Abstract
Most naturally occurring biofilms contain a vast majority of microorganisms which have not yet been cultured, and therefore we have little information on the genetic information content of these communities. Therefore, we initiated work to characterize the complex metagenome of model drinking water biofilms grown on rubber-coated valves by employing three different strategies. First, a sequence analysis of 650 16S rRNA clones indicated a high diversity within the biofilm communities, with the majority of the microbes being closely related to the Proteobacteria. Only a small fraction of the 16S rRNA sequences were highly similar to rRNA sequences from Actinobacteria, low-G+C gram-positives and the Cytophaga-Flavobacterium-Bacteroides group. Our second strategy included a snapshot genome sequencing approach. Homology searches in public databases with 5,000 random sequence clones from a small insert library resulted in the identification of 2,200 putative protein-coding sequences, of which 1,026 could be classified into functional groups. Similarity analyses indicated that significant fractions of the genes and proteins identified were highly similar to known proteins observed in the genera Rhizobium, Pseudomonas, and Escherichia. Finally, we report 144 kb of DNA sequence information from four selected cosmid clones, of which two formed a 75-kb overlapping contig. The majority of the proteins identified by whole-cosmid sequencing probably originated from microbes closely related to the alpha-, beta-, and gamma-Proteobacteria. The sequence information was used to set up a database containing the phylogenetic and genomic information on this model microbial community. Concerning the potential health risk of the microbial community studied, no DNA or protein sequences directly linked to pathogenic traits were identified.
Current estimates indicate that more than 99% of the microorganisms present in many natural environments are not readily culturable and therefore not accessible for biotechnology or basic research (1). In fact, most of the species in many environments have never been described, and this situation will not change until new culture technologies are developed (1). Additionally, many approaches currently used to explore the diversity and potential of microbial communities are biased because of the limitations of cultivation methods.
To overcome the difficulties and limitations associated with cultivation techniques, several DNA-based molecular methods have been developed. In general, methods based on 16S rRNA gene analysis provide extensive information about the taxa and species present in an environment. However, these data usually provide little information about the functional role of any of the different microbes within the community and the genetic information they contain.
Metagenomics is a new and rapidly developing field that tries to analyze the complex genomes of microbial niches. Although the term metagenome has been introduced only recently to describe the genomes of noncultivated microbes present within a soil microbial community (10), earlier studies used a similar approach. In one such study, the approach was employed for the isolation of cellulases from a thermophilic environment (11), and in a second study the approach was used for the phylogenetic characterization of marine picoplankton (27).
Since then, an increasing number of publications have applied similar techniques to study the metagenomes of diverse microbial communities. The microbial niches addressed within these studies included the characterization of a wide range of different microbial communities ranging from soil and rather extreme environments to laboratory enrichments (2-5, 7, 19, 22, 24, 25, 32). The goal of these studies was to increase our understanding of ecological and molecular processes in the microbial communities, and several of these studies also aimed at an increased understanding of the genome information of individual microbes within the complex communities. In addition, the approach has been used to identify a number of novel biocatalysts and other interesting biomolecules from noncultivated microbes (8, 9, 11-13, 16, 17, 32). Altogether, these studies have led to an increased knowledge of the genetic structure of the microbial communities studied. Despite the number of metagenome studies, the amount of DNA information generated for individual niches is still very limited if one takes into account that the DNA information of several thousand different microbial genomes may be stored within a single microbial habitat (31). Thus, conclusions on the functional role of the microbes and sequences identified within these highly diverse bacterial communities cannot easily be made.
Since it can be assumed that microbial biofilms commonly found in drinking water distribution systems typically consist of fewer bacterial species than soil samples, they are ideal models to study metagenomes in combination with a phylogenetic analysis. The microbial communities that build drinking water biofilms have been characterized to some extent by 16S rRNA gene analyses. While these studies have mostly focused on the detection of bacterial species causing infectious diseases, such as Legionella and indicator organisms for fecal contamination, such as coliform bacteria (30), a number of more recent studies have led to the identification of novel nonpathogenic bacterial species (14, 15). Thus, the metagenomes of drinking water biofilms represent distinct and highly intriguing ecological niches, and their analysis is of significance to both the water suppliers and the consumers.
The aim of this study was to give insight into the metagenomes of drinking water biofilms grown on rubber-coated valves. For this purpose we characterized the phylogenetic structure of bacterial biofilms derived from rubber-coated drinking water valves by sequencing 16S rRNA clones. Additionally, we generated and analyzed about 2.0 Mb of DNA sequence information with a snapshot genome sequencing approach. With this sequence information, we analyzed the DNA sequence of four cosmid clones. This information has been used to set up a database to link the phylogenetic information with the genomic and functional information and to shed new light on the fine structure and evolution of the metagenomes of such complex microbial communities.
MATERIALS AND METHODS
Total DNA extraction.
For the analysis, three biofilm samples were collected within the drinking water networks of a town in the northwestern part of Germany in the state of North Rhine-Westphalia, and the samples were all obtained from the surfaces of identical ethylene-propylene-diene monomer-coated valves. Prior to removal, the rubber-coated valves were submerged in nonchlorinated drinking water for 4 to 7 months. Samples were frozen at −70°C until processing. For library construction, three samples were collected from the surface of the rubber-coated valves (Fig. 1), and the samples were designated BioI, BioII, and BioIII. Total nucleic acids were extracted from the biofilms by standard protocols (8).
FIG. 1.
Bacterial biofilm observed on the surface of a rubber-coated drinking water valve. The arrow indicates the bacterial biofilm. The drinking water valve was obtained from a drinking water pipe with an internal diameter of 15 cm. The valves are normally submerged in the drinking water.
Cosmid and small insert libraries were constructed as previously published (8). After collection, bacteria were resuspended in TE-sucrose (20%, wt/vol) buffer and lysed in DNA extraction buffer (100 mM Tris HCl, 100 mM EDTA, 100 mM Na2HPO4, 1.5 M NaCl, 1% SDS) for several hours. RNA was degraded with RNase A (10 mg/ml). The resulting DNA extracts were incubated with protease and Sarcosyl (5%, wt/vol) in TE buffer overnight. Total genomic DNA was then repeatedly extracted with chloroform-phenol (1:1, vol/vol), washed once with chloroform, and dialyzed against 2 liters of TE buffer at 4°C overnight. Finally, an aliquot of the DNA was analyzed on a 0.8% agarose gel to ensure that the DNA was not degraded.
Cosmid libraries were prepared in pWE15 (Stratagene, La Jolla, Calif.) with standard protocols (8). DNA fragments (20 to 40 kb) obtained after partial Sau3A digestion were ligated into the BamHI restriction sites of the cosmid vector. Phage packaging mixes were obtained from Stratagene (La Jolla, Calif.), and infection of Escherichia coli VCS257 was performed according to the manufacturer's protocol. For the construction of the snapshot libraries, DNA fragments with inserts of 3 to 7 kb were ligated into the sequencing vector pTZ19R (Amersham-Pharmacia, Essex, United Kingdom) and transformed into E. coli. For the construction of cosmid and small-insert libraries, the DNAs of the three samples were pooled. This was necessary because the amounts of DNA obtained from each individual sample were not sufficient to allow construction of the different samples. Therefore, the DNA of the three samples is considered a pool of biofilm genomes throughout this work, and the data summarize the possible microbes and genes occurring in these microbial niches.
