Abstract
Objective
To systematically review randomized controlled trials comparing the effect of supplementation with multiple micronutrients versus iron and folic acid on pregnancy outcomes in developing countries.
Methods
MEDLINE and EMBASE were searched. Outcomes of interest were birth weight, low birth weight, small size for gestational age, perinatal mortality and neonatal mortality. Pooled relative risks (RRs) were estimated by random effects models. Sources of heterogeneity were explored through subgroup meta-analyses and meta-regression.
Findings
Multiple micronutrient supplementation was more effective than iron and folic acid supplementation at reducing the risk of low birth weight (RR: 0.86, 95% confidence interval, CI: 0.79–0.93) and of small size for gestational age (RR: 0.85; 95% CI: 0.78–0.93). Micronutrient supplementation had no overall effect on perinatal mortality (RR: 1.05; 95% CI: 0.90–1.22), although substantial heterogeneity was evident (I2 = 58%; P for heterogeneity = 0.008). Subgroup and meta-regression analyses suggested that micronutrient supplementation was associated with a lower risk of perinatal mortality in trials in which > 50% of mothers had formal education (RR: 0.93; 95% CI: 0.82–1.06) or in which supplementation was initiated after a mean of 20 weeks of gestation (RR: 0.88; 95% CI: 0.80–0.97).
Conclusion
Maternal education or gestational age at initiation of supplementation may have contributed to the observed heterogeneous effects on perinatal mortality. The safety, efficacy and effective delivery of maternal micronutrient supplementation require further research.
ملخص
الغرض
مراجعة منهجية للتجارب العشوائية ذات الشواهد لمقارنة تأثير تعزيز المغذيات المتعددة الزهيدة المقدار مقابل تعزيز الحديد وحمض الفوليك على نتائج الحمل في البلدان النامية.
الطريقة
أُجري بحث في خط استرجاع النشريات الطبية MEDLINE وقواعد المعطيات الطبية EMBASE. وكانت الحصيلة موضع الاهتمام هي وزن الوليد، وانخفاض وزن الوليد، وقلة حجم الوليد مقابل عمره الحملي، والوفيات المحيطة بالولادة، ووفيات الولدان. وحُسِبَ الخطر النسبي المُجمّع من خلال نماذج تأثيرات عشوائية. واستكشفت مصادر التغايرية عن طريق تحليل تلوي وتحوّف تلوي للمجموعات الفرعية.
النتائج
كان تعزيز المغذيّات المتعددة الزهيدة المقدار أكثر فعاليّة عن تعزيز الحديد وحمض الفوليك في خفض خطر انخفاض وزن الوليد (الخطر النسبي= 0.86، فاصلة الثقة 95%= 0.79-0.93) وقلة الحجم الوليد مقابل عمره الحملي (الخطر النسبي= 0.85؛ فاصلة الثقة 95%=0.78-0.93). لم يكن لتعزيز المغذيّات الزهيدة المقدار تأثير إجمالي على الوفيات المحيطة بالولادة (الخطر النسبي= 1.05؛ فاصلة الثقة 95%: 0.90-1.22)، وذلك مع وجود بينة على تغايرية كبيرة (I2 =58%؛ قوة الاحتمال للتغايرية= 0.008). دلت تحليلات الفئات الفرعية والتحوّف التلوي أن تعزيز المغذيات الزهيدة المقدار ارتبط باحتمال أقل لخطر حدوث وفيات الولدان في التجارب التي كان نسبة التعليم الرسمي لدى الأمهات فيها تزيد عن 50% (الخطر النسبي= 0.93؛ فاصلة الثقة 95%= 0.82-1.06) أو التي بدأ تعزيز المغذيات فيها بعد 20 أسبوعاً من الحمل في المتوسط (الخطر النسبي= 0.88؛ فاصلة الثقة 95%= 0.80-0.97).
الاستنتاج
قد يساهم تعليم الأمهات أو العمر الحملي عند الشروع في التعزيز بالمغذيات في التأثيرات التغايرية الملاحظة على الوفيات المحيطة بالولادة. وتحتاج معرفة سلامة، ونجاعة، وفعالية توصيل المغذيّات إلى الأمهات إلى المزيد من البحث.
Résumé
Objectif
Étudier systématiquement les essais contrôlés randomisés comparant l’effet de l’apport en micronutriments multiples à l’apport en fer et en acide folique sur les issues de la grossesse dans les pays en voie de développement.
Méthodes
MEDLINE et EMBASE ont fait l’objet d’une recherche. Les résultats d’intérêt étaient le poids de l’enfant à la naissance, un faible poids à la naissance, une taille trop petite pour l’âge gestationnel, la mortalité périnatale et la mortalité néonatale. Les risques relatifs (RR) globaux étaient estimés par des modèles à effets aléatoires. Les sources d’hétérogénéité étaient étudiées par le biais de la méta-régression et les méta-analyses de sous-groupes.
Résultats
La supplémentation en micronutriments multiples s’est révélée plus efficace que la supplémentation en fer et en acide folique dans la diminution du risque de faible poids à la naissance (RR = 0,86, 95% IC = 0,79–0,93) et du risque de taille trop petite pour l’âge gestationnel (RR = 0,85; 95% IC = 0,78–0,93). L’apport en micronutriments n’a pas eu d’effet général sur la mortalité périnatale (RR = 1,05; 95% IC = 0,90–1,22) et ce, malgré une hétérogénéité évidente (I2 = 58%; P pour hétérogénéité = 0,008). Les analyses des sous-groupes et de la méta-régression laissaient à penser que l’apport en micronutriments était associé à un risque inférieur de mortalité périnatale dans les essais pour lesquels > 50% des mères avaient eu une éducation structurée (RR = 0,93; 95% IC = 0,82–1,06) ou pour lesquels la supplémentation avait débuté en moyenne après 20 semaines de gestation (RR = 0,88; 95% IC = 0,80–0,97).
Conclusion
L’éducation de la mère ou l’âge gestationnel en début de supplémentation a pu contribuer aux effets hétérogènes observés sur la mortalité périnatale. La sécurité, l’efficacité et l’assimilation effective de l’apport maternel en micronutriments nécessitent des recherches supplémentaires.
Resumen
Objetivo
Realizar una revisión sistemática de ensayos aleatorizados y controlados en los que se compara el efecto de la administración de múltiples micronutrientes con el de la administración de hierro y ácido fólico sobre los resultados de los embarazos en los países en vías de desarrollo.
Métodos
Se realizaron búsquedas en MEDLINE y EMBASE. Los resultados de interés fueron: peso del neonato, bajo peso neonatal, neonatos con una talla baja para la edad gestacional, mortalidad perinatal y mortalidad neonatal. Se calcularon los riesgos relativos (RR) agrupados, empleando modelos de efectos aleatorios. Se investigaron las fuentes de heterogeneidad del metanálisis y la metarregresión de los subgrupos.
Resultados
La administración de múltiples micronutrientes fue más eficaz que la administración de hierro y ácido fólico a la hora de reducir el riesgo del peso bajo neonatal (RR = 0,86, IC del 95%= 0,79–0,93) y la talla baja para la edad gestacional (RR = 0,85; IC del 95% = 0,78–0,93). La administración de micronutrientes no tuvo un efecto global en la mortalidad perinatal (RR = 1,05; IC del 95%= 0,90–1,22), si bien la heterogeneidad fue importante y evidente (I2 = 58%; p de heterogeneidad = 0,008). Los análisis de los subgrupos y de la metarregresión sugirieron que la administración de micronutrientes estaba asociada a un menor riesgo de mortalidad perinatal en aquellos estudios en los que más del 50% de las madres tenía formación universitaria (RR = 0,93; IC del 95%= 0,82–1,06) o en los que la administración se inició después de una media de 20 semanas de gestación (RR = 0,88; IC del 95%= 0,80–0,97).
