Abstract
Background
The availability of human papillomavirus (HPV) DNA testing and vaccination against HPV types 16 and 18 (HPV-16,18) motivates questions about the cost-effectiveness of cervical cancer prevention in the United States for unvaccinated older women and for girls eligible for vaccination.
Methods
An empirically calibrated model was used to assess the quality-adjusted life years (QALYs), lifetime costs, and incremental cost-effectiveness ratios (2004 US dollars per QALY) of screening, vaccination of preadolescent girls, and vaccination combined with screening. Screening varied by initiation age (18, 21, or 25 years), interval (every 1, 2, 3, or 5 years), and test (HPV DNA testing of cervical specimens or cytologic evaluation of cervical cells with a Pap test). Testing strategies included: 1) cytology followed by HPV DNA testing for equivocal cytologic results (cytology with HPV test triage); 2) HPV DNA testing followed by cytology for positive HPV DNA results (HPV test with cytology triage); and 3) combined HPV DNA testing and cytology. Strategies were permitted to switch once at age 25, 30, or 35 years.
Results
For unvaccinated women, triennial cytology with HPV test triage, beginning by age 21 years and switching to HPV testing with cytology triage at age 30 years, cost $78 000 per QALY compared with the next best strategy. For girls vaccinated before age 12 years, this same strategy, beginning at age 25 years and switching at age 35 years, cost $41 000 per QALY with screening every 5 years and $188 000 per QALY screening triennially, each compared with the next best strategy. These strategies were more effective and cost-effective than screening women of all ages with cytology alone or cytology with HPV triage annually or biennially.
Conclusions
For both vaccinated and unvaccinated women, age-based screening by use of HPV DNA testing as a triage test for equivocal results in younger women and as a primary screening test in older women is expected to be more cost-effective than current screening recommendations.
In the United States, cervical cancer screening has reduced mortality from invasive cancer, although disparities exist in access to screening and outcomes (1). Since the last clinical guidelines for screening were developed (2–6), an increasing number of studies have been published that support the high sensitivity of human papillomavirus (HPV) DNA testing, relative to cytologic evaluation of cervical cells with a Pap test (cytology), for detecting high-grade cervical intraepithelial neoplasia (CIN) (7–10). In addition, clinical trials of two vaccines designed to prevent infections with HPV-16 and HPV-18, which are responsible for approximately 70% of cervical cancer cases, have shown high efficacy in preventing infections with HPV-16 and HPV-18 and associated precancerous changes in women not previously infected with these types of HPV (11–15). Ideally, new screening and vaccination options would be used in a way that improves cancer outcomes, reduces disparities, and enhances the cost-effectiveness of cervical cancer prevention.
Challenges to identifying optimal prevention policies for cervical cancer are formidable. Reduction in cancer mortality as a result of preadolescent HPV vaccination or the introduction of a new screening strategy will not be observable for decades. Inevitably, therefore, decision making in the immediate future will rely on studies reporting intermediate outcomes. Moreover, because vaccination and screening take place at different stages of cervical carcinogenesis, no single study will be able to evaluate all possible strategies. Mathematical models that synthesize the best available data while ensuring consistency with epidemiologic observations can project outcomes beyond those reported in clinical trials, provide insight into key drivers of cost-effectiveness, and be revised as new information emerges (16).
We conducted a cost-effectiveness analysis that addressed two general questions about cervical cancer screening. For women who are not vaccinated, what recommendations can be made regarding cervical cancer screening guidelines, taking into account new data on the performance of HPV DNA testing? For girls who are eligible to be vaccinated, does the optimal approach to cervical cancer prevention include preadolescent vaccination and should screening use more sensitive HPV DNA testing and age-based screening protocols?
Model and Methods
Analytic Approach
We use an empirically calibrated model of cervical cancer in the United States to assess the health and economic outcomes associated with alternative screening strategies for adult women who have not been vaccinated and for those women who will have received HPV-16,18 vaccination as preadolescent girls between the ages of 9 and 12 years. Details of model development, calibration, and evaluation (eg, internal consistency and predictive validity) are available elsewhere (17). As recommended for economic evaluations that are intended to provide information for resource allocation, we adopted a societal perspective, included all costs and benefits regardless of to whom they accrue, incorporated patient time costs, and discounted future costs and life years by 3% annually (18–20). The relative performance of strategies was measured by use of the incremental cost-effectiveness ratio, which is defined as the additional cost of a strategy divided by its additional benefit compared with the next most expensive strategy. Strategies were excluded if they were more costly and less effective (ie, strongly dominated) or more costly and less cost-effective (ie, weakly dominated) than an alternative strategy. We referred to strategies that were not dominated as efficient or preferred. We evaluated parameter uncertainty by conducting one- and two-way sensitivity analyses and a probabilistic sensitivity analysis.
There is no universal criterion that defines a threshold cost-effectiveness ratio below which an intervention would be considered to be cost-effective. However, a benchmark often used in the United States is that interventions that cost less than $50 000 per quality-adjusted life year (QALY) and potentially between $50 000 and $100 000 per QALY are comparable to other interventions society has elected to adopt, which implies that they are considered to be good value for the resources invested (21).
Model
We used an individual-based stochastic microsimulation model in which 1 000 000 girls were followed from age 9 years throughout their lifetime. The natural history of disease in an individual was characterized as a sequence of monthly transitions between mutually exclusive health states (Supplementary Figure 1, available online in Supplementary Appendix, p. 4). Health states distinguished HPV infection with type 16, type 18, other high-risk types, and other low-risk types; CIN grade 1 (CIN1) and CIN grades 2 and 3 (CIN2,3); and local, regional, and distant cervical cancer. The model was stochastic in that a random number generator and a set of estimated probabilities were used to determine the sequence of clinical pathways that each individual followed until death. Each woman’s clinical course was tracked, with a running tally maintained for all events, length of time spent in each health state, and the cost and quality of life associated with each health state. Simulating 1 000 000 individuals one at a time provided stable estimates of long-term outcomes for each strategy. Summary statistics for each individual were compiled to compute population measures such as average lifetime costs and quality-adjusted life expectancy.
CONTEXT AND CAVEATS.
Prior knowledge
Cervical cancer screening by cytology has reduced mortality from invasive cancer, although disparities exist in access to screening and outcomes. Human papillomavirus (HPV) DNA testing and vaccination against HPV-16,18 have recently become available.
Study design
A simulation model was used to assess quality-adjusted life years, lifetime costs, and incremental cost-effectiveness ratios of screening, vaccination of preadolescent girls (girls younger than 12 years), and vaccination combined with screening. Screening was varied by start age (18, 21, or 25 years), interval between screenings (1, 2, 3, or 5 years), test (HPV DNA testing or cytologic testing with a Pap test, or both combined), and age at which tests switched (25, 30, or 35, or no switch).
Contribution
For both vaccinated and unvaccinated women, age-based screening with HPV DNA testing as a triage test for equivocal results in younger women and as a primary screening test in older women is expected to be more cost-effective than current screening recommendations.
Implications
Achieving this potential will likely depend on high vaccine coverage in preadolescent girls, communication of clear messages to both vaccinated and unvaccinated women about continuing screening and following appropriate age-based guidelines, and targeted efforts to recruit and screen women with historically poor access to cervical cancer prevention.
Limitations
Vaccine uptake, vaccine effectiveness, duration of HPV-type–specific immunity, screening compliance, and behavior of subgroups of women defined by vaccination status, race, access to preventive care, and other factors are uncertain.
In our model, movement between health states occurred according to transition probabilities (ie, model input parameters) that depended on HPV type, age, history of previous HPV infection, type-specific natural immunity, previously treated CIN, and screening pattern. Incidence of HPV infection was a function of age and individual-level characteristics but did not change over time directly in response to sexual activity or population prevalence. Instead, we explored indirect effects on risk of HPV infection, which are potentially conferred via herd immunity, by modifying incidence rates on the basis of output from a dynamic transmission model (22,23) in a sensitivity analysis. The input parameters used in the model represent current knowledge about the natural history and epidemiology of HPV infection and cervical carcinogenesis. That is, most women with HPV infection will develop cervical abnormalities reflective of a productive HPV infection, and, although some will progress to high-grade CIN, the majority will regress on their own. Similarly, women with persistent high-risk HPV infection and high-grade CIN may progress to invasive cancer. Women with cancer may be identified as a result of eventual symptoms that prompt medical attention or abnormalities discovered via screening tests, whereas others remain undetected and progress to more advanced stages of cancer. Women with cancer are subject to stage-specific survival rates (24), and all women face competing mortality risks from other causes (25). The data and methods used in this analysis have been described previously (17).
