Abstract
Recurrence of multiple myeloma (MM) after therapy suggests the presence of tumor-initiating subpopulations. In our study, we performed flow cytometry–based Hoechst 33342 staining to evaluate the existence of a MM population with stem-like features known as side population (SP) cells. SP cells exhibit substantial heterogeneity in MM cell lines and primary MM cells; express CD138 antigen in MM cell lines; display higher mRNA expression and functional activity of ABCG2 transporter; and have a higher proliferation index compared with non-SP cells. We observed evidence for clonogenic potential of SP cells, as well as the ability of SP cells to regenerate original population. Moreover, SP cells revealed higher tumorigenicity compared with non-SP cells. Importantly, lenalidomide decreased the percentage and clonogenicity of SP cells, and also induced phosphorylation changes in Akt, GSK-3α/β, MEK1, c-Jun, p53, and p70S6K in SP cells. Adherence to bone marrow stromal cells (BMSCs) increased the percentage, viability, and proliferation potential of SP cells. Lenalidomide and thalidomide abrogated this stimulatory effect of BMSCs and significantly decreased the percentage of SP cells. Our studies demonstrate a novel mechanism of action for lenalidomide, namely targeting SP fraction, providing the framework for new therapeutic strategies targeting subpopulations of MM cells including presumptive stem cells.
Introduction
Multiple myeloma (MM), a malignancy hallmarked by accumulation of malignant plasma cells in the bone marrow, remains largely incurable despite the use of conventional and novel therapies.1 The bone marrow (BM) microenvironment promotes tumor cell growth, survival, and confers drug resistance against conventional agents.2 Although currently available anti-MM strategies have been effective in targeting the bulk of tumor cells, it has been postulated that a tumor-initiating subpopulation or cancer stem cell persists, which may be responsible for eventual relapses.3 Side population (SP) cells are an enriched source of cancer-initiating cells with stem cell properties, which have been identified in solid tumors, as well as in hematopoietic malignancies.4–8 The SP cells show a distinct “low-staining pattern” with the Hoechst 33342 dye.9 Importantly, SP cells possess the ability to generate non-SP cells both in vitro and in vivo, and are associated with chemoresistance and tumorigenicity in vivo.4,10 The prevalence and biologic function of SP cells in MM are not fully defined.
In the late 1990s, thalidomide was introduced to the treatment of relapsed/refractory MM; however, its effect in patients is associated with dose- and duration-dependent side effects.11,12 Since then, more potent immunomodulatory drugs (IMiDs), such as lenalidomide, have been introduced. Lenalidomide has been approved for the treatment of both myelodysplasia with deletion of chromosome 5q and for relapsed MM, specifically in combination with dexamethasone.12,13 Although IMiDs act directly on tumor cells, block adherence to bone marrow stromal cells (BMSCs), modulate angiogenesis and cytokines, and up-regulate host antitumor immunity, the molecular mechanism for their action remains largely undefined, and it is unclear whether they target SP cells in MM.14–18
In this study, we identified SP cells in MM cell lines as well as in primary MM tumor cells by flow cytometry–based Hoechst 33342 staining, and showed heterogeneity in the percentage of SP cells, as well as the lack of strict correlation between SP fraction and CD138− status. SP cells exhibited clonogenic and tumorigenic potential; and importantly, lenalidomide significantly decreased the percentage and clonogenicity of SP cells at clinically relevant concentrations. Moreover, lenalidomide only slightly altered expression of drug-resistant transporter ABCG2 with no effect on functional activity of BCRP1 efflux pump. Modulation of diverse signaling cascades in SP cells by lenalidomide, including changes in Akt, GSK-3α/β, MEK1, c-Jun, p53, and p70S6K phosphorylation was observed. Adherence to BMSCs increased the percentage, viability, and proliferation potential of SP cells. Interestingly, both lenalidomide and thalidomide attenuated this stimulatory effect of BMSCs by significantly decreasing SP cell percentages. Therefore, our studies provide insight toward developing novel strategies targeting SP cells to overcome conventional drug resistance and improve patient outcome in MM.
Methods
Culture of MM cell lines, primary MM cells, and stromal cells
We used a panel of MM cell lines (U266, NCI-H929, OPM-1, OPM-2, OPM-6, delta47, OCIMy5, XG-1, JJN3, KMS-11, KMS-18, KMS-34, MM.1S, MM.1R, RPMI 8226-S [also referred to as RPMI-S], RPMI-Dox40, RPMI-MR20, and RPMI-LR5) and the human bone marrow stromal cell line HS-5 (ATCC), which were cultured as described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Reagents
Thalidomide and lenalidomide (CC-5013, Revlimid) were provided by Celgene and dissolved in dimethyl sulfoxide (DMSO). Reserpine, verapamil, and fumitremorgin C were obtained from Sigma-Aldrich. Other chemicals were purchased from Sigma-Aldrich, unless specifically mentioned.
Cell-based assays
Viability of MM cells was evaluated by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) colorimetric survival assay. Clonogenicity of SP and main population (MP) cells was determined using colony-forming cell (CFC) assay. Cell-surface CD138 antigen expression was evaluated by fluorescence immunophenotyping, as described in supplemental Methods. Flow cytometry–based evaluation of MM cell proliferation was performed by BrdU proliferation assay, as well as labeling MM cells with carboxyfluorescein diacetate succinimidyl ester (CFSE) for studies in both the presence and absence of BMSCs, as described in supplemental Methods.
Functional assays
Side population analysis was performed with Hoechst 33342 dye using methods described by Goodell et al9 with modifications, as described in supplemental Methods. Functional activity of ABC transporters was evaluated using rhodamine 123 (Rh123) for P-glycoprotein transporter and mitoxantrone (Mit) for BCRP1 transporter, as described in supplemental Methods.
Molecular profiling analyses
The molecular sequelae of treatment with lenalidomide on SP and MP cells were evaluated by multiplex evaluation of levels of total and phosphorylated proteins using the xMAP luminex platform (Luminex), as described in supplemental Methods. Quantification of ABC transporter mRNA expression in SP and MP cells was determined by Quantigene 7-plex assay (Panomics), as described in supplemental Methods.
Tumor cell implantation in mouse models
In vivo experiments were done in accordance with the institutional guidelines for the use of laboratory animals; details are included in supplemental Methods. All in vivo experiments were approved by the Dana-Farber Cancer Institute Animal Care and Use Committee.
Statistical analysis
Data presented are mean plus or minus SD. The statistical significance of differences was assessed using the unpaired t test. P values < .05 were considered significant.
Results
Characterization of SP heterogeneity in a panel of MM cell lines and primary MM cells
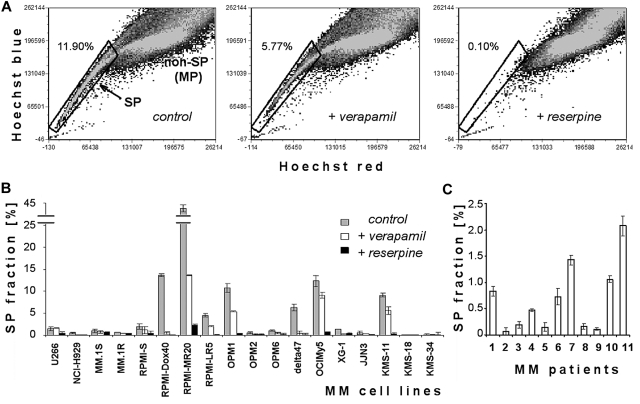
We examined SP cells in human MM cell lines and primary MM cells based on their ability to efflux Hoechst 33342 dye (Figure 1). Flow cytometry identifies low-fluorescence SP cells in the tail region of the dot plot relative to Hoechst bright-fluorescence cells (non-SP or MP). Figure 1A depicts SP cells in the OPM1 cell line: low intracellular accumulation of Hoechst is characteristic of SP cells (left panel), and disappears when cells are treated with ABC transporter inhibitors such as verapamil (100μM; middle panel) or reserpine (50μM; right panel). Because higher concentrations of verapamil (> 100μM) were too toxic (data not shown), we used reserpine in all subsequent experiments to confirm the appropriate gating for SP cells. Indeed, reserpine (50μM) completely abolished the detection of SP cells in the OPM1 cell line (Figure 1A right panel), without significant toxicity to MM cells (data not shown).