Conclusión
La educación de la madre o la edad gestacional en la que se inició la administración pueden haber contribuido a los efectos heterogéneos observados en la mortalidad perinatal. Se debe seguir investigando la seguridad, la eficacia y la efectividad de la administración de micronutrientes a mujeres embarazadas.
Резюме
Цель
Провести систематический обзор рандомизированных контролируемых испытаний, сравнивающих воздействие назначения поливитаминных-мультиминеральных комплексов и пищевых добавок железа и фолиевой кислоты на исходы беременности в развивающихся странах.
Методы
Был проведен поиск в базах данных MEDLINE и EMBASE. Исходами, представлявшими интерес, были: масса тела при рождении, низкая масса тела при рождении, малый размер плода для данного срока гестации, перинатальная смертность и неонатальная смертность. Совокупные относительные риски (ОР)) оценивались при помощи моделей со случайными эффектами. Источники гетерогенности исследовались при помощи мета-анализа и мета-регрессии в подгруппах.
Результаты
Назначение поливитаминных и мультиминеральных комплексов было более эффективным, чем назначение пищевых добавок железа и фолиевой кислоты, для снижения риска низкой массы тела при рождении (ОР = 0.86, доверительный интервал (ДИ) 95% = 0,79–0,93) и малого размера плода для данного срока гестации (ОР = 0,85; ДИ 95% = 0,78–0,93). В целом назначение поливитаминных и мультиминеральных комплексов не оказывало воздействия на перинатальную смертность (ОР = 1,05; ДИ 95% = 0,90–1,22), хотя была заметна значительная гетерогенность (I2 = 58%; P для гетерогенности = 0,008). Анализ в подгруппах и метарегрессионный анализ позволили сделать вывод, что назначение поливитаминных и мультиминеральных комплексов коррелировало с низким риском перинатальной смертности в тех испытаниях, где более 50% матерей имело тот или иной уровень формального образования (ОР = 0,93; ДИ 95% = 0,82–1,06) или где назначение было применено при среднем сроке гестации в 20 недель (ОР = 0,88; ДИ 95% = 0,80–0,97).
Вывод
На наблюдаемую гетерогенность воздействия на перинатальную смертность могли повлиять уровень образования матерей и срок гестации при назначении пищевых добавок. Вопросы безопасности, результативности и эффективности приема поливитаминных и мультиминеральных комплексов матерями требуют дальнейшей научной проработки.
摘要
目的
旨在系统回顾发展中国家通过补充多种微量营养素与补充铁和叶酸对妊娠结果所产生影响的随机对照试验。
方法
对联机医学文献分析和检索系统(MEDLINE)与荷兰医学文摘数据库(EMBASE)进行搜索。检索结果中受到关注的是出生体重、低出生体重、小于胎龄儿、围产期死亡率和新生儿死亡率。混合相对危险度(RRs)通过随机效果模型进行估测。另外,运用分组荟萃分析和元回归分析探讨异质性的根源。
结果
在降低低出生体重风险(相对危险度:0.86;95%可信区间:0.79-0.93)和降低小于胎龄儿(相对危险度:0.85;95%可信区间:0.78-0.93)风险方面,补充多种微量营养素比补充铁和叶酸更有效。尽管异质性十分明显(I2 = 58%; 异质性P = 0.008),但是补充微量营养素对围产期死亡率(相对危险度:1.05;95%可信区间:0.90-1.22)总体没有什么影响。分组和元回归分析表明在下述试验中微量营养素补充与较低围产期死亡率相关:超过50%的母亲受过正规教育(相对危险度:0.93;95%可信区间:0.82-1.06)或微量营养素在平均妊娠20周后进行补充(相对危险度:0.88;95%可信区间:0.80-0.97)。
结论
母亲教育程度或开始补充微量营养素时的孕龄这两项指标可能对所观察到的围产期死亡率产生影响。母体微量营养素补充的安全性、功效和有效传递需要进一步研究。
Introduction
Every year more than 20 million infants are born with low birth weight worldwide1. About 3.6 million infants die during the neonatal period.2 Two thirds of these deaths occur in southern Asia and sub-Saharan Africa. More than one third of child deaths are thought to be attributable to maternal and child undernutrition.3 Deficiencies in micronutrients such as folate, iron and zinc and vitamins A, B6, B12, C, E and riboflavin are highly prevalent and may occur concurrently among pregnant women.3 Micronutrient deficiencies result from inadequate intake of meat, fruits and vegetables, and infections can also be a cause. Multiple micronutrient supplementation in pregnant women may be a promising strategy for reducing adverse pregnancy outcomes through improved maternal nutritional and immune status.4,5 The World Health Organization (WHO) currently recommends iron and folic acid supplementation to reduce the risk of iron deficiency anaemia among pregnant women. Since many developing countries already have systems in place for the delivery of iron and folic acid supplements, micronutrient supplements could be provided at little additional cost.6
Several systematic reviews of trials examining the effects of maternal multiple micronutrient supplementation have been conducted,7–10 but they have had limitations. Although some researchers have raised concerns that micronutrient supplementation may increase perinatal mortality, none of the previous review articles has adequately addressed this issue.7–9,11 None has examined the potential sources of heterogeneity in the effect of supplementation on perinatal mortality. The effects of maternal micronutrient supplementation on perinatal mortality and other pregnancy outcomes can differ depending on trial characteristics and study population. An updated systematic review is essential to provide the basis for future research and for a discussion of policy implications. We conducted a systematic review of trials comparing the effect of maternal multiple micronutrient supplementation with that of iron and folic acid supplementation on pregnancy outcomes in developing countries. We also conducted subgroup meta-analysis and meta-regression to explore sources of heterogeneity.
Methods
Search strategy and inclusion criteria
We searched MEDLINE and EMBASE up to 1 August 2010 and identified potentially relevant published trials using the combination of medical subject headings (MeSH) and text words denoting micronutrient supplements and pregnancy outcomes. We used the terms micronutrient, multivitamin, vitamin, mineral and supplement in combination with pregnancy, birth, newborn, infant, low birth weight, preterm, fetal growth, small-for-gestational-age, perinatal mortality and neonatal mortality. We also searched the references cited by the retrieved articles for additional references.
We applied the following inclusion criteria: (i) only randomized controlled trials conducted in developing countries; (ii) only trials that compared an intervention group receiving multiple micronutrient supplements (defined as more than three micronutrients) with a control group receiving iron and folic acid, and (iii) only trials that examined any of the following outcomes: birth weight, low birth weight (birth weight < 2500 g), preterm birth (birth before the 37th week of gestation), small size for gestational age (birth weight below the 10th percentile of weight for gestational age), perinatal death (death from the 28th week of gestation through the first week after delivery) and neonatal death (death within 28 days of delivery).
We included only trials in developing countries because micronutrient malnutrition among pregnant women is much more common in developing countries than in industrialized countries. Furthermore, no recommendations for prenatal micronutrient supplements exist in most developing countries, whereas such supplements are routinely recommended in some developed countries.12
We defined a “multiple micronutrient supplement” as a single tablet containing more than three different micronutrients; micronutrient-fortified powders, foods and beverages were not included in the definition.