Model Calibration
After deriving initial estimates and ranges for each model parameter from published literature, the model was empirically calibrated to epidemiologic data from North America (17). Briefly, 1 000 000 unique natural history parameter sets were generated by sampling values from the ranges defined for each parameter. Numerical simulations were undertaken with each set of sampled parameter values. Model outcomes produced by each parameter set were scored according to their fit with multiple calibration targets, including age-specific and type-specific prevalence of HPV, age-specific prevalence of CIN, HPV type distribution within different grades of CIN and cervical cancer, and age-specific cancer incidence. A subset of these parameter sets was then selected by use of a likelihood-based goodness-of-fit criterion obtained from the 95% confidence intervals of the calibration data. Examples of the model output from a random sample of 50 good-fitting parameter sets, compared with the 95% confidence intervals of empirical data on prevalence of infection with high-risk HPV; age-specific cancer incidence; and type distribution of HPV within CIN1, CIN2,3, and cervical cancer are shown in Supplementary Figure 2 (available online in the Supplementary Appendix, pp. 6,7).
To explicitly incorporate the effect of parameter uncertainty (ie, second-order uncertainty), we conducted cost-effectiveness analyses that used each of the 50 good-fitting parameter sets. Using these results, we have reported the mean reduction in lifetime risk of cancer as well as the projected range of reduction across the good-fitting parameter sets. As recommended (26), incremental cost-effectiveness ratios have been reported as the ratio of the mean costs divided by the mean effects for the goodfitting parameter sets.
Strategies
Screening strategies varied by primary screening test, triage test conducted for abnormal results, age of screening initiation, screening frequency, and HPV vaccination (Supplementary Table 1, available online in the Supplementary Appendix, pp. 15,16). Specific screening protocols included: 1) cytologic evaluation of cervical cells with a Pap test (cytology) followed by HPV DNA testing of cervical specimens (HPV test) for atypical squamous cells of uncertain significance (ASCUS) (cytology with HPV test triage); 2) HPV DNA testing followed by cytology for positive HPV DNA results (HPV test with cytology triage); and 3) HPV DNA testing and cervical cytology in combination. Strategies were permitted to differ by age group, allowing for the screening protocol to change one time, at age 25, 30, or 35 years. Vaccination was assumed to occur before age 12 years (referred to as preadolescent vaccination in this article) and could occur alone or in combination with different screening strategies.
We conducted two general analyses. The first analysis considered all screening strategies in the absence of vaccination. This analysis was intended to provide information for screening guidelines for unvaccinated women. The second analysis considered all screening strategies with and without HPV vaccination. This analysis was intended to provide information for future cervical cancer prevention strategies for girls who are eligible to be vaccinated now.
We made the following assumptions: 1) All abnormal cytology results, except for those of ASCUS, are subject to colposcopy and biopsy. 2) Women with cytology results of ASCUS are managed by use of triage HPV DNA testing (2). 3) Women with cytology results of ASCUS who test positive for high-risk HPV types receive colposcopy and biopsy examinations, whereas those who test negative for high-risk HPV types return to routine screening. 4) Among women with true underlying CIN2,3, colposcopy and biopsy examinations determine the actual histology of the cervix. 5) All women with a confirmed histology of CIN2,3 or worse are treated according to standard guidelines (2). 6) Women with histologically confirmed CIN1 are not treated but are monitored every 6–12 months until they have three negative screening test results (2). 7) Women with any confirmed abnormality, even if treated successfully for CIN, are screened at least annually until there are three consecutive negative results. 8) Women who are positive for high-risk HPV on their primary screening test but who have a negative cytology triage test result are rescreened at 6 and 12 months and returned to routine screening after three consecutive negative results (6).
For strategies that incorporate vaccination, we assumed that 1) preadolescent vaccination occurs by age 12 years and is 100% effective in preventing infection with HPV-16 and HPV-18, 2) all girls receive the recommended three doses of vaccine, 3) vaccinated girls could be infected with non-16,18 types of HPV according to the same probabilities that would have governed these infections in the absence of vaccination, and 4) duration of vaccine-induced immunity is lifelong. Given the uncertainty about the long-term efficacy of HPV vaccination, we evaluated the implications of alternative assumptions in sensitivity analyses.
In the base case, we assumed 100% coverage with screening and vaccination to enable unbiased comparison of primary and secondary prevention strategies, although lower coverage levels were considered in sensitivity analyses. We also evaluated a number of alternative scenarios that included: 1) simulation of current screening practice in the United States, in which screening patterns were based on those observed in large population-based studies with various levels of screening coverage and frequency for different subpopulations; 2) scenarios in which there was differential vaccine uptake by women of different ages (12, 21–24, and 25–29 years); and 3) scenarios of reduced screening coverage and frequency in the setting of vaccination. Further details of these exploratory analyses are presented in the Supplementary Appendix (pp. 22,122, available online).
Although the main analyses considered the effect of cervical cancer prevention on the health of all women in the United States, we also conducted exploratory analyses to provide insight into the potential differential impact of cervical cancer prevention technologies for subpopulations of women who were less likely to have access to high-quality secondary cancer prevention services. By use of data from population-based screening programs and surveys of past screening history (27–30), we modeled various levels of screening coverage and frequency for different subpopulations to reflect observed differences in screening practices in racial and ethnic subgroups (Supplementary Appendix, pp. 22,123, available online). We then projected outcomes under alternative hypothetical scenarios in which use of HPV DNA testing, vaccine coverage, and screening frequency with new strategies differed by subgroup.
Data
Data used in the natural history model have been documented previously (17). Data for costs, screening test performance, and quality of life (7–10,31–51) are shown in Table 1. Direct medical costs associated with screening strategies and cancer treatment were obtained from the published literature (31–36). Costs associated with vaccination included the costs of three doses of the vaccine, which were based on manufacturer prices (37), and of provider time for administration and counseling, disposable supplies, equipment, and facilities, which were based on published data for other vaccines and HIV testing programs (38–43). For all strategies, we included direct nonmedical costs, such as transportation and patient time costs (36,44,45). Screening test characteristics were based on published studies (7–10,46,47). We used age-specific quality of life weights derived from population-based data (48) and stage-specific weights for cervical cancer (49), as described previously (50). We assumed a multiplicative relationship between age- and stage-specific quality weights for women with cervical cancer.
Table 1.
Selected model variables*
| Variable (reference) | Base case | Plausible range
|
|
|---|---|---|---|
| Minimum | Maximum | ||
| Direct medical and nonmedical costs (31–45)† | |||
| Screening test, US$ | |||
| Office visit | 25 | 12 | 115 |
| Cytology screening test (Pap smear) | 30 | 6 | 87 |
| HPV DNA test (Hybrid Capture II) | 55 | 14 | 217 |
| Patient time and transport | 24 | 12 | 217 |
| Diagnostic follow-up, US$ | |||
| Office visit | 57 | 29 | 115 |
| Colposcopy | 341 | 62 | 651 |
| Biopsy | 50 | 18 | 71 |
| Patient time and transport | 48 | 23 | 84 |
| Treatment of CIN2,3, US$ | |||
| Office visit | 105 | 105 | 105 |
| Procedures and follow-up‡ | 3116 | 198 | 12 925 |
| Cancer treatment, US$ | |||
| Local invasive cervical cancer | 24 477 | 16 740 | 29 225 |
| Regional invasive cervical cancer | 26 197 | 18 768 | 35 623 |
| Distant invasive cervical cancer | 41 959 | 22 041 | 55 964 |
| HPV vaccine cost per dose, US$§ | |||
| Vaccine and wastage | 134 | 100 | 300 |
| Supplies and administration | 9 | 4.5 | 27 |
| Patient time and transport | 24 | 12 | 72 |
| Test characteristics (7–10,46,47) | |||
| Cervical cytology|| | |||
| Sensitivity, % | |||
| CIN1 | 70.0 | 18.6 | 99.0 |
| CIN2,3 and cancer | 80.0 | 18.6 | 99.0 |
| Specificity, % | 95.0 | 87.0 | 99.6 |
| HPV DNA test (Hybrid Capture II)¶ | |||
| Sensitivity (CIN2,3 and cancer), % | 83.0 | 70.0 | 85.0 |
| Specificity, % | 93.0 | 79.0 | 94.0 |
| Quality of life weights (48–50) | |||
| Age-related quality weight | |||
| <20 y | 1.000 | ||
| 20–29 y | 0.913 | ||
| 30–49 y | 0.893 | ||
| 50–59 y | 0.837 | ||
| 60–69 y | 0.811 | ||
| 70–79 y | 0.771 | ||
| >79 y | 0.724 | ||
| Cancer-related quality weight | |||
| Local invasive cervical cancer | 0.680 | ||
| Regional invasive cervical cancer | 0.560 | ||
| Distant invasive cervical cancer | 0.480 | ||
HPV = human papillomavirus; CIN = cervical intraepithelial neoplasia.
