulations bear this out. Figures 1 and 2 show that we can account for most of the experimental differences in El(t) in mice obtained with VV vs. CV on the basis of our computational model of recruitment/derecruitment dynamics. In addition, while the model readily yields a prediction of the measurements with VV, it is only by assuming progression of lung injury during mechanical ventilation that we are able to account for the CV results (Fig. 1), which further supports the relatively noninjurious nature of VV compared with CV. Indeed, IL-1β, a cytokine that is known to accumulate in lung injury, was found to be increased in the lungs of mice ventilated with CV compared with those ventilated with VV (39).
Experimentation with the parameters of the computational model suggests further insights into the mechanisms underlying the efficacy of VV relative to CV. First, although the relative efficacy remains when PEEP is varied between 1 and 6 cmH2O, the effect is less pronounced at the higher PEEP levels (Figs. 3 and 4). This is not surprising, given that more of the lung becomes derecruited at the lower PEEP levels, so there is more room for improvement with an efficacious method of mechanical ventilation such as VV. Even a single pressure boundary between recruitment and derecruitment (i.e., Po = Pc) maintains the improvement of VV over CV, at least at <6 cmH2O PEEP. On the other hand, the benefits of VV disappear altogether and are even slightly reversed, when the speeds at which airways open and close are made the same (i.e., So = Sc). This can be understood again in terms of the transient nature of lung recruitment. If a recruitment maneuver opens the lung more quickly than the subsequent closure rate, then occasional DIs will be effective at maintaining the lung open, provided each maneuver occurs before the effects of the previous maneuver have completely worn off.
More rapid lung opening than closing is also supported by experimental observation. For example, Bellardine et al. (12) found in saline-lavaged excised calf lungs that the recruitment associated with a large Vt during VV lasted >200 s, which was an order of magnitude greater than the average time interval between large Vt. By contrast, they noted that the recruitment following a large breath during VV was virtually instantaneous. Similarly, in vitro experiments (31) have shown that airway reopening occurs more rapidly and at a higher pressure than does closure. Of course, our dynamic recruitment-derecruitment model is based on a purely empirical recruitment/derecruitment mechanism. Nevertheless, this mechanism is reminiscent of the processes of liquid bridge formation and breakage that have been shown to occur in small airways (17, 18, 29–33, 42). It thus seems likely that the experimental data used in the present study (39) reflect a rate of recruitment that was more rapid than derecruitment and that such differences may be critical to the relative benefits of VV over CV. Importantly, as the speeds of recruitment and derecruitment in the lung are likely to vary with the nature and degree of injury (2), the relative benefits of VV may be disease-specific. Interestingly, we also found that although the opening speed was greater than the closing speed for the best-fit CV model simulation (Fig. 1), the speed difference was substantially smaller than for the best-fit VV simulation. The reason for this difference may be that we had to invoke increasing mean critical pressures in the CV simulation to achieve a reasonable fit, which is an ad hoc means of accounting for injury progression and would likely have affected the best-fit values of the other model parameters. The opening-closing speed difference was also smaller than we found in a previous experimental study in control and injured mice (21), but this previous study considered only recruitment/derecruitment dynamics over 3 min, rather than 1 h, which makes comparison problematic.
If the benefits of VV accrue in large part from the transient recruitment effects of the occasional large Vt, then these benefits should be related primarily to the intermittency of large Vt, and not to the randomness of the ventilatory pattern per se. We tested this idea with the computational model by reordering the random Vt to make them highly correlated in two ways, as shown in Fig. 5, B and C. The results on the progression of El(t) were similar to those found with the random Vt and, indeed, were even similar to those found with regular Vt interspersed with regular DI (Fig. 5D). On their own, therefore, these results suggest that the judicious placement of DIs on a background of regular ventilation should be just as efficacious as VV. Other experimental findings, however, do not support this view, as significantly lower El and airway pressures, better gas exchange, and lower IL-1β levels have been found with VV than with CV complemented with DIs (39). This is not to say that ventilation necessarily has to be truly random; it has been shown, for example, that ventilation of pigs with white and correlated noise provides no difference in gas exchange (15). Rather, the crucial factor may simply be a sufficiently large number of different Vt values within a given time period. Thus the benefits of VV are likely not related simply to the effects of recruitment/derecruitment dynamics but also reflect other mechanisms related to the generation of lung injury. One possibility is the mechanism that originally motivated the use of VV (19, 23, 25–27); namely, it varies the sites of airway reopening stress from breath to breath, rather than concentrating it at the same sites with every breath. Another possibility is that a sufficiently rich variation in Vt is required to optimize surfactant function (7). Since these mechanisms were not uncovered in the experimental study (39), we could not build them into the model; hence, this remains a limitation of the current study.