Data extraction and quality assessment
We extracted information on the characteristics of the trials and their participants, interventions, outcome measures and methodological quality. Our criteria for assessing methodological quality included randomization, allocation concealment, blinding, completeness of follow-up and compliance with study regimens. We also extracted the results of randomized controlled trials included in previous reviews.
Statistical analysis
We applied random effects models to estimate the pooled relative risks (RRs) or mean differences and their respective 95% confidence intervals (CIs).13 The natural logarithms of the RRs and their corresponding standard errors from individual trials were used to compute the pooled estimates. We employed the I2 statistic to determine the proportion of the total variation among studies due to heterogeneity rather than chance.14 To evaluate possible publication bias, we visually inspected the funnel plot for asymmetry and performed the Begg’s rank correlation test and Egger’s linear regression test.
To investigate the sources of heterogeneity, we conducted subgroup meta-analysis and meta-regression analysis.15 We examined the effects of micronutrient supplementation according to the following dichotomized characteristics: prevalence of maternal underweight (body mass index [BMI, or weight in kg divided by the square of the height in metres] < 18.5), < 20% or ≥ 20%; average height, < 155 cm or ≥ 155 cm; proportion of primiparous women, < 35% or ≥ 35%) and proportion of women without formal education, < 50% or ≥ 50%. Additionally, we examined the effect modification of different iron doses as a function of treatment group and average gestational age at initiation of supplementation (< 20 weeks or ≥ 20 weeks). For perinatal mortality, we also examined the effect of micronutrient supplementation on the risk of large size for gestational age (RR < 1.10 or ≥ 1.10) and birth weight (mean difference in birth weight by treatment group, < 50 g or ≥ 50 g). We performed random effects meta-regression using weights proportional to the inverse variance. We employed residual I2 to estimate residual heterogeneity between studies after adjusting for the covariate of interest in the meta-regression. All statistical analyses were performed using STATA version 11 (StataCorp. LP, College Station, United States of America).
Results
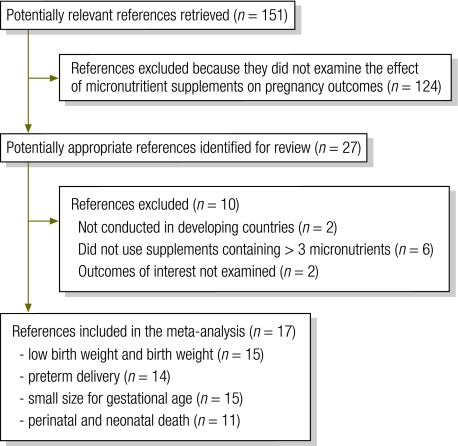
We identified 27 potentially relevant articles for detailed review (Fig. 1). We excluded 10 articles because they were conducted in developed countries, used supplements containing fewer than three micronutrients or did not assess outcomes of interest. The remaining 17 articles provided data for meta-analysis.9,10,16–31 Three of them were systematic reviews9,10,31 from which we extracted the results of trials in Indonesia (Indramayu) and Bangladesh.9,10
Fig. 1.
Selection of studies included in systematic review of randomized controlled trials on maternal multiple micronutrient supplementation and pregnancy outcomes in developing countries
Table 1 shows the characteristics of the 15 trials (n = 64 244) that compared the effect of micronutrient supplementation with that of iron and folic acid supplementation on pregnancy outcomes. All trials were double-blinded and showed high compliance with the study regimens. While loss to follow-up was minimal in most trials, in Mexico, Niger and Zimbabwe more than 20% of trial participants were lost to follow-up. A hospital-based trial in India was restricted to only undernourished women and two trials conducted in the United Republic of Tanzania were restricted to either HIV-positive or HIV-negative women. Most of the trials used the international multiple micronutrient preparation (UNIMMAP) of the United Nations Children’s Fund/World Health Organization/United Nations University, which contains the recommended dietary allowance (RDA) of 15 vitamins and minerals. Because vitamins can enhance iron absorption and utilization, the UNIMMAP contains 30 mg of iron, or half the dose of iron in the iron and folic acid supplements. The trials conducted in Mexico, Nepal (Sarlahi district) and Zimbabwe used the RDA of multiple micronutrients, like the UNIMMAP, whereas two trials conducted in the United Republic of Tanzania used multivitamin supplements containing multiples of the RDA. In the trial conducted in India, supplements contained the RDA of 29 micronutrients.
Table 1. Characteristics of randomized controlled trials examining the effect of maternal micronutrient supplementation on pregnancy outcomes in developing countries.
| Study | Country | No. of women | Intervention | Control | Compliance (%) | Loss to follow-up (%) | Weeks of gestation at enrolment (mean ± SD) |
|---|---|---|---|---|---|---|---|
| Fawzi et al., 199816 | United Republic of Tanzania | 1075 HIV+ | 8 vitamins in multiples of RDAa | Iron (60 mg) and folic acid (5 mg) | 91 | 6 | 20.3 ± 3.4 |
| Christian et al., 200317,18 | Nepal (Sarlahi) | 1978 | 15 micronutrientsb | Iron (60 mg) and folic acid (400 µg) | 75 | 6 | 11.4 ± 5.1 |
| Ramakrishnan et al., 200319 | Mexico | 873 | 13 micronutrientsc | Iron (60 mg) | 95 | 26 | 9.2 ± 3.0 |
| Friis 200420 | Zimbabwe | 1669 (1 135 HIV− and 534 HIV+) | 13 micronutrientsd | Iron and folic acid | NA | 34 | 29.2 |
| Kaestel et al.,200521 | Guinea Bissau | 2100 | UNIMMAP in 1 or 2 doses of RDAe | Iron (60 mg) and folic acid (400 µg) | 76 | 19 | 22 ± 7 |
| Osrin et al., 200522 | Nepal (Janakpur) | 1200 | UNIMMAPe | Iron (60 mg) and folic acid (400 µg) | NA | 3 | 16.2 ± 3.0 |
| Gupta et al., 200723 | India | 200 undernourished | 29 micronutrientsf | Iron (60 mg) and folic acid (500 µg) | 85 | 15 | 27.9 |
| Zagré et al., 200724 | Niger | 3670 | UNIMMAPe | Iron (60 mg) and folic acid (400 µg) | 79 | 31 | 10 |
| Fawzi et al., 200725 | United Republic of Tanzania | 8468 HIV− | 8 vitamins in multiples of RDAa | Iron (60 mg) and folic acid (5 mg) | 88 | 1 | 21.3 ± 3.5 |
| Shankar et al., 200826 | Indonesia | 31 290 | UNIMMAPe | Iron (30 mg) and folic acid (400 µg) | 85 | 6 | 20.9 |
| Zeng et al., 200827 | China | 3811 | UNIMMAPe | Iron (60 mg) and folic acid (400 µg) | 92 | 8 | 13.8 ± 5.6 |
| Roberfroid et al., 200828 | Burkina Faso | 1426 | UNIMMAPe | Iron (60 mg) and folic acid (400 µg) | NA | 4 | 17.3 ± 8.0 |
| Bhutta et al., 200929 | Pakistan | 2378 | UNIMMAPe | Iron (60 mg) and folic acid (400 µg) | 76 | 11 | 12.2 ± 3.0 |
| Sunawang et al., 20099,10,30 | Indonesia (Indramayu) | 1694 | UNIMMAPe | Iron (60 mg) and folic acid (400 µg) | NA | NA | 14.4 |
| Tofail et al., 20099,10 | Bangladesh | 2412 | UNIMMAPe | Iron (30 mg) and folic acid (400 µg) | NA | NA | 14.6 |
HIV, human immunodeficiency virus; NA, not available; RDA, recommended dietary allowance; SD, standard deviation; UNIMMAP, UNICEF/WHO/United Nations University international multiple micronutrient preparation.