Costs were inflation adjusted to constant 2004 US dollars by use of the medical component of the Consumer Price Index (51).
The costs associated with CIN2,3 represent an average estimate based on the mixture of procedures currently performed to diagnose and treat CIN2,3. Costs include staff time, supplies, equipment, anesthetic, and facilities used during the procedures, as well as the costs of the small number of complications and any hospitalization needed. Costs also include follow-up screening visits. Additionally, the patient time and transport costs associated with all procedures and visits are also included.
The full course of HPV vaccination requires three doses. The total cost of vaccination is therefore three times the per-dose costs presented in the table.
The distribution of positive cytology results for women with true CIN2,3 was as follows: low-grade squamous intraepithelial lesion = 45.0%; high-grade squamous intraepithelial lesion (HSIL) = 17.0%; atypical squamous cells of uncertain significance = 26.5%; atypical squamous cells that cannot exclude HSIL = 11.5%.
Probability of high-risk HPV DNA being detected by an HPV DNA test, given the presence of high-risk HPV DNA, was assumed to be 100%; however, we defined the clinically relevant sensitivity of HPV DNA testing to be the probability of high-risk HPV DNA being detected by an HPV DNA test, given CIN2,3 or cancer.
Model Performance
The model’s face validity and external consistency were assessed by comparing modeled outcomes with data not used to parameterize or calibrate the model. These results are available in a previous publication (17) and the Supplementary Appendix (pp. 8–12, available online).
Results
Cancer Prevention Effectiveness
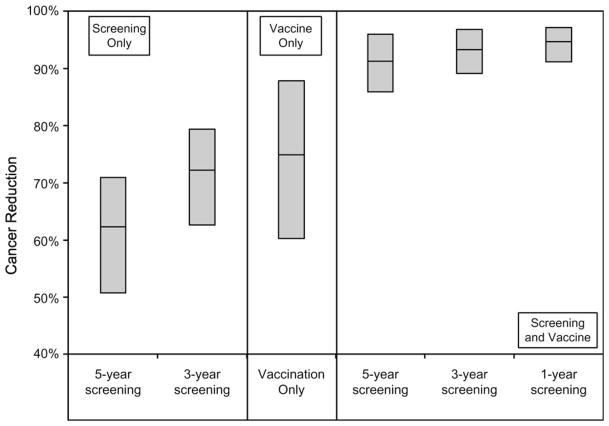
We first modeled the reduction in lifetime risk of cervical cancer associated with all screening and vaccination strategies (results shown for selected strategies in Figure 1). For women screened with cytology with HPV DNA triage for ASCUS starting at age 25 years and switching to HPV DNA testing with cytology triage starting at age 35 years, the expected reductions in lifetime risk of cervical cancer for every-3-year screening alone (71%) was similar to the reductions for preadolescent HPV vaccination alone (75%). However, the uncertainty around the projected benefit was greater for vaccination alone (range = 60%–88%) than for screening alone (range = 61%–77%). Preadolescent vaccination followed by every-3-year screening was more effective than either modality alone and reduced the uncertainty (93%; range = 89%–97%) (for results from all strategies evaluated, see the Supplementary Appendix, pp. 25–34, available online).
Figure 1.
Reduction in the lifetime risk of cervical cancer incidence for selected screening strategies. The range (top and bottom edges of the shaded boxes) represents the minimum and maximum reductions achieved for each strategy when a random sample of 50 good-fitting parameter sets was analyzed; the central horizontal line in each box represents the mean reduction achieved. Strategies depicted are as follows: screening alone every 3 or 5 years; vaccination of preadolescent girls alone; and screening every 1, 3, or 5 years combined with vaccination of preadolescent girls. The screening component of the strategies shown uses cytology with human papillomavirus test triage for atypical squamous cells of uncertain significance starting at age 25 years and HPV DNA testing with cytology triage for women older than 35 years.
Cost-Effectiveness Analysis
The comparative performance of alternative cervical cancer prevention strategies was assessed by calculating incremental cost-effectiveness ratios associated with the efficient (ie, nondominated) set of strategies (Table 2). The costs and benefits associated with strategies that were excluded because they were more costly and less effective (ie, strongly dominated) or more costly and less cost-effective (ie, weakly dominated) than an alternative strategy are included in the Supplementary Appendix (pp. 35–54, available online).
Table 2.
Cost-effectiveness results*
| Screening frequency, y | Screening strategy in younger women† | Start age, y | Screening strategy in older women† | Switch age, y | Preadolescent vaccination | Cost‡, $|| | QALE gains§, QALDs|| | ICER¶, $/QALY |
|---|---|---|---|---|---|---|---|---|
| Evaluation of cervical cancer screening strategies for women who are not vaccinated | ||||||||
| – | Natural history | – | – | – | – | 153 | ** | – |
| 5 | Cytology with HPV triage | 25 | Cytology with HPV triage | None | No | 471 | 16.966 | 7000 |
| 5 | Cytology with HPV triage | 25 | HPV with cytology triage | 35 | No | 562 | 2.747 | 12 000 |
| 5 | Cytology with HPV triage | 25 | HPV with cytology triage | 30 | No | 598 | 0.453 | 29 000 |
| 3 | Cytology with HPV triage | 25 | HPV with cytology triage | 35 | No | 787 | 1.841 | 37 000 |
| 3 | Cytology with HPV triage | 25 | HPV with cytology triage | 30 | No | 844 | 0.391 | 53 000 |
| 3 | Cytology with HPV triage | 21 | HPV with cytology triage | 30 | No | 960 | 0.544 | 78 000 |
| 2 | Cytology with HPV triage | 21 | HPV with cytology triage | 30 | No | 1297 | 1.012 | 122 000 |
| 2 | Cytology with HPV triage | 21 | HPV with cytology triage | 25 | No | 1423 | 0.183 | 252 000 |
| 2 | Cytology with HPV triage | 18 | HPV with cytology triage | 25 | No | 1533 | 0.139 | 291 000 |
| 1 | Cytology with HPV triage | 21 | HPV with cytology triage | 30 | No | 2228 | 0.449 | 564 000 |
| 1 | Cytology with HPV triage | 18 | HPV with cytology triage | 30 | No | 2458 | 0.142 | 592 000 |
| 1 | Cytology with HPV triage | 18 | HPV with cytology triage | 25 | No | 2603 | 0.069 | 736 000 |
| 1 | HPV with cytology triage | 18 | HPV with cytology triage | None | No | 2965 | 0.040 | 3 242 000 |
| 1 | Cytology with HPV triage | 18 | HPV–cytology combination | 25 | No | 3319 | 0.033 | 3 986 000 |
| Evaluation of cervical cancer prevention strategies (screening and/or preadolescent vaccination) for girls eligible to be vaccinated | ||||||||
| – | Natural history | – | – | – | – | 153 | ** | – |
| N/A | Vaccination only | N/A | N/A | N/A | Yes | 534 | 21.557 | 6000 |
| 5 | Cytology with HPV triage | 25 | Cytology with HPV triage | None | Yes | 875 | 3.755 | 33 000 |
| 5 | Cytology with HPV triage | 25 | HPV with cytology triage | 35 | Yes | 942 | 0.595 | 41 000 |
| 5 | Cytology with HPV triage | 25 | HPV with cytology triage | 30 | Yes | 962 | 0.062 | 126 000 |
| 3 | Cytology with HPV triage | 25 | HPV with cytology triage | 35 | Yes | 1161 | 0.384 | 188 000 |
| 3 | Cytology with HPV triage | 25 | HPV with cytology triage | 30 | Yes | 1199 | 0.058 | 243 000 |
| 3 | HPV with cytology triage | 25 | HPV with cytology triage | None | Yes | 1255 | 0.044 | 467 000 |
| 3 | Cytology with HPV triage | 21 | HPV with cytology triage | 25 | Yes | 1325 | 0.044 | 571 000 |
| 2 | Cytology with HPV triage | 25 | HPV with cytology triage | 30 | Yes | 1482 | 0.095 | 596 000 |
| 2 | Cytology with HPV triage | 21 | HPV with cytology triage | 30 | Yes | 1627 | 0.058 | 944 000 |
| 2 | Cytology with HPV triage | 21 | HPV with cytology triage | 25 | Yes | 1698 | 0.022 | 1 092 000 |
| 2 | Cytology with HPV triage | 18 | HPV with cytology triage | 25 | Yes | 1811 | 0.026 | 1 607 000 |
| 2 | Cytology with HPV triage | 18 | HPV–cytology combination | 30 | Yes | 2120 | 0.040 | 2 765 000 |
| 2 | Cytology with HPV triage | 18 | HPV–cytology combination | 25 | Yes | 2243 | 0.011 | 4 092 000 |
| 1 | Cytology with HPV triage | 18 | HPV with cytology triage | 25 | Yes | 2858 | 0.051 | 4 703 000 |
| 1 | Cytology with HPV triage | 18 | HPV–cytology combination | 30 | Yes | 3300 | 0.026 | 6 239 000 |
| 1 | Cytology with HPV triage | 18 | HPV–cytology combination | 25 | Yes | 3529 | 0.004 | 12 749 000 |
QALE = quality-adjusted life expectancy; QALD = quality-adjusted life day; ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life year; HPV = human papillomavirus; N/A = not applicable. Strategies shown are those that remained after excluding the strategies that were more costly and less effective (ie, strongly dominated) or less costly and less cost-effective (ie, weakly dominated) than an alternative strategy.
