Abstract
Many factors have been shown to influence bacterial transfer between surfaces, including surface type, bacterial species, moisture level, pressure, and friction, but the effect of inoculum size on bacterial transfer has not yet been established. Bacterial cross contamination rates during performance of common food service tasks were previously determined in our laboratory using nalidixic acid-resistant Enterobacter aerogenes. Eight different transfer rates were determined, each involving a minimum of 30 volunteers. The influence of source inoculum level on the percentage of bacteria transferred (percent transfer rates) and log10 CFU per recipient surface was determined using statistical analysis. The effect of inoculum size on transfer rate was highly statistically significant (P < 0.0001) for all transfer rate data combined (352 observations) and for each individual cross contamination rate, except for data on contamination via transfer from chicken to hand through a glove barrier (P = 0.1643). Where inoculum size on the source was greater, transfer rates were lower, and where inoculum size on the source was less, transfer rates were higher. The negative linear trend was more obvious for activities that had a larger range of inoculum sizes on the source surface. This phenomenon has serious implications for research seeking to determine bacterial cross contamination rates, since the different transfer efficiencies that were previously shown to be associated with certain activities may actually be the result of differing initial inoculum levels. The initial inoculum size on the source and the amount of bacteria transferred must both be considered to accurately determine bacterial transfer rates.
Microbial concentration plays an essential role in many microbial systems. It plays an essential role in regulating bioluminescence (8), antibiotic biosynthesis (1), virulence determination (20), catalase activity (6), and initiation of chromosomal replication (29). Bacillus megaterium spores germinate faster when present at higher concentrations (4). The inoculum size of Clostridium botulinum affects time to detection and the fraction of samples that show growth (30). A threshold inoculum size for Listeria monocytogenes to initiate growth at suboptimal conditions has been established (19, 23). Whether due to interaction between cells, statistical effects, or sensitivity of microbiological methods, initial inoculum levels can drastically affect experimental results.
Many factors that influence the transfer of bacteria from surface to surface have been identified. Type of bacteria (14, 24), source and destination surfaces (5, 10, 24), time postinoculation (26), and moisture level (10, 25) have all been shown to affect cross contamination rates. However, the effect of the initial inoculum level on transfer efficiency has not been established.
Research conducted in our laboratory to determine the effectiveness of gloves as a barrier to cross contamination identified inoculum size as a possible factor influencing the percent transfer rate (16). When inoculum size on hands was small, the percent transfer rate through gloves to lettuce was high, and when inoculum size on hands was large, the percent transfer rate was correspondingly low. This is in contrast to other cross contamination rates determined in our laboratory where inoculum size had no obvious effect (5). Through further analysis of data from both studies, we sought to determine whether a connection exists between inoculum size and the percent of bacteria transferred for other cross contamination rates and, if so, the nature of that connection.
MATERIALS AND METHODS
Preparation of cultures.
The methods used for preparation of cultures were as previously published (5, 16). These were based on methods originally proposed by Zhao et al. (31) and are briefly described below. A nonpathogenic, food-grade microorganism, Enterobacter aerogenes B199A (31), was used for all experiments. This E. aerogenes strain is resistant to nalidixic acid, which allows it to be enumerated in the presence of other microorganisms in food and resident bacteria on the hands of study participants. Raw, skinless, boneless, chicken breast meat and iceberg lettuce were obtained from a local supermarket. Control experiments showed that nalidixic acid-resistant E. aerogenes cells were not initially present on any of the test surfaces.