PCR and cloning of 16S rRNA sequences.
Bacterial biofilm ribosomal DNAs (rRNAs) were amplified by PCR from DNA in reaction mixtures containing (as final concentrations) 1× PCR buffer (Perkin-Elmer), 2.5 mM MgCl2, 200 μM each deoxynucleoside triphosphate, 300 nM each forward and reverse primer, and 0.25 U of Taq DNA polymerase (Perkin-Elmer) per ml. Reaction mixtures were incubated in a gradient thermal cycler (MJ Research, Boston, Mass.) at 96°C for 5 min for initial denaturation, followed by 25 to 35 cycles at 94°C for 30 s, 50°C for 45 s, and 72°C for 1.5 min, followed by a final extension period of 10 min at 72°C. For the clone library, rRNA genes were amplified with the universal reverse oligonucleotide primer 5′-CGGCCTCTACCTTGTTACGAC-3′ and the universal forward primer 5′-AGAGTTTGATCCTCACTGGCTCAG-3′. The resulting PCR products (of 1.5 kb) were cloned with a Topo TA cloning kit in accordance with the manufacturer's instructions (Invitrogen Corp., Karlsruhe, Germany). Plasmid DNAs containing inserts were sequenced with standard protocols for ABI 377 automated sequencing.
Assignment of cloned sequences to established phylogenetic divisions.
The phylogenetic diversity was assessed with clone libraries of the 16S rRNA gene sequences of the different biofilm samples. The cloned 16S rRNA gene sequences were compared with reference sequences contained in the NCBI nucleotide sequence database with the FASTA program. For calculation of a phylogenetic tree, all ambiguous positions were excluded from similarity calculations. Sequences were screened for chimeras with the Check_Chimera program of the Ribosome Database Project and by manual alignments of secondary structure. As a final check for chimeras, each sequence was split into 5′ and 3′ fragments, which were analyzed separately by Blast searching of GenBank. Sequences for which either the 5′ or 3′ fragment had significantly different closest relatives were considered probable chimeras and were removed from the data set.
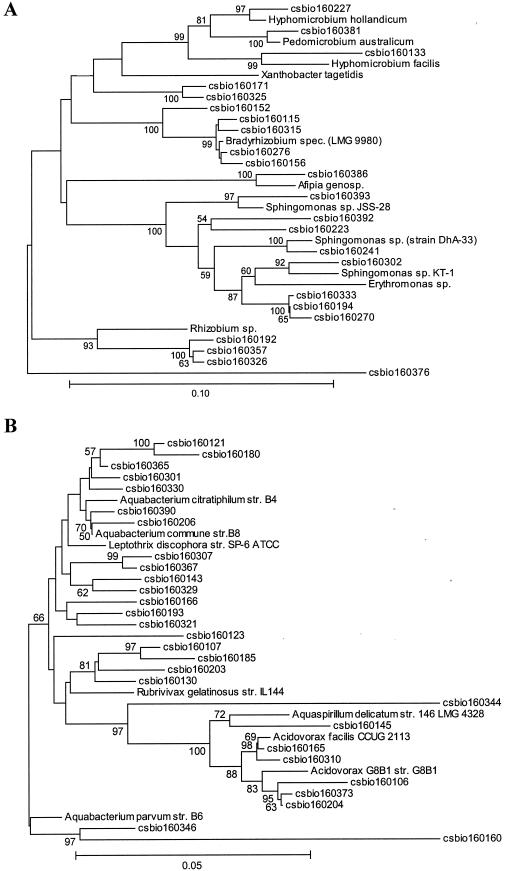
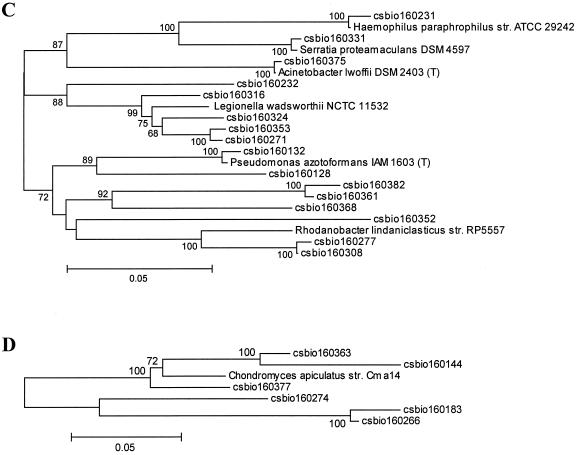
For calculation of the dendrogram shown in Fig. 2, cloned sequences were aligned with 16S rRNA gene sequences representative of the main bacterial divisions. Sequences were aligned with 16S rRNA sequences of other bacteria obtained from the Ribosomal Database Project (RPD-II) (18). Matrices of evolutionary distance were computed from the sequence alignment with the program DNADIST implemented in the software package Phylip (http://evolution.genetics.washington.edu/phylip.html) (version 3.5). For calculations of a phylogenetic tree from the distance matrices, the program applies the neighbor-joining method described by Saitou and Nei (23).
FIG. 2.
Dendrogram of the 16S rRNA clones identified within the drinking water bacterial community DNA, showing the relationship to the closest known relatives. Only the proteobacterial lineages species are depicted. (A) Alpha-proteobacterial lineage; (B) beta-proteobacterial lineage; (C) gamma-protebacterial lineage; (D) delta-proteobacterial lineage. The phylogenetic trees were calculated with the software package MEGA version 2.1 (Molecular Evolutionary Genetics Analysis software, Arizona State University, Tempe, Ariz.) and verified with the Phylip software package from the Ribosomal Database Project (RDP) (18). Only high-quality sequences from the 16S rRNA gene clones were included in the calculations, and the hypervariable regions in the 16S rRNA molecule were excluded from the calculations. Numbers indicate data from a bootstrap analysis, and values below 50% are not indicated.
Single-strand conformation polymorphism analysis.
The single-strand conformation polymorphism analyses of the biofilm communities were done following standard protocols (26, 29). After PCR amplification of the partial the 16S rRNA genes with primers COM1 (5′CAGCAGCCGCGGTAATAC3′, positions 519 to 536) and reverse primer COM2-Ph (5′CCGTCAATTCCTTTGAGTTT3′, positions 907 to 926) (29), the phosphorylated strand of the amplified PCR fragments was removed by λ exonuclease digestion. The fragment size of the amplified V4 and V5 regions of the 16S rRNA gene was 390 bp. To introduce specific secondary structures in the strands, samples were heat denatured, quickly chilled on ice, and then electrophoresed on nondenaturing gels; bands visualized by silver staining. For determining the band numbers, the gels were digitized to create TIF files. Analysis of the 16S rRNA fingerprints was performed with the software package GelCompare II (Applied Maths, Kortrijk, Belgium). The background was subtracted with rolling-circle correction (circle diameter, 30 points), and lanes were normalized. Only bands with an intensity of 2% or more of the total lane intensity were considered.
Nucleotide sequence data analysis.