Figure 1.
Characterization of SP heterogeneity in the panel of MM cell lines. (A) Representative flow cytometric dot plots of SP analysis. Dot plots show control OPM1 cells incubated in Hoechst 33342 alone (left), Hoechst 33342 accumulation in the presence of 100μM verapamil (middle), and Hoechst 33342 accumulation in the presence of 50μM reserpine (right). Abscissa is Hoechst red fluorescence intensity and ordinate is Hoechst blue fluorescence intensity, with gate representing the SP fraction of OPM1 cells. (B) Quantification of SP fraction at 1 day in MM cell lines alone and in the presence of 100μM verapamil or 50μM reserpine. (C) Percentage of SP fraction in 11 primary samples of patient with MM.
We performed SP analysis of 18 MM cell lines (Figure 1B). Frequency of SP cells was heterogeneous: most cell lines (U266, MM.1S, MM.1R, RPMI-S, OPM2, OPM6, XG-1, and JJN3) had a minor population of SP cells (∼ 1%-2%); KMS-18, KMS-34 and NCI-H929 lines had < 0.5% SP cells; and some cell lines (Delta47, KMS-11, OCIMy5, OPM1, RPMI-Dox40, RPMI-LR5, and RPMI-MR20) had higher levels of SP cells. The proportion of SP cells was decreased by verapamil (100μM) and was lost with reserpine (50μM). When analyzing bone marrow samples of patients with MM (Figure 1C), we identified SP cells in all 11 samples, with a variable percentage of the SP fraction (range, 0.07%-2.08%). We also confirmed that these SP cells contain MM malignant clonal cells (details in supplemental Figure 1). Moreover, supplemental Figure 2 shows 4 MM cell lines (KMS-11, OCIMy5, RPMI-Dox40, and RPMI-MR20) with highest SP stained with Hoechst dye alone (left column dot blots panel), gated on SP cells and treated with ABC transport inhibitors verapamil (100μM; middle) or reserpine (50μM; right). Reserpine completely depleted SP cells in all MM cell lines. Our data therefore indicate that MM cell lines and primary MM cells contain SP cells. Some of these cell lines were used in further experiments.
Lack of correlation between SP and CD138−/low+ subpopulations in MM cell lines
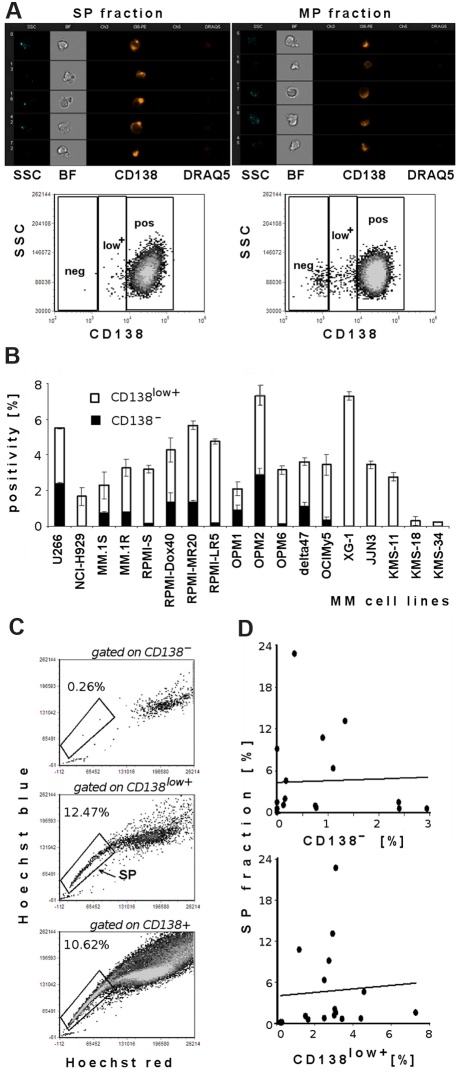
Previous studies have shown a clonogenic MM cell population with CD138− status in both in vitro colony-formation assays in methylcellulose and in vivo experiments in immunodeficient mice.19 Therefore, we next examined whether there is correlation between the proportion of CD138− or CD138low+ and SP cells in the MM cell lines tested. Hoechst 33342–labeled unsorted OPM1 cells, as well as sorted SP or MP, were costained with anti-CD138, and viability was assessed using 7-AAD dye. We showed that some tested cell lines had distinct CD138− and CD138low+ subpopulations, whereas the majority of MM cell lines tested were CD138+. ImageStream analysis (Figure 2A) on viable sorted SP and MP cells demonstrated CD138 antigen on both subpopulations. Similarly, flow cytometric data depict quantification of 3 different subpopulations of CD138 expression (CD138−, CD138low+, and CD138+) in unsorted OPM1 cell line costained with Hoechst 33342 and gated on SP and MP fractions. SP fractions were preferentially within CD138low+ and CD138+ populations.
Figure 2.
Lack of correlation of SP and CD138− or CD138low+ subpopulations in MM cell lines. (A) ImageStream analysis of sorted viable DRAQ5− SP and MP cell fractions from OPM1 cell line shows expression of the CD138 antigen. Imaging analysis results are depicted in the following channels: side scatter (SSC), bright-field image (BF), channel 3 empty, CD138-PE, channel 5 empty, DRAQ5. OPM1 cells were imaged at 40×/0.75 magnification and analyzed by IDEAS analytical software. Representative flow cytometric evaluation of CD138 expression in sorted viable 7-AAD− SP and MP cells: abscissa is fluorescence intensity of CD138, and ordinate is SSC. Dot plots show quantification of CD138 expression gated on SP fraction (left) and quantification of CD138 expression gated on MP cells (right): CD138− population, CD138low+ population, and CD138+ population. (B) Quantification of CD138− and CD138low+ cell fractions in MM cell lines (U266, NCI-H929, MM.1S, MM.1R, RPMI 8226-S, RPMI-Dox40, RPMI-MR20, RPMI-LR5, OPM1, OPM2, OPM6, delta47, OCIMy5, XG-1, JJN3, KMS-11, KMS-18, and KMS-34. (C) Representative dot plots of SP analysis of OPM1 cells, costained with Hoechst 33342 and CD138-PE mAb. Dot plot depicts Hoechst 33342–stained viable cells gated on CD138−, CD138low+, and CD138+ populations; gates represent the population of SP cells. (D) Correlation between SP fraction and CD138− or CD138low+ phenotype in 17 MM cell lines tested (U266, NCI-H929, MM.1S, MM.1R, RPMI 8226-S, RPMI-Dox40, RPMI-LR5, OPM1, OPM2, OPM6, delta47, OCIMy5, XG-1, JJN3, KMS-11, KMS-18, and KMS-34).