Screening strategies for younger and older women included cytology with HPV triage (cytology followed by HPV DNA testing for atypical squamous cells of unknown significance results), HPV with cytology triage (HPV DNA testing followed by cytology for high-risk HPV-positive results), and HPV–cytology combination (combined HPV DNA testing and cytology).
Costs are expressed in discounted 2004 US dollars. Costs represent the total expected lifetime cost of a given strategy.
QALE is expressed as discounted QALDs. QALE gains are expressed as incremental benefits, gains compared with the next most costly strategy on the efficient frontier.
Costs and QALYs represent expected costs and expected QALYs across 50 good-fitting parameter sets.
The ICER was calculated as the difference in costs divided by the difference in QALEs between a given strategy and the next most expensive strategy.
The discounted QALE for natural history (no screening and no vaccination) for 9-year-old females entering the model is 26.67212 discounted QALYs or 9741.992 discounted QALDs.
Evaluating Cervical Cancer Screening Strategies for Unvaccinated Women
In the absence of vaccination, cytology screening with HPV triage for ASCUS every 3 years starting at age 21–25 years and HPV DNA testing with cytology triage in women older than 30 years was consistently identified as an efficient strategy (Table 2). Depending on whether screening was started at age 25 or 21 years, this strategy cost $53 000 or $78 000 per QALY, respectively, compared with the next best alternative. Variants of this strategy were less cost-effective. For example, if screening began at age 18 years and was conducted every 2 years thereafter and if the switch to HPV DNA testing with cytology triage was made at age 25 years rather than at age 30 years, then the strategy would cost nearly $300 000 per QALY. For comparison, annual cytology screening with HPV triage beginning at age 18 years followed by a switch to combined HPV DNA testing and cytology at age 25 years provided an expected QALY gain of 46 minutes at a cost of approximately $4 million per QALY, compared with the next best strategy.
Evaluating Future Cervical Cancer Prevention Strategies for Girls Eligible To Be Vaccinated Now
We next considered the costs and effects of all strategies, including screening only, preadolescent vaccination only, and screening and vaccination used in combination (Table 2). If we assumed equivalent coverage with vaccination and screening and lifelong immunity with vaccination, then strategies that used both screening and vaccination generally dominated strategies that used screening alone. Preadolescent vaccination followed by screening with cytology and HPV triage every 5 years beginning at age 25 years and switching to HPV DNA testing with cytology triage at age 35 years had an expected incremental cost-effectiveness ratio of $41 000 per QALY compared with the next best strategy. Accelerating the screening schedule in this strategy to every 3 years instead of every 5 years provided an additional 2% reduction in cancer but had an incremental cost of $188 000 per QALY compared with the next best option. For women vaccinated as preadolescents, screening more frequently and beginning screening at earlier ages with this same age-based protocol were projected to cost more than $500 000 per QALY and to exceed $1 million per QALY for several variants of this strategy.
Sensitivity Analyses
We capitalized on the random sample of 50 unique good-fitting input parameter sets that were identified through our calibration procedure to assess the frequency with which strategies having certain attributes were preferred, given a cost-effectiveness threshold between $50 000 and $100 000 per QALY. We considered the following scenarios: 1) a lifelong vaccine efficacy of 100% (base case); 2) a lifelong vaccine efficacy of 75%; and 3) a 15-year duration of 100% vaccine-induced immunity (Table 3). The results were more sensitive to waning immunity than to lower vaccine efficacy. For example, by use of our 50 good-fitting input parameter sets, vaccination was included as a preferred cervical cancer prevention strategy 51% of the time with a vaccine efficacy of 75% but only 15% of the time if vaccine-induced immunity lasted only 15 years. Screening more frequently (every 3 vs every 5 years) and, to a lesser degree, beginning screening earlier (age 21 vs 25 years) compensated for lower vaccine efficacy and waning immunity. The use of cytology as a primary screening test for women who start screening between ages 21 and 25 years and continue screening until the ages of 30–35 years was identified as part of a preferred efficient strategy more than 90% of the time for all three scenarios. Similarly, for women older than 30–35 years, using HPV DNA testing as a primary screening test with triage cytology in HPV-positive women was preferred 87% of the time when vaccine efficacy was 100% and was always preferred with lower vaccine efficacy. The exact optimal age between ages 30 and 35 years to switch to an HPV-based screening strategy was less certain, although under the scenarios considered, switching at age 25 years was never preferred more than 10% of the time.
Table 3.
Frequency that strategies are preferred under the assumption of a cost-effectiveness threshold of $50 000–$100 000 per quality-adjusted life year under alternative scenarios of vaccine effectiveness and duration of protection*
| Variable | Vaccine effectiveness, duration of protection
|
||
|---|---|---|---|
| 100%, lifelong | 75%, lifelong | 100%, 15 y | |
| Use of vaccination | |||
| No | 0 | 49 | 85† |
| Yes | 100† | 51 | 15 |
| Screening frequency | |||
| None | 4 | 0 | 0 |
| Every 5 y | 96† | 13 | 4 |
| Every 3 y | 0 | 87† | 95† |
| Every 2 y | 0 | 0 | 1 |
| Every 1 y | 0 | 0 | 0 |
| Starting age for screening | |||
| None | 4 | 0 | 0 |
| 25 y | 96† | 83 | 66 |
| 21 y | 0 | 17 | 34 |
| 18 y | 0 | 0 | 0 |
| Primary screening technology for younger women | |||
| None | 4 | 0 | 0 |
| Cytology | 92† | 97† | 99† |
| HPV DNA testing | 4 | 3 | 1 |
| Primary screening technology for older women | |||
| None | 4 | 0 | 0 |
| Cytology | 9 | 0 | 0 |
| HPV DNA testing | 87† | 100† | 100† |
| Cytology–HPV testing combination | 0 | 0 | 0 |
| Age at screening technology switch | |||
| No switch | 18 | 3 | 1 |
| 35 y | 66 | 31 | 34 |
| 30 y | 16 | 60 | 55 |
| 25 y | 0 | 5 | 10 |
HPV = human papillomavirus. Data are frequency (or percentage of time) that strategies with a given characteristic were preferred across a random sample of 50 good-fitting parameter sets for cost-effectiveness thresholds of $50 000–$100 000 per quality-adjusted life year (QALY). Frequencies shown were estimated by use of 11 thresholds between $50 000 and $100 000 per QALY in $5000 increments and then averaged.
Strategy characteristics with high levels of certainty (≥85% of the time part of the cost-effective strategy).
Simulating indirect herd immunity effects by reducing the underlying risk of infection with both HPV-16 and HPV-18 by 10%–30% in unvaccinated women lowered the incremental cost-effectiveness ratios by 15%–30% for the preferred strategies identified in the base case (see the Supplementary Appendix, pp. 21,118,119, available online), although no plausible scenarios were identified in which vaccination alone would be more effective than vaccination plus screening. When we included the averted costs associated with CIN1 and genital warts attributable to HPV-6 and -11, which are expected with use of the quadrivalent vaccine (13), the rank ordering of strategies remained unchanged, although the incremental cost-effectiveness ratio for vaccination was 10%–30% lower. When we included vaccine-conferred cross protection against other high-risk HPV types not targeted by the vaccine, as recently reported (14,15,52), incremental cost-effectiveness ratios changed by less than 10%, and the main results remained stable (see the Supplementary Appendix, p. 23; available online).