Automated DNA sequencing was performed with ABI377 and dye terminator chemistry following the manufacturer's instructions; when required, gaps in the DNA sequences were filled by PCR. The nucleotide sequences obtained for larger contigs or complete cosmids have been deposited at GenBank, and accession numbers are listed in Tables 4 to 6. The sequence data of the cloned 16S rRNA genes were deposited at GenBank, and all 81 accession numbers (AY187312 to AY187393) are available at www.gwdg.de/∼biofilm/ together with the corresponding sequences; the snapshot genome sequences are available at the same web pages together with the BlastX results. Also, the sequences of the completely sequenced cosmids are available together with the GenBank accession numbers and other useful information on this web site. The GC contents of the nucleic acid sequences from the cosmids and the snapshot library was calculated with the program Geecee from the free open-source software package for sequence analysis, Emboss (http://www.hgmp.mrc.ac.uk/Software/EMBOSS/) running on a local Linux server.
TABLE 4.
Genes identified and observed similarities for ORFs identified on pBioVa
| ORF | Size of putative protein (AA) | Function, closest match | GenBank accession no. of closest match | ORF alignment region (AA) | Alignment region (AA range/total AA) | % Identity |
|---|---|---|---|---|---|---|
| csv001 | 258 | d-Pantothenate synthesis, panC Ralstonia solanacearum | NP520508.1 | 1-256 | 1-247/283 | 58 |
| csv002 | 315 | d-Pantothenate synthesis, panB Neisseria meningitides | NP283858.1 | 48-308 | 2-255/263 | 56 |
| csv003 | 288 | Sterol desaturase Shewanella oneidensis | AAN56787.1 | 15-280 | 11-280/287 | 52 |
| csv004 | 265 | Hypothetical protein Burkholderia fungorum | ZP00029113.1 | 2-265 | 28-291/291 | 73 |
| csv005 | 324 | Hypothetical protein Pseudomonas fluorescens | ZP00083568.1 | 2-315 | 3-317/456 | 29 |
| csv006 | 627 | Sulfate transporter Chlorobium tepidum | AAM71951.1 | 78-625 | 40-586/618 | 41 |
| csv007 | 701 | Methionyl-tRNA synthetase Ralstonia solanacearum | CAD16088.1 | 3-701 | 4-688/688 | 58 |
| csv008 | 375 | MRP ATP binding protein Ralstonia solanacearum | CAD16086.1 | 1-369 | 1-359/362 | 57 |
| csv009 | 875 | Histidine kinase Nostoc sp. | BAB73836.1 | 205-822 | 309-912/944 | 22 |
| csv010 | 213 | Response regulator Mycobacterium tuberculosis | AAK45932.1 | 10-201 | 18-200/208 | 25 |
| csv011 | 357 | Hypothetical protein Geobacter metallireducens | ZP00081330.1 | 113-337 | 181-396/397 | 47 |
| csv012 | 1,248 | Hypothetical protein Magnetococcus sp. | ZP0004555.1 | 300-1186 | 159-1045/1202 | 34 |
| csv013 | 742 | Hypothetical protein Magnetococcus sp. | ZP0043181.1 | 3-727 | 28-733/733 | 32 |
| csv014 | 584 | Hypothetical protein Xanthomonas axonopodis | AAM35315.1 | 167-580 | 129-537/542 | 30 |
| csv015 | 505 | Hypothetical protein Rhodopseudomonas palustris | ZP00012497 | 6-499 | 3-491/499 | 52 |
| csv016 | 553 | Hypothetical protein Pseudomonas fluorescens | ZP0084879.1 | 161-551 | 19-407/410 | 60 |
| csv017 | 524 | ABC transporter Pseudomonas putida | AAN67853.1 | 1-520 | 96-617/617 | 68 |
| csv018 | 151 | Hypothetical protein Ralstonia metallidurans | ZP00022071.1 | 1-150 | 1-150/150 | 66 |
| csv019 | 165 | Hypothetical protein Pseudomonas syringae | ZP00125646.1 | 15-164 | 15-165/169 | 37 |
| csv020 | 615 | p-Aminobenzoate-synthase component Listeria innocua | CAC98119.1 | 28-603 | 15-566/568 | 39 |
| aroC | 380 | Chorismate synthase Ralstonia solanacearum | CAD15268.1 | 14-379 | 1-366/366 | 78 |
| csv022 | 427 | Ribonuclease Ralstonia solanacearum | NP519680.1 | 36-402 | 8-375/441 | 39 |
AA, amino acids. DNA sequences were submitted to GenBank under accession number AY280634. Identified ORFs were designated a gene name if the observed e value was below 10−80.
TABLE 6.
Genes and observed similarities for ORFs identified on the 75-kb DNA fragment formed by overlapping cosmids pbioX and pbioYa
| ORF | Size of putative protein (AA) | Function, closest match | GenBank accession no. of closest match | ORF alignment region (AA range) | Alignment region (AA range/total AA) | % Identity |
|---|---|---|---|---|---|---|
| csx001 | 327 | Hypothetical protein ORF48 Photorhabdus luminescens | AAL18484.1 | 31-279 | 7-263/277 | 28 |
| csx002 | 547 | Possible lipase ORF47 Photorhabdus luminescens | AAL18483.1 | 1-540 | 2-535/537 | 40 |
| csx003 | 1,050 | Possible VgrG related protein Ralstonia solanacearum | CAD17919.1 | 35-1047 | 16-999/1006 | 39 |
| csx004 | 398 | Possible invertase Arabidopsis thaliana | AAL08305.1 | 23-394 | 134-537/617 | 20 |
| csx005 | 350 | Hypothetical protein Azotobacter vinelandii | ZP00092060.1 | 146-332 | 32-249/687 | 30 |
| csx006 | 1132 | Possible alpha-amylase Pseudomonas syringae | AAO56261.1 | 2-673 | 5-653/1108 | 61 |
| csx007 | 390 | Alpha-amylase family protein Chlorobium tepidum | AAM73306.1 | 21-341 | 18-337/670 | 47 |
| csx008 | 568 | 1,4-Alpha-glucan branching enzyme Aquifex aeolicus | AAC06895.1 | 12-520 | 52-612/630 | 61 |
| csx009 | 274 | Hypothetical protein Xanthomonas campestris | NP636161.1 | 1-267 | 1-271/274 | 39 |
| csx010 | 472 | Proton glutamate symport protein Ralstonia metallidurans | ZP000247776.1 | 24-454 | 1-422/430 | 75 |
| csx011 | 241 | Transcriptional regulator Magnetospirillum magnetotacticum | ZP00055867.1 | 49-219 | 12-181/202 | 30 |
| csx012 | 334 | Multidrug resistance protein A Nostoc punctiforme | ZP001111687.1 | 2-319 | 56-382/393 | 37 |
| csx013 | 492 | Multidrug resistance protein B Nostoc punctiforme | ZP001111685.1 | 18-491 | 12-485/526 | 54 |
| csx014 | 509 | Multidrug resistance protein C Burkholderia funghorum | ZP00032009.1 | 12-508 | 5-510/516 | 35 |
| csx015 | 212 | Hypothetical protein Burkholderia fungorum | ZP00034179.1 | 1-206 | 1-204/210 | 41 |
| csx016 | 867 | FhuE, transport protein Xanthomonas campestris | AAM39477.1 | 125-867 | 1-746/746 | 43 |
| csx017 | 268 | Hypothetical protein Rhodobacter sphaeroides | ZP00005910.1 | 21-226 | 3-208/373 | 42 |
| csx018 | 404 | Hypothetical protein Mesorhizobium loti | BAB50278.1 | 26-402 | 27-403/404 | 58 |
| csx019 | 341 | Transcriptional regulator Mesorhizobium loti | BAB50279.1 | 1-326 | 1-319/328 | 55 |