a Used vitamins B1 (20 mg), B2 (20 mg), B6 (25 mg), B12 (50 µg), C (500 mg), E (30 mg) and niacin (100 mg) plus iron (60 mg) and folic acid (60.8 mg).
b Used vitamins A (1000 µg), D (10 µg), E (10 mg), B1 (1.6 mg), B2 (1.8 mg), B6 (2.2 mg), B12 (2.6 µg), C (100 mg) and niacin (20 mg) plus zinc (30 mg), copper (2 mg), vitamin K (65 µg), magnesium (100 mg), iron (60 mg) and folic acid (400 µg).
c Used vitamins A (2150 IU), D (309 IU), E (5.73 IU), B1 (0.93 mg), B2 (1.87 mg), B6 (1.94 mg), B12 (2.04 µg), C (66.5 mg) and niacin (15.5 mg) plus zinc (12.9 mg), magnesium (252 mg), iron (62.4 mg) and folic acid (215 µg).
d Used β-carotene (3.5 mg), vitamins A (3000 IU), D (5 µg), E (10 mg), B1 (1.5 mg), B2 (1.6 mg), B6 (2.2 mg), B12 (4.0 µg), C (80 mg) and niacin (17 mg) plus zinc (15 mg), copper (1.2 µg), selenium (65 µg) and folic acid (5 mg).
e International multiple micronutrient preparation containing vitamins A (800 µg), D (5 µg), E (10 mg), B1 (1.4 mg), B2 (1.4 mg), B6 (1.9 mg), B12 (2.6 µg) C (70 mg) and niacin (18 mg) plus zinc (15 mg), copper (2 mg), selenium (65 µg), iodine (150 µg), iron (30 mg) and folic acid (400 µg).
f Used β-carotene (2500 IU), vitamins B1 (1 mg), B2 (1.5 mg), B6 (1 mg), B12 (1 µg), C (50 mg), D (200 IU), E (7.5 mg), calcium (5 mg), nicotinamide (20 mg), biotin (30 µg), zinc (15 mg), potassium iodide (0.15 mg), magnesium oxide (100 mg), magnese sulfate (2.5 mg), copper (2 mg), calcium (162 mg), phosphorus (125 mg), potassium (40 mg), chloride (36.3 mg), chromium (25 µg), molybdenum (25 µg), spdium selenate (30 µg), nickel (5 µg), silicon dioxide (2 mg), vanadium (10 µg), boron (150 µg), iron (70 mg) and folic acid (900 µg).
Pooled analyses of 15 trials showed that maternal micronutrient supplementation increased birth weight (pooled mean difference: 44 g; 95% CI: 28–60; Table 2). Pregnant women who received micronutrient supplements were less likely to deliver low-birth-weight infants (pooled RR: 0.86, 95% CI: 0.79–0.93) or small-for-gestational-age infants (pooled RR: 0.85; 95% CI: 0.78–0.93) than women who received iron and folic acid supplements. Micronutrient supplementation had no effect on preterm delivery (pooled RR: 0.99; 95% CI: 0.95–1.03). We examined sources of heterogeneity for effects on birth weight, low birth weight and small size for gestational age (Table 3, Table 4 and Table 5, available at: http://www.who.int/bulletin/volumes/89/6/10-083758). None of these three outcomes appeared to be modified by maternal education, underweight, height, parity, the average timing of initiation of supplements or iron dosage (P for test for heterogeneity > 0.05). However, trials in which micronutrient supplementation was initiated after an average gestational age of 20 weeks appeared to show a greater beneficial effect of supplementation with respect to low birth weight (RR: 0.76; 95% CI: 0.64–0.89) and small size for gestational age (RR: 0.77; 95% CI: 0.65–0.91), although the test for heterogeneity did not yield statistical significance.
Table 2. Summary of pooled estimates for the effect of maternal micronutrient supplementation on pregnancy outcomes.
| Pregnancy outcome | No. of trials | Pooled RR or mean difference (95% CI) | I2 (P-value) |
|---|---|---|---|
| Low birth weighta | 15 | 0.86 (0.79–0.93) | 41% (0.05) |
| Birth weight | 15 | 44 g (28–60) | 57% (0.003) |
| Preterm birthb | 14 | 0.99 (0.95–1.03) | 0% (0.63) |
| Small size for gestational agec | 15 | 0.85 (0.78–0.93) | 47% (0.02) |
| Perinatal deathd | 11 | 1.05 (0.90–1.22) | 58% (0.008) |
| Neonatal deathe | 11 | 1.08 (0.92–1.26) | 28% (0.17) |
CI, confidence interval; RR, relative risk.
a Birth weight < 2500 g.
b Birth before the 37th week of gestation.
c Birth weight below the 10th percentile of weight for gestational age.
d Death from the 28th week of gestation through the first week postpartum.
e Death within 28 days of delivery.
Table 3. Subgroup analysis and meta-regression for the effect of maternal micronutrient supplements on birth weight in developing countries .
| Characteristic | No. of trials | Mean difference in birth weight in g (95% CI) | Univariate meta-regression |
||
|---|---|---|---|---|---|
| P-value for test for heterogeneity by covariate | Residual I2 after adjusting for covariate (P-value) | Reduction in I2 after adjusting for covariate (%) | |||
| Average gestational age at initiation of supplements | 0.22 | 57% (0.003) | 0 | ||
| < 20 weeks | 9 | 35 (14–57) | – | – | – |
| ≥ 20 weeks | 6 | 60 (33–86) | – | – | – |
| No maternal education | 0.43 | 56% (0.01) | 7 | ||
| < 50% | 9 | 39 (18–60) | – | – | – |
| ≥ 50% | 4 | 50 (23–78) | – | – | – |
| Iron dose in treatment and control groups | 0.99 | 57% (0.003) | 0 | ||
| Same | 7 | 45 (17–73) | – | – | – |
| Different | 8 | 45 (23–66) | – | – | – |
| Maternal underweighta | 0.37 | 56% (0.01) | 1 | ||
| < 20% | 11 | 47 (30–63) | – | – | – |
| ≥ 20% | 4 | 43 (−5–90) | – | – | – |
| Average maternal height | 0.08 | 43% (0.05) | 25 | ||
| < 155 cm | 7 | 27 (6–49) | – | – | – |
| ≥ 155 cm | 8 | 59 (43–75) | – | – | – |
| Primiparas | 0.20 | 51% (0.01) | 11 | ||
| < 35% | 8 | 53 (29–78) | – | – | – |
| ≥ 35% | 7 | 37 (15–58) | – | – | – |
CI, confidence interval.
a Body mass index (weight in kg divided by square of height in metres) < 18.5.
