Abstract
Objective
To assess 12-month survival, pharmacokinetics, immunologic and virologic efficacy, tolerance, compliance and drug resistance in HIV-infected children in Bobo-Dioulasso, Burkina Faso, receiving once-daily highly-active antiretroviral therapy as a combination of didanosine (DDI), lamivudine (3TC) and efavirenz (EFV).
Methods
In the ANRS 12103 open phase II trial, HIV-infected children were examined at inclusion and monthly thereafter. CD4+ T-lymphocyte (CD4) count, plasma concentration of ribonucleic acid (RNA) of human immunodeficiency virus type 1 (HIV-1) and haematologic and biochemical parameters were measured at baseline and every trimester. HIV-1 resistance testing was performed in case of viral escape. Drug plasma concentrations were determined with high-performance liquid chromatography.
Findings
From February 2006 to November 2007, 51 children (39% girls) with a mean age of 6.8 years were enrolled and treated for 12 months. At baseline, Z scores for mean weight-for-age and mean height-for-age were −2.01 and −2.12, respectively. Mean CD4% was 9.0. Median plasma HIV-1 RNA viral load was 5.51 log10 copies per millilitre (cp/ml). Two children (3.9%) died and another 11 (22%) suffered 13 severe clinical events. At month 12, mean WAZ had improved by 0.63 (P < 0.001) and mean HAZ by 0.57 (P < 0.001). Mean CD4% had risen to 24 (P < 0.001). Viral load was below 300 RNA cp/ml in 81% of the children; HIV resistance mutations were detected in 11 (21.6%).
Conclusion
The once-a-day combination of DDI + 3TC + EFV is an alternative first-line treatment for HIV-1-infected children. Dose adjustment should further improve efficacy.
Résumé
Objectif
Évaluer sur 12 mois la survie, la pharmacocinétique, l'efficacité immunologique et virologique, la tolérance, l'observance et la résistance au médicament chez des enfants infectés par le VIH à Bobo-Dioulasso, Burkina Faso, et qui reçoivent une fois par jour une thérapie antirétrovirale hautement active par une association de didanosine (DDI), de lamivudine (3TC) et d'éfavirenz (EFV).
Méthodes
Dans l'essai ouvert ANRS 12103 phase II, des enfants infectés par le VIH ont été examinés au moment de l'inclusion et ensuite chaque mois. Le taux de lymphocytes T CD4 (CD4), la concentration plasmatique d'acide ribonucléique (ARN) du virus de l'immunodéficience humaine type 1 (VIH-1), ainsi que des paramètres hématologiques et biochimiques ont été mesurés au début de l'étude et chaque trimestre. Le test de résistance au VIH-1 a été effectué dans les cas d'évasion virale. Les concentrations plasmatiques de médicament ont été déterminées par chromatographie liquide à haute performance.
Résultats
De février 2006 à novembre 2007, 51 enfants (39% de filles), avec une moyenne d'âge de 6,8 ans, ont été sélectionnés et traités pendant 12 mois. Au début de l'étude, les écarts réduits pour le poids moyen par âge et la taille moyenne par âge étaient respectivement de -2,01 et -2,12. Le CD4 moyen était 9,0%. La charge virale plasmatique médiane de l'ANR VIH-1 était de 5,51 log10 copies par millilitre (cp/ml). Deux enfants (3,9%) sont décédés et 11 autres (22%) ont souffert 13 événements cliniques sévères. Au 12e mois, le WAZ moyen s'était amélioré de 0,63 (P<0,001) et le HAZ moyen de 0,57 (P<0,001). Le CD4 moyen s'était élevé à 24% (P<0,001). La charge virale était en-dessous de 300 cp/ml ARN pour 81% des enfants ; des mutations de résistance au VIH ont été détectées chez 11 enfants (21,6%).
Conclusion
L'association DDI + 3TC + EFV en une prise quotidienne est un traitement alternatif de première intention pour les enfants infectés au VIH-1. L'adaptation de la dose devrait encore améliorer l'efficacité.
Resumen
Objetivo
Evaluar a 12 meses la supervivencia, la farmacocinética, la eficacia inmunológica y virológica, la tolerancia, el cumplimiento terapéutico y la resistencia a los medicamentos en niños infectados con el VIH en Bobo-Dioulasso (Burkina Faso), que reciben una vez al día un tratamiento antirretrovírico muy activo, constituido por la asociación de didanosina (DDI), lamivudina (3TC) y efavirenz (EFV).
Métodos
En el ensayo clínico ANRS 12103, abierto y de fase II, se examinó a los niños infectados con el VIH en el momento de la inclusión y, a continuación, mensualmente. Se midieron el recuento de linfocitos T CD4+ (CD4), la concentración plasmática de ácido ribonucleico (ARN) del virus de la inmunodeficiencia humana de tipo 1 (VIH-1) y los parámetros hematológicos y bioquímicos, al inicio y cada trimestre. Las pruebas de resistencia al VIH-1 se realizaron en el caso de escape vírico. Las concentraciones plasmáticas del fármaco se determinaron con cromatografía en fase líquida de alta resolución.
Resultados
Desde febrero del 2006 a noviembre del 2007, 51 niños (39% niñas) con una edad media de 6,8 años fueron registrados y tratados durante 12 meses. Al inicio del estudio, las puntuaciones Z del peso medio por edad y la altura media por edad fueron -2,01 y -2,12 respectivamente. El % medio de CD4 fue 9,0. La carga vírica media de RNA del HIV-1 en plasma fue de 5,51 log10 copias por mililitro (cp/ml). Dos niños (3,9%) murieron y otros 11 (22%) sufrieron 13 acontecimientos clínicos graves. En el mes 12, el WAZ medio (peso por edad según la puntuación Z) había mejorado en un 0,63 (p < 0,001) y el HAZ medio (altura por edad según la puntuación Z) en 0,57 (p < 0,001). El % medio de CD4 había subido a 24 (p < 0,001). La carga vírica estaba por debajo de 300 ARN cp/ml en el 81% de los niños; se detectaron mutaciones de resistencia al VIH en 11 (21,6%).