| csx020 | 647 | Hypothetical protein Ralstonia metallidurans | ZP00025947.1 | 61-485 | 171-607/935 | 36 |
| csx021 | 426 | OruR regulator Pseudomonas aeruginosa | AAB94774.1 | 141-423 | 57-338/339 | 30 |
| csx022 | 603 | Hypothetical protein Caulobacter crescentus | AAK22724.1 | 35-583 | 537-1219/1245 | 25 |
| csx023 | 184 | Hypothetical protein Pseudomonas aeruginosa | AAK22724.1 | 8-184 | 3-187/195 | 31 |
| csx024 | 311 | Lactonizing lipase Pseudomonas mendocina | AAM14701 | 64-311 | 33-301/311 | 41 |
| csx026 | 647 | HSP70 Ralstonia solanacearum | CAD16342.1 | 1-644 | 37-684/688 | 83 |
| csx027 | 180 | HSP24 Burkholderia fungorum | ZP00030660.1 | 25-180 | 43-194/194 | 65 |
| csx028 | 649 | Lipoprotein Ralstonia solanacearum | NP519830.1 | 24-641 | 43-648/657 | 37 |
| bdhA | 284 | Beta-hydroxybutyrate dehydrogenase Ralstonia eutropha | AAD33952.1 | 25-284 | 1-259/259 | 78 |
| csx030 | 865 | Pyrophosphokinase Ralstonia solanacearum | CAD15278.1 | 131-865 | 6-746/746 | 50 |
| csx031 | 450 | Transposase Burkholderia fungorum | CAD17735.1 | 12-411 | 11-438/440 | 66 |
| csx032 | 483 | Hypothetical protein/chitinase Deinococcus radiodurans | AAF12325.1 | 159-332 | 451-641/818 | 31 |
| csx033 | 440 | Sensor histidine kinase Pseudomonas putida | AAN68322.1 | 5-412 | 2-429/454 | 30 |
| csx034 | 228 | Two-component response regulator Pseudomonas aeruginosa | AAG08162.1 | 1-222 | 1-216/221 | 54 |
| csx035 | 223 | Hypothetical protein Shewanella oneidensis | AAN56949.1 | 9-204 | 22-223/264 | 34 |
| csx036 | 615 | Hypothetical protein Pseudomonas aeruginosa | AAG05360.1 | 77-613 | 5-538/538 | 43 |
| csx038 | 433 | Phospoglycerate dehydrogenase Pseudomonas putida | AAN70720.1 | 25-433 | 1-409/409 | 69 |
| csx039 | 467 | Oxidoreductase Pseudomonas aeruginosa | NP249008.1 | 13-465 | 10-462/464 | 69 |
| csx040 | 499 | Hypothetical protein Geobacter metallireducens | ZP00081903.1 | 6-404 | 6-385/638 | 29 |
| csx041 | 213 | Antioxidant peroxidase Ralstonia solanacearum | CAD14284.1 | 3-213 | 2-212/212 | 84 |
| csx042 | 217 | Hypothetical protein/esterase Burkholderia fungorum | ZP00031463.1 | 22-196 | 83-250/271 | 44 |
| csx043 | 431 | Homocysteine synthase Xanthomonas campestris | AAM42339.1 | 4-426 | 3-425/428 | 81 |
| csx044 | 208 | Hypothetical protein Novosphingobium aromaticivorans | ZP00093590.1 | 3-204 | 89-293/297 | 51 |
| csx045 | 237 | Hypothetical protein Agrobacterium tumefaciens | AAK87332.1 | 1-223 | 30-238/321 | 36 |
| rbiA | 223 | Ribose 5-phosphate isomerase Ralstonia metallidurans | ZP00026886.1 | 9-223 | 1-211/211 | 62 |
| csx047 | 681 | Oligopeptidase A Burkholderia fungorum | ZP00028805.1 | 5-659 | 40-706/732 | 60 |
| csx048 | 281 | Methylenetetrahydrofolate dehydrogenase Ralstonia solanacearum | CAD15298.11 | 1-280 | 1-280/289 | 76 |
| csx049 | 215 | Two-component response regulator Ralstonia metallidurans | ZP00021742.1 | 5-201 | 53-250/254 | 69 |
| csx050 | 860 | Two-component sensor protein Burkholderia fungorum | ZP00028811.1 | 6-850 | 13-834/841 | 42 |
| csx051 | 848 | Pyruvate dehydrogenase E1 complex Ralstonia eutropha | AAA21598.1 | 9-823 | 12-821/895 | 63 |
RESULTS
Phylogenetic analyses.
The samples used for total nucleic acid extraction were taken from the surfaces of the rubber-coated drinking water valves and used for DNA extraction (Fig. 1). DNAs of three biofilms, which were grown on the rubber coated-surfaces were pooled. The phylogenetic diversity of the bacterial biofilm community was assessed with the cloned and pooled rRNA gene sequences of the pooled biofilm samples. For this purpose, 650 clones were analyzed, and this resulted in the identification of 81 different clones. These sequences are phylogenetically highly diverse and include numerous bacterial lineages (Fig. 2).
Interestingly, no single phylogenetic group of bacteria dominated the clone collection. Instead, common bacterial phylotypes that occurred in the sample included members of the alpha-, beta-, delta-, and gamma-Proteobacteria, the Cytophaga-Flavobacterium-Bacteroides group, the Actinobacteria, and the low G+C gram-positive group (Fig. 2A to D and Table 1). Altogether, the Proteobacteria constituted 86% of the clones identified and thus represented the largest fraction of microbes within the bacterial community. The Actinobacteria, the low G+C gram-positives, the Cytophaga-Flavobacterium-Bacteroides group, and the Acidobacteria constituted only minor fractions of the clones. Finally, a small number of sequences were highly similar to unclassified bacteria (Table 1). While several of the isolates were highly similar to previously described microbial species within drinking water bacterial communities, a novel observation was that a limited number of the clones identified were closely related to the microbes which belong to the genera Rhizobium and Bradyrhizobium.
TABLE 1.
Different phylogenetic groups and clones observed in the 16S rRNA clone library derived from a drinking water biofilm community DNAa
| Bacterial division | No. of clones | % of total |
|---|---|---|
| Total | 81 | 100 |
| Proteobacteria | 70 | 86 |
| Alpha subdivision | 23 | 28 |
| Beta subdivision | 29 | 36 |
| Gamma subdivision | 15 | 19 |
| Delta subdivision | 3 | 4 |
| Other groups | 11 | 14 |
| Actinobacteria | 1 | 1.2 |
| Low G+C gram-positives | 1 | 1.2 |
| Cytophaga-Flavobacterium-Bacteroides | 4 | 4.9 |
| Acidobacteria | 3 | 3.7 |
| Unclassified bacteria | 2 | 2.5 |
Clones were significantly different when DNA similarities were lower than 97%. Data were generated by sequence analysis of 450 clones, resulting in the identification of 81 different clones.
Further tests were employed to verify the high phylogenetic diversity within the microbial communities studied. For this purpose, single-strand conformation polymorphism genetic profiles of the different drinking water biofilm microbial communities DNA were analyzed. Primers designed to amplify the bacterial 16S rRNA gene sequences, including the variable V4 and V5 regions, yielded complex single-strand conformation polymorphism patterns on polyacrylamide gels. In these tests, the observed profiles consisted of more than 35 different product bands for each of the samples tested (data not shown).