For women between 25 and 64 years old who were vaccinated as preadolescents, the estimated positive predictive value of an abnormal cytology result (CIN2,3 or worse) was reduced by 45%–64%, compared with positive predictive value of cytology for women of similar ages who had not been vaccinated (see the Supplementary Appendix, pp. 23,124, available online). If the sensitivity of cytologic evaluation was reduced by more than 15% (eg, below 70% for CIN2,3), then HPV DNA testing with cytology triage became increasingly attractive (see the Supplementary Appendix, pp. 21,120, available online). Changes in the specificity of HPV DNA testing were more influential than changes in test sensitivity. For example, compared with cytology-based strategies, HPV DNA testing with cytology triage every 5 years remained an efficient strategy even when the sensitivity for CIN2,3 was as low as 70%. In contrast, when the specificity of HPV DNA testing was less than 85%, cytology-based screening with HPV DNA testing when ASCUS was detected was preferred (see the Supplementary Appendix, pp. 23,125,126, available online).
Exploring Uncertainties Associated with Adding Vaccination to Current Screening Practice
When we used current US screening practice as a comparator and hypothetically assumed that screening behavior would not change in future birth cohorts, adding preadolescent vaccination provided an additional 36% reduction in cancer risk (see the Supplementary Appendix, p. 121, available online). It is important to note that this average reduction assumed 1) a homogeneous population where all women were equally likely to benefit from screening and vaccination, 2) the screening behavior of the vaccinated birth cohort would exactly mirror current patterns of screening, and 3) screening would not include primary testing with HPV DNA testing or with combined HPV DNA testing and cytology. To examine these assumptions, we conducted a series of exploratory analyses intended to provide qualitative insight into several alternative scenarios, as detailed below.
Impact of Different Patterns of Vaccine Uptake by Age and Changes in Screening Behavior
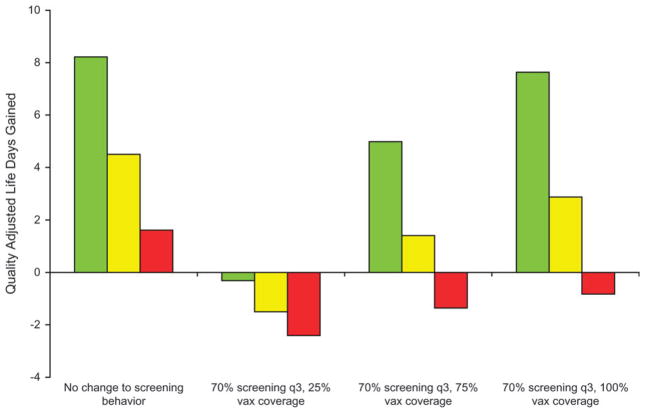
We first used our model to project the expected incremental benefit (QALYs) associated with opportunistic vaccination of women aged 21–24 or 25–29 years, rather than a targeted program that covered all preadolescents, and the influence of their subsequent screening behavior on overall outcomes. The marginal benefit of vaccinating women aged 25–29 years was approximately 80% lower than the benefit of vaccinating girls as preadolescents (before age 12 years) (Figure 2). To explore the impact of changes in population screening behavior that might be directly or indirectly attributable to the introduction of a type-specific vaccine, we considered the hypothetical scenario that 30% of all women would not be screened or would be screened much less frequently after they reached the recommended age to begin screening (presumably because of the misconception that they were otherwise protected or because of general confusion about cervical cancer screening messages) and that 70% would receive at least triennial screening. The results of this scenario are shown in Figure 2 for three levels of vaccine coverage (25%, 75%, and 100%). The marginal benefit of HPV vaccination was more easily diminished for women vaccinated at age 21–24 or 25–29 years than for girls vaccinated as preadolescents (before age 12 years) if regular screening could not be maintained, even at vaccine coverage levels that were greater than 75%. Given these assumptions about age of vaccine uptake and changes in screening behavior after the introduction of vaccination, we identified conditions in which the quality-adjusted life expectancy was lower than with current screening practice—for example, when vaccination coverage was less than 75% among women aged 21–29 years and was accompanied by decreased screening (Figure 2).
Figure 2.
Potential impact of opportunistic human papillomavirus 16,18 (HPV-16,18) vaccine uptake in women aged 21–29 years and changes in cervical cancer screening behavior. The incremental benefit shown is associated with opportunistic vaccination of women aged 21–24 years (yellow bars) and 25–29 years (red bars), rather than with a targeted program that covers all preadolescent girls aged 9–12 years (green bars), and the influence of their subsequent screening behavior on overall outcomes. For three levels of vaccine coverage (25%, 75%, and 100%), expected incremental changes in quality-adjusted life expectancy associated with less frequent screening, compared with current screening practice, are shown. q3 = triennial screening; vax = HPV vaccination.
Potential Impact of HPV DNA Testing and HPV-16,18 Vaccination on Disparities
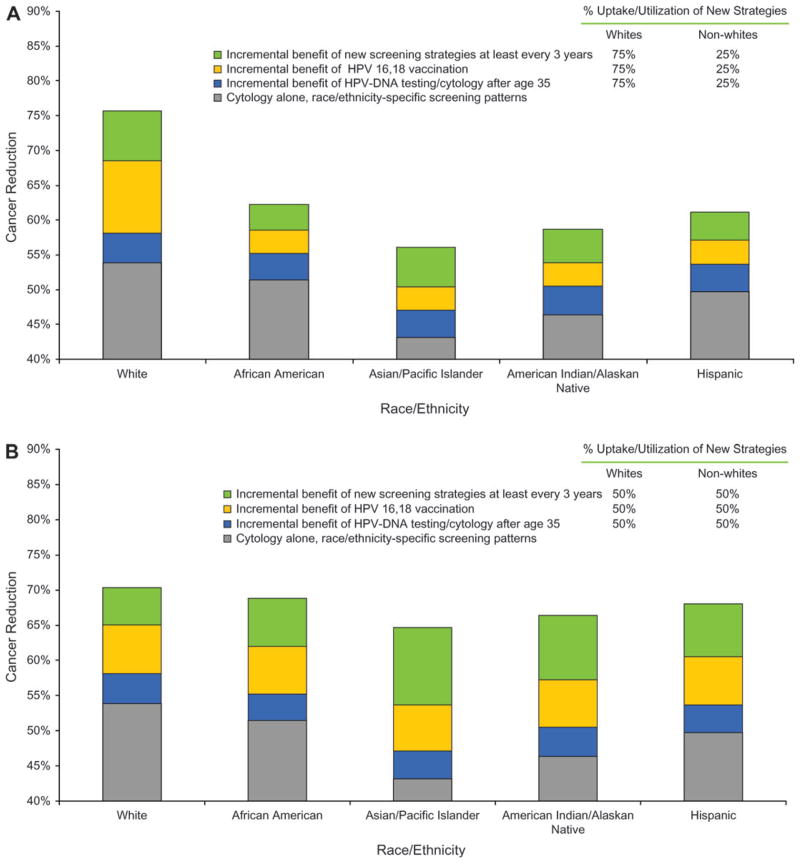
Higher proportions of women in minority racial and ethnic groups than white women have developed invasive cervical cancer in the last two decades, in part because of barriers to access of screening services and their underuse (24). If screening strategies with more sensitive tests and HPV-16,18 vaccination were preferentially used by those most likely to have been screened regularly in the past or for adolescents most likely to be screened in the future, disparities could widen. Specifically, if the lowest-risk subpopulation of white women preferentially accessed these newer services (75% shift from current screening to new services and recommendations) compared with 25% uptake by racial and ethnic minorities, existing differences in expected reduction in cancer widened between these groups (Figure 3, A), with minimal overall change in population-based cervical cancer incidence. If, however, there were specific efforts to ensure equal access and utilization of HPV vaccination and new screening strategies among all racial and ethnic subgroups, disparities in cervical cancer reduction could be reduced (Figure 3, B).
Figure 3.
Potential impact of human papillomavirus (HPV) DNA testing and HPV-16,18 vaccination on disparities in cervical cancer risk reduction among racial and ethnic groups in the United States. We modeled differential uptake and use of HPV DNA testing and cytology triage in women older than 35 years, of HPV-16,18 vaccination uptake, and of increased adherence to a triennial screening schedule. A) Hypothetical scenario that would worsen disparities. In this scenario, there is preferential uptake (75%) of newer prevention strategies by the lowest-risk subpopulation of white women compared with reduced uptake (25%) in racial and ethnic minority women. B) Hypothetical scenario that would lessen disparities. In this scenario, there is equal level of uptake (50%) in all women, regardless of racial and ethnic group, of newer prevention strategies. In both A and B, gray = the expected reduction in cancer with racial- and ethnicity-specific screening patterns and frequencies by use of cytology alone; blue = the additional incremental benefit conferred by HPV DNA testing and cytology triage in women older than 35 years; orange = the additional incremental benefit conferred by preadolescent HPV vaccination; and green = the additional incremental benefit conferred by increased adherence to a triennial screening schedule.