Conclusión
La asociación de DDI + 3TC + EFV una vez al día es un tratamiento alternativo de primera línea para los niños infectados con el VIH-1. Un ajuste de la dosis debería mejorar todavía más la eficacia.
ملخص
الغرض
قياس البقاء على قيد الحياة لمدة 12 شهراً، والحرائك الدوائية، والنجاعة المناعية والفيروسية، والتحمّل، والامتثال، ومقاومة الفيروس للدواء في الأطفال المصابين بفيروس العوز المناعي البشري في بوبو ديولاسو، وبوركينا فاسو، الذين يتلقون مرة واحدة يومياً معالجة نشطة للغاية بمضادات الفيروسات القهقرية كتوليفة مكوّنة من ديدانوزين، ولاميفيودين، وإيفافيرينز.
الطريقة
في المرحلة الثانية المفتوحة من تجربة الوكالة الوطنية الفرنسية لبحوث الإيدز 12103، جرى فحص الأطفال المصابين بفيروس العوز المناعي البشري عند وقت إدراجهم في العلاج وبعد مرور شهر على ذلك. وقيس عدد اللمفاويات التائية CD4+، وتركيز حمض الريبونوكلييك المصلي للنمط الأول من فيروس العوز المناعي البشري، والمتثابتات الدميّة والكيميائية البيولوجية عند المستوى القاعدي وكل ثلاث شهور. أجري اختبار المقاومة لفيروس العوز المناعي البشري من النمط الأول في حال فرار الفيروس. وحددت تركيزات الدواء في المصل عن طريق جهاز تخطيط كروماتوغرافي عالي الأداء.
النتائج
من شباط/فبراير 2006 حتى تشرين الثاني/نوفمبر 2007، أُدرج 51 طفلاً (منهم 39% فتيات) ومتوسط عمرهم 6.8 سنة وعولجوا لمدة 12 شهراً. كان الحرز Z القاعدي لمتوسط الوزن لقاء العمر -2.01 ولمتوسط الطول لقاء العمر -2.12. كان متوسط النسبة المئوية للمفاويات CD4 9.0. وكان وسيط الحمل الفيروسي المصلي للنمط الأول من فيروس العوز المناعي البشري 5.51 لوغاريتم 10 نسخ لكل ملي متر. توفي طفلان (3.9%) وعاني 11طفلاً آخراً (22%) من 13 حادثة إكلينيكية وخيمة. في الشهر الثاني عشر، تحسن متوسط حرز Z للوزن لقاء العمر بمقدار0.63 (قوة الاحتمال P أقل من 0.001) وتحسن متوسط حرز Z للطول لقاء العمر بمقدار0.57 (قوة الاحتمال P أقل من 0.001). وارتفع متوسط النسبة المئوية للمفاويات CD4 إلى 24 (الاحتمال P أقل من 0.001). وكان الحمل الفيروسي أقل من 300 نسخة من حمض الريبونوكلييك في الملي لتر في 81% من الأطفال؛ واكتشفت طفرات لمقاومة فيروس العوز المناعي البشري في 11 طفلاً (21.6%).
الاستنتاج
التوليفة التي تُعطى مرة واحدة يومياً والمكوّنَة من ديدانوزين، ولاميفيودين، وإيفافيرينز تشكل بديلاً لعلاج الخط الأول للأطفال المصابين بالنمط الأول من فيروس العوز المناعي البشري. وسيزيد ضبط الجرعة من تحسّن النجاعة.
Резюме
Цель
Оценить 12-месячную выживаемость, фармакокинетику, иммунологическую и вирусологическую эффективность, толерантность, соблюдение предписаний врача и устойчивость вируса к лекарственным средствам у ВИЧ-инфицированных детей в Бобо-Диуласо (Буркина-Фасо), получавших в режиме однократного суточного приема высокоактивную антиретровирусную терапию (ВААРТ) в виде сочетания диданозина (DDI), ламувидина (3TC) и эфавиренза (EFV).
Методы
В ходе II фазы открытого клинического испытания 12103, выполнявшегося ANRS (Французским национальным агентством по изучению СПИДа), осмотр ВИЧ-инфицированных детей проводился при включении в испытание и далее ежемесячно. Содержание CD 4+ Т-лимфоцитов (CD4), концентрация в плазме крови рибонуклеиновой кислоты вируса иммунодефицита человека типа 1 (РНК ВИЧ-1), а также гематологические и биохимические показатели измерялись на базовом уровне и далее каждый квартал. В случае ускользания вируса из-под иммунологического контроля проводилось тестирование резистентности ВИЧ-1. Содержание лекарственных средств в плазме крови определялось методом высокоэффективной жидкостной хроматографии.
Результаты
В период с февраля 2006 года по ноябрь 2007 года 51 ребенок (39% – девочки, средний возраст – 6,8 лет) были включены в испытание и получали лечение в течение 12 месяцев. На базовом уровне Z-счет для среднего индекса «масса тела – возраст» и среднего индекса «длина тела/рост – возраст» составляли −2,01 и −2,12, соответственно. Среднее процентное содержание CD4 составляло 9,0. Медианная вирусная нагрузка в плазме, измеренная при помощи РНК ВИЧ-1, составляла 5,51 лог10 копий на миллилитр (копий/мл). Два ребенка (3,9%) умерли, а еще 11 (22%) перенесли 13 тяжелых клинических событий. На 12-ом месяце среднее значение WAZ (Z-счет для индекса «длина тела/рост – возраст») улучшилось на 0,63 (P < 0,001), а среднее значение HAZ (Z-счет для индекса «масса тела – возраст») на 0,57 (P < 0.001). Среднее процентное содержание CD4 выросло до 24 (P < 0,001). У 81% детей вирусная нагрузка была ниже 300 РНК копий/мл; мутации резистентности ВИЧ отмечены у 11 детей (21,6%).