Random sequencing of 2,500 small insert clones containing biofilm DNA.
Total genomic DNA of the drinking water biofilms was used to construct a small insert library with inserts ranging in size from 1 to 5 kb. Of the 5,000 random sequences obtained, 2,496 produced high-quality DNA sequences (Table 2); and 2,504 sequences (50.1%) were not included in further analyses because of poor sequence quality, short length of the reads, or vector contaminations. In this way, more than 2.0 Mb of high-quality nucleotide sequence were collected and analyzed. The G+C content of the high-quality sequences was 62%.
TABLE 2.
Overview of snapshot genome sequence analysis of a small insert library of drinking water biofilm DNAa
| Sequence type | No. | % of total |
|---|---|---|
| Sequences generated | 5,000 | 100.0 |
| High-quality sequences over 800 bp in length | 2,496 | 49.9 |
| Low-quality sequences (not included in further analysis) | 2,504 | 50.1 |
| Sequences with significant similarity (E value < 10−4) | 1,344 | 26.9 |
| Sequences with weak similarity (E value >10−4) | 856 | 17.0 |
| Sequences with no hit in database | 296 | 6.0 |
High-quality sequences refers to sequence more than 800 bp in length and a confidence value of >15. Sequences were analyzed by automated BlastX search at the NCBI nonredundant databases. An E value of 10−4 was arbitrarily chosen as the cutoff for similarity searches.
To assign putative functions to the cloned DNA fragments, sequences were compared to the NCBI protein and nucleotide databases. BlastX analyses indicated that 1,344 of the 2,496 high-quality sequences matched known protein-coding ORFs (Table 2). Of the 1,344 putative protein-coding sequences, 318 (24%) were similar to hypothetical genes with no known function (Table 3). BlastX searches with 296 of the sequences did not return any significant similarities. To provide an overview of the genetic organization of the biofilm metagenome, 1,344 predicted protein-coding sequences, based on BlastX searches, were grouped into nine classes according to their putative function (Table 3). Also, all BlastX results are available at http://www.gwdg.de/∼biofilm.de together with the corresponding sequences and other information on the metagenome analyzed.
TABLE 3.
Functional classes and possible ORFs identified in random biofilm genome sequences after automated BlastX searchesa
| Functional class | No. of complete or partial proteins identified | % of total |
|---|---|---|
| Regulatory function | 112 | 8.4 |
| Metabolism and catabolism | 455 | 34.0 |
| Cell processes and structure | 132 | 9.0 |
| Elements of external origin | 22 | 1.7 |
| DNA/RNA-modifying enzymes | 117 | 8.8 |
| Protection responses | 28 | 2.1 |
| Transport proteins | 112 | 8.4 |
| Hypothetical proteins | 318 | 23.9 |
| Miscellaneous | 48 | 3.7 |
| Total | 1,344 | 100.0 |
All BlastX results and corresponding sequences are publicly available together with other information at http//www.gwdg/∼biofilm/.
Catabolic and metabolic abilities stored in the biofilm metagenome.
A total of 455 (34%) sequences were found to encode putative proteins involved in catabolic or metabolic activities of the microbial biofilm community (Table 3). Of these, the majority encoded genes involved in classical pathways such as the tricarboxylic acid cycle, 2-keto-3-deoxy-6-phosphogluconate pathway, glycolysis, and the glyoxylate cycle. Interestingly, quite a large number of possible genes involved in lipid hydrolysis could be identified. Altogether, 21 partial genes coding for possible lipases were identified, suggesting that lipolytic activities are probably of importance for this biofilm community. Most of the putative lipases were highly similar to lipases known to be present in Pseudomonas fluorescens.
Furthermore, a number of genes were identified which encoded proteins involved in the degradation of aromatic compounds. These included mostly genes involved in the degradation of toluate and benzoate or related compounds. The partial proteins were highly similar to corresponding proteins from gram-positive and gram-negative microbes. Also, 14 possible ORFs were identified encoding genes involved in the degradation or modification of polysaccharides (i.e., starch and cellulose). Surprisingly, 21 putative protease genes were identified and 12 ORFs possibly involved in the catabolism of amino acids were found. Altogether, these findings suggest that the microbial community analyzed in this study is nutritionally highly diverse and able to catabolize a wide range of different carbon and energy sources.
Other remarkable features included the identification of 28 (2.1%) sequences encoding genes that are involved in protection response, such as antibiotic resistance or metal detoxification. Eight clones carried possible tetracycline resistance genes, and seven clones were possibly involved in resistance to β-lactam antibiotics. Two ORFs were identified that might be linked to bacterial polyketide synthesis. Other features identified included possible ORFs involved in bacterial photosynthesis and light emission. Finally, it is noteworthy that none of the sequences of the snapshot analysis encoded proteins specifically related to pathogenic mechanisms. A complete list of all the possible ORFs identified and their possible functions is available at http://www.gwdg.de/∼biofilm/overviewtable.htm.
Statistical and phylogenetic analysis of the BlastX hits.
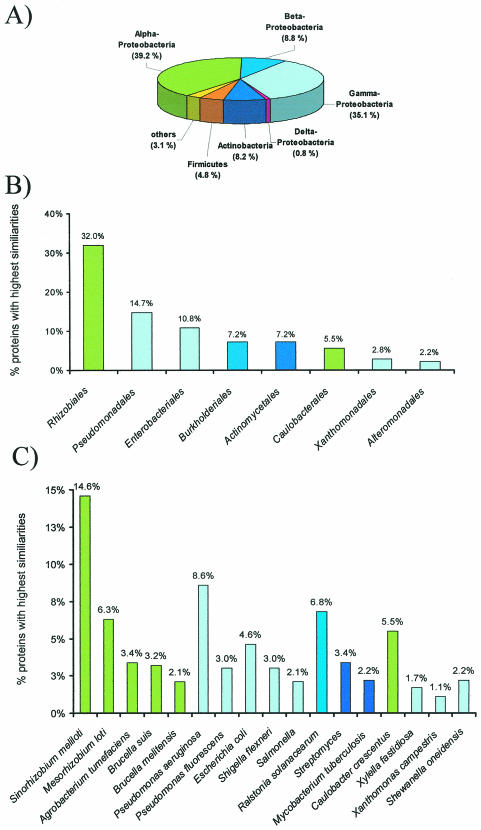
To further exploit the DNA snapshot sequences, we analyzed the distribution of BlastX hits over different bacterial groups. For this purpose, the results of 1,026 BlastX similarity searches were evaluated. The statistical analysis of the BlastX searches indicated that the major fraction (84%) of all proteins were highly similar to proteins derived from the Proteobacteria (Fig. 3A). Among these, most were highly similar to the group of the alpha- and gamma-Proteobacteria (74.3%). Among the proteins most similar to proteins originating from the alpha-Proteobacteria, the largest fraction were highly similar to rhizobial proteins (i.e., Rhizobiales) (Fig. 3B). Interestingly, within the Rhizobiales most deduced proteins were highly similar to Sinorhizobium meliloti and Mesorhizobium loti proteins (Fig. 3C). Also, a significant fraction of proteins (5.5%) were highly similar to proteins originating from microbes closely related to the typical freshwater microbe Caulobacter crescentus.