Next, we analyzed CD138 expression on 18 MM cell lines (Figure 2B). The majority of cells for all MM cell lines were CD138+ (data not shown). However, all cell lines contained a distinct proportion of CD138low+ (0.23%-7.3% cells). The CD138− subpopulation ranged from 0.13% to 2.9% in U266, MM.1S, MM.1R, RPMI-S, and its parental sublines, as well as OPM1, OPM2, OPM6, delta47, and OCIMy5 cells. On the other hand, some MM cell lines (NCI-H929, XG-1, JJN3, KMS-11, KMS-18, and KMS-34) lacked the CD138− subpopulation. Next we used Hoechst 33342–labeled viable cells gated on CD138− population, CD138low+ population, and CD138+ cells to assess correlation between SP and CD138−/low+ subpopulations (Figure 2C). SP fractions were mainly present in CD138low+ and CD138+ populations (middle and bottom dot plots). Therefore, analysis of 17 MM cell lines (excluding the outlier MM cell line RPMI-MR20), as well as representative dot plots (Figure 2A,C), confirmed the lack of correlation between SP and CD138− or CD138low+ subpopulations (correlation coefficients < 0.10; Figure 2D).
Viability, proliferation, and clonogenicity of sorted SP and MP cells
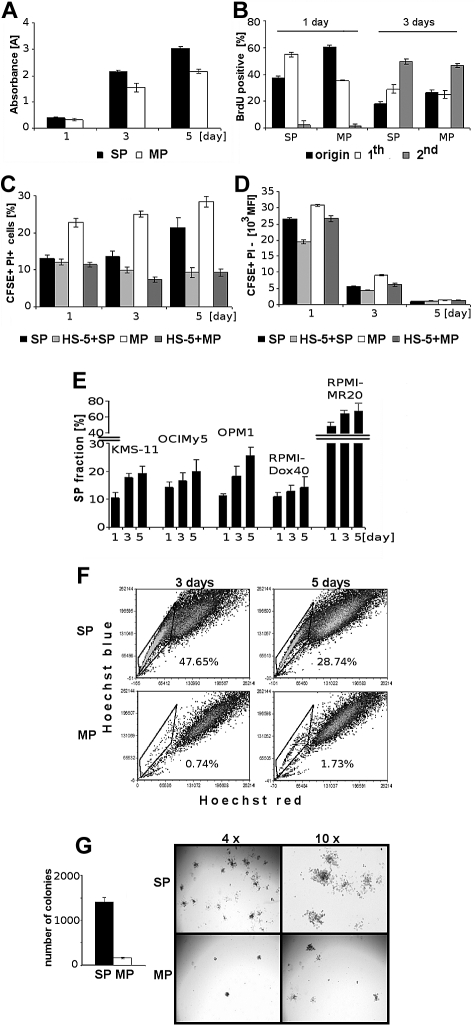
The quantification of changes in viability, proliferation, cell cycle, and clonogenicity of Hoechst-stained cells in comparison to untreated control cells did not reveal any significant toxic effects (supplemental Figure 3). We next evaluated the viability of sorted SP and MP cells from OPM1 cells after 1, 3, and 5 days of culture using MTT assay (Figure 3A), and observed a high rate of SP cell growth. Assessment of BrdU incorporation after 1 and 3 days revealed significantly increased proliferation of SP cells at day 1, as evidenced by increased ratio of cells in the 1st generation. The distribution of cells in successive generations at 3 days was higher for SP cells compared with MP cells (Figure 3B).
Figure 3.
Viability, proliferation, and clonogenicity of sorted SP and MP cells. (A) Viability of sorted SP and MP cells at 1, 3, and 5 days was assessed by MTT. (B) Proliferation of sorted SP and MP cells was assessed by BrdU incorporation at 1 and 3 days. Percentage of cells in undivided generation (origin; undivided cells immediately after sorting measured), “1st” generation (once divided), and “2nd” generation (twice divided) are shown. (C) Viability of sorted CFSE-labeled SP and MP cells both alone or with unlabeled HS-5 BMSCs at 1, 3, and 5 days, was measured using PI staining and analyzed using a FACSCanto II flow cytometer. (D) Proliferation of sorted CFSE-labeled viable SP and MP cells is indirectly proportional to the number of cell divisions, quantified as mean of cell membrane-bound CFSE fluorescent intensity. Fluorescence intensity of CFSE+PI−-labeled SP and MP cells decreases in a time-dependent manner (1, 3, and 5 days). (E) Percentage of SP fraction in 5 MM cell lines (KMS-11, OCIMy5, OPM1, RPMI-Dox40, and RPMI-MR20) at 1, 3, and 5 days. (F) Sorted SP and MP cells cultured for 3 and 5 days were restained with Hoechst 33342. (G) The number of colonies of sorted SP and MP cells was assessed at day 14. Representative images of colonies of sorted SP and MP are shown (using an inverted Leica microscope with a Leica DFC300Fx camera (4×/0.1 and 10×/0.22; Leica IM50 image-acquisition software Version 4).
To evaluate the effect of the BM microenvironment, CFSE-stained and sorted SP and MP cells from OPM1 cells were cultured, alone or with HS-5 BMSCs. Cell division was assessed by CFSE content, and the proportion of nonviable SP and MP cells (CFSE+/PI+) was measured. The viability of MP cells decreased compared with SP cells, but BMSCs enhanced the viability of both populations (Figure 3C). Because there is an inverse correlation between CFSE staining intensity and number of cell divisions, our results confirmed the higher proliferation of SP than MP cells, as seen in the BrdU assay. Furthermore, the proliferation of both sorted subpopulations was enhanced in the presence of HS-5 BMSCs (Figure 3D).
Next we analyzed MM cell lines with a high percentage of SP cells (KMS-11, OCIMy5, OPM1, RPMI-Dox40, and RPMI-MR20) and confirmed that the percentage of SP cells in unsorted cell populations, cultured in vitro, gradually increased with time in all MM cell lines (Figure 3E). To test for self-renewal and to compare the repopulation ability of SP and MP cells from OPM1 cells, the sorted SP and MP cells were cultured under similar conditions. After 3 and 5 days, both populations were restained with Hoechst 33342 dye and reanalyzed (Figure 3F). Cultured SP cells generated a significant fraction of MP cells (top row), whereas MP cells (bottom row) produced mainly MP cells. We also performed colony-forming unit/clonogenic assays in methylcellulose using sorted SP and MP cells from OPM1 cells. After 14 days, sorted SP cells generated 9 times more colonies in comparison to MP cells. The shape and size of colonies differs significantly between SP and MP cells (Figure 3G). These results confirm that SP cells contain clonogenic cells with high proliferation rates, which can generate a significant proportion of cells with MP phenotype.
Tumorigenic potential of SP cells compared with MP cells
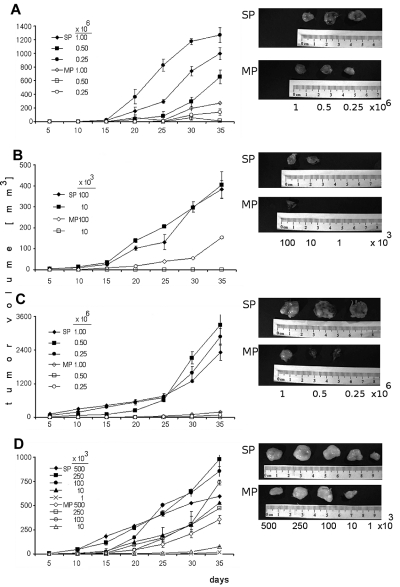
To evaluate tumorigenicity of SP cells, several dilutions of sorted SP and MP cells from OPM1 and KMS11 were subcutaneously injected into immunocompromised mice and monitored for tumor development (Figure 4). In CB17/SCID mice, sorted SP cells (injection doses, 0.25 × 106, 0.5 × 106, and 106 cells/mouse) from KMS11 (Figure 4A) induced significantly greater tumor growth compared with MP cells (90%-70% less tumor mass than SP tumors). However, both SP and MP fractions eventually developed tumors. Therefore, lower number of SP and MP (range, 1-100 × 103 cells/mouse) were injected in the same mouse model (Figure 4B). At lower innocula of cells (10 × 103 cells/mouse), SP cells resulted in tumors, whereas no tumors were produced by MP cells. The same CB17/SCID model was used to test for tumorigenic potential of SP cells from OPM1 cell line (Figure 4C). The tumor generated by SP cells (2888 mm3) was 34-fold larger in volume than that of the MP cells (85 mm3) at the injection dose (0.25 × 106 cells/mouse) at 35 days. In NOD/SCID mice, overall SP cells (500-10 × 103 cells/mouse) produced greater tumor mass than MP cells (Figure 4D). Interestingly, the lowest numbers of SP cells (1 × 103 cells/mouse) gave rise to tumors, whereas MP cells did not. These data strongly support the tumorigenic potential of SP cells.