The frequency of DI is also a factor that contributes to the difference between our current modeling study and previous experimental studies of VV. In previous experiments on pigs, for example, DIs were used every 6 min (16) and every hour (26), neither of which is likely to be frequent enough to have a significant effect on the mean amount of open lung. Even the greater DI frequency (from once every 25–400 breaths) used previously in mice (39) may not have been sufficient to maintain the same degree of recruitment as VV. Of course, keeping the lung as open as possible is not the only important consideration; if DIs are too frequent, they may cause lung injury themselves, and, in fact, we previously demonstrated the existence of an optimum DI frequency that balances recruitment against the production of injury (6). The factors leading to tissue injury were not taken into account in the present study, nor were any possible biological effects of VV considered. One potentially important biological effect is stimulation of surfactant release (7, 9). Effectiveness of surfactant in reducing airway opening pressure can also have a biophysical origin via alterations in rates of transport to and from the surface, something that has been shown to be facilitated by oscillatory relative to steady airflow (34). Indeed, improved surfactant function would be expected to decrease critical opening pressures through its effect on surface tension (17). On the other hand, the stresses related to airway opening may cause further injury, which would tend to adversely affect surfactant function. These stresses depend markedly on the frequency of ventilation (36), so it is conceivable that, under some conditions, the ongoing development of tissue injury might cancel out any improvements in surfactant function, leaving the dynamics of recruitment and derecruitment unchanged.
Surfactant dynamics are also affected by ventilation history. Pillert and Gaver (34) recently showed that opening airways using high-frequency low-amplitude oscillations substantially reduces mean reopening pressure compared with steady flow because of increases in surfactant transport and adsorption. Similarly, the modeling study of Smith and Gaver (36) on propagation of a finger of air through a liquid-filled cylindrical rigid tube showed that the normal stress at the tube wall is highly dynamic and reaches a minimum value at moderate oscillation frequencies. These experimental and modeling studies suggest that airway reopening and the associated mechanical stresses on the epithelium depend on breathing frequency in complex ways, which might explain the efficacy of VV, since the breathing frequency in VV varies from cycle to cycle.
Some other limitations of our modeling study must also be considered. For example, the scale factor (So) for the probability distribution of opening speeds was assumed to be constant over time and not to vary with airway generation. Because dx/dt depends on (P − Po) (Eq. 1) and Po increases with generation number, opening speed will also decrease with generation number. In other words, small airways will tend to open during inspiration more slowly than large airways, a prediction that requires experimental verification. Another model limitation is that although the flows in different airway segments interact because flow is conserved, the effects of interdependence on airway opening via the surrounding parenchyma is not taken into account.
Finally, our conclusions are based on data collected from acid-injured mice, and we found in previous studies that rodents exhibit marked derecruitability when injury is severe (2, 4, 5). Whether larger animals and humans behave in the same way under similar circumstances is uncertain. Pigs, for example, have been shown to exhibit little variation in elastance with time under CV (16, 37). This might reflect species differences in chest wall stiffness or alveolar size or, perhaps, differences in the nature and degree of the lung injury involved. Nevertheless, it is clear that ALI leads to significant instability of the lung and an exaggerated tendency for derecruitment, so we believe it is likely that our present results extend in principle to larger animals and humans.
In conclusion, we have developed a computational model to compare the efficacy of CV and VV on lung mechanics. While several distinct mechanisms have been proposed to account for the improved overall lung function during VV, our results show that the dynamics of recruitment and derecruitment in the lung may also be important determinants in the relative efficacy of VV over CV in terms of maintaining the lung open, decreasing elastance, and avoiding the development of lung injury.