E. aerogenes cells were grown overnight (18 to 24 h) at 37°C with shaking (150 rpm) in tryptic soy broth (Difco, Detroit, Mich.) containing 50 μg of nalidixic acid/ml (Sigma Chemical Co., St. Louis, Mo.). Cells were harvested by centrifugation (Micro 7; Fisher Scientific, Pittsburgh, Pa.) at 5,000 × g for 3.5 min and washed three times in phosphate-buffered saline (PBS; 0.1 M, pH 7.2) (Fisher Scientific Co.). Cell pellets were resuspended in PBS and adjusted by a spectrophotometer (model UV160; Shimadzu Scientific Instruments, Columbia, Md.) to an A660 of approximately 0.5, corresponding to ∼108 CFU/ml. Appropriate 10-fold dilutions in PBS were made to determine the cell density of the inoculum and enumerate samples collected from various surfaces. One-tenth milliliter of the two lowest dilutions was then plated in duplicate on MacConkey agar (Difco) containing 50 μg of nalidixic acid/ml. Pour plating was done in duplicate by mixing 1 ml of a sample with 10 ml of warm agar for samples containing low levels of E. aerogenes. Agar plates were incubated at 37°C for 24 h prior to enumeration.
Study participants.
More than 60 Rutgers University students and staff participated in the study to produce at least 30 different data points for each transfer rate evaluated. Both hands of each participant were sampled so that handedness would not be a factor.
Biosafety and human subject assurance.
While Enterobacter species have been linked to disease outbreaks, the victims of such disease have been in a weakened or immunocompromised state. Outbreaks have been linked to contaminated IV fluid (15), infant formula (17), hemodialysis (13), and vaporizers and whirlpools (2).
Immunocompromised individuals and anyone with obvious cuts or abrasions on the hands were not allowed to participate in these studies. Each participant was informed about the general nature of the experimental procedures and signed a consent form prior to taking part in the experiments. University biosafety committee and human subject approvals were obtained prior to the initiation of this study. The Food and Drug Administration has suggested the use of E. aerogenes as a surrogate to study Salmonella and E. coli in food systems, and the strain used in this study was originally developed by the U.S. Department of Agriculture to ferment reducing sugars in egg whites prior to drying to prevent Maillard browning.
Volunteers' hands were also sampled at the end of the experiment, and less than 1 log CFU of bacteria was left on the hands (21). After the sampling process, volunteers were instructed to wash their hands before leaving the laboratory, which further reduced the amount of bacteria present. As part of our experimental protocol, samples were taken in various sites around the laboratory. Sink faucets, doorknobs, soap dispensers, a countertop, a pen the volunteers had used after the experiment, and the buttons on the elevator in the hall were sampled. In all cases, the amount of E. aerogenes was below the limit of detection.
Contamination of chicken and hands.
We inoculated 150-g portions of chicken with 1.0 ml of E. aerogenes suspension gradually, one drop at a time, over the entire surface of the breast. Samples were then held for 15 min at room temperature to facilitate attachment. Prior studies with this organism used a 30-min time period to facilitate attachment (31), but controls in our lab indicated no difference between the use of 15- and 30-min periods. The participant then cut the chicken into small cubes (approximately 1 by 1 by 1 cm) on a clean, sterile plastic cutting board (American Chef, Bentonville, Ark.), which transferred E. aerogenes from the chicken to the hands of the participant. One of the participant's hands was sampled using the glove juice method (21) after completion of this step. The fingers of a sterile surgical glove (Fisher Scientific Co.) were filled with PBS (20 ml), and the glove was then fitted onto the volunteer's hand. The hand was rubbed for 1 min by an investigator, and the sample was collected for enumeration. The participant handled three sterile spigots to simulate turning on a water faucet using the hand not sampled by the glove juice method. To standardize the level of hand contamination, the participant transferred the diced chicken from the cutting board to a tray back and forth three times prior to handling each of the three spigots.
To determine the number of E. aerogenes cells on the spigots, one of the three spigots was sampled by the alginate swab method, which was reported to be more sensitive than other sampling methods (3, 12, 18, 27). Briefly, an alginate swab (Fisher Scientific Co.) was moistened in 0.8% saline and swabbed over the entire spigot surface (∼25 cm2). Two swabs were used to sample each spigot, and the swabs were dissolved in 4 ml of sodium citrate (1%) for 5 min while being intermittently agitated on a vortex. The sample was then diluted in PBS, and E. aerogenes cells were enumerated.