Вывод
Применение сочетания DDI + 3TC + EFV в режиме однократного суточного приема является альтернативным методом лечения первого выбора для детей, инфицированных ВИЧ-1. При корректировке дозы эффективность должна повыситься.
摘要
目的
旨在评估布基纳法索博博迪乌拉索艾滋病病毒感染儿童接受每天一次由地达诺新(DDI)、拉米夫定(3TC)和依法韦仑(EFV)组合而成的高活性抗逆转录病毒治疗过程中12个月间的存活率、药物与人体间的相互作用、免疫和病毒学功效、耐受性、依从性和药物抗药性。
方法
法国国家艾滋病研究院(ANRS)12103第二阶段公开试验中,艾滋病病毒感染儿童在纳入研究时以及之后的每个月接受一次检查。人类免疫缺陷病毒1型(HIV-1)核糖核酸的CD4+T-淋巴细胞(CD4)数量和血浆浓度,以及生化参数均进行了基线测量并且每三个月测量一次。在发生病毒逃逸的情况下,则进行HIV-1抗药性测试。血药浓度运用高效液相色谱法确定。
结果
从2006年2月至2007年11月,平均年龄为6.8岁的51名儿童(39%女孩)登记参加试验并接受为期12个月的治疗。基线处平均年龄别体重和平均年龄别身高的Z分数分别为2.01和2.12。平均CD4%为9.0。每毫升血浆HIV-1核糖核酸病毒载量中值为5.51 log10。12个月的治疗过程中,两名(3.9%)儿童死亡,另外11名(22%)儿童遭受13例严重临床事件。治疗的第十二个月,平均年龄别体重Z分数增加了0.63(P<0.001),而平均年龄别身高Z分数增加了0.57(P<0.001)。平均CD4%上升至24(P<0.001)。81%的儿童病毒载量低于300核糖核酸cp/ml;11名儿童(21.6%)出现HIV耐药变异。
结论
由地达诺新(DDI)、拉米夫定(3TC)和依法韦仑(EFV)组成的每天一次治疗是HIV-1感染儿童替代治疗方案的首选。剂量调整可以进一步改善治疗功效。
Introduction
Worldwide, over two million children are infected by human immunodeficiency virus type 1 (HIV-1).1 Ninety per cent of them live in Africa, and only 38% of those who need treatment receive highly active antiretroviral therapy (HAART).2 Several studies have shown that HAART can save children’s lives, both in Africa and elsewhere.3–7 In 2008, the CHER study showed that paediatric HAART should be started as early as possible.8 Compliance is essential for therapeutic success and complex antiretroviral therapy (ART) regimens are difficult to follow in paediatric settings. Simplified HAART regimens administered once a day can improve compliance and efficacy in children.
A combination of didanosine (DDI), lamivudine (3TC) and efavirenz (EFV) is one option for once-daily dosing, having yielded good clinical and virologic efficacy and better compliance in adults.9 In addition, long-term tolerance appears to be better than with HAART regimens requiring multiple daily intakes.10,11 However, the pharmacokinetics, tolerance and immunologic and virologic efficacy of such a once-a-day HAART regimen in children remain to be assessed.
Here we report the 12-month results of a phase-II trial of a once-a-day paediatric combination regimen of DDI + 3TC + EFV in Burkina Faso whose primary objective was to assess pharmacokinetics, immunologic and virologic efficacy and tolerance. The secondary objectives were to evaluate compliance and drug resistance.
Methods
Study design and population
The French National Agency for AIDS Research (Agence nationale de recherches sur le SIDA et les hépatites virales, ANRS) 12103 trial was an open phase-II trial evaluating the pharmacokinetics, immunologic and virologic efficacy and toxicity of a once-a-day combination of DDI + 3TC + EFV in HIV-1-infected children during a 12-month period. The trial was conducted in Bobo-Dioulasso, the second largest city in Burkina Faso (western Africa), by the Centre Muraz and Bobo Dioulasso University Hospital, with the support of French institutions.
Recruitment took place in two paediatric outpatient units (Bobo-Dioulasso University Hospital and the Mother and Child Health Department of the National Social Security Clinic). Follow-up visits took place at the Paediatrics Department of University Hospital. The study was approved by the Burkina Faso Ethics Committee for Health Research and recorded in the clinical trials registry of the United States National Institutes of Health (reference NCT00122538 at www.clinicaltrials.gov). Children were only included in the trial after the mother or legal representative had signed an informed consent form.
We aimed to recruit 50 HIV-1-infected children ranging in age from 30 months to 15 years and naive to antiretroviral drugs (except as prophylaxis against mother-to-child transmission). The eligibility criteria were as follows: (i) children ≤ 5 years of age: stage C infection and/or a CD4 percentage (CD4%) ≤ 15, or stage A, B or N infection and 15% ≤ CD4% ≤ 20%; (ii) children > 5 years of age: absolute CD4 count ≤ 200/mm3, regardless of the plasma HIV-1 ribonucleic acid (RNA) concentration, or 200/mm3 ≤ CD4 ≤ 500/mm3 and HIV-1 RNA > 100 000 copies per millilitre (cp/ml). The following baseline laboratory values were also required for enrolment: haemoglobin ≥ 7 g/dl, platelets ≥ 50 000/μl, amylase < 2.5 times the upper limit of normal, and aspartate aminotransferase and alanine aminotransferase < 5 times the upper limit of normal. All the eligibility criteria were checked 4 weeks before inclusion and initiation of DDI + 3TC + EFV, and were validated by the study team at weekly meetings.