Figure 4.
Tumorigenic potential of SP and MP cells. (A) CB17/SCID mice were subcutaneously inoculated with SP or MP cells from KMS11 cell line at 0.25 × 106, 0.5 × 106, and 106 cells/mouse. (B) CB17/SCID mice were inoculated with SP or MP cells from KMS11 cell line at lower numbers 1 × 103, 10 × 103, and 100 × 103 cells/mouse. (C) CB17/SCID mice were subcutaneously inoculated with SP or MP cells from OPM1 cell line at 0.25 × 106, 0.5 × 106, and 106 cells/mouse. (D) NOD/SCID mice were subcutaneously inoculated with SP and compared with MP cells from KMS11 cell line at 500 × 103, 250 × 103, 100 × 103, 10 × 103, and 1 × 103 cells/mouse. Caliper measurements of tumor diameters were done every 5 days. Representative pictures were performed using camera IVIS 1327 in the Xenogen system.
Gene expression and functional activity of ABC protein transporters in SP and MP cells
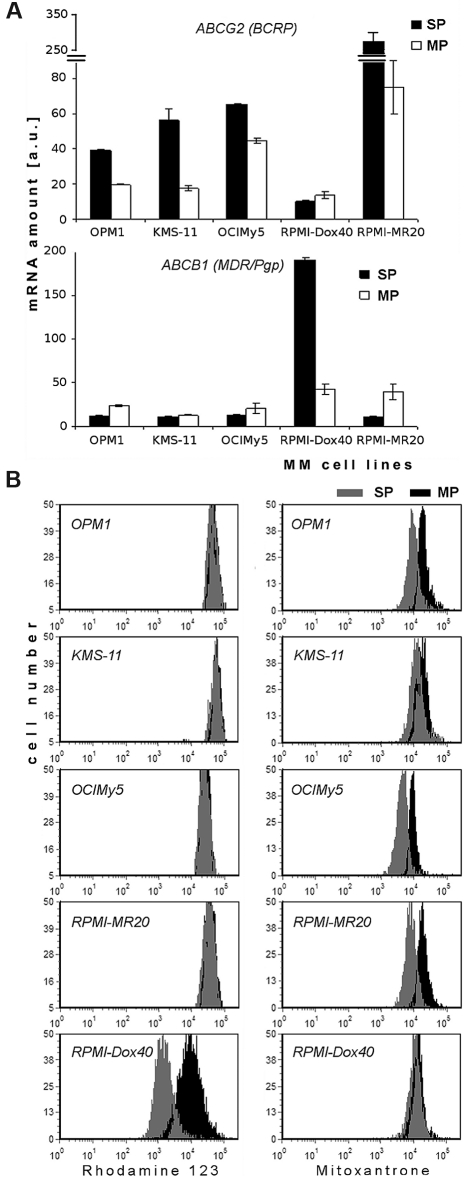
Recent studies attributed the SP phenotype to expression of ATP-binding cassette (ABC) transporters, especially ABCG2, which is located at the surface of cells.20 Using the QuantiGene Plex assay system, we determined the quantitative amount of mRNA of selected human ABC transporters in SP and MP cells of human MM cell lines (Figure 5A and supplemental Figure 4). Five ABC transporters studied included ABCB1 (MDR/TAP1/Pgp), ABCC1 (CFTR/MRP1), ABCC2 (CFTR/MRP2), ABCC3 (CFTR/MRP3), and ABCG2 (WHITE/BCRP1), and their expression was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). ABCG2 transcripts were significantly elevated in SP cells in comparison to MP cells in all MM cell lines, except in doxorubicin-resistant RPMI-Dox40 cells, which express very high levels of ABCB1 (Figure 5A). Similarly, high levels of ABCC1 were detected in SP cells of KMS-11 cell line in comparison to other cell lines. Of note, high levels of ABCC2 or ABCC3 (4/5 lines; OPM1, OCIMy5, RPMI-Dox40, and RPMI-MR20) were expressed in all MP cells (supplemental Figure 4). The highest level of ABCG2 transcripts was detected in mitoxantrone-resistant RPMI-MR20 cells.
Figure 5.
Expression of mRNA and functional activity of ABC protein transporters in SP and MP cells. (A) Expression of mRNA of ABCG2 (BCRP) and ABCB1 (MDR/Pgp) in sorted SP and MP cells of MM (OPM1, KMS-11, OCIMy5, RPMI-Dox40, and RPMI-MR20) cell lines, as determined by QuantiGene Plex assay system. Fluorescence intensity of ABC transporters was normalized to fluorescence intensity of the housekeeping gene GAPDH. All experiments were performed in triplicate, P < .05, t test, statistical significance. (B) Efflux of Rh123, ie, functional activity of P-glycoprotein transporter (left column) and Mitoxantrone, ie, functional activity of BCRP1 transporter (right column) in SP and MP cells. MM cell lines (OPM1, KMS-11, OCIMy5, RPMI-MR20, and RPMI-Dox40) were dual stained with either Rh123 and Hoechst 33342 or Mitoxantrone and Hoechst 33342: SP cells (gray) and MP cells (black). Data are representative of 3 independent experiments; all experiments were performed in triplicate.
To validate these findings, we evaluated the functional activity of P-glycoprotein and BCRP1 protein transporters in SP and MP fraction of cells using Rh123 (P-glycoprotein substrate) or mitoxantrone (BCRP1 substrate) dye efflux, and costaining with Hoechst 33342 (Figure 5B). Most cell lines (OPM1, KMS-11, OCIMy5, and RPMI-MR20) had similar levels of Rh123 efflux, but significantly higher levels of mitoxantrone efflux in SP cells than in MP cells. Conversely, efflux of Rh123 dye occurred in SP fractions of RPMI-Dox40 cells.