The other two spigots were used to recontaminate the hands of each participant in a manner simulating normal use. The rate of cross contamination between metal spigot surfaces and hands (see below) was evaluated under two conditions: (i) when the participant's hands had some level of E. aerogenes contamination, and (ii) when the participant had clean hands (i.e., E. aerogenes negative). Under the first condition, a participant handled the spigots that they contaminated in the previous step of the experiment; under the second condition, a participant started the experiment by handling spigots contaminated by a previous participant.
Contamination of lettuce.
Volunteers diced a 25-g portion of lettuce on a fresh cutting board and then placed the lettuce in a filter bag. After the lettuce was cut, both hands were sampled using the glove juice technique. Lettuce was homogenized in a stomacher (Cooke Laboratory Products, Alexandria, Va.) at 230 rpm for 2 min with 225 ml of tryptic soy broth. The solid lettuce pieces were discarded, and samples were then centrifuged at 8 × g for 20 min. Supernatant was decanted, and cells were enumerated by pour plating in MacConkey agar containing 50 μg of nalidixic acid/ml.
Effectiveness of a glove barrier.
In experiments to determine the effectiveness of a glove barrier, new polyethylene gloves (Fisher Scientific) were donned without the technician's assistance to better simulate a real-world situation. Volunteers diced the chicken into 1-cm cubes on a sterile plastic cutting board (American Chef) with either bare or gloved hands and then transferred chicken pieces from the cutting board to a container three times. Fresh gloves were donned, and lettuce was sliced. Both hands were sampled using the glove juice technique after the lettuce was cut. All other methods were as described above.
Data analysis.
The total numbers of CFU per source were determined for chicken (150 g), hands, and lettuce (25 g). The number of E. aerogenes on hands before cutting the lettuce was calculated by adding the number of cells isolated on both hands (after lettuce cutting) to the number of cells isolated from the lettuce. The limit of detection for the hand was 100 CFU/hand. The detection limit for lettuce was dependent on the amount of concentrate remaining after centrifugation but was on average around 30 CFU/sample. A comparison was made between data sets where “none detected” values were not included in data analysis and those where “none detected” values were replaced with the detection limit, and no appreciable differences in the distributions were noted. Transfer rates were calculated using the following equation: (CFU on destination/CFU on source) × 100 = transfer rate (percent).
Data were compiled and logarithmically transformed in Excel (Microsoft Corporation, Redmond, Wash.) spreadsheets. Data from the two published studies were combined where appropriate. For example, the results for transfer from bare hands to lettuce included data from bare hands that were contaminated and washed as well as data from hands contaminated through gloves. The effect of initial inoculum level on source was examined for both log10 percent transfer and log10 CFU per surface (amount) transferred. Regression analysis, analysis of variance (ANOVA), and Duncan's multiple-range tests were performed using SAS software (SAS Institute, Cary, N.C.).
RESULTS
The inoculum on the source surface ranged from 1.90 (on hands) to 9.37 (on chicken) log10 CFU/object (Table 1). The range of inoculum sizes was smallest for transfer from chicken to cutting board (∼0.5 log10 CFU/chicken breast), which was artificially inoculated and had fewer than 10 replicates, and was largest for transfer from gloved hands to lettuce (∼5 log10 CFU/hand). The smallest range of log10 percent transfer was also observed for transfer from chicken to cutting board (∼1 log10 percent CFU transferred), and the largest was observed for transfer from hand to lettuce through a glove (∼5.5 log10 percent CFU transferred). Mean inoculum sizes were similar for transfer from chicken to bare hands and from chicken to gloved hands, for transfer from cutting board to lettuce and from bare hand to spigot, and for transfer from bare hand to lettuce and from spigot to hand (Table 1). Percent transfer rates for transfer from bare hand to lettuce or from hand to lettuce through glove (P < 0.0001) and for hand or board to lettuce (P < 0.0001) were significantly different by ANOVA and Duncan's multiple-range test. However, transfer rates for bare hand to lettuce and for spigot to hand were not significantly different (P = 0.8153). A comparison of mean log10 percent transfer for all rates is shown in Table 1. The log10 percent transfer rates for chicken to cutting board, cutting board to lettuce, and chicken to bare hands were all similar, as were those for chicken to bare hand, bare hand to lettuce, and spigot to bare hand. The only transfer activity that produced a rate significantly different from those of all others was chicken to hand through a glove barrier.