FIG. 3.
Distribution of BlastX similarities among bacterial phyla (A), bacterial families (B), and bacterial genera and species (C). The results indicate the distribution of the highest similarities observed after 1,026 BlastX searches. The DNA sequences were derived from the snapshot genome sequencing project, and only those sequences which resulted in the identification of functional proteins were included. In B, only those bacterial families for which more than 20 hits (2%) could be observed were included; and in C, only the bacterial species for which more than 10 hits (1%) could be observed were included.
Within the group of the gamma-Proteobacteria, the majority of proteins were highly similar to proteins derived from bacteria closely related to the Pseudomonadales (14.7%) and Enterobacteriales (10.8%) (Fig. 3B). The possible ORFs identified within the Pseudomonadales were highly similar to proteins derived from Pseudomonas aeruginosa and P. fluorescens. Furthermore, 6.8% of all proteins analyzed appeared to be highly similar to proteins originating from microbes related to Ralstonia solanacearum. Finally, it is noteworthy that 7.2% of all proteins analyzed were highly similar to known proteins from the Actinomycetales (i.e., Streptomyces and Mycobacterium) (Fig. 3B and C). Altogether, the statistical analysis of the putative ORFs supports the idea that the biofilm community studied is highly diverse. The data also suggest that a significant number of the proteins possibly expressed in the bacterial community originates from microbes closely related to the Rhizobiales.
Sequence analysis of large insert clones.
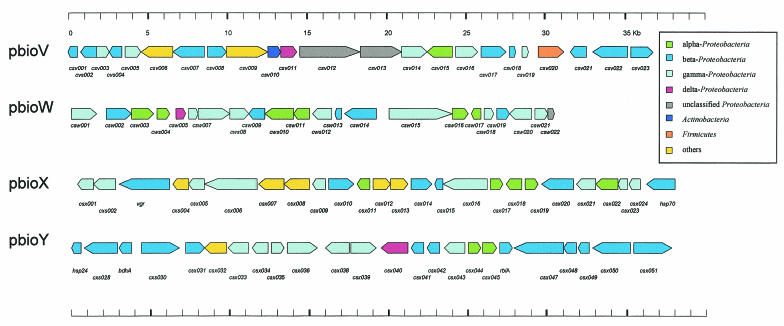
To further exploit the genomic information contents of drinking water biofilms, the complete DNA sequences of four cosmid clones were determined. Three of the sequenced cosmid clones were randomly selected from a library containing approximately 2,500 clones, and the sequenced clones were designated pbioW, pbioV, and pbioX. Cosmid clone pbioY was selected because it overlapped cosmid pbioX. In total, 144 kb of additional DNA sequence information was generated, and this resulted in the identification of 94 ORFs. The G+C content was highly similar for all the cosmids and ranged between 65 and 67%. The nucleotide sequences obtained for the cosmids have been deposited at GenBank, and the accession numbers are listed in Tables 4 to 6. All ORFs identified on the sequenced cosmids are summarized in Fig. 4.
FIG. 4.
Physical maps of the central parts of four cosmid clones isolated from the biofilm metagenome library. Arrows indicate the locations and directions of transcription of the identified open reading frames (ORFs) on the different cosmids. Observed similarities for the indicated ORFs are listed in Tables 4 to 6, together with the GenBank accession numbers. Color codes indicate the highest similarities of the deduced protein sequences to proteins of known bacterial species and their phylogenetic positions within the Proteobacteria, Actinobacteria, and Firmicutes. Only the highest similarities were considered for this analysis; color coding is identical to the color coding used in Fig. 3. The clones pbioX and pbioY form a 75-kb overlapping DNA fragment, and the DNA sequence was submitted to GenBank in two parts (contig1, csx001 to csx024; contig2, csx026 to csx051).
The insert size of pbioV was 37.8 kb, and the cosmid encoded 22 ORFs; 13 ORFs encoded hypothetical proteins, and many of these were probably involved in cellular processes. Two genes were identified which were involved in the biosynthesis of panthothenate (panB and panC), and two ORFs possibly involved in amino acid biosynthesis (csv020 and aroC) were identified (Table 4).
Cosmid clone pbioW encoded 22 ORFs in its 30.8-kb insert. Among these was a cluster of ORFs possibly involved in nitrogen regulatory circuits. Other possible genes encoded included a heme oxygenase and two proteins possibly involved in DNA modification. In addition, a number of hypothetical proteins were identified (Table 5).
TABLE 5.
Genes and observed similarities for ORFs identified on pBioWa
| ORF | Size of putative protein (AA) | Function, closest match | GenBank accession no. of closest match | ORF alignment region (AA range) | Alignment region (AA range/total AA) | % Identity |
|---|---|---|---|---|---|---|
| csw001 | 491 | ABC transporter Azotobacter vinelandii | ZP00092800.1 | 1-485 | 69-548/561 | 71 |
| csw002 | 474 | Hypothetical protein Achromobacter xylosoxidans | CAD24029.1 | 2-474 | 49-478/478 | 47 |
| csw003 | 508 | Hypothetical protein Caulobacter cresentus | AAK24385.1 | 5-496 | 3-514/524 | 37 |
| csw004 | 215 | Hypothetical protein Rhodobacter sphaeroides | ZP00004258.1 | 37-213 | 116-280/282 | 46 |
| csw005 | 203 | Hypothetical protein Desulfovibrio desulfuricans | ZP00129685.1 | 50-180 | 19-147/163 | 28 |
| csw006 | 212 | Oxireductase Xanthomonas axonopodis | AAM37740.1 | 6-212 | 9-222/222 | 67 |
| csw007 | 441 | Oxireductase Xanthomonas axonopodis | AAM37739.1 | 126-441 | 1-316/316 | 76 |
| csw008 | 762 | Oxireductase Xanthomonas campestris | AAM41999.1 | 27-759 | 1-730/735 | 73 |
| csw009 | 353 | Hypothetical protein Ralstonia metallidurans | ZP00024178.1 | 1-341 | 1-332/332 | 52 |
| csw010 | 579 | Hypothetical protein Bradyrhizobium japonicum | NP769440.1 | 5-578 | 7-580/580 | 55 |
| csw011 | 270 | Hypothetical protein Bradyrhizobium japonicum | AAO07326.1 | 1-267 | 1-288/300 | 74 |
| csw012 | 438 | Nitrate regulatory protein Klebsiella pneumoniae | AAA25101.2 | 11-425 | 17-389/396 | 31 |
| csw013 | 112 | Nitrite reductase (small subunit) Ralstonia solanacearum | NP522782.2 | 7-110 | 5-111/113 | 53 |
| csw014 | 848 | Nitrite reductase (large subunit) Ralstonia solanacearum | NP522783.1 | 1-848 | 3-842/852 | 76 |
| csw015 | 1,387 | DNA helicase Xanthomonas axonopodis | AAM37301.1 | 1-1374 | 79-1449/1480 | 67 |
| csw016 | 278 | Hypothetical protein Rhodobacter sphaeroides | ZP00008199.1 | 91-233 | 40-193/203 | 28 |
| csw017 | 185 | Repressor protein Agrobacterium tumefaciens | NP356453.1 | 7-183 | 11-187/198 | 53 |
| csw018 | 301 | LysR regulator Pseudomonas putida | AAN68114.1 | 1-299 | 1-299/308 | 64 |
| csw019 | 194 | Putative ligase protein Ralstonia solanacearum | NP519564.1 | 4-193 | 3-192/198 | 50 |
| csw020 | 676 | Oligopeptidase Shewanella oneidensis | AAN57578.1 | 43-664 | 19-641/645 | 38 |
| csw021 | 320 | Heme oxygenase Pseudomonas syringae | AAM00281.1 | 130-312 | 13-196/204 | 32 |
DNA sequences were submitted to GenBank under accession number AY280635. Identified ORFs were designated a gene name if the observed e value was below 10−80.