GRANTS
This study was supported by National Institutes of Health National Center for Research Resources Centers of Biomedical Research Excellence Grant RR-15557 and National Heart, Lung, and Blood Institute Grants R01 HL-75593 and HL-098976.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The simulations in this work were performed on the Linux cluster at the Vermont Advanced Computing Center.
Glossary
- CV
Constant ventilation
- DI
Deep inflation
- Egas
Ventilator gas elastance
- El
Total lung elastance
- El0
Baseline total lung elastance (when all airways are open)
- f
Breathing frequency
- O
Fraction of peripheral lung units in communication with the trachea
- Pl
Air pressure at the opening of the lung (proximal end of the trachea)
- Pc
Critical airway closing pressure
- Po
Critical airway opening pressure
- Req
Ventilator tubing resistance
- Rl
Total lung resistance
- Rl0
Baseline total lung resistance (when all airways are open)
- so
Airway opening speed proportionality factor
- sc
Airway closing speed proportionality factor
- So
Scale factor for probability distribution of so values
- Sc
Scale factor for probability distribution of sc values
- t
Time
- Vl
Total lung volume in communication with the trachea
- Vt
Tidal volume
- Vtmean
Mean tidal volume
- Vmin
Minimum tidal volume
- Vp
Parameter defining a probability density function
- Vmax
Maximum tidal volume
- VV
Variable ventilation
- x
Virtual trajectory (dimensionless variable associated with each airway)
- μc
Mean critical closing pressure
- σc
Standard deviation of critical closing pressure
- μo
Mean critical opening pressure
- σo
Standard deviation of critical opening pressure
- Δt
Time step size
- ρ
Probability density function for distribution of tidal volume
- ν0
Normalization constant in probability density function
- V̇l
Total flow to the lung
REFERENCES
- 1. Albert SP, DiRocco J, Allen GB, Bates JHT, Lafollette R, Kubiak BD, Fischer J, Maroney S, Nieman GF. The role of time and pressure on alveolar recruitment. J Appl Physiol 106: 757–765, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen G, Bates JH. Dynamic mechanical consequences of deep inflation in mice depend on type and degree of lung injury. J Appl Physiol 96: 293–300, 2004. [DOI] [PubMed] [Google Scholar]
- 3. Allen G, Lundblad LK, Parsons P, Bates JH. Transient mechanical benefits of a deep inflation in the injured mouse lung. J Appl Physiol 93: 1709–1715, 2002. [DOI] [PubMed] [Google Scholar]
- 4. Allen GB, Leclair T, Cloutier M, Thompson-Figueroa J, Bates JH. The response to recruitment worsens with progression of lung injury and fibrin accumulation in a mouse model of acid aspiration. Am J Physiol Lung Cell Mol Physiol 292: L1580–L1589, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Allen GB, Pavone LA, DiRocco JD, Bates JH, Nieman GF. Pulmonary impedance and alveolar instability during injurious ventilation in rats. J Appl Physiol 99: 723–730, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Allen GB, Suratt BT, Rinaldi L, Petty JM, Bates JH. Choosing the frequency of deep inflation in mice: balancing recruitment against ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 291: L710–L717, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Arold SP, Bartolak-Suki E, Suki B. Variable stretch pattern enhances surfactant secretion in alveolar type II cells in culture. Am J Physiol Lung Cell Mol Physiol 296: L574–L581, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arold SP, Mora R, Lutchen KR, Ingenito EP, Suki B. Variable tidal volume ventilation improves lung mechanics and gas exchange in a rodent model of acute lung injury. Am J Respir Crit Care Med 165: 366–371, 2002. [DOI] [PubMed] [Google Scholar]