Lenalidomide decreased SP fractions but increased CD138−/low+ population of MM cells in both the absence and presence of BMSCs
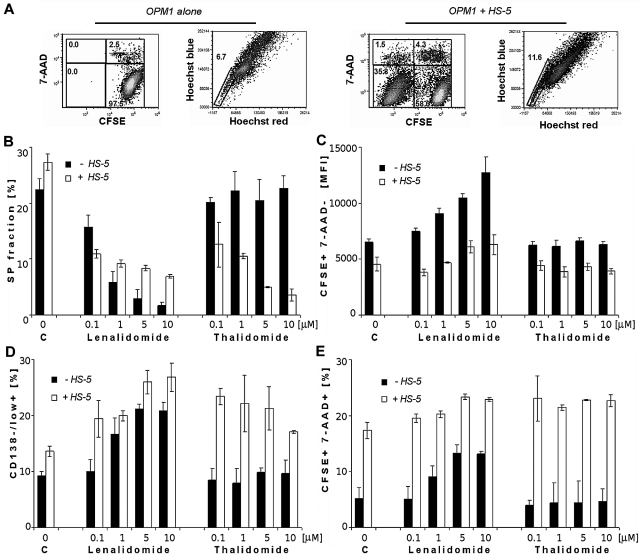
Representative dot plots of CFSE-stained OPM1 cells alone (first 2 dot plots) or in coculture with HS-5 BMSCs (last 2 dot plots) were analyzed for viability and cell division. Hoechst 33342 uptake on CFSE+/7-AAD− population of OPM1 cells at 24 hours are shown in Figure 6A. Although no effect of IMiDs on SP cells was detected at 24 hours (data not shown), lenalidomide significantly decreased the percentage of SP cells in a dose-dependent manner at 72 hours (Figure 6B). In contrast, thalidomide did not affect SP cells. Bone marrow stromal cells significantly increased the percentage of SP control cells at 24 hours (data not shown) and at 72 hours, but treatment with lenalidomide at 72 hours abrogated this stimulatory effect. Interestingly, thalidomide in coculture with BMSCs significantly decreased the percentage of SP cells.
Figure 6.
Effect of lenalidomide and thalidomide on viability, proliferation, and stemness markers (SP cell fraction and CD138−/low+ population) of OPM1 in the absence and presence of BMSCs. (A) Cytometric dot plots represent CFSE-labeled OPM1 cells alone (first 2 dot plots) or in coculture with unlabeled-HS-5 BMSCs (last 2 dot plots), showing CFSE profiles together with 7-AAD+ cells, and percentage of SP cells by Hoechst 33342. Analysis gated only on CFSE+/7-AAD− OPM1 cells at 1 day. (B) Percentage of SP fraction in control and lenalidomide and thalidomide treated OPM1 cells, both alone and with BMSCs, decreased in a dose-dependent manner at 3 days. (C) Fluorescence intensity of CFSE+ 7-AAD−–labeled OPM1 cells, both alone or with HS-5 BMSCs, is shown after treatment with lenalidomide and thalidomide (0.1, 1, 5, and 10μM). (D) Percentage of CD138−/low+ OPM1 cells at 3 days is shown in control cultures; as well as after treatment with lenalidomide and thalidomide alone, or in coculture with BMSCs. (E) Viability of CFSE-labeled OPM1 cells at 3 days decreased after drug treatment in a dose-dependent manner (0.1, 1, 5, and 10μM), assessed using 7-AAD staining and a FACSAria Special Sorter UV laser flow cytometer.
The increased fluorescent intensity of CFSE staining in viable cells observed with lenalidomide treatment indicated inhibition of cell proliferation of OPM1 cells either alone or with BMSCs, whereas no effects were shown in thalidomide-treated cells (Figure 6C). The percentage of CD138−/low+ population was increased after addition of lenalidomide to OPM1 cells, alone or cocultured with BMSCs (Figure 6D). Thalidomide, in contrast to lenalidomide, did not increase percentage of CD138+/− population of cells in the absence of BMSCs. In addition, BMSCs and lenalidomide slightly increased the proportion of CFSE+/7-AAD+ cells at 72 hours, whereas the effect of thalidomide was seen only in the presence of BMSCs (Figure 6E).
In KMS-11 cells, alone or with BMSCs, treatment with lenalidomide significantly decreased the percentage of SP fractions (supplemental Figure 5A) and inhibited proliferation (supplemental Figure 5B), whereas thalidomide had no effect. Lenalidomide and thalidomide had no effect on the CD138+/− population (supplemental Figure 5C) as well as on the viability of cells (supplemental Figure 5D). However, higher concentrations of lenalidomide increased both the CD138+/− population and dead cells (data not shown). These effects were qualitatively similar in the other MM cell lines including OCIMy5 and RPMI-MR20 (data not shown).
Lenalidomide, not thalidomide, significantly affected clonogenic potential, decreased ABCG2 transcripts, and modulated signaling pathways of SP cells
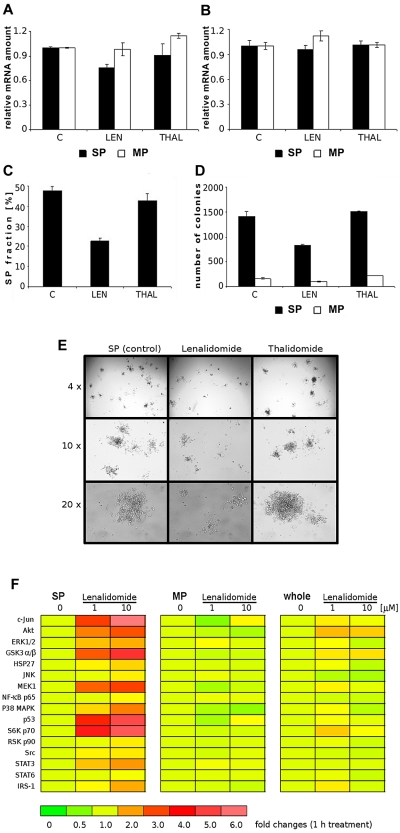
Using the QuantiGene Plex assay system, we simultaneously quantified mRNA levels of 5 human ABC transporters in the sorted SP and MP cells, with or without treatment with lenalidomide and thalidomide for 6 hours (Figure 7A, Figure 7B and supplemental Figure 6). The relative signal intensities (normalized to corresponding GAPDH values) revealed only slight decreases of ABCG2 in sorted SP cells treated with lenalidomide, but not thalidomide. No changes were found in all treated MP cells (Figure 7A). Similarly, no significant changes were detected in relative ABCB1 mRNA expression in SP and MP cells treated with lenalidomide and thalidomide (Figure 7B). The other 3 ABC transporters (ABCC1, ABCC2 and ABCC3) in sorted SP and MP cells also were not significantly affected by lenalidomide and thalidomide (supplemental Figure 6). Therefore, we analyzed the effect of lenalidomide and thalidomide on the intracellular accumulation as well as efflux of mitoxantrone as a substrate of the ABCG2/BCRP1 efflux pump. Similar to ABCG2 transcript, we did not reveal any significant modulation of functional activity of BCRP1 by lenalidomide and thalidomide (supplemental Figure 7).
Figure 7.
Effect of lenalidomide and thalidomide on mRNA of ABCG2 transcript, clonogenic potential, and signaling pathways of SP cells. (A) Quantification of the relative mRNA expression of ABCG2 and (B) ABCB1 (MDR/Pgp) in SP or MP cells treated with lenalidomide and thalidomide (10μM) for 6 hours was assessed using QuantiGene Plex assay. Fluorescence intensity of ABC transporters was normalized to fluorescence intensity of the housekeeping gene GAPDH. All experiments were performed in triplicate, P < .05, t test, statistical significance. (C) Sorted SP cells, with or without treatment with lenalidomide (LEN; 1μM) and thalidomide (THAL, 1μM) for 72 hours, were restained with Hoechst 33342. (D) Clonogenic assay of sorted SP and MP cells treated with lenalidomide and thalidomide (10μM) is shown at day 14. (E) Images of colonies derived from SP cells, both control and treated with lenalidomide and thalidomide, were obtained using a Leica inverted microscope (with a Leica DFC300 Fx camera [4×/0.1, 10×/0.22, and 20×/0.35] Leica IM50 image-acquisition software Version 4). (F) Multiplex analysis of lenalidomide-induced changes in phosphorylation of signaling pathways in sorted SP and MP fractions or control OPM1 cells. The different populations of cells were treated with lenalidomide (1 or 10μM) for 1 hour. Protein concentrations of whole-cell lysates were measured using a Bradford protein assay kit and normalized to a panel of 5 total proteins. A multiplex panel of 16 phosphoproteins (Akt, c-Jun, ERK1/2, GSK-3α/β, HSP27, IRS-1, JNK, MEK1, NF-κB p65, p38 MAPK, p53, p70 S6 kinase, p90RSK, Src, STAT3, STAT6) was analyzed in a 96-well format using the Bio-Plex suspension array system. Relative amounts of phosphorylation in treated vs control untreated cells are shown, and fold changes in expression are depicted in a color-coded scale.