Drugs
The treatments used in this trial were purchased through the Burkina Faso national drugs distribution office. Antiretroviral drug supplies were also secured for post-trial therapy. The three drugs were administered simultaneously once a day. EFV, a non-nucleoside reverse transcriptase inhibitor, was used in the form of 200-mg and 50-mg capsules according to body weight; 3TC, a nucleoside reverse transcriptase inhibitor, was administered at a dose of 8 mg/kg in the form of 150-mg pills or syrup; and the DDI dose was 240 mg/m2 (25-, 50-, 100- or 200-mg tablets of Videx®). Only DDI tablets were available and they had to be taken with an antacid (aluminum hydroxide, Maalox®) because they lacked an enteric coating. Since DDI should be taken 1 hour before or 2 hours after a meal, the parents were invited to administer the three drugs simultaneously each day at 18:00. After day 15 some families chose to give the treatments early in the morning (04:00 to 05:00) in the fasting state because of their work schedules.
Clinical follow-up
Physical examinations were carried out every month by physicians from the paediatric department. The children were also seen for intercurrent illnesses as necessary. All visits, hospitalizations, treatments and laboratory tests were provided free of charge. The children’s medical files were discussed at weekly team meetings.
Compliance was evaluated in three different ways. First, the two practitioners who followed the children estimated compliance semiquantitatively as follows: < 2 missed pills per month: compliant; 2 to 4 missed pills per month: moderately compliant; ≥ 5 missed pills per month: non-compliant. Second, a health worker recorded the number of blisters returned by the parents at each monthly visit to the hospital dispensary. Third, after 12 months of follow-up, the legal representatives were interviewed to explore how well they had managed to give the drugs to their children.
Laboratory procedures
Laboratory tests during follow-up
Virologic, immunologic, blood and biochemical tests were performed at baseline and every quarter during follow-up. All the tests were carried out at Centre Muraz laboratories, except for HIV-1 resistance testing, which was performed by the Virology Laboratory of Montpellier University Hospital (France).
Plasma HIV-1 RNA was measured with Generic HIV Viral Load® (Biocentric, Bandol, France), a real-time polymerase chain reaction assay based on long-terminal repeat with a detection limit of 300 RNA cp/ml in 0.2 ml of plasma.12 All month-12 samples that contained < 300 RNA cp/ml were re-tested with the same HIV-1 RNA assay but using an ultrasensitive (US) protocol with a detection limit of 50 RNA cp/ml.
HIV-1 resistance testing was based on AC11 Resistance Group recommendations (ANRS). RNA was extracted with the MagNa pure compact apparatus (Roche Applied Science, Indianapolis, United States of America) from samples containing > 1000 HIV-1 RNA cp/ml. The HIV-1 reverse transcriptase and protease coding regions were amplified and sequenced with primer sets described by the ANRS Resistance Group. Drug susceptibility was deduced from the ANRS algorithm (http://www.hivfrenchresistance.org). The HIV-1 genotype was determined from both the reverse transcriptase and protease sequences, as previously described.13
CD4 counts were conducted with a FACSCountTM flow cytometer (Becton Dickinson, BD Biosciences, San Jose, USA), which gave the results in absolute numbers and percentages. Total blood cells were counted with the SYSMEX KX21N haematology instrument (Sysmex Corporation, Kobe, Japan). The laboratory participated successfully in the UKNEQAS External Quality Assurance Program, based on two samples once a month. Blood tests were conducted with a Lisa 300 Plus analyser (Hycel Diagnostics, Massy, France).
Pharmacokinetic study
The pharmacokinetic study was performed after 15 days of treatment. Blood samples for pharmacokinetic analyses were taken from the children just before and 1 and 3 hours after drug administration. Additional samples were obtained from 9 children between months 2 and 5 of treatment before drug administration and 1, 2, 3, 6, 12 and 24 hours afterwards. The time elapsed since the administration was carefully recorded, along with age, body weight and height. Plasma samples were immediately prepared and stored at −20 °C until analysis.
The three drugs were assayed in two different laboratories (Hôpital Cochin-Saint-Vincent-de-Paul, France, and Hôpital Lapeyronie, Centre Hospitalier Universitaire Montpellier, France). DDI and 3TC plasma concentrations were determined with a modified version of a validated reverse-phase high-performance liquid chromatography (HPLC) assay based on ultraviolet wave absorbance. The limits of quantification were 0.01 mg/l for DDI,14,15 0.025 mg/l for 3TC,16 and 0.5 mg/l for efavirenz.17,18
Statistical analysis
As weight and height vary with age and sex, we calculated weight-for-age Z scores (WAZ) and height-for-age Z scores (HAZ). Epinut software from EpiInfo 6.04cfr was used for this purpose (Centres for Disease Control and Prevention, Atlanta, USA & World Health Organization, Geneva, Switzerland). WAZ and HAZ scores at inclusion and 12 months were compared by using a t test.
Only three children older than 5 years had a CD4+ T-lymphocyte count in excess of 15%. We therefore analysed the CD4 percentage (CD4%) rather than the absolute count, as the former changes little during childhood.19 CD4% and viral load values at baseline and at 12 months were also compared by using a t test Stata version X (StataCorp. LP, College Station, USA). Significance was set at P < 0.05.