- 9. Arold SP, Suki B, Alencar AM, Lutchen KR, Ingenito EP. Variable ventilation induces endogenous surfactant release in normal guinea pigs. Am J Physiol Lung Cell Mol Physiol 285: L370–L375, 2003. [DOI] [PubMed] [Google Scholar]
- 10. Bates JHT. Lung Mechanics: An Inverse Modeling Approach. Cambridge, UK: Cambridge University Press, 2009, p. 236. [Google Scholar]
- 11. Bates JHT, Irvin CG. Time dependence of recruitment and derecruitment in the lung: a theoretical model. J Appl Physiol 93: 705–713, 2002. [DOI] [PubMed] [Google Scholar]
- 12. Bellardine CL, Hoffman AM, Tsai L, Ingenito EP, Arold SP, Lutchen KR, Suki B. Comparison of variable and conventional ventilation in a sheep saline lavage lung injury model. Crit Care Med 34: 439–445, 2006. [DOI] [PubMed] [Google Scholar]
- 13. Boker A, Graham MR, Walley KR, McManus BM, Girling LG, Walker E, Lefevre GR, Mutch WA. Improved arterial oxygenation with biologically variable or fractal ventilation using low tidal volumes in a porcine model of acute respiratory distress syndrome. Am J Respir Crit Care Med 165: 456–462, 2002. [DOI] [PubMed] [Google Scholar]
- 14. Brewster JF, Graham MR, Mutch WAC. Convexity, Jensen's inequality and benefits of noisy mechanical ventilation. J R Soc Interface 2: 393–396, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Froehlich KF, Graham MR, Buchman TG, Girling LG, Scafetta N, West BJ, Walker EK, McManus BM, Mutch WA. Physiological noise versus white noise to drive a variable ventilator in a porcine model of lung injury. Can J Anaesth 55: 577–586, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Funk DJ, Graham MR, Girling LG, Thliveris JA, McManus BM, Walker EK, Rector ES, Hillier C, Scott JE, Mutch WA. A comparison of biologically variable ventilation to recruitment manoeuvres in a porcine model of acute lung injury. Respir Res 5: 22, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gaver DP, Samsel RW, Solway J. Effects of surface-tension and viscosity on airway reopening. J Appl Physiol 69: 74–85, 1990. [DOI] [PubMed] [Google Scholar]
- 18. Heil M, Hazel AL, Smith JA. The mechanics of airway closure. Respir Physiol Neurobiol 163: 214–221, 2008. [DOI] [PubMed] [Google Scholar]
- 19. Lefevre GR, Kowalski SE, Girling LG, Thiessen DB, Mutch WA. Improved arterial oxygenation after oleic acid lung injury in the pig using a computer-controlled mechanical ventilator. Am J Respir Crit Care Med 154: 1567–1572, 1996. [DOI] [PubMed] [Google Scholar]
- 20. Ma B, Bates JHT. Modeling the complex dynamics of derecruitment in the lung. Ann Biomed Eng 38: 3466–3477, 2010. [DOI] [PubMed] [Google Scholar]
- 21. Massa CB, Allen GB, Bates JHT. Modeling the dynamics of recruitment and derecruitment in mice with acute lung injury. J Appl Physiol 105: 1813–1821, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mutch WA, Buchman TG, Girling LG, Walker EK, McManus BM, Graham MR. Biologically variable ventilation improves gas exchange and respiratory mechanics in a model of severe bronchospasm. Crit Care Med 35: 1749–1755, 2007. [DOI] [PubMed] [Google Scholar]
- 23. Mutch WA, Eschun GM, Kowalski SE, Graham MR, Girling LG, Lefevre GR. Biologically variable ventilation prevents deterioration of gas exchange during prolonged anaesthesia. Br J Anaesth 84: 197–203, 2000. [DOI] [PubMed] [Google Scholar]
- 24. Mutch WA, Graham MR, Girling LG, Brewster JF. Fractal ventilation enhances respiratory sinus arrhythmia. Respir Res 6: 41, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mutch WA, Harms S, Lefevre GR, Graham MR, Girling LG, Kowalski SE. Biologically variable ventilation increases arterial oxygenation over that seen with positive end-expiratory pressure alone in a porcine model of acute respiratory distress syndrome. Crit Care Med 28: 2457–2464, 2000. [DOI] [PubMed] [Google Scholar]