Discussion
For both vaccinated and unvaccinated women, age-based screening protocols that use HPV DNA testing as a triage test for equivocal results in younger women and as a primary screening test in older women have the potential to be more effective than current screening recommendations. For women who will not be vaccinated, screening by cervical cytology with HPV triage starting between the ages of 21 and 25 years and HPV DNA testing with cytology triage for women older than 30 years was found to be more cost-effective than screening all ages by cervical cytology alone, cervical cytology with HPV DNA testing as a triage for equivocal results, or by combination cytology and HPV DNA testing, when a threshold of $50 000–$100 000 per QALY was used. For girls vaccinated by age 12 years, screening by cervical cytology with HPV triage every 5 years starting at age 25 years and switching at age 35 years to HPV testing with cytology triage was also found to be cost-effective at the same threshold. Ensuring that vaccination is preferentially targeted to achieve high coverage in preadolescent females, especially those who may be at a future disadvantage for regular screening, and emphasizing the need for continued screening even in vaccinated women will be critical to achieving population reductions in cervical cancer.
There is great promise in the availability of accurate HPV diagnostics, new screening strategies, and a preventive vaccine against both HPV-16 and HPV-18 for cervical cancer prevention in the United States. As consensus guidelines are developed, decision analyses of how best to use these new options alone or in combination can provide insight for policy deliberations and identify priority research areas. Given current data limitations, particularly with respect to the long-term effects of HPV-16,18 vaccination, analyses should reflect uncertainties related to the natural history of type-specific HPV infections, the performance of HPV-16,18 vaccination and of new screening strategies, and the response of women in the general population to new screening guidelines in the context of an overwhelming amount of new information about HPV-16,18 vaccination and HPV DNA testing. Because not all women in the United States have benefited equally from cervical cancer screening, it is also important to highlight the potential for widening or narrowing of disparities that could accompany changes in cervical cancer prevention policies.
Reports from the last major update to US cervical cancer screening guidelines were published between 2001 and 2003 (2–6). These reports recommended 1) initiating screening 3 years after women become sexually active or between the ages of 18 and 21 years, 2) screening yearly with conventional cytology methods or every 2–3 years with liquid-based cytology methods, 3) using HPV DNA testing only to determine whether diagnostic follow-up is necessary for women with equivocal cytology results (ie, ASCUS), and 4) discontinuing screening between ages 65 and 70 years for women without indicators of elevated risk. Food and Drug Administration approval was expanded to include the use of cytologic evaluation and HPV DNA testing in combination for women older than 30 years, although only interim guidelines have been published that recommend 3-year screening intervals and repeat screening at 6- to 12-month intervals for women whose results are positive on both screening tests (6). Our findings indicate that it may be worthwhile to consider strategies that differ according to age and vaccination status and to revisit screening options recommended for older women with specific consideration of the role of HPV DNA testing with cytology as triage for HPV-positive results.
If we assume that newly available HPV vaccines provide protection in the community that is commensurate with efficacy results from clinical trials, then preadolescent vaccination of girls before beginning sexual activity followed by cytologic screening every 3–5 years, to begin in a woman aged 25 years, and then switching to HPV DNA testing with cytology triage in a woman aged 35 years was more effective and cost-effective than current screening. This strategy was the most efficient identified for a cost-effectiveness threshold of less than $100 000 per QALY. If the sensitivity of cytology testing were to drop substantially in a vaccinated population but the specificity of HPV testing were to remain stable, then HPV DNA testing with cytology triage every 5 years may eventually be an attractive option for women of all ages.
If high levels of vaccine coverage are attainable in all preadolescents, and in particular for those at greater risk for inadequate screening in their future, we would expect that cervical cancer mortality in this country would decrease and that the current disparities in cervical cancer outcomes could decrease. However, in the hypothetical scenario of low vaccine coverage in preadolescents, opportunistic vaccination of women aged 21–29 years who are already adherent to screening recommendations, and no change in the access to improved screening technology (eg, HPV DNA testing) for women not currently being screened, disparities would be expected to worsen, with little change in overall rates of cervical cancer. In select scenarios that are admittedly based on extreme assumptions about age of vaccine uptake, vaccine efficacy, and screening behavior in a partially vaccinated population, quality-adjusted life expectancy might actually be worse than that under current screening practice. We emphasize that the hypothetical scenarios that we constructed are not based on empiric data but were developed to simulate plausible situations that could occur. Our intent was to provide qualitative insight to policy makers about the consequences, both negative and positive, of differential uptake and utilization of new screening technology and HPV vaccination. The results of these exploratory analyses highlight the importance of careful deliberation and responsible planning for introducing HPV vaccination in the United States and for providing clear messages to women about appropriate screening strategies that will be conditional on their vaccination status and age.
Previous modeling studies have found that HPV DNA testing, whether used to triage cervical cytologic abnormalities as a primary screening test in combination with cytologic examination or as a primary screening test alone, can be cost-effective when compared with primary screening with cytologic examination, provided there are longer intervals between screenings (31,32,36,50,53–56). Several previous modeling studies considered the potential benefits of type-specific vaccination and the cost-effectiveness of vaccination in both developed (57–63) and developing (64,65) countries. Our model expands on this previous work in light of questions raised in the development of the last cervical cancer prevention guidelines. We incorporate new epidemiologic data from multiple sources, account for HPV types not targeted by the vaccine, reflect complexities such as HPV-type–specific natural immunity and vaccine-induced immunity, and consider multiple policy-relevant screening and vaccination technologies, including the previously unevaluated strategy of HPV DNA testing with cytology triage. By using systematic, empirical calibration methods, we explicitly incorporated the uncertainty about the natural history of HPV and cervical cancer into the policy results.
This analysis has several important limitations. Key influential uncertainties include the nature and duration of natural vs vaccine-induced type-specific immunity, whether clearance and reinfection or reactivation predominates in older women, the nature of interactions between HPV types, the behavior of non–vaccine-targeted HPV types over time as an increasing number of birth cohorts achieve high vaccination coverage rates and prevalence of HPV-16 and HPV-18 declines, and the presence of cross protection to non-16 and non-18 types of HPV (66,67). In addition to monitoring long-term outcomes, it will be imperative to monitor the HPV type distribution and changes, if any, in screening test performance within a vaccinated population. Our exploratory analyses of the effect of screening behavior illustrate how the benefits of HPV vaccination could potentially be eroded by lower adherence to screening. It will be important to repeat these analyses as data become available from studies of vaccine uptake, screening compliance, and behavior in subgroups of women defined by vaccination status, race, access to preventive care, and other factors. Additionally, a number of methodologic limitations have already been described in a previous publication (17). For this analysis, we made a deliberate trade-off in choosing a detailed stochastic simulation model that accommodates complex screening strategies and individual history and that represents all HPV types instead of a model that reflects the transmission dynamics of HPV-16 and HPV-18. Although our group has also developed a transmission model that can be linked to this microsimulation model (22,23), it increased the complexity considerably and did not add substantially to our main goal of assessing screening guidelines for both unvaccinated and vaccinated women. Other analyses that focus on assessing the cost-effectiveness of immunizing boys in addition to girls, the optimal age range of a catch-up program in the United States, and the incremental benefits of vaccinating older women (22,23) used this additional component of our transmission model, although they did not include all possible screening strategies. By design, we aimed to obtain results that would be relevant to both the bivalent and quadrivalent HPV vaccines, focusing on the prevention of HPV-16 and HPV-18 infections, which are responsible for more than half of the invasive cervical cancers worldwide. Including the costs and benefits of preventing noncervical cancers caused by HPV-16 and HPV-18 will improve estimates of cost-effectiveness. Finally, although the price of the vaccine is available, the true cost associated with delivering this vaccine to adolescents in the United States—including education, counseling, and delivery mechanisms—is not yet known. Similarly, no estimates are available yet for the costs of monitoring and surveillance. As these data become available, this analysis should be revisited.
There are more than 75 million women in the United States at risk of developing invasive cervical cancer who may not benefit directly from vaccination because they are older than the currently recommended age range for routine vaccination. They do, however, have the opportunity to benefit from new technology and improved screening strategies. For these women, screening using cervical cytology with HPV triage starting between the ages of 21 and 25 years and switching to HPV DNA testing with cytology triage for women older than 30 years is more cost-effective than a single recommendation to use cervical cytology, HPV DNA testing, or both in women of all ages. For the nearly 40 million girls who could be vaccinated in the next 20 years (68), if HPV-16,18 vaccines provide long-lasting immunity, vaccination of preadolescent girls combined with less frequent screening that begins by the age of 25 years would provide comparable protection from invasive cancer and would be considered to be cost-effective. Achieving this potential is likely to depend on high vaccine coverage in preadolescent girls, communication of clear messages to both vaccinated and unvaccinated women about continuing screening and following appropriate age-based guidelines, and targeted efforts to recruit and screen women with historically poor access to cervical cancer prevention.