DNA restriction analysis and sequencing indicated that cosmids pbioX and pbioY formed a 75-kb contig of biofilm DNA. Altogether, 51 ORFs were identified through the DNA sequence analysis. Among the possible genes identified were mostly genes involved in cellular processes. Also, one possible transposase (csx031) and several regulatory genes were identified. Additionally, we encountered at least five different ORFs with potential value for biotechnological application. ORFs csx002 and csx024 encoded putative novel lipases, and ORFs csx006, csx007, and csx008 encoded putative amylolytic enzymes. Finally, three ORFs encoding a possible drug resistance transporter were identified (csx012 to csx014) (Table 6).
Of the 94 identified proteins, three were highly similar to proteins derived from delta-Proteobacteria, 14 were highly similar to proteins derived from the alpha-Proteobacteria, 34 were highly similar to the beta-proteobacterial proteins, and 30 were highly similar to proteins derived from gamma-proteobacterial species. Only 13 proteins were highly similar to known proteins from gram-positive microbes or other microbial species (Fig. 4). Altogether, the analysis of large insert clones also supports the concept that the studied biofilm is mainly constructed of microbes closely related to known species of the alpha-, beta-, and gamma-proteobacterial lineages.
In summary, all these data give a first insight into the complex metagenome of biofilms derived from rubber-coated valves used in drinking water networks.
DISCUSSION
The primary focus of the present paper was to provide high-resolution information on the genome information stored within the metagenome of drinking water biofilms grown on rubber-coated valves. This was achieved with three different strategies. Our first approach included an analysis of the 16S rRNA genes of the microbes present within the microbial community. The phylogenetic data indicated that the microbial community is constructed out of a significant number of different and mostly nonpathogenic proteobacterial species. Of these many are probably novel and have not yet been cultured. Drinking water biofilms are well known to carry diverse microbial communities. Many of the microbes identified in this work as part of the studied biofilm are indeed very closely related to typical drinking water or fresh water microbes, and their presence in biofilms has been described earlier (14, 15, 20, 21, 28, 30).
Surprisingly, many of the 16S rRNA clones analyzed in this work were highly similar to microbes closely related to rhizobial species. Microorganisms from the gram-negative genera Rhizobium, Sinorhizobium, Bradyrhizobium, Mesorhizobium, and Azorhizobium, collectively termed rhizobia, are well known for their capacity to establish N2-fixing symbioses with legume plants (6). The observation here that rhizobial species or closely related microbes are possibly present within the biofilm community is a novel finding and might suggest an ecological role for these microbes in these nutrient-deprived environments.
In the second approach applied in this work, we analyzed and evaluated the genome information of 2,496 high-quality snapshot sequences (Table 2), which encode approximately 2.0 Mb of raw DNA sequence information. We speculate that the overall biofilm metagenome of the studied drinking water biofilm has a size of at least 324 to 648 Mb. This is based on the finding that the biofilm communities of the analyzed samples consisted of more than 81 different microbial species (Fig. 2), each with a genome size of 4 to 8 Mb. Thus, the amount of genomic sequences generated corresponds to approximately 0.3 to 0.6% of the genomic information stored in the samples analyzed.
Although the available sequences do not allow a complete analysis of the physiological and metabolic functions within this bacterial community, the sequences give a first insight into the biofilm genome structure and its metabolic potential. The genomic information suggests that the biofilm community is able to metabolize and catabolize a wide range of complex nutrients. Possible carbon sources available to the biofilm bacteria might be derived from the additives within the rubber coating, namely fatty acids, solubilizers, paraffin oils, and other compounds. However, additional experiments are necessary to correlate the occurrence and frequency of the catabolic genes identified through the snapshot sequencing with the in vivo catabolism of such compounds.
Our third strategy focused on the DNA analysis of large cosmid clones. The information on the DNA sequence has led to the identification of 94 ORFs (Fig. 4). The data obtained by whole cosmid sequencing supported the concept that our model microbial community is constructed of novel uncultured microbes closely related to Proteobacteria, and these findings support the data obtained through the phylogenetic analysis (Fig. 2A to D) and the snapshot sequencing analysis (Fig. 3). Although the observed similarities were surprisingly high for several of the identified genes, we have no evidence indicating from which species the sequenced cosmids were derived.
It is further noteworthy that the whole-cosmid sequencing as well as the snapshot genome sequencing did not result in the identification of genes encoding potential virulence factors. Therefore, we conclude that the microbial community within the studied microbial niche has only negligible pathogenic potential. This speculation is further supported by the phylogenetic data (Fig. 2). Although the phylogenetic analysis indicated the presence of several potentially pathogenic microbes, the majority of clones were similar to nonpathogenic microbial species.
Lastly, the sequencing data have been used to set up a publicly accessible database. Together with this information, a Blast server has been set up to allow in silico gene mining in the accumulated DNA sequences. Thus, one of the strengths of this report is that all the data generated are available in a searchable database, giving insight into the fine structure of the metagenome studied and other features of this unique biofilm community.
Acknowledgments
This work was supported by the BMBF within the framework Genomforschung an Bakterien für die Analyze der Biodiversität und die Nutzung zur Entwicklung neuer Produktionsverfahren and the EU project GEMINI.