Pharmacokinetic analyses
Pharmacokinetic data were analysed using the nonlinear mixed-effects modelling program NONMEM version VI, level 1.0 (Icon Development Solutions, Ellicott City, USA) with the Digital Fortran compiler. A pharmacokinetic model was built for each drug.15,18 For 3TC, a two-compartment model in which the slope of the distribution was assumed to be equal to the absorption rate constant adequately described the data, an allometric scaling was added.
Main study endpoints
The pharmacokinetic study focused on the minimal concentration (Cmin) or trough level, the maximal concentration (Cmax) or peak level and the area under the curve for 24 hours (AUC0→24), indicative of exposure to the three drugs. Immunologic efficacy was defined as the percentage of children with CD4 ≥ 25% at 12 months, and virologic efficacy as the percentages of children with viral load < 300 RNA cp/ml and < 50 RNA cp/ml at 12 months. Tolerance was evaluated in terms of the frequency and type of severe adverse effects during the 12 months of follow-up. Viral resistance was identified from the percentage and type of mutations known to cause resistance to the three drugs, in patients with a viral load > RNA 1000 RNA cp/ml at 12 months. Compliance was measured as the percentage of patients who missed at least one intake in the last three days. The rates of loss to follow-up, death and treatment cessation were also main study endpoints.
Results
Characteristics at enrolment
Between February and November 2006, 97 children (46 girls, 47.4%) were pre-included in the trial, but 45 were not eligible for the following reasons: CD4% > 20 (n = 31), hyperamylasaemia (n = 4), HIV-2 infection (n = 3), loss to follow-up between pre-inclusion and inclusion visits (n = 2), death before recruitment (n = 2), severe anaemia (n = 1), active tuberculosis (n = 1) and severe failure to thrive (n = 1). One child was included by error, as he had previously received ART.
Thus, the ANRS 12103 trial involved 51 children (20 girls, 39%) who were followed up for 12 months. Mean age was 6.8 years (median: 6; range: 2.5–15). At baseline, 17 children were at CDC stage A, 29 at stage B and five at stage C. WAZ and HAZ scores before treatment were −2.01 (95% confidence interval, CI: −2.25 to −1.76) and −2.12 (95% CI: −2.47 to −1.77), respectively.
The median CD4% was 8.0 (interquartile range, IQR: 6–12%) (mean: 8.9; range: 0.4–26) and the median absolute CD4 count was 260/mm3 (IQR: 126–566) (mean: 378; range: 2–1510). The median plasma HIV-1 RNA level was 5.51 log10 cp/ml (IQR: 3.61–6.75). The mean haemoglobin, alanine aminotransferase, aspartate aminotransferase and amylase levels were 10 g/dl, 53 IU/l, 34 IU/l and 99 IU/l, respectively.
Outcomes
Mortality, morbidity and Z scores
During the 12-month follow-up period, 2 children died (3.9%). One died early in the study from probable tuberculous meningitis and a possible immune reconstitution syndrome in a context of severe immunosuppression; the other died with severe varicella at 11 months of follow-up in a context of resistance to antiretrovirals. The 12-month probability of survival was 96% (95% CI: 85–99%).
Besides these two deaths, 13 severe clinical events occurred in 11 children, six of them during the first three months. The events included tuberculosis (n = 3, including one case with another bacterial infection superimposed on pulmonary tuberculosis and one with peritoneal tuberculosis), pneumonia (n = 2 without bacterial identification), severe fever of unknown origin (n = 2), varicella (n = 1), malaria (n = 1), abdominal pain (n = 1), cardiopathy (n = 1), transitory oedema (n = 1) and genital bleeding (n = 1). The last four conditions were considered unlikely to be related to the drugs.
Among the three cases of tuberculosis, two occurred during the first 90 days of HAART in children with severe immunosuppression.
WAZ and HAZ scores were −1.35 and −1.46, respectively, at 12 months (n = 49). Between inclusion and month 12, the mean WAZ score improved by 0.63 (P < 0.001) and the mean HAZ score, by 0.57 (P < 0.001).
Immunologic and virologic responses
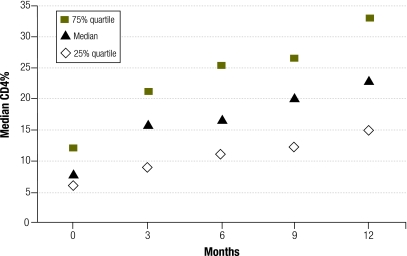
Between baseline and month 12, the mean absolute CD4 count increased from 378 to 822 cells/mm3 (difference: 444; P < 0.001) and the mean CD4% rose from 8.9 to 24 (difference: 15.1; P < 0.001) (Fig. 1).
Fig. 1.
CD4+ T-lymphocyte percentage (CD4%) over 12 months, ANRS 12103 trial, Bobo-Dioulasso, Burkina Faso, 2006–2007
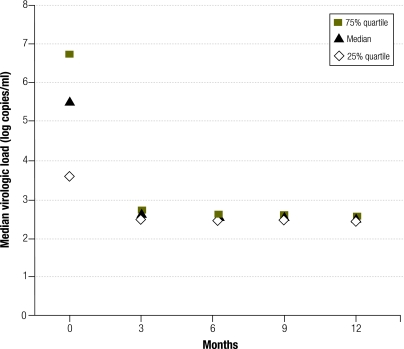
At month 12, plasma HIV-1 RNA was undetectable (< 300 cp/ml) in 81% (95% CI: 70–92) of the patients (Fig. 2). Thirty-six samples with a viral load < 300 RNA cp/ml at month 12 were re-tested with the ultrasensitive protocol. Twenty-five (69.4%) samples contained < 50 RNA cp/ml, 7 (19.4%) contained between 50 and 300 RNA cp/ml and 4 (11.1%) contained between 415 and 1068 RNA cp/ml. Therefore, virologic efficacy at 12 months with a 50 RNA cp/ml threshold was obtained in 52% (25/48) of cases (95% CI: 38–66).