To examine for self-renewal and repopulation ability, sorted SP cells from OPM1 cells were treated with lenalidomide and thalidomide for 3 days, and then restained with Hoechst 33342 dye (Figure 7C). Lenalidomide treatment significantly reduced the proportion of SP cells, but no change was observed after thalidomide treatment. To assess the effects of lenalidomide and thalidomide on clonogenic potential of SP and MP cells from OPM1 cells, sorted populations were treated for 14 days. Decreased colonies were observed in lenalidomide-treated cells, whereas thalidomide had no effect (Figure 7D). In contrast, the number of colonies of MP cells was low and unchanged by treatment. Figure 7E depicts representative images of colonies derived from control versus lenalidomide-treated and thalidomide-treated SP cells. Colony numbers and shapes in control and thalidomide-treated SP cells were similar, but differed significantly from the lower colony numbers in lenalidomide-treated SP cells.
To better understand the molecular mechanisms of lenalidomide, we compared the gene expression profiles of lenalidomide-treated versus control SP cells, as well as the profiles of lenalidomide-treated versus control MP cells. We did not detect major changes in gene expression profiles in either of the 2 comparisons (data not shown), which suggested that the effect of lenalidomide at early time-points may be primarily related with posttranslational modifications, rather than transcriptional profile changes. Therefore, we have analyzed the effect of lenalidomide on posttranslational modifications, such as activation of phosphoproteins implicated in key cellular-signaling pathways in SP, MP, and whole OPM1 populations (Figure 7F). After treatment with 2 concentrations of lenalidomide, we observed significant differences in phosphoprotein profiles of SP and MP cells, as depicted in respective rainbow heat maps; in contrast, there were no changes in total protein levels (Akt, ERK1/2, IκB-α, JNK, p38 MAPK, data not shown). In the SP fraction treated with lenalidomide, phosphorylation was significantly increased in Akt, GSK-3α/β, MEK1, c-Jun, p53, and p70 S6K; modestly increased in ERK1/2, p38 MAPK, HSP27, STAT3, and IRS-1; and unchanged in JNK, NF-κB, STAT6, p90 RSK, and Src. In contrast, no activation of phosphoproteins was induced by lenalidomide in MP cells; although, decreased c-Jun, Akt, and p38 MAPK phosphorylation in lenalidomide-treated cells was noted. Because SP cells represent a low fraction of OPM1 cells, these changes induced by lenalidomide in SP cells were only faintly visible on analysis of unsorted OPM1 cells.
Discussion
The heterogeneity of cancer cell populations, with different proliferative, differentiative and tumor-initiating potential, has led to the development of the cancer stem cell (CSC) concept. According to this concept, CSCs express stem cell–like features, including asymmetric cell division, and can both undergo self-renewal and give rise to the entire tumor population.21 Importantly, even in patients who achieved complete clinical remission after treatment with systemic chemotherapy, a small fraction of resistant tumor cells persist after treatment and drive the recurrence of disease. This phenomenon has been attributed to the existence of CSCs.22
Two different approaches to study CSCs have been proposed. The first approach exploits functional characteristics to identify stem-like cells by staining with Hoechst 33342 dye to identify SP cells that fall, in flow cytometric analysis, to the side of the bulk of the positively Hoechst 33342–stained cells.9 This SP is considered an enriched source of tumor-initiating cells with stem cell properties, associated with chemoresistance and tumorigenicity in vivo. It has been identified in a variety of tumor types, including lung, gastric, esophageal, squamous, and ovarian carcinoma cell lines.4,23–26 In our study, we performed a flow cytometry–based Hoechst 33342 staining assay of MM cells to identify this subpopulation with stem-like features. We demonstrated that MM cell lines and part of the malignant clone from primary MM samples showed heterogeneity in the percentage of SP cells. Moreover, we showed that modification of cell culture conditions, such as duration of culture or exogenous factors, alter the percentage of SP cells in MM cell lines. We also found that prolonged culture can increase the proportion of SP cells. This increase suggests that the percentage of SP cells within the tumor population can be modified, depending on the context of the tumor cell growth, which is compatible with the finding that serum starvation and hypoxia enriches SP cells in cancer cell lines.27 In fact, we detected ABCG2 activity in our SP cells, similar to a prior study that associated ABCG2 with high rates of proliferation.28
The second approach is based on specific surface markers that are expressed selectively on CSCs but not on the bulk of tumor cells. Different CSC markers are used for different cancer types: CD34+/CD38− cells are highly enriched in leukemic stem cells, CD133+ cells in brain and colon tumors, and CD44high/CD24low/Linneg cells in breast cancer.6,7,29,30 Several studies have attempted to identify and study MM stem cells. Earlier studies identified that a proportion of circulating CD19+ B lymphocytes is monoclonal, although there are still many unanswered questions about the relationship of these clonotypic B cells and the MM clone.31,32 Other studies have identified clonogenic properties of MM CD138− fraction including CD19+, CD20+, and CD27+ cells, but not CD138+ cells.19,33,34 On the other hand, earlier studies show that only malignant plasma cells based on their phenotype CD38+/CD45− could engraft into a human bone implant and produce xenogenic myeloma.35
The expression of these markers, which have been proposed to characterize the treatment-resistant CSCs, may overlap, but not completely, with the SP cell phenotype.36 In the case of MM, it has been proposed that the CD138− population of MM cell lines (RPMI 8226 and NCI-H929) and clinical MM samples show greater clonogenic potential in vitro and in vivo than CD138+ plasma,19 consistent with the criteria for CSC. Here we extended the analysis using a panel of 18 MM cell lines that revealed phenotypic heterogeneity: some cell lines contained distinct subpopulations of CD138low+ cells and CD138−, whereas the majority of MM cell lines tested had a main population of CD138+ cells. In contrast, 6 cell lines of our panel did not contain CD138− subpopulation. Therefore, our findings suggest that the proposed overlap between SP phenotype and CD138− population33 may vary, because the SP fractions in our MM cell line panel expressed the CD138 antigen as confirmed both by flow cytometry and ImageStream analysis. In addition, we have found no correlation between expression of CD19, CD20, or CD27 and the proportion of SP cells (data not shown). Nonetheless, our results on MM patient samples indicate that SP cells are heterogeneous for CD138 antigen expression (J.J., unpublished observations, September 2009), predominantly with lower expression of CD138, similar to a previous report.37 Another study shows correlation of the CD138− phenotype in MM cells with their trend to become apoptotic and fail to grow in vitro,38 which raises the possibility that the status of CD138 expression in SP cells may exhibit differences depending on the conditions to which the tumor cells are exposed.