Supplementary Material
Acknowledgments
Funding
National Science Foundation (Graduate Research Fellowship to J.D.G.-F.); National Cancer Institute (R01 CA093435 to S.J.G. and K.M.K.); Agency for Healthcare Research and Quality (RO1 HSO15570-01A1 to S.J.G. and K.M.K.); Bill and Melinda Gates Foundation (to S.J.G., K.M.K., J.A.S., and N.K.S.); Harvard Center for Risk Analysis (to N.K.S.).
The authors wish to thank Phil Castle at the National Cancer Institute for his thoughtful comments and advice at critical junctures of the analytic work. The authors would like to warmly acknowledge the contributions of the entire cervical cancer prevention team at the Program in Health Decision Science (Harvard School of Public Health) including Jane Kim, Henri Folse, Katie Kobus, Meredith O’Shea, Steven Sweet, Nicole Gastineau Campos, Mireia Diaz Sanchis, and Bethany Andres-Beck. Particular acknowledgment is due to Jesse Ortendahl for his wide-reaching and thorough research assistance.
Footnotes
Author contributions: J. D. Goldhaber-Fiebert had access to the data used in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acquisition of data: J. D. Goldhaber-Fiebert, S. J. Goldie, and K. M. Kuntz.
Analysis and interpretation of the data: J. D. Goldhaber-Fiebert, S. J. Goldie, K. M. Kuntz, and N. K. Stout.
Drafting of the manuscript: J. D. Goldhaber-Fiebert.
Critical revision of the manuscript for important intellectual content: J. D. Goldhaber-Fiebert, S. J. Goldie, K. M. Kuntz, J. A. Salomon, and N. K. Stout.
Statistical analysis: J. D. Goldhaber-Fiebert, K. M. Kuntz, J. A. Salomon, and N. K. Stout.
Obtaining funding: S. J. Goldie.
Administrative, technical, or material support: J. D. Goldhaber-Fiebert and S. J. Goldie.
Study supervision: S. J. Goldie.
Financial disclosure: None reported.
The authors declare that they have no competing interests.
None of the funding agencies had any role in the conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
References
- 1.Wang SS, Sherman ME, Hildesheim A, Lacey JV, Jr, Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000. Cancer. 2004;100(5):1035–1044. doi: 10.1002/cncr.20064. [DOI] [PubMed] [Google Scholar]
- 2.Wright TC, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. 2001 ASCCP-Sponsored Consensus Conference. 2001 Consensus guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287(16):2120–2129. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- 3.Saslow D, Runowicz C, Solomon D, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52(6):342–362. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 4.ACOG Committee on Practice Bulletins. ACOG Practice Bulletin: clinical management guidelines for obstetrician-gynecologists. Number 45, August 2003. Cervical cytology screening (replaces committee opinion 152, March 1995) Obstet Gynecol. 2003;102(2):417–427. doi: 10.1016/s0029-7844(03)00745-2. [DOI] [PubMed] [Google Scholar]
- 5.US Preventive Services Task Force. Guide to Clinical Preventive Services. 3. Washington, DC: US Department of Health and Human Services; 2003. [Google Scholar]
- 6.Wright TC, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103(2):304–309. doi: 10.1097/01.AOG.0000109426.82624.f8. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119(5):1095–1101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 8.Franco EL. Chapter 13: primary screening of cervical cancer with human papillomavirus tests. J Natl Cancer Inst Monogr. 2003;31:89–96. doi: 10.1093/oxfordjournals.jncimonographs.a003488. [DOI] [PubMed] [Google Scholar]
- 9.Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: clinical applications of HPV testing: a summary of meta-analyses. Vaccine. 2006;24(suppl 3):S78–S89. doi: 10.1016/j.vaccine.2006.05.117. [DOI] [PubMed] [Google Scholar]
- 10.Cuzick J, Mayrand MH, Ronco G, Snijders P, Wardle J. Chapter 10: new dimensions in cervical cancer screening. Vaccine. 2006;24(suppl 3):S90–S97. doi: 10.1016/j.vaccine.2006.05.122. [DOI] [PubMed] [Google Scholar]
- 11.Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(29):1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 12.Ault KA Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369(9576):1861–1868. doi: 10.1016/S0140-6736(07)60852-6. [DOI] [PubMed] [Google Scholar]
- 13.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 14.Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367(9518):1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 15.Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 16.Goldie SJ, Goldhaber-Fiebert JD, Garnett GP. Chapter 18: public health policy for cervical cancer prevention: the role of decision science, economic evaluation, and mathematical modeling. Vaccine. 2006;24(suppl 3):S155–S163. doi: 10.1016/j.vaccine.2006.05.112. [DOI] [PubMed] [Google Scholar]
- 17.Goldhaber-Fiebert JD, Stout NK, Ortendahl J, Kuntz KM, Goldie SJ, Salamon JA. Modeling human papillomavirus and cervical cancer in the United States for analyses of screening and vaccination. Popul Health Metr. 2007;5(1):11. doi: 10.1186/1478-7954-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 19.Kamlet MS. The Comparative Benefits Modeling Project: A Framework for Cost-Utility Analysis of Government Health Care Program. Washington, DC: Public Health Service, US Department of Health and Human Services; 1992. [Google Scholar]
- 20.Weinstein MC. From cost-effectiveness ratios to resource allocation: where to draw the line? In: Sloan FA, editor. Valuing Health Care: Costs, Benefits and Effectiveness of Pharmaceuticals and Other Medical Technologies. Cambridge, UK: Cambridge University Press; 1995. pp. 77–98. [Google Scholar]
- 21.Neumann PJ, Sandberg EA, Bell CM, Stone PW, Chapman RH. Are pharmaceuticals cost-effective? A review of the evidence. Health Aff (Millwood) 2000;19(2):92–109. doi: 10.1377/hlthaff.19.2.92. [DOI] [PubMed] [Google Scholar]
- 22.Kim JJ, Andres-Beck B, Goldie SJ. The value of including boys in an HPV vaccination programme: a cost-effectiveness analysis in a low resource setting. Br J Cancer. 2007;97(9):1322–1328. doi: 10.1038/sj.bjc.6604023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JJ, Andres-Beck B, Goldie SJ. Health and economic implications of CDC recommendations for HPV vaccination in the U.S. Abstract presented at the 29th annual SMDM meeting; October 2007; Pittsburgh, PA. [Google Scholar]
- 24.Ries LAG, Melbert D, Krapcho M, et al., editors. Bethesda, MD: National Cancer Institute; 2007. [Accessed January 28, 2008]. SEER Cancer Statistics Review, 1975–2004 based on November 2006 SEER data submission, posted to the SEER web site. http://seer.cancer.gov/csr/1975_2004/ [Google Scholar]
- 25.US Lifetables for Females. National Center for Health Statistics. Centers for Disease Control and Prevention; 2005. [Accessed January 28, 2008]. http://www.cdc.gov/nchs/products/pubs/pubd/lftbls/lftbls.htm. [Google Scholar]
- 26.Stinnett AA, Paltiel AD. Estimating CE ratios under second-order uncertainty: the mean ratio versus the ratio of means. Med Decis Making. 1997;17(4):483–489. doi: 10.1177/0272989X9701700414. [DOI] [PubMed] [Google Scholar]
- 27.Adams EK, Breen N, Joski PJ. Impact of the National Breast and Cervical Cancer Early Detection Program on mammography and Pap test utilization among white, Hispanic, and African American women: 1996–2000. Cancer. 2007;109(2 suppl):348–358. doi: 10.1002/cncr.22353. [DOI] [PubMed] [Google Scholar]
- 28.Benard VB, Lee NC, Piper M, Richardson L. Race-specific results of Papanicolaou testing and the rate of cervical neoplasia in the National Breast and Cervical Cancer Early Detection Program, 1991–1998 (United States) Cancer Causes Control. 2001;12(1):61–68. doi: 10.1023/a:1008959019019. [DOI] [PubMed] [Google Scholar]