Table 4. Subgroup analysis and meta-regression for the effect of maternal micronutrient supplements on the risk of low birth weight in developing countries .
| Characteristic | No. of trials | Pooled RR (95% CI) | Univariate meta-regression |
||
|---|---|---|---|---|---|
| P-value for test for heterogeneity by covariate | Residual I2 after adjusting for covariate (P-value) | Reduction in I2 after adjusting for covariate (%) | |||
| Average gestational age at initiation of supplements | 0.08 | 22% (0.22) | 46 | ||
| < 20 weeks | 9 | 0.92 (0.85–0.99) | – | – | – |
| ≥ 20 weeks | 6 | 0.76 (0.64–0.89) | – | – | – |
| No maternal education | 0.12 | 12% (0.32) | 33 | ||
| < 50% | 9 | 0.83 (0.76–0.91) | – | – | – |
| ≥ 50% | 4 | 0.95 (0.87–1.05) | – | – | – |
| Iron dose in treatment and control groups | 0.66 | 41% (0.05) | 0 | ||
| Same | 7 | 0.86 (0.75–0.99) | – | – | – |
| Different | 8 | 0.87 (0.80–0.95) | – | – | – |
| Maternal underweighta | 0.96 | 41% (0.05) | 0 | ||
| < 20% | 11 | 0.86 (0.80–0.92) | – | – | – |
| ≥ 20% | 4 | 0.79 (0.61–1.02) | – | – | – |
| Average maternal height | 0.44 | 41% (0.05) | 0 | ||
| < 155 cm | 7 | 0.91 (0.82–1.00) | – | – | – |
| ≥ 155 cm | 8 | 0.79 (0.69–0.91) | – | – | – |
| Primiparas | 0.70 | 41% (0.05) | 0 | ||
| < 35% | 8 | 0.83 (0.72–0.96) | – | – | – |
| ≥ 35% | 7 | 0.85 (0.77–0.92) | – | – | – |
CI, confidence interval; RR, relative risk.
a Body mass index (weight in kg divided by square of height in metres) < 18.5.
Table 5. Subgroup analysis and meta-regression for the effect of maternal multiple micronutrient supplements on the risk of small size for gestational age in developing countries.
| Characteristic | No. of trials | Pooled RR (95% CI) | Univariate meta-regression |
||
|---|---|---|---|---|---|
| P-value for test for heterogeneity by covariate | Residual I2 after adjusting for covariate (P-value) | Reduction in I2 after adjusting for covariate (%) | |||
| Average gestational age at initiation of supplements | 0.12 | 9% (0.35) | 81 | ||
| < 20 weeks | 9 | 0.95 (0.89–1.02) | – | – | – |
| ≥ 20 weeks | 6 | 0.77 (0.65–0.91) | – | – | – |
| No maternal education | 0.22 | 29% (0.16) | 34 | ||
| < 50% | 9 | 0.84 (0.76–0.92) | – | – | – |
| ≥ 50% | 4 | 0.95 (0.84–1.08) | – | – | – |
| Iron dose in treatment and control groups | 0.86 | 47% (0.02) | 0 | ||
| Same | 7 | 0.82 (0.70–0.97) | – | – | – |
| Different | 8 | 0.85 (0.67–1.08) | – | – | – |
| Maternal underweighta | 0.47 | 34% (0.04) | 28 | ||
| < 20% | 11 | 0.85 (0.78–0.91) | – | – | – |
| ≥ 20% | 4 | 0.96 (0.82–1.14) | – | – | – |
| Average maternal height | 0.21 | 34% (0.10) | 28 | ||
| < 155 cm | 7 | 0.98 (0.91–1.06) | – | – | – |
| ≥ 155 cm | 8 | 0.80 (0.73–0.89) | – | – | – |
| Primiparas | 0.54 | 47% (0.02) | 0 | ||
| < 35% | 8 | 0.82 (0.70–0.97) | – | – | – |
| ≥ 35% | 7 | 0.85 (0.79–0.92) | – | – | – |
CI, confidence interval; RR, relative risk.
a Body mass index (weight in kg divided by square of height in metres) < 18.5.
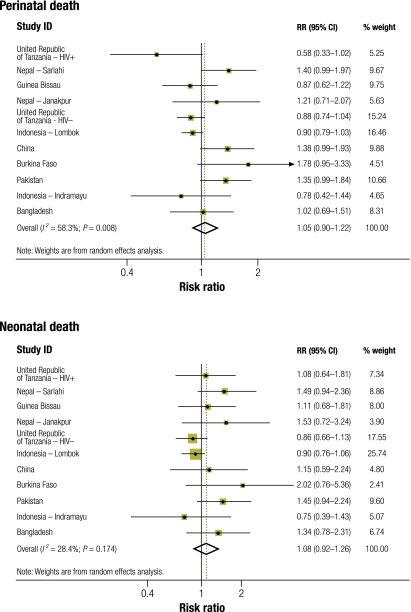
The effect of micronutrient supplementation on perinatal and neonatal mortality was assessed in 11 randomized controlled trials (Table 6). A pooled estimate showed no overall effect of micronutrient supplementation on perinatal mortality (RR: 1.05; 95% CI: 0.90–1.22) or neonatal mortality (RR: 1.08; 95% CI: 0.92–1.26; Fig. 2). However, substantial heterogeneity of effect estimates on perinatal mortality was evident (I2 = 58%; 95% CI: 19–79; P for heterogeneity = 0.008). Subgroup and meta-regression analyses showed that differences between trials in maternal underweight, maternal height, parity and iron dosage did not explain the heterogeneity of effect estimates on perinatal mortality (Table 7). Furthermore, differences in the effect of supplementation on the risk of large size for gestational age did not explain the heterogeneity. In univariate meta-regression analysis, adverse effects on perinatal mortality were found in trials conducted in settings where most mothers had no formal education (P = 0.009). All three trials conducted in populations in which more than 50% of mothers had no formal education (Burkina Faso, Sarlahi in Nepal and Pakistan) showed adverse effects on perinatal mortality (pooled RR: 1.41; 95% CI: 1.14–1.75). The average gestational age at the initiation of supplementation may have contributed to the heterogeneity in the effect estimates, and early initiation was associated with harmful effects (univariate meta-regression, P = 0.003). The pooled RR was 1.28 (95% CI: 1.10–1.49) for trials in which supplementation was on average initiated before the 20th weeks of gestation and 0.88 (95% CI: 0.80, 0.97) for trials in which supplementation was initiated after the 20th week. Maternal education and average gestational age at supplement initiation were correlated; trials conducted among mothers with no education were also those that initiated supplementation early. When we constructed a multivariate meta-regression model including maternal education and gestational age at initiation of supplementation, both covariates became non-significant (timing of initiation, P = 0.09; maternal education, P = 0.30; residual I2 = 0%; residual P for heterogeneity = 0.51). We also conducted meta-regression analysis to explore the effect of micronutrient supplementation on neonatal mortality. Similarly, we found that maternal educational level (univariate meta-regression; P = 0.01) or average gestational age at initiation of supplementation (P = 0.02) may have contributed to the heterogeneous effect on neonatal mortality.