- 26. Mutch WA, Harms S, Ruth Graham M, Kowalski SE, Girling LG, Lefevre GR. Biologically variable or naturally noisy mechanical ventilation recruits atelectatic lung. Am J Respir Crit Care Med 162: 319–323, 2000. [DOI] [PubMed] [Google Scholar]
- 27. Mutch WAC, Lefevre GR, Cheang MS. Biologic variability in mechanical ventilation in a canine oleic acid lung injury model. Am J Respir Crit Care Med 163: 1756–1757, 2001. [DOI] [PubMed] [Google Scholar]
- 28. Nam AJ, Brower RG, Fessler HE, Simon BA. Biologically variable mechanical ventilation (BV) does not improve oxygenation or lung mechanics in dogs after acute lung injury (ALI). Anesthesiology 89: U1041–U1041, 1998. [Google Scholar]
- 29. Naureckas ET, Dawson CA, Gerber BS, Gaver DP, Gerber HL, Linehan JH, Solway J, Samsel RW. Airway reopening pressure in isolated rat lungs. J Appl Physiol 76: 1372–1377, 1994. [DOI] [PubMed] [Google Scholar]
- 30. Otis DR, Johnson M, Pedley TJ, Kamm RD. Role of pulmonary surfactant in airway closure—a computational study. J Appl Physiol 75: 1323–1333, 1993. [DOI] [PubMed] [Google Scholar]
- 31. Otis DR, Petak F, Hantos Z, Fredberg JJ, Kamm RD. Airway closure and reopening assessed by the alveolar capsule oscillation technique. J Appl Physiol 80: 2077–2084, 1996. [DOI] [PubMed] [Google Scholar]
- 32. Perun ML, Gaver DP. An experimental-model investigation of the opening of a collapsed untethered pulmonary airway. J Biomech Eng 117: 245–253, 1995. [DOI] [PubMed] [Google Scholar]
- 33. Perun ML, Gaver DP. Interaction between airway lining fluid forces and parenchymal tethering during pulmonary airway reopening. J Appl Physiol 79: 1717–1728, 1995. [DOI] [PubMed] [Google Scholar]
- 34. Pillert JE, Gaver DP., 3rd Physicochemical effects enhance surfactant transport in pulsatile motion of a semi-infinite bubble. Biophys J 96: 312–327, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Slutsky AS. Lung injury caused by mechanical ventilation. Chest 116: 9S–15S, 1999. [DOI] [PubMed] [Google Scholar]
- 36. Smith BJ, Gaver DP. The pulsatile propagation of a finger of air within a fluid-occluded cylindrical tube. J Fluid Mech 601: 1–23, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spieth PM, Carvalho AR, Pelosi P, Hoehn C, Meissner C, Kasper M, Hubler M, von Neindorff M, Dassow C, Barrenschee M, Uhlig S, Koch T, de Abreu MG. Variable tidal volumes improve lung protective ventilation strategies in experimental lung injury. Am J Respir Crit Care Med 179: 684–693, 2009. [DOI] [PubMed] [Google Scholar]
- 38. Suki B, Alencar AM, Sujeer MK, Lutchen KR, Collins JJ, Andrade JS, Jr, Ingenito EP, Zapperi S, Stanley HE. Life-support system benefits from noise. Nature 393: 127–128, 1998. [DOI] [PubMed] [Google Scholar]
- 39. Thammanomai A, Hueser LE, Majumdar A, Bartolak-Suki E, Suki B. Design of a new variable-ventilation method optimized for lung recruitment in mice. J Appl Physiol 104: 1329–1340, 2008. [DOI] [PubMed] [Google Scholar]
- 40. The ARDS Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000. [DOI] [PubMed] [Google Scholar]
- 41. Wiesenfeld K, Moss F. Stochastic resonance and the benefits of noise—from ice ages to crayfish and squids. Nature 373: 33–36, 1995. [DOI] [PubMed] [Google Scholar]
- 42. Yap DYK, Liebkemann WD, Solway J, Gaver DP. Influences of parenchymal tethering on the reopening of closed pulmonary airways. J Appl Physiol 76: 2095–2105, 1994. [DOI] [PubMed] [Google Scholar]