Conflicting results have also been obtained regarding the SP cell division kinetics, because some groups identify the SP fraction as slowly proliferating cells26,39 whereas others observe a high proliferation rate in SP cells.25,40 Our data revealed that MM SP cells had a higher proliferation index/rate than MP cells. This finding is similar to previous studies, which also identified high Ki67 expression associated with a high proliferation rate of MM stem cells with a CD138−/CD19+/CD20 surface phenotype.19 These observed differences could be attributed, at least in part, to the differentiation stage of cells from which the SP fraction is derived. Specifically, the SP fraction may originate from slowly proliferative progenitors in some tumors and from more cycling progenitors in others.
Several criticisms have been raised concerning the use of Hoechst 33342 because of its cytotoxic and mutagenic effect due to its DNA binding. We confirmed that the quantification of changes in viability, proliferation, cell cycle, and clonogenicity of Hoechst-stained cells versus untreated control cells did not reveal any significant toxic effects. Our results also showed that both SP and MP cells in MM had similar viability in MTT assay, and that the observed decrease in MP cells was due to a lower rate of proliferation in both BrdU and in CFSE-label assays. However, recent data show that sorted non-SP cells of primary mesenchymal tumors are able to form tumors.10 Furthermore, unstained but sorted MCF-7 breast carcinoma cells and stained non-SP cells by Hoechst have similar clonogenic potential in vitro and in vivo.41 In our studies, we confirmed both the higher potential of SP cells than MP cells to generate colonies in MM, as well as the ability of SP cells to regenerate a population resembling the original unsorted population. Moreover, SP cells revealed significantly higher tumorigenicity compared with MP cells.
The efflux of Hoechst dye is attributed to members of the ABC (ATP-binding cassette) family of membrane pumps, with ABCG2 and ABCB1 identified as likely mediators of Hoechst efflux.20,42 BCRP1 protein plays an important role in the drug resistance of normal stem cells and tumor stem cells, and is a molecular determinant of the SP phenotype.42 In our study, we found elevated expression of ABCG2 transcripts in sorted SP of MM cell lines, with the exception of RPMI-Dox40 cells that overexpressed Pgp. Our data are in accordance with previous studies43,44 showing increased expression of ABCG2 transporters in SP cells compared with MP cells. Moreover, our mRNA profiling results were further corroborated by functional assays demonstrating the association of the SP phenotype with expression of ABCG2.
Because of the clonogenic and tumorigenic potential of SP cells,4,23,25 they might contribute to the aggressive behavior of some tumors or drug-resistant phenotype in others, making them a promising target for novel therapeutics. Importantly, we found that lenalidomide significantly decreased the percentage and clonogenicity of SP cells, as well as their repopulation ability, at clinically achievable concentrations. In contrast, thalidomide did not change the proportion of the SP fraction and did not affect the clonogenicity of SP cells. Importantly, in the OPM1 cell line a small but concentration-dependent decrease of cell viability, as well as an increase of CD138−/low+ cells, was induced by lenalidomide, but not thalidomide. This simultaneous increase of cells with a CD138− phenotype and a decrease of viability is in accord with previous findings.38 Finally, proliferation was inhibited by lenalidomide treatment in both OPM1 and KMS-11 cell lines, but not by thalidomide.
It has been shown that IMiDs inhibit MM cell growth through direct pro-apoptotic and antiproliferative effects on MM cells, antiangiogenic activities, as well as by reduction of adhesion molecule expression and prosurvival cytokine signaling.14–16,45 Our study extends the spectrum of these anti-MM effects of IMiDs, by showing that lenalidomide can influence the behavior of the SP cells. Strategies directed at SP tumor cells may target molecular pathways overactivated in this fraction of the tumor cell population. For example, ABCG2 activity of SP cells was modulated by Akt signaling.46,47 In glioma tumor stem-like cells, Akt, but not its downstream target mTOR, regulates ABCG2 activity, and loss of PTEN increased SP cells.48 Here we found that lenalidomide, but not thalidomide, modestly decreased ABCG2 transcript, but neither of them modulated functional activity of ABCG2/BCRP-1 as well as other ABC transporters.49 In addition, we observed modulation of diverse signaling pathways, mainly in SP cells (including phosphorylation changes in Akt, GSK-3α/β, MEK1, c-Jun, p53, and p70S6K). The kinetics of this activation of signaling pathways and its biologic sequelae is currently under investigation.
The bidirectional interactions between CSCs and microenvironment are critical to sustain equilibrium between quiescence and self-renewal. The stem cell niche provides protection as well as nourishment to CSCs, with exclusion from molecules that may cause differentiation or mutation.50 In our studies, MM cell adherence to BMSCs increased the percentage, viability, and proliferation potential of SP cells. Conversely, lenalidomide and thalidomide attenuated this stimulatory effect of stromal cells, thereby significantly decreasing SP cell percentages.
Identifying the presence of MM stem-like/tumor-initiating cells responsible for tumor regrowth will facilitate the development of new strategies to halt progression of the disease. Moreover, defining their biologic features and molecular characteristics will allow for the assessment of their sensitivity to conventional and novel therapies that may be used to adequately predict MM patient response. Our study raises the intriguing possibility that lenalidomide could target SP cells with clonogenic potential, providing the framework for development of new treatment strategies targeting presumptive MM stem/tumor-initiating cells.
Supplementary Material
Acknowledgments
We thank Suzan Lazo-Kallanian for flow cytometry and Alexei Protopopov for FISH analysis.
This work was supported in part by the Dunkin Donuts Rising Stars Program at the Dana-Farber Cancer Institute (C.S.M.), the Chambers Medical Foundation (P.G.R, C.S.M.), the Stepanian Fund (P.G.R, C.S.M.), the Richard J. Corman Fund (P.G.R., C.S.M.), National Institute of Health grants R01-50947 (K.C.A, C.S.M.) and P0-1-78378 (K.C.A.), and the Slovak R&D Agency grant VVCE-0001-07 (J.S.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.J. designed and performed research, analyzed data, and wrote the paper; D. Cervi contributed to the writing of the paper; S.A., M.K.-A., J.F.D., and D. Cholujova performed research and analyzed data; S.K., M.L., S.B., M.O., S.-Y.K., and J.D. performed research; J.L. and P.G.R. contributed research specimens; and J.S., K.C.A., and C.S.M. designed research and contributed to the writing of the paper.
Conflict-of-interest disclosure: P.G.R. was in the Speakers' Bureau (until 7/1/09) of Millennium and Celgene and received honoraria from Millennium and Celgene. K.C.A. has received grants and honoraria from Millennium and Celgene and was in the bureau (until 7/1/09) of Millennium and Celgene. C.S.M. has received in the past consultant honoraria from Millennium Pharmaceuticals, Novartis Pharmaceuticals, Bristol-Myers Squibb, Merck & Co, Kosan Pharmaceuticals and Pharmion, licensing royalties from PharmaMar, and research funding from Amgen Pharmaceuticals, AVEO Pharma, EMD Serono, Sunesis, Gloucester, Genzyme, and Johnson & Johnson. The remaining authors declare no competing financial interests.
Correspondence: Constantine S. Mitsiades, MD, PhD, Jerome Lipper Multiple Myeloma Center, Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, 44 Binney St, Boston, MA 02115; e-mail: Constantine_Mitsiades@dfci.harvard.edu; or Kenneth C. Anderson, MD, Jerome Lipper Multiple Myeloma Center, Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, 44 Binney St, Boston, MA 02115; e-mail: Kenneth_Anderson@dfci.harvard.edu.