Fig. 2.
Plasma concentrations of human immunodeficiency virus (HIV) ribonucleic acid (RNA) over 12 months, ANRS 12103 trial, Bobo-Dioulasso, Burkina Faso, 2006–2007
Compliance and adverse effects
As evaluated by the practitioners, 29 children were considered compliant, 13 moderately compliant and 8 non compliant. Five non-compliant children were shown to harbour resistant HIV-1 strains acquired during the study.
Based on the pharmacy files, about one third (32%) of children were compliant more than 95% of the time, whereas the other two thirds were compliant less than 95% of the time at least once during follow-up. More than 80% of the children were compliant at least 95% of the time based on returned blister counts. Between 10% and 15% of children were compliant between 80% and 95% of the time. Less than 5% of the children were considered compliant less than 80% of the time. After 12 months of follow-up, 41 adults in charge of the children (parents or tutors) declared that 3TC and EFV were easy or very easy to administer, and only five expressed difficulties. Six adults expressed difficulties with administering DDI.
Four reversible adverse events were considered possibly related to the study drugs. They comprised two cases of hyperamylasaemia (grade 2) and two liver enzyme elevations (grade 2 and grade 3). At month 12 the mean haemoglobin, alanine aminotransferase, aspartate aminotransferase and amylase values were 11 g/dl, 53 IU/l, 36 IU/l and 104 IU/l, respectively.
Resistance to antiretrovirals
Fifteen children had at least one plasma HIV-1 RNA value of ≥ 1000 RNA cp/ml during follow-up. Of these children, 13 could be tested for viral resistance. Genotypic resistance was identified in 11 children, whereas in two children the virus was susceptible to all three drugs (Table 1). Thus, the overall rate of resistance was 21.6% (11/51; 95% CI: 10.3–32.9) at 12 months. Among these 11 children, all but one harboured susceptible strains at baseline. One child harboured a strain resistant to zidovudine, possibly related to prophylaxis for mother-to-child transmission.
Table 1. Resistance to lamivudine (3TC), didanosine (DDI) and efavirenz (EFV), ANRS 12103 trial, Bobo-Dioulasso, Burkina Faso, 2006–2007.
| Patient ID | Month of follow-up | HIV genotype | Mutation positions, NRTI | Mutation positions, NNRTI | Drug resistance |
|---|---|---|---|---|---|
| B03 | 6 | CRF-02 | K103N | EFV | |
| B05 | 9 | CRF-02 | M184I, L210G | V90I, K103N, V108I, V179Ia | 3TC, EFV |
| B07 | 6 | CRF-02 | L74V, M184V, K219E | K103N, K101P | 3TC, DDI, EFV |
| B09 | 6 | CRF-06 | M184V | K103N, V108I | 3TC |
| B13 | 6 | CRF-02 | M184V | A106V, G190A, P225H | 3TC, EFV |
| B21 | 9 | CRF-02 | M184I | K103N, L100I, V179I,a P225H | 3TC, EFV |
| B26 | 3 | CRF-06 | M184V | K101E, G190E | 3TC, EFV |
| B33 | 3 | CRF-02 | M184V | K103N | 3TC, EFV |
| B39 | 12 | CRF-02 | M184V | K103N | 3TC, EFV |
| B44 | 3 | CRF-06 | K65R, D67G,a K70E | G190E | 3TC,b DDI,b EFV |
| B52 | 3 | CRF-02 | K65N,a T215S | K103N, M230L | EFV |
HIV, human immunodeficiency virus; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor.
a Polymorphism on known resistance position.
b Likely resistance.
Because of documented resistance, one child was switched to abacavir + DDI + lopinavir/ritonavir (Kaletra) and plasma HIV-1 RNA rapidly became undetectable. Only 7 of the 11 children with resistant strains met the WHO criterion for clinical resistance (viral load > 10 000 RNA cp/ml) at 12 months.
Pharmacokinetics
Between 150 and 200 plasma samples from 49 children were available for the pharmacokinetic evaluation of each drug. The DDI, EFV and 3TC pharmacokinetic models were validated by the visual predictive check method, so that Cmin, Cmax, AUC could be derived from individual pharmacokinetic parameters (Table 2). Target concentrations of each drug associated with efficacy or toxicity were chosen either from our data or from the literature, and different doses were simulated in each child with individual parameters to achieve threshold concentrations.
Table 2. Median plasma concentrations of didanosine (DDI), lamivudine (3TC) and efavirenz (EFV) and proposed doses to reach effective target concentrations, ANRS 12103 trial, Bobo-Dioulasso, Burkina Faso, 2006–2007.
| Drug | Cmin (mg/l) Median (IQR) | Cmax (mg/l) Median (IQR) | AUC0→24 (mg/l.h) Median (IQR) | Target (mg/l) | Recommended dose | Proposed dose to reach target interval |
|---|---|---|---|---|---|---|
| DDI | < 0.01 | 0.35 (0.23–0.67) | 0.61 (0.35–1.70) | This study AUC = 0.60 | 240 mg/m2 | 360 mg/m2 |
| EFV | 1.64 (1.08–2.56) | 3.71 (2.88–5.22) | 65.2 (48.1–91.9) | This study: 1.1 < Cmin | 13–15 kg: 200 mg | 2–6 y: 25 mg/kg |
| Literature: Cmin < 4 | 15–20 kg: 250 mg | 6–10 y: 15 mg/kg | ||||
| 20–25 kg: 300 mg | 10–15 y: 10 mg/kg | |||||
| 25–32.5 kg: 350 mg | ||||||
| 3TC | 0.04 (0.01–0.14) | 1.7 (1.34–2.01) | 7.8 (4.72–14.27) | Literature: AUCadult around 8.9 | 8 mg/kg | < 17 kg: 10 mg/kg > 17 kg: 8 mg/kg |
AUC, area under the curve; Cmax, maximal concentration; Cmin, minimal concentration; IQR, interquartile range; y, year.