Table 6. Study population and setting of randomized controlled trials examining the effect of maternal micronutrient supplementation on perinatal and neonatal mortality in developing countries.
| Study location | Setting | Maternal BMI < 18.5 (%) | Maternal height (cm) | Women without education (%) | Effect of micronutrient supplements on perinatal mortality, RR (95% CI) | Perinatal mortalitya in controls | Low birth weight in controls (%) |
|---|---|---|---|---|---|---|---|
| United Republic of Tanzania, HIV+16 | Urban, Dar es Salaam | 10–20 | 157 | 8 | 0.58 (0.33–1.02) | 103 | 15.8 |
| Nepal, Sarlahi18 | Rural villages in Sarlahi | > 20 | 150 | 81 | 1.40 (0.99–1.96) | 62 | 34.3 |
| Guinea Bissau21 | Semi-urban setting in Bissau | < 10 | 160 | 22 | 0.87 (0.62–1.22) | 88 | 13.6 |
| Nepal, Janakpur22 | Janakpur, urban and rural settings | > 20 | 151 | 45 | 1.21 (0.71–2.08) | 40 | 25.4 |
| United Republic of Tanzania, HIV−25 | Urban, Dar es Salaam | < 10 | 157 | 8 | 0.88 (0.74–1.04) | 66 | 9.4 |
| Indonesia, Lombok26 | Periurban and rural villages in Lombok island | 10–20 | 150 | 12 | 0.90 (0.79–1.03) | 38 | 10.5 |
| China27 | Two poor rural north-western counties | < 10 | 159 | 6 | 1.38 (0.99–1.93) | 37 | 4.5 |
| Burkina Faso28 | Two rural health centres in Hounde | 10–20 | 162 | 81 | 1.78 (0.95–3.32) | 21 | 15.6 |
| Pakistan28 | Urban and rural Sindh | 10–20 | 153 | 79 | 1.35 (0.99–1.83) | 75 | 19.6 |
| Indonesia, Indramayu9 | Two rural subdistricts in Indramayu | 10–20 | 151 | 10 | 0.78 (0.42–1.43) | 42 | 7 |
| Bangladesh9 | A rural subdistrict in Matlab | > 20 | 150 | 31 | 1.02 (0.69–1.52) | 44 | 35.7 |
BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; RR, relative risk.
a Perinatal deaths per 1000 live births.
Fig. 2.
Effect of maternal micronutrient supplementation on perinatal and neonatal mortality in randomized controlled trials in developing countries
CI, confidence interval; HIV, human immunodeficiency virus; RR, relative risk.
Table 7. Results of subgroup analysis and meta-regression for the effect of maternal micronutrient supplementation on perinatal mortality in randomized controlled trials conducted in developing countries.
| Characteristic | No. of trials | Pooled RR (95% CI) | Univariate meta-regression |
||
|---|---|---|---|---|---|
| P-value for test for heterogeneity by covariate | Residual I2 after adjusting for covariate (P-value) | Reduction in I2 after adjusting for covariate (%) | |||
| Average gestational age at initiation of supplements | 0.003 | 0% (0.49) | 100 | ||
| < 20 wk | 7 | 1.28 (1.10–1.49) | – | – | – |
| ≥ 20 wk | 4 | 0.88 (0.80–0.97) | – | – | – |
| No maternal education | 0.009 | 17% (0.31) | 71 | ||
| < 50% | 8 | 0.93 (0.82–1.06) | – | – | – |
| ≥ 50% | 3 | 1.41 (1.14–1.75) | – | – | – |
| Iron dose in treatment and control groups | 0.19 | 47% (0.05) | 19 | ||
| Same | 5 | 0.94 (0.79–1.12) | – | – | – |
| Different | 6 | 1.18 (0.95–1.47) | – | – | – |
| Maternal underweighta | 0.36 | 55% (0.01) | 5 | ||
| < 20% | 8 | 1.00 (0.84–1.19) | – | – | – |
| ≥ 20% | 3 | 1.22 (0.97–1.54) | – | – | – |
| Average maternal height | 0.90 | 58% (0.008) | 0 | ||
| < 155 cm | 6 | 1.09 (0.89–1.34) | – | – | – |
| ≥ 155 cm | 5 | 1.00 (0.75–1.33) | – | – | – |
| Primiparas | 0.91 | 58% (0.008) | 0 | ||
| < 35% | 6 | 1.10 (0.85–1.44) | – | – | – |
| ≥ 35% | 5 | 0.97 (0.83–1.14) | – | – | – |
| Effect of supplementation on risk of LGA | 0.86 | 56% (0.02) | 0 | ||
| < 10% (RR < 1.10) | 4 | 1.19 (0.92–1.54) | – | – | – |
| ≥ 10% (RR ≥ 1.10) | 5 | 1.06 (0.81–1.38) | – | – | – |
| Effect of supplementation on birth weight (mean difference) | 0.34 | 58% (0.008) | 0 | ||
| ≥ 50 g | 5 | 0.96 (0.76–1.22) | – | – | – |
| < 50 g | 6 | 1.14 (0.90–1.44) | – | – | – |
CI, confidence interval; LGA, large size for gestational age; RR, relative risk.
a Body mass index (weight in kg divided by square of height in metres) < 18.5.
Neither the Egger test nor the Begg test for publication bias showed statistical significance (P > 0.05 for both tests) for an effect on low birth weight, birth weight, small size for gestational age, preterm delivery and perinatal mortality. In the case of neonatal mortality, we found some evidence of publication bias based on the Egger test (P = 0.02) but not on the Begg test (P = 0.28).
Discussion
Our study is consistent with recent systematic reviews in showing that maternal micronutrient supplementation can reduce the risk of having an infant with low birth weight.8,31 While micronutrient supplementation showed no overall effect on perinatal mortality, several trials reported non-significant adverse effects. We found that maternal educational level or gestational age at initiation of supplementation may have contributed to the heterogeneous effects on perinatal mortality.
Several biological mechanisms can explain the beneficial effects of micronutrient supplementation on fetal growth. Women require more vitamins and minerals during pregnancy and supplements can improve their nutritional and haemoglobin status. Supplements also help improve and maintain functional immunity.32 Deficiencies of B-complex vitamins and folate are prevalent and may be a major cause of homocysteinaemia.4 Elevated homocysteine levels can lead to endothelial cell dysfunction and affect placental function. Thus, micronutrient supplements can help maintain normal homocysteine levels. Many vitamins and minerals also play important roles in gene regulation as well as in cellular metabolism and fetal growth.33
Why does micronutrient supplementation appear to be associated with increased risk of perinatal death in some trials? Christian et al. have hypothesized that such adverse effects may be due to an increased risk of birth asphyxia or of cephalopelvic disproportion among infants who are large for gestational age.18 However, in our study the heterogeneity in the effect on perinatal mortality was not explained by the prevalence of maternal underweight, average maternal height or the percentage of primiparous women. In a trial in Burkina Faso, half of the perinatal deaths were due to prematurity. This led the researchers to conclude that an increase in perinatal mortality cannot be entirely due to the complications associated with delivering an infant too large for gestational age.28 Furthermore, large head size can increase the risk of obstructed delivery, but a previous review found no effect of micronutrient supplementation on neonatal head circumference.10 Alternatively, the effects on perinatal mortality could stem from the use of a lower dose of iron in the micronutrient group than in the iron and folic acid group.27 However, differences in the dose of iron did not explain heterogeneity in our analysis.
We found that all trials that reported an adverse effect on perinatal mortality were conducted in poor rural settings where most mothers had no education. Maternal educational level is likely to be a proxy for unmeasured characteristics. Low maternal education may be a correlate of a greater likelihood of delivering at home, limited access to health facilities, limited availability of skilled birth attendants and maternal and newborn care of lesser quality.34 Mothers with limited education are less likely to seek neonatal care and more likely to be at risk of adverse pregnancy outcomes. Furthermore, in most developing countries access to quality perinatal health care differs substantially between rural and urban areas.35 Our findings suggest that micronutrient supplements may need to be delivered under adequate obstetric and postnatal care. Large cluster randomized trials with a stepped wedge design may need to be conducted in rural settings to assess the safety and efficacy of micronutrient supplementation in the context of programmes for improving obstetric and postnatal care. In many parts of the developing world, enhancing access to obstetric care, improving postnatal care and empowering mothers through community health workers are essential measures for reducing perinatal and neonatal mortality.36,37
There is no clear explanation for the association between multiple micronutrient supplementation and a higher risk of perinatal mortality found in trials in which supplementation began in the first and early second trimester of pregnancy. This finding needs to be interpreted with caution because the meta-regression explored the potential relationship between treatment effect and the timing of supplement initiation (e.g. average gestational week) across trials. Thus, we are unable to assess whether a relationship exists or not between treatment effect and the timing of supplement initiation within trials.15 It is possible that supplementation for a longer period increased the risk of complications during labour by increasing infants’ size for gestational age. Alternatively, early supplementation could have altered metabolic regulation and led to complications during pregnancy and consequently to perinatal death. Another possibility is that micronutrient supplementation prevented early spontaneous abortion and allowed mothers to carry frail fetuses to much later stages of pregnancy, with a resulting spurious increase in the number of perinatal deaths. Minerals can interact with desoxyribonucleic acid (DNA) and disrupt ligand binding or protein function, or induce oxidative damage on embryotic tissue.38 There is clearly a need to elucidate the biological mechanisms behind the potentially detrimental effects of micronutrient supplementation.