- 29.Hewitt M, Devesa SS, Breen N. Cervical cancer screening among U.S. women: analyses of the 2000 National Health Interview Survey. Prev Med. 2004;39(2):270–278. doi: 10.1016/j.ypmed.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 30.Sirovich BE, Welch HG. The frequency of Pap smear screening in the United States. J Gen Intern Med. 2004;19(3):243–250. doi: 10.1111/j.1525-1497.2004.21107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bidus MA, Maxwell GL, Kulasingam S, et al. Cost-effectiveness analysis of liquid-based cytology and human papillomavirus testing in cervical cancer screening. Obstet Gynecol. 2006;107(5):997–1005. doi: 10.1097/01.AOG.0000210529.70226.0a. [DOI] [PubMed] [Google Scholar]
- 32.Kulasingam SL, Kim JJ, Lawrence WF, et al. Cost-effectiveness analysis based on the atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesion Triage Study (ALTS) J Natl Cancer Inst. 2006;98(2):92–100. doi: 10.1093/jnci/djj009. [DOI] [PubMed] [Google Scholar]
- 33.Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus-related disease. Am J Obstet Gynecol. 2004;191(1):114–120. doi: 10.1016/j.ajog.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 34.Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics. 2005;23(11):1107–1122. doi: 10.2165/00019053-200523110-00004. [DOI] [PubMed] [Google Scholar]
- 35.Goldie SJ, Kohli M, Grima D, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst. 2004;96(8):604–615. doi: 10.1093/jnci/djh104. [DOI] [PubMed] [Google Scholar]
- 36.Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol. 2004;103(4):619–631. doi: 10.1097/01.AOG.0000120143.50098.c7. [DOI] [PubMed] [Google Scholar]
- 37.HPV Vaccine Questions and Answers. Atlanta, GA: Centers for Disease Control and Prevention; 2006. [Accessed January 28, 2008]. http://www.cdc.gov/std/hpv/STDFact-HPV-vaccine.htm. [Google Scholar]
- 38.Wallace LA, Young D, Brown A, et al. Costs of running a universal adolescent hepatitis B vaccination programme. Vaccine. 2005;23(48–49):5624–5631. doi: 10.1016/j.vaccine.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 39.Turner DA, Wailoo AJ, Cooper NJ, Sutton AJ, Abrams KR, Nicholson KG. The cost-effectiveness of influenza vaccination of healthy adults 50–64 years of age. Vaccine. 2006;24(7):1035–1043. doi: 10.1016/j.vaccine.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 40.Iskedjian M, Walker JH, Hemels ME. Economic evaluation of an extended acellular pertussis vaccine programme for adolescents in Ontario, Canada. Vaccine. 2004;22(31–32):4215–4227. doi: 10.1016/j.vaccine.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 41.De Wals P, Petit G, Erickson LJ, et al. Benefits and costs of immunization of children with pneumococcal conjugate vaccine in Canada. Vaccine. 2003;21(25–26):3757–3764. doi: 10.1016/s0264-410x(03)00361-x. [DOI] [PubMed] [Google Scholar]
- 42.Mangtani P, Roberts JA, Hall AJ, Cutts FT. An economic analysis of a pneumococcal vaccine programme in people aged over 64 years in a developed country setting. Int J Epidemiol. 2005;34(3):565–574. doi: 10.1093/ije/dyh341. [DOI] [PubMed] [Google Scholar]
- 43.HIV Counseling and Testing System Version 4.0. Atlanta, GA: National Center for HIV, STD, and TB Prevention: Centers for Disease Control and Prevention; 1999. [Google Scholar]
- 44.Cantor SB, Levy LB, Cardenas-Turanzas M, et al. Collecting direct non-health care and time cost data: application to screening and diagnosis of cervical cancer. Med Decis Making. 2006;26(3):265–272. doi: 10.1177/027298906288679. [DOI] [PubMed] [Google Scholar]
- 45.Shireman TI, Tsevat J, Goldie SJ. Time costs associated with cervical cancer screening. Int J Technol Assess Health Care. 2001;17(1):146–152. doi: 10.1017/s0266462301104137. [DOI] [PubMed] [Google Scholar]
- 46.Sherman ME. Chapter 11: future directions in cervical pathology. J Natl Cancer Inst Monogr. 2003;31:72–79. doi: 10.1093/oxfordjournals.jncimonographs.a003486. [DOI] [PubMed] [Google Scholar]
- 47.Solomon D. Chapter 14: role of triage testing in cervical cancer screening. J Natl Cancer Inst Monog. 2003;31:97–101. doi: 10.1093/oxfordjournals.jncimonographs.a003489. [DOI] [PubMed] [Google Scholar]
- 48.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006;26(4):391–400. doi: 10.1177/0272989X06290497. [DOI] [PubMed] [Google Scholar]
- 49.Center for the Evaluation of value and Risk in Health. Tufts New England Medical Center; Boston, MA: [Accessed January 25, 2008]. Catalog of Preference Scores: the CEA Registry. http://research.tufts-nemc.org/cear/default.aspx. [Google Scholar]
- 50.Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. JAMA. 2002;287(18):2382–2390. doi: 10.1001/jama.287.18.2382. [DOI] [PubMed] [Google Scholar]
- 51.Medical Component of the Consumer Price Index. Washington, DC: Census Bureau; 2006. [Accessed January 28, 2008]. http://www.census.gov/prod/2005pubs/06statab/prices.pdf. [Google Scholar]
- 52.Cross-protection data for Gardasil submitted to FDA. AIDS Patient Care STDS. 2007;21(5):370. [PubMed] [Google Scholar]
- 53.Kulasingam SL, Myers ER, Lawson HW, et al. Cost-effectiveness of extending cervical cancer screening intervals among women with prior normal pap tests. Obstet Gynecol. 2006;107(2 pt 1):321–328. doi: 10.1097/01.AOG.0000196500.50044.ce. [DOI] [PubMed] [Google Scholar]
- 54.Sherlaw-Johnson C, Philips Z. An evaluation of liquid-based cytology and human papillomavirus testing within the UK cervical cancer screening programme. Br J Cancer. 2004;91(1):84–91. doi: 10.1038/sj.bjc.6601884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of human papillomavirus DNA testing in the United Kingdom, The Netherlands, France, and Italy. J Natl Cancer Inst. 2005;97(12):888–895. doi: 10.1093/jnci/dji162. [DOI] [PubMed] [Google Scholar]
- 56.Goldie SJ, Kim JJ, Myers E. Cost-effectiveness of cervical cancer screening. Vaccine. 2006;24(suppl 3):S164–S170. doi: 10.1016/j.vaccine.2006.05.114. [DOI] [PubMed] [Google Scholar]
- 57.Barnabas RV, Laukkanen P, Koskela P, Kontula O, Lehtinen M, Garnett GP. Epidemiology of HPV 16 and cervical cancer in Finland and the potential impact of vaccination: mathematical modelling analyses. PLoS Med. 2006;3(5):e138. doi: 10.1371/journal.pmed.0030138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kulasingam SL, Myers ER. Potential health and economic impact of adding a human papillomavirus vaccine to screening programs. JAMA. 2003;290(6):781–789. doi: 10.1001/jama.290.6.781. [DOI] [PubMed] [Google Scholar]
- 59.Sanders GD, Taira AV. Cost-effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis. 2003;9(1):37–48. doi: 10.3201/eid0901.020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brisson M, Van de Velde N, De WP, Boily MC. The potential cost-effectiveness of prophylactic human papillomavirus vaccines in Canada. Vaccine. 2007;25(29):5399–5408. doi: 10.1016/j.vaccine.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 61.Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13(1):28–41. doi: 10.3201/eid1301.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kohli M, Ferko N, Martin A, et al. Estimating the long-term impact of a prophylactic human papillomavirus 16/18 vaccine on the burden of cervical cancer in the UK. Br J Cancer. 2007;96(1):143–150. doi: 10.1038/sj.bjc.6603501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garnett G, Kim JJ, French K, Goldie SJ. Chapter 21: modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine. 2006;24(suppl 3):S178–S186. doi: 10.1016/j.vaccine.2006.05.116. [DOI] [PubMed] [Google Scholar]
- 64.Goldie SJ, Kim JJ, Kobus K, et al. Cost-effectiveness of HPV 16, 18 vaccination in Brazil. Vaccine. 2007;25(33):6257–6270. doi: 10.1016/j.vaccine.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 65.Goldie SJ. A public health approach to cervical cancer control, considerations of screening and vaccination strategies. Int J Gynaecol Obstet. 2006;94(suppl 1):S93–S103. doi: 10.1016/S0020-7292(07)60016-2. [DOI] [PubMed] [Google Scholar]
- 66.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 67.Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007;7(1):11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- 68.Department of Economic and Social Affairs, Population Division, United Nations. World Population Prospects: The 2004 Revision. New York: United Nations publications; 2005. CD-ROM ed.—Extended Dataset. Sales No. E.05.XIII.12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.