REFERENCES
- 1.Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beja, O., L. Aravind, E. V. Koonin, M. T. Suzuki, A. Hadd, L. P. Nguyen, S. B. Jovanovich, C. M. Gates, R. A. Feldman, J. L. Spudich, E. N. Spudich, and E. F. DeLong. 2000. Bacterial rhodopsin: evidence for a new type of phototrophy in the sea. Science 289:1902-1906. [DOI] [PubMed] [Google Scholar]
- 3.Beja, O., E. V. Koonin, L. Aravind, L. T. Taylor, H. Seitz, J. L. Stein, D. C. Bensen, R. A. Feldman, R. V. Swanson, and E. F. DeLong. 2002. Comparative genomic analysis of archaeal genotypic variants in a single population and in two different oceanic provinces. Appl. Environ. Microbiol. 68:335-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beja, O., E. N. Spudich, J. L. Spudich, M. Leclerc, and E. F. DeLong. 2001. Proteorhodopsin phototrophy in the ocean. Nature 411:786-789. [DOI] [PubMed] [Google Scholar]
- 5.Beja, O., M. T. Suzuki, E. V. Koonin, L. Aravind, A. Hadd, L. P. Nguyen, R. Villacorta, M. Amjadi, C. Garrigues, S. B. Jovanovich, R. A. Feldman, and E. F. DeLong. 2000. Construction and analysis of bacterial artificial chromosome libraries from a marine microbial assemblage. Environ. Microbiol. 2:516-529. [DOI] [PubMed] [Google Scholar]
- 6.Broughton, W. J., and X. Perret. 1999. Genealogy of legume-Rhizobium symbioses. Curr. Opin. Plant Biol. 2:305-311. [DOI] [PubMed] [Google Scholar]
- 7.Courtois, S., C. M. Cappellano, M. Ball, F. X. Francou, P. Normand, G. Helynck, A. Martinez, S. J. Kolvek, J. Hopke, M. S. Osburne, P. R. August, R. Nalin, M. Guerineau, P. Jeannin, P. Simonet, and J. L. Pernodet. 2003. Recombinant environmental libraries provide access to microbial diversity for drug discovery from natural products. Appl. Environ. Microbiol. 69:49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Entcheva, P., W. Liebl, A. Johann, T. Hartsch, and W. R. Streit. 2001. Direct cloning from enrichment cultures, a reliable strategy for isolation of complete operons and genes from microbial consortia. Appl. Environ. Microbiol. 67:89-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta, R., Q. K. Beg, and P. Lorenz. 2002. Bacterial alkaline proteases: molecular approaches and industrial applications. Appl. Microbiol. Biotechnol. 59:15-32. [DOI] [PubMed] [Google Scholar]
- 10.Handelsman, J., M. R. Rondon, S. F. Brady, J. Clardy, and R. M. Goodman. 1998. Molecular biological access to the chemistry of unknown soil microbes: a new frontier for natural products. Chem. Biol. 5:R245-R249. [DOI] [PubMed] [Google Scholar]
- 11.Healy, F. G., R. M. Ray, H. C. Aldrich, A. C. Wilkie, L. O. Ingram, and K. T. Shanmugam. 1995. Direct isolation of functional genes encoding cellulases from the microbial consortia in a thermophilic, anaerobic digester maintained on lignocellulose. Appl. Microbiol. Biotechnol. 43:667-674. [DOI] [PubMed] [Google Scholar]
- 12.Henne, A., R. Daniel, R. A. Schmitz, and G. Gottschalk. 1999. Construction of environmental DNA libraries in Escherichia coli and screening for the presence of genes conferring utilization of 4-hydroxybutyrate. Appl. Environ. Microbiol. 65:3901-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henne, A., R. A. Schmitz, M. Bomeke, G. Gottschalk, and R. Daniel. 2000. Screening of environmental DNA libraries for the presence of genes conferring lipolytic activity on Escherichia coli. Appl. Environ. Microbiol. 66:3113-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalmbach, S., W. Manz, and U. Szewzyk. 1997. Isolation of new bacterial species from drinking water biofilms and proof of their in situ dominance with highly specific 16S rRNA probes. Appl Environ Microbiol. 63:4164-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalmbach, S., W. Manz, J. Wecke, and U. Szewzyk. 1999. Aquabacterium gen. nov., with description of Aquabacterium citratiphilum sp. nov., Aquabacterium parvum sp. nov. and Aquabacterium commune sp. nov., three in situ dominant bacterial species from the Berlin drinking water system. Int. J. Syst. Bacteriol. 49:769-777. [DOI] [PubMed] [Google Scholar]
- 16.Knietsch, A., T. Waschkowitz, S. Bowien, A. Henne, and R. Daniel. 2003. Construction and screening of metagenomic libraries derived from enrichment cultures: Generation of a gene bank for genes conferring alcohol oxidoreductase activity on Escherichia coli. Appl. Environ. Microbiol. 69:1408-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacNeil, I. A., C. L. Tiong, C. Minor, P. R. August, T. H. Grossman, K. A. Loiacono, B. A. Lynch, T. Phillips, S. Narula, R. Sundaramoorthi, A. Tyler, T. Aldredge, H. Long, M. Gilman, D. Holt, and M. S. Osburne. 2001. Expression and isolation of antimicrobial small molecules from soil DNA libraries. J. Mol. Microbiol. Biotechnol. 3:301-308. [PubMed] [Google Scholar]
- 18.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, R. J. Farris, G. M. Garrity, G. J. Olsen, T. M. Schmidt, and J. M. Tiedje. 2001. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 29:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochsenreiter, T., F. Pfeifer, and C. Schleper. 2002. Diversity of Archaea in hypersaline environments characterized by molecular-phylogenetic and cultivation studies. Extremophiles 6:267-274. [DOI] [PubMed] [Google Scholar]
- 20.Poindexter, J. S., K. P. Pujara, and J. T. Staley. 2000. In situ reproductive rate of freshwater Caulobacter spp. Appl. Environ. Microbiol. 66:4105-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribas, F., J. Perramon, A. Terradillos, J. Frias, and F. Lucena. 2000. The Pseudomonas group as an indicator of potential regrowth in water distribution systems. J. Appl. Microbiol. 88:704-710. [DOI] [PubMed] [Google Scholar]
- 22.Rondon, M. R., P. R. August, A. D. Bettermann, S. F. Brady, T. H. Grossman, M. R. Liles, K. A. Loiacono, B. A. Lynch, I. A. MacNeil, C. Minor, C. L. Tiong, M. Gilman, M. S. Osburne, J. Clardy, J. Handelsman, and R. M. Goodman. 2000. Cloning the soil metagenome: a strategy for accessing the genetic and functional diversity of uncultured microorganisms. Appl. Environ. Microbiol. 66:2541-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 24.Schleper, C., E. F. DeLong, C. M. Preston, R. A. Feldman, K. Y. Wu, and R. V. Swanson. 1998. Genomic analysis reveals chromosomal variation in natural populations of the uncultured psychrophilic archaeon Cenarchaeum symbiosum. J. Bacteriol. 180:5003-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schleper, C., R. V. Swanson, E. J. Mathur, and E. F. DeLong. 1997. Characterization of a DNA polymerase from the uncultivated psychrophilic archaeon Cenarchaeum symbiosum. J. Bacteriol. 179:7803-7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmalenberger, A., and C. C. Tebbe. 2003. Bacterial diversity in maize rhizospheres: conclusions on the use of genetic profiles based on PCR-amplified partial small subunit rRNA genes in ecological studies. Mol. Ecol. 12:251-262. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt, T. M., E. F. DeLong, and N. R. Pace. 1991. Analysis of a marine picoplankton community by 16S rRNA gene cloning and sequencing. J. Bacteriol. 173:4371-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz, T., S. Hoffmann, and U. Obst. 1998. Formation and bacterial composition of young, natural biofilms obtained from public bank-filtered drinking water systems. Water Res. 32:2787-2797. [Google Scholar]
- 29.Schwieger, F., and C. C. Tebbe. 1998. A new approach to utilize PCR-single-strand-conformation polymorphism for 16S rRNA gene-based microbial community analysis. Appl. Environ. Microbiol. 64:4870-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szewzyk, U., R. Szewzyk, W. Manz, and K. H. Schleifer. 2000. Microbiological safety of drinking water. Annu. Rev. Microbiol. 54:81-127. [DOI] [PubMed] [Google Scholar]
- 31.Torsvik, V., and L. Ovreas. 2002. Microbial diversity and function in soil: from genes to ecosystems. Curr. Opin. Microbiol. 5:240-245. [DOI] [PubMed] [Google Scholar]
- 32.Voget, S., C. Leggewie, A. Uesbeck, C. Raasch, K. E. Jaeger, and W. R. Streit. Prospecting for novel biocatalysts in a soil metagenome. Appl. Environ. Microbiol. 69:6236-6242. [DOI] [PMC free article] [PubMed]