References
- 1.Greipp PR, San MJ, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 2.Mitsiades CS, Mitsiades NS, Richardson PG, Munshi NC, Anderson KC. Multiple myeloma: a prototypic disease model for the characterization and therapeutic targeting of interactions between tumor cells and their local microenvironment. J Cell Biochem. 2007;101(4):950–968. doi: 10.1002/jcb.21213. [DOI] [PubMed] [Google Scholar]
- 3.Donnenberg VS, Donnenberg AD. Multiple drug resistance in cancer revisited: the cancer stem cell hypothesis. J Clin Pharmacol. 2005;45(8):872–877. doi: 10.1177/0091270005276905. [DOI] [PubMed] [Google Scholar]
- 4.Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67(10):4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 6.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer–initiating cells. Nature. 2007;445(7123):111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 8.Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24(1):3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- 9.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183(4):1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C, Wei Q, Utomo V, et al. Side population cells isolated from mesenchymal neoplasms have tumor initiating potential. Cancer Res. 2007;67(17):8216–8222. doi: 10.1158/0008-5472.CAN-07-0999. [DOI] [PubMed] [Google Scholar]
- 11.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341(21):1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 12.Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108(10):3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352(6):549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 14.Mitsiades N, Mitsiades CS, Poulaki V, et al. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99(12):4525–4530. doi: 10.1182/blood.v99.12.4525. [DOI] [PubMed] [Google Scholar]
- 15.Dredge K, Marriott JB, Macdonald CD, et al. Novel thalidomide analogues display anti-angiogenic activity independently of immunomodulatory effects. Br J Cancer. 2002;87(10):1166–1172. doi: 10.1038/sj.bjc.6600607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta D, Treon SP, Shima Y, et al. Adherence of multiple myeloma cells to bone marrow stromal cells up-regulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001;15(12):1950–1961. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 17.Chang DH, Liu N, Klimek V, et al. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Blood. 2006;108(2):618–621. doi: 10.1182/blood-2005-10-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies FE, Raje N, Hideshima T, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98(1):210–216. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- 19.Matsui W, Huff CA, Wang Q, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103(6):2332–2336. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonker JW, Freeman J, Bolscher E, et al. Contribution of the ABC transporters Bcrp1 and Mdr1a/1b to the side population phenotype in mammary gland and bone marrow of mice. Stem Cells. 2005;23(8):1059–1065. doi: 10.1634/stemcells.2005-0150. [DOI] [PubMed] [Google Scholar]
- 21.Jordan CT. Cancer stem cells: controversial or just misunderstood? Cell Stem Cell. 2009;4(3):203–205. doi: 10.1016/j.stem.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda K, Saikawa Y, Ohashi M, et al. Tumor initiating potential of side population cells in human gastric cancer. Int J Oncol. 2009;34(5):1201–1207. [PubMed] [Google Scholar]
- 24.Huang D, Gao Q, Guo L, et al. Isolation and identification of cancer stem-like cells in esophageal carcinoma cell lines. Stem Cells Dev. 2009;18(3):465–473. doi: 10.1089/scd.2008.0033. [DOI] [PubMed] [Google Scholar]
- 25.Loebinger MR, Giangreco A, Groot KR, et al. Squamous cell cancers contain a side population of stem-like cells that are made chemosensitive by ABC transporter blockade. Br J Cancer. 2008;98(2):380–387. doi: 10.1038/sj.bjc.6604185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci U S A. 2006;103(30):11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tavaluc RT, Hart LS, Dicker DT, El-Deiry WS. Effects of low confluency, serum starvation and hypoxia on the side population of cancer cell lines. Cell Cycle. 2007;6(20):2554–2562. doi: 10.4161/cc.6.20.4911. [DOI] [PubMed] [Google Scholar]
- 28.Patrawala L, Calhoun T, Schneider-Broussard R, et al. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005;65(14):6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 30.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergsagel PL, Masellis SA, Belch AR, Pilarski LM. The blood B-cells and bone marrow plasma cells in patients with multiple myeloma share identical IgH rearrangements. Curr Top Microbiol Immunol. 1995;194:17–24. doi: 10.1007/978-3-642-79275-5_3. [DOI] [PubMed] [Google Scholar]
- 32.Pilarski LM, Masellis-Smith A, Szczepek A, Mant MJ, Belch AR. Circulating clonotypic B cells in the biology of multiple myeloma: speculations on the origin of myeloma. Leuk Lymphoma. 1996;22(5–6):375–383. doi: 10.3109/10428199609054775. [DOI] [PubMed] [Google Scholar]
- 33.Matsui W, Wang Q, Barber JP, et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008;68(1):190–197. doi: 10.1158/0008-5472.CAN-07-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peacock CD, Wang Q, Gesell GS, et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci U S A. 2007;104(10):4048–4053. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yaccoby S, Epstein J. The proliferative potential of myeloma plasma cells manifest in the SCID-hu host. Blood. 1999;94(10):3576–3582. [PubMed] [Google Scholar]
- 36.Burkert J, Otto WR, Wright NA. Side populations of gastrointestinal cancers are not enriched in stem cells. J Pathol. 2008;214(5):564–573. doi: 10.1002/path.2307. [DOI] [PubMed] [Google Scholar]
- 37.Loh YS, Mo S, Brown RD, et al. Presence of Hoechst low side populations in multiple myeloma. Leuk Lymphoma. 2008;49(9):1813–1816. doi: 10.1080/10428190802272676. [DOI] [PubMed] [Google Scholar]
- 38.Khotskaya YB, Dai Y, Ritchie JP, et al. Syndecan-1is required for robust growth, vascularization, and metastasis of myeloma tumors in vivo. J Biol Chem. 2009;284(38):26085–26095. doi: 10.1074/jbc.M109.018473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadnagy A, Gaboury L, Beaulieu R, Balicki D. SP analysis may be used to identify cancer stem cell populations. Exp Cell Res. 2006;312(19):3701–3710. doi: 10.1016/j.yexcr.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 40.Moserle L, Indraccolo S, Ghisi M, et al. The side population of ovarian cancer cells is a primary target of IFN-alpha antitumor effects. Cancer Res. 2008;68(14):5658–5668. doi: 10.1158/0008-5472.CAN-07-6341. [DOI] [PubMed] [Google Scholar]
- 41.Zhou J, Wulfkuhle J, Zhang H, et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci U S A. 2007;104(41):16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou S, Schuetz JD, Bunting KD, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7(9):1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 43.Chiba T, Kita K, Zheng YW, et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44(1):240–251. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 44.Haraguchi N, Utsunomiya T, Inoue H, et al. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24(3):506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 45.Hideshima T, Chauhan D, Shima Y, et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96(9):2943–2950. [PubMed] [Google Scholar]
- 46.Hu C, Li H, Li J, et al. Analysis of ABCG2 expression and side population identifies intrinsic drug efflux in the HCC cell line MHCC-97L and its modulation by Akt signaling. Carcinogenesis. 2008;29(12):2289–2297. doi: 10.1093/carcin/bgn223. [DOI] [PubMed] [Google Scholar]
- 47.Mogi M, Yang J, Lambert JF, et al. Akt signaling regulates side population cell phenotype via Bcrp1 translocation. J Biol Chem. 2003;278(40):39068–39075. doi: 10.1074/jbc.M306362200. [DOI] [PubMed] [Google Scholar]
- 48.Bleau AM, Hambardzumyan D, Ozawa T, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4(3):226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trussardi-Regnier A, Lavenus S, Gorisse MC, Dufer J. Thalidomide alters nuclear architecture without ABCB1 gene modulation in drug-resistant myeloma cells. Int J Oncol. 2009;35(3):641–647. doi: 10.3892/ijo_00000376. [DOI] [PubMed] [Google Scholar]
- 50.Chepko G, Dickson RB. Ultrastructure of the putative stem cell niche in rat mammary epithelium. Tissue Cell. 2003;35(2):83–93. doi: 10.1016/s0040-8166(02)00107-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.