The significant viral load decline after 12 months of treatment correlated with the DDI AUC. An AUC of 0.60 mg/l.h was the most discriminatory pharmacokinetic parameter for predicting a significant reduction in viral load. On simulating various doses in children, the probability of reaching an AUC of 0.60 mg/l.h was higher with 360 mg/m2 DDI chewable tablets than with 240 mg/m2, and a DDI dose > 360 mg/m2 would not have significantly increased this probability. We were unable to show whether a dose of 360 mg/m2 would have increased adverse effects.
A target EFV Cmin of 1.1 mg/l was related to efficacy and a target Cmin of 4 mg/l was considered for toxicity, based on adult data. Children weighing < 15 kg were more likely to have inadequate concentrations. Simulating various doses, the highest percentage of children with a Cmin in this target interval was obtained with once-daily EFV doses of 25 mg/kg from ages 2 to 6 years, 15 mg/kg from ages 6 to 10 years and 10 mg/kg from ages 10 to 15 years.
No significant relationship between 3TC concentration and effect was found. A mean AUC of 8.9 mg /l.h, obtained elsewhere in adults, was thus considered. A dose of 10 mg/kg once daily for children weighing < 17 kg and of 8 mg/kg for children weighting > 17 kg seemed more appropriate for obtaining the same drug exposure in children than the effective dose in adults.
Discussion
This trial shows that DDI + 3TC + EFV given simultaneously once a day is an interesting alternative to other first-line treatments. It is well tolerated and effective in terms of immunologic recovery (more than a twofold increase in CD4 count after one year of treatment). Even if only 50% of children had a viral load < 50 RNA cp/ml, 80% of the children had < 300 RNA cp/ml. These results are better than previously reported in African paediatric cohorts3–5 and similar to those obtained in adults.9 This may be related to the satisfactory plasma concentrations of the three drugs and to the low rate of attrition.
Tolerance was excellent: only five transient mild to moderate adverse effects were attributed to the study drugs during the 12 months of follow-up. Only two deaths occurred and neither was attributed to adverse effects. As in other reports,6,7 weight and height gain was satisfactory throughout the study period.
The immune reconstitution inflammatory syndrome (IRIS) is difficult to identify in children, but the three cases of tuberculosis that occurred during the first 90 days of HAART in severely immunocompromised children may have involved IRIS. Its frequency (6%) was far lower than described in South Africa,20 although some less severe cases of IRIS were probably missed in our study.
Despite the satisfactory pharmacokinetics of DDI, 3TC and EFV,15,18 target DDI plasma concentrations were not always reached in some of the youngest children, probably because the pills were not enteric-coated. Dose adaptation of the three drugs for the youngest children (< 15–17 kg in weight) would probably improve efficacy.
The mean absolute CD4 count increased by more than twofold and the mean CD4% by nearly threefold after one year. Twenty (41%) of the 49 children had a CD4% of more than 25 at 12 months, while 39/49 children (81%) had a viral load < 300 RNA cp/ml at 12 months.
Viral resistance was detected in 21% of the children after 12 months of follow-up. Of the children with a viral load ≥ 1000 RNA cp/ml, all except one had strains resistant to EFV. The situation was similar for 3TC, whereas only two strains were resistant to DDI. In Côte d’Ivoire we observed a similar overall frequency (23%) of viruses with genotypic resistance to at least one antiretroviral drug.21 This highlights the need for antiretroviral resistance testing of paediatric patients to switch to a second-line regimen in a timely manner.
Since we performed this study, other simple paediatric HAART regimens, including fixed-dose combinations, have been produced.22 However, the regimen tested here has the advantage of being very well tolerated. The only drawback to this “once-a-day” regimen is that several pills have to be ingested in one sitting and one of them has to be an antacid.
In conclusion, once-a-day HAART as a first-line treatment for HIV-infected children proved safe and as efficacious as other regimens in this African paediatric population. Such regimens are particularly advantageous when multiple daily dosing is difficult, as is the case with children attending school. However, vigilance is required to ensure good compliance throughout treatment. Longer follow-up is necessary to confirm the good clinical, immunologic and virologic results and excellent tolerance of this once-a-day antiretroviral paediatric regimen.
Funding:
This clinical trial was funded by the Agence nationale de recherches sur le SIDA et les hépatites virales, Paris, France.
Competing interests:
None declared.
References
- 1.AIDS epidemic update Geneva: United Nations Joint Programme on HIV/AIDS; 2009.
- 2.Children and AIDS: fourth stocktaking report New York: United Nations Children’s Fund; 2009.