Our study has several limitations. Factors that we did not examine, such as the prevalence of maternal infections, including malaria and hookworm, and dietary nutrient intake, may have accounted for the heterogeneity of effect estimates. As shown by a trial in Nepal, zinc can reduce the beneficial effects of iron supplements through biochemical interactions that are possibly influenced by whether or not supplements are taken with a meal.18,39,40 The heterogeneity of effect estimates could also depend on differences in the quality of the trials; however, most trials showed good compliance with the study regimen and high participant retention. We were unable to verify the composition and dosage of the micronutrient supplements. Most trials used the RDA of each micronutrient, an amount considered sufficient to meet the requirements of most healthy individuals in industrialized countries. In developing countries pregnant women may require higher doses because of their poorer nutritional status and higher rates of infection. However, a recent trial found that multivitamin supplements containing the RDA of each component may be as effective as those containing multiple doses of the RDA in reducing the risk of adverse pregnancy outcomes among HIV-infected women.41
There is insufficient evidence to recommend routine prenatal multiple micronutrient supplementation for women in developing countries. Although our study provides valuable insights into the heterogeneous effects of micronutrient supplements on perinatal mortality, more research is needed to explain why some trials found multiple micronutrient supplementation to be associated with higher perinatal mortality. Few studies have examined the effects of micronutrient supplementation on long-term child health outcomes, such as child mortality, morbidity, growth and cognitive development. A large trial in Indonesia showed that prenatal micronutrient supplementation was associated with a significant 18% reduction in early infant mortality.26 Examining long-term outcomes is important because greater infant survival or other beneficial effects may not be reflected in birth weight. Micronutrient supplementation before pregnancy also warrants further research.42,43 Among Bhutanese refugees dependent on food aid, the incidence of low birth weight declined from 16% to 8% after 2 to 3 years of implementation of micronutrient-fortified blended foods.44
In conclusion, we found that maternal micronutrient supplementation can reduce the risk of low birth weight but has no overall effect on perinatal mortality in developing countries. Non-significant detrimental effects on perinatal mortality were reported in some trials conducted in poor rural settings. This suggests that in such settings supplements may need to be delivered in the context of programmes for improving obstetric and postnatal care. More research is needed to address the safety, efficacy and effective delivery of maternal micronutrient supplementation.
Competing interests:
None declared.
References
- 1.Low birthweight: country, regional and global estimates New York: United Nations Children’s Fund & World Health Organization; 2004.
- 2.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Child Health Epidemiology Reference Group of WHO and UNICEF Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 3.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and Child Undernutrition Study Group Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 4.Allen LH. Multiple micronutrients in pregnancy and lactation: an overview. Am J Clin Nutr. 2005;81:1206S–12S. doi: 10.1093/ajcn/81.5.1206. [DOI] [PubMed] [Google Scholar]
- 5.Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, et al. Maternal and Child Undernutrition Study Group What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371:417–40. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- 6.Shrimpton R, Shrimpton R, Schultink W. Can supplements help meet the micronutrient needs of the developing world? Proc Nutr Soc. 2002;61:223–9. doi: 10.1079/PNS2002163. [DOI] [PubMed] [Google Scholar]
- 7.Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2006;4:CD004905. doi: 10.1002/14651858.CD004905.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Shah PS, Ohlsson A, Knowledge Synthesis Group on Determinants of Low Birth Weight and Preterm Births Effects of prenatal multimicronutrient supplementation on pregnancy outcomes: a meta-analysis. CMAJ. 2009;180:E99–108. doi: 10.1503/cmaj.081777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronsmans C, Fisher DJ, Osmond C, Margetts BM, Fall CH, Maternal Micronutrient Supplementation Study Group Multiple micronutrient supplementation during pregnancy in low-income countries: a meta-analysis of effects on stillbirths and on early and late neonatal mortality. Food Nutr Bull. 2009;30(Suppl):S547–55. doi: 10.1177/15648265090304S409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fall CH, Fisher DJ, Osmond C, Margetts BM, Maternal Micronutrient Supplementation Study Group Multiple micronutrient supplementation during pregnancy in low-income countries: a meta-analysis of effects on birth size and length of gestation. Food Nutr Bull. 2009;30(Suppl):S533–46. doi: 10.1177/15648265090304S408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christian P, Osrin D, Manandhar DS, Khatry SK, de L Costello AM, West KP., Jr Antenatal micronutrient supplements in Nepal. Lancet. 2005;366:711–2. doi: 10.1016/S0140-6736(05)67166-8. [DOI] [PubMed] [Google Scholar]
- 12.Picciano MF, McGuire MK. Use of dietary supplements by pregnant and lactating women in North America. Am J Clin Nutr. 2009;89:663S–7S. doi: 10.3945/ajcn.2008.26811B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 16.Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, McGrath N, Mwakagile D, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet. 1998;351:1477–82. doi: 10.1016/S0140-6736(98)04197-X. [DOI] [PubMed] [Google Scholar]
- 17.Christian P, Khatry SK, Katz J, Pradhan EK, LeClerq SC, Shrestha SR, et al. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ. 2003;326:571. doi: 10.1136/bmj.326.7389.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christian P, West KP, Khatry SK, Leclerq SC, Pradhan EK, Katz J, et al. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster-randomized trial in Nepal. Am J Clin Nutr. 2003;78:1194–202. doi: 10.1093/ajcn/78.6.1194. [DOI] [PubMed] [Google Scholar]
- 19.Ramakrishnan U, González-Cossío T, Neufeld LM, Rivera J, Martorell R. Multiple micronutrient supplementation during pregnancy does not lead to greater infant birth size than does iron-only supplementation: a randomized controlled trial in a semirural community in Mexico. Am J Clin Nutr. 2003;77:720–5. doi: 10.1093/ajcn/77.3.720. [DOI] [PubMed] [Google Scholar]
- 20.Friis H, Gomo E, Nyazema N, Ndhlovu P, Krarup H, Kaestel P, et al. Effect of multimicronutrient supplementation on gestational length and birth size: a randomized, placebo-controlled, double-blind effectiveness trial in Zimbabwe. Am J Clin Nutr. 2004;80:178–84. doi: 10.1093/ajcn/80.1.178. [DOI] [PubMed] [Google Scholar]
- 21.Kaestel P, Michaelsen KF, Aaby P, Friis H. Effects of prenatal multimicronutrient supplements on birth weight and perinatal mortality: a randomised, controlled trial in Guinea-Bissau. Eur J Clin Nutr. 2005;59:1081–9. doi: 10.1038/sj.ejcn.1602215. [DOI] [PubMed] [Google Scholar]
- 22.Osrin D, Vaidya A, Shrestha Y, Baniya RB, Manandhar DS, Adhikari RK, et al. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: double-blind, randomised controlled trial. Lancet. 2005;365:955–62. doi: 10.1016/S0140-6736(05)71084-9. [DOI] [PubMed] [Google Scholar]
- 23.Gupta P, Ray M, Dua T, Radhakrishnan G, Kumar R, Sachdev HP. Multimicronutrient supplementation for undernourished pregnant women and the birth size of their offspring: a double-blind, randomized, placebo-controlled trial. Arch Pediatr Adolesc Med. 2007;161:58–64. doi: 10.1001/archpedi.161.1.58. [DOI] [PubMed] [Google Scholar]
- 24.Zagré NM, Desplats G, Adou P, Mamadoultaibou A, Aguayo VM. Prenatal multiple micronutrient supplementation has greater impact on birthweight than supplementation with iron and folic acid: a cluster-randomized, double-blind, controlled programmatic study in rural Niger. Food Nutr Bull. 2007;28:317–27. doi: 10.1177/156482650702800308. [DOI] [PubMed] [Google Scholar]
- 25.Fawzi WW, Msamanga GI, Urassa W, Hertzmark E, Petraro P, Willett WC, et al. Vitamins and perinatal outcomes among HIV-negative women in Tanzania. N Engl J Med. 2007;356:1423–31. doi: 10.1056/NEJMoa064868. [DOI] [PubMed] [Google Scholar]
- 26.Shankar AH, Jahari AB, Sebayang SK, Aditiawarman, Apriatni M, Harefa B, et al. Supplementation with Multiple Micronutrients Intervention Trial (SUMMIT) Study Group Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: a double-blind cluster-randomised trial. Lancet. 2008;371:215–27. doi: 10.1016/S0140-6736(08)60133-6. [DOI] [PubMed] [Google Scholar]
- 27.Zeng L, Dibley MJ, Cheng Y, Dang S, Chang S, Kong L, et al. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ. 2008;337(nov07 4):a2001. doi: 10.1136/bmj.a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberfroid D, Huybregts L, Lanou H, Henry MC, Meda N, Menten J, et al. MISAME Study Group Effects of maternal multiple micronutrient supplementation on fetal growth: a double-blind randomized controlled trial in rural Burkina Faso. Am J Clin Nutr. 2008;88:1330–40. doi: 10.3945/ajcn.2008.26296. [DOI] [PubMed] [Google Scholar]
- 29.Bhutta ZA, Rizvi A, Raza F, Hotwani S, Zaidi S, Moazzam Hossain S, et al. A comparative evaluation of multiple micronutrient and iron-folic acid supplementation during pregnancy in Pakistan: impact on pregnancy outcomes. Food Nutr Bull. 2009;30(Suppl):S496–505. doi: 10.1177/15648265090304S404. [DOI] [PubMed] [Google Scholar]
- 30.Sunawang UB, Utomo B, Hidayat A, Kusharisupeni, Subarkah Preventing low birthweight through maternal multiple micronutrient supplementation: a cluster-randomized, controlled trial in Indramayu, West Java. Food Nutr Bull. 2009;30(Suppl):S488–95. doi: 10.1177/15648265090304S403. [DOI] [PubMed] [Google Scholar]
- 31.Margetts BM, Fall CH, Ronsmans C, Allen LH, Fisher DJ, Maternal Micronutrient Supplementation Study Group Multiple micronutrient supplementation during pregnancy in low-income countries: review of methods and characteristics of studies included in the meta-analyses. Food Nutr Bull. 2009;30(Suppl):S517–26. doi: 10.1177/15648265090304S406. [DOI] [PubMed] [Google Scholar]
- 32.Wintergerst ES, Maggini S, Hornig DH. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab. 2007;51:301–23. doi: 10.1159/000107673. [DOI] [PubMed] [Google Scholar]
- 33.Ashworth CJ, Antipatis C. Micronutrient programming of development throughout gestation. Reproduction. 2001;122:527–35. doi: 10.1530/rep.0.1220527. [DOI] [PubMed] [Google Scholar]
- 34.Wagstaff A, Bustreo F, Bryce J, Claeson M, WHO–World Bank Child Health and Poverty Working Group Child health: reaching the poor. Am J Public Health. 2004;94:726–36. doi: 10.2105/AJPH.94.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houweling TA, Ronsmans C, Campbell OM, Kunst AE. Huge poor-rich inequalities in maternity care: an international comparative study of maternity and child care in developing countries. Bull World Health Organ. 2007;85:745–54. doi: 10.2471/BLT.06.038588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhutta ZA, Ali S, Cousens S, Ali TM, Haider BA, Rizvi A, et al. Interventions to address maternal, newborn, and child survival: what difference can integrated primary health care strategies make? Lancet. 2008;372:972–89. doi: 10.1016/S0140-6736(08)61407-5. [DOI] [PubMed] [Google Scholar]
- 37.Barber SL, Gertler PJ. The impact of Mexico’s conditional cash transfer programme, Oportunidades, on birthweight. Trop Med Int Health. 2008;13:1405–14. doi: 10.1111/j.1365-3156.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanna LA, Peters JM, Wiley LM, Clegg MS, Keen CL. Comparative effects of essential and nonessential metals on preimplantation mouse embryo development in vitro. Toxicology. 1997;116:123–31. doi: 10.1016/S0300-483X(96)03534-2. [DOI] [PubMed] [Google Scholar]
- 39.Fischer Walker C, Kordas K, Stoltzfus RJ, Black RE. Interactive effects of iron and zinc on biochemical and functional outcomes in supplementation trials. Am J Clin Nutr. 2005;82:5–12. doi: 10.1093/ajcn.82.1.5. [DOI] [PubMed] [Google Scholar]
- 40.Christian P, Stewart CP, LeClerq SC, Wu L, Katz J, West KP, Jr, et al. Antenatal and postnatal iron supplementation and childhood mortality in rural Nepal: a prospective follow-up in a randomized, controlled community trial. Am J Epidemiol. 2009;170:1127–36. doi: 10.1093/aje/kwp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawai K, Kupka R, Mugusi F, Aboud S, Okuma J, Villamor E, et al. A randomized trial to determine the optimal dosage of multivitamin supplements to reduce adverse pregnancy outcomes among HIV-infected women in Tanzania. Am J Clin Nutr. 2010;91:391–7. doi: 10.3945/ajcn.2009.28483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ronnenberg AG, Goldman MB, Chen D, Aitken IW, Willett WC, Selhub J, et al. Preconception homocysteine and B vitamin status and birth outcomes in Chinese women. Am J Clin Nutr. 2002;76:1385–91. doi: 10.1093/ajcn/76.6.1385. [DOI] [PubMed] [Google Scholar]
- 43.Timmermans S, Jaddoe VW, Hofman A, Steegers-Theunissen RP, Steegers EA. Periconception folic acid supplementation, fetal growth and the risks of low birth weight and preterm birth: the Generation R Study. Br J Nutr. 2009;102:777–85. doi: 10.1017/S0007114509288994. [DOI] [PubMed] [Google Scholar]
- 44.Shrimpton R, Thorne-Lyman A, Tripp K, Tomkins A. Trends in low birthweight among the Bhutanese refugee population in Nepal. Food Nutr Bull. 2009;30(Suppl):S197–206. doi: 10.1177/15648265090302S203. [DOI] [PubMed] [Google Scholar]