- 3.Rouet F, Fassinou P, Inwoley A, Anaky MF, Kouakoussui A, Rouzioux C, et al. Long-term survival and immuno-virologic response of African HIV-1-infected children to highly active antiretroviral therapy regimens containing a protease or a non-nucleoside reverse transcriptase inhibitor. AIDS. 2006;20:2315–9. doi: 10.1097/QAD.0b013e328010943b. [DOI] [PubMed] [Google Scholar]
- 4.Eley B, Davies MA, Apolles P, Cowburn C, Buys H, Zampoli M, et al. Antiretroviral treatment for children. S Afr Med J. 2006;96:988–93. [PubMed] [Google Scholar]
- 5.Song R, Jelagat J, Dzombo D, Mwalimu M, Mandaliya K, Shikely K, et al. Efficacy of highly active antiretroviral therapy in HIV-1 infected children in Kenya. Pediatrics. 2007;120:e856–61. doi: 10.1542/peds.2006-1122. [DOI] [PubMed] [Google Scholar]
- 6.Ellis J, Molyneux EM. Experience of anti-retroviral treatment for HIV-infected children in Malawi: the 1st 12 months. Ann Trop Paediatr. 2007;27:261–7. doi: 10.1179/146532807X245643. [DOI] [PubMed] [Google Scholar]
- 7.Kabue MM, Kekitiinwa A, Maganda A, Risser JM, Chan W, Kline MW. Growth in HIV-infected children receiving antiretroviral therapy at a pediatric infectious diseases clinic in Uganda. AIDS Patient Care STDS. 2008;22:245–51. doi: 10.1089/apc.2007.0049. [DOI] [PubMed] [Google Scholar]
- 8.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. CHER Study Team Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landman R, Schiemann R, Thiam S, Vray M, Canestri A, Mboup S, et al. Imea 011/ANRS 12-04 Study Group Once-a-day highly active antiretroviral therapy in treatment-naive HIV-1-infected adults in Senegal. AIDS. 2003;17:1017–22. doi: 10.1097/00002030-200305020-00010. [DOI] [PubMed] [Google Scholar]
- 10.Ena J, Pasquau F. Once-a-day highly active antiretroviral therapy: a systematic review. Clin Infect Dis. 2003;36:1186–90. doi: 10.1086/374602. [DOI] [PubMed] [Google Scholar]
- 11.Maggiolo F, Ripamonti D, Gregis G, Quinzan G, Callegaro A, Arici C, et al. Once-a-day therapy for HIV infection: a controlled, randomized study in antiretroviral-naive HIV-1-infected patients. Antivir Ther. 2003;8:339–46. [PubMed] [Google Scholar]
- 12.Rouet F, Chaix ML, Nerrienet E, Ngo-Giang-Huong N, Plantier JC, Burgard M, et al. Impact of HIV-1 genetic diversity on plasma HIV-1 RNA Quantification: usefulness of the Agence Nationale de Recherches sur le SIDA second-generation long terminal repeat-based real-time reverse transcriptase polymerase chain reaction test. J Acquir Immune Defic Syndr. 2007;45:380–8. doi: 10.1097/QAI.0b013e3180640cf5. [DOI] [PubMed] [Google Scholar]
- 13.Pasquier C, Millot N, Njouom R, Sandres K, Cazabat M, Puel J, et al. HIV-1 subtyping using phylogenetic analysis of pol gene sequences. J Virol Methods. 2001;94:45–54. doi: 10.1016/S0166-0934(01)00272-5. [DOI] [PubMed] [Google Scholar]
- 14.Knupp CA, Stancato FA, Papp EA, Barbhaiya RH. Quantitation of didanosine in human plasma and urine by high-performance liquid chromatography. J Chromatogr A. 1990;533:282–90. doi: 10.1016/S0378-4347(00)82215-X. [DOI] [PubMed] [Google Scholar]
- 15.Hirt D, Bardin C, Diagbouga S, Nacro B, Hien H, Zoure E, et al. Didanosine population pharmacokinetics in West African HIV-infected children administered once daily tablets in relation to efficacy after one year of treatment. Antimicrob Agents Chemother. 2009;53:4399–406. doi: 10.1128/AAC.01187-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harker AJ, Evans GL, Hawley AE, Morris DM. High-performance liquid chromatographic assay for 2’-deoxy-3’-thiacytidine in human serum. J Chromatogr B Biomed Appl. 1994;657:227–32. doi: 10.1016/0378-4347(94)80092-8. [DOI] [PubMed] [Google Scholar]
- 17.Cociglio M, Hillaire-Buys D, Peyrière H, Alric R. Performance analysis of a rapid HPLC determination with the solvent demixing extraction of HIV antiproteases and efavirenz in plasma. J Chromatogr Sci. 2003;41:80–6. doi: 10.1093/chromsci/41.2.80. [DOI] [PubMed] [Google Scholar]
- 18.Hirt D, Urien S, Olivier M, Peyrière H, Nacro B, Diagbouga S, et al. Is the recommended dose of efavirenz optimal in young West African HIV-infected children? Antimicrob Agents Chemother. 2009;53:4407–13. doi: 10.1128/AAC.01594-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antiretroviral therapy of HIV infection in infants and children in resource-limited settings: towards universal access. Recommendations for a public health approach Geneva: World Health Organization; 2006. [PubMed] [Google Scholar]
- 20.Smith K, Kuhn L, Coovadia A, Meyers T, Hu CC, Reitz C, et al. Immune reconstitution inflammatory syndrome among HIV-infected South African infants initiating antiretroviral therapy. AIDS. 2009;23:1097–107. doi: 10.1097/QAD.0b013e32832afefc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaix ML, Rouet F, Kouakoussui KA, Laguide R, Fassinou P, Montcho C, et al. Genotypic human immunodeficiency virus type 1 drug resistance in highly active antiretroviral therapy-treated children in Abidjan, Côte d’Ivoire. Pediatr Infect Dis J. 2005;24:1072–6. doi: 10.1097/01.inf.0000190413.88671.92. [DOI] [PubMed] [Google Scholar]
- 22.L’Homme RF, Kabamba D, Ewings FM, Mulenga V, Kankasa C, Thomason MJ, et al. Nevirapine, stavudine and lamivudine pharmacokinetics in African children on paediatric fixed-dose combination tablets. AIDS. 2008;22:557–65. doi: 10.1097/QAD.0b013e3282f4a208. [DOI] [PubMed] [Google Scholar]