TABLE 1.
Summary of transfer data for a variety of cross contamination tasks
| Transfer type | No. of observations | Range on source (log10 CFU/source)
|
Mean inoculum size (log10 CFU/ source)a | Log10 transfer (%)
|
Range on recipient surface (log10 CFU/ recipient)
|
Mean amt transferred (log10 CFU/ recipient)a | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Min | Max | Meana | Min | Max | ||||
| Chicken to cutting board | 7 | 6.04 | 6.45 | 6.18 | 0.48 | 1.49 | 1.05 A | 4.61 | 5.59 | 5.30 |
| Cutting board to lettuce | 32 | 4.61 | 5.59 | 5.33 B | −0.47 | 1.73 | 0.79 AB | 3.01 | 5.33 | 4.12 A |
| Chicken to bare hand | 66 | 8.10 | 9.37 | 8.37 A | −0.44 | 2.00 | 0.59 ABC | 5.94 | 8.38 | 6.97 |
| Bare hand to lettuce | 62 | 1.90 | 6.46 | 3.97 C | −2.54 | 2.00 | 0.21 BC | 0.00 | 3.87 | 2.19 B |
| Spigot to bare hand | 32 | 2.78 | 5.74 | 3.95 C | −1.70 | 2.00 | 0.16 C | <1b | 4.10 | 2.12 B |
| Bare hand to spigot | 30 | 5.94 | 8.38 | 7.16 B | −2.95 | 1.09 | −1.08 D | 2.43 | 5.74 | 4.08 A |
| Gloved hand to lettuce | 61 | 2.33 | 7.27 | 5.08 | −3.98 | 1.53 | −1.26 D | 0.84 | 5.45 | 1.81 B |
| Chicken to gloved hand | 61 | 7.67 | 8.93 | 8.34 A | −4.40 | −0.62 | −2.94 | 2.32 | 5.45 | 3.41 |
Values in the same column that are followed by the same uppercase letter are not statistically significantly different.
Limit of detection was used.
A comparison of the mean amounts of E. aerogenes transferred by each cross contamination task is also presented in Table 1. The range of log10 CFU transferred to recipient surfaces spanned fewer orders of magnitude than did that of log10 percent transfer. Again, transfer from chicken to cutting board had the smallest range of values (less than 1 log10 CFU/chicken breast) and transfer from hand to lettuce through a glove had the greatest range (greater than 4 log10 CFU/hand). The amounts of E. aerogenes transferred from cutting board to lettuce and from bare hand to spigot were similar, despite significantly different log10 percent transfer rates. Bare hand to lettuce, spigot to bare hand, and hand to lettuce through a glove all resulted in similar amounts of bacteria being transferred to the recipient surface (means of 2.19, 2.12, and 1.81 log10 CFU transferred, respectively).
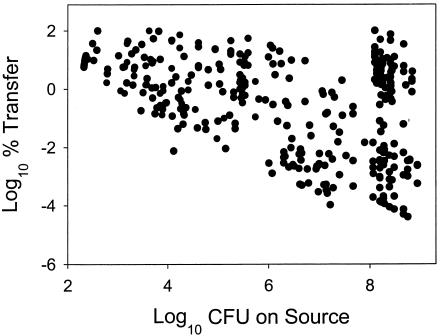
All data (352 observations) for all transfer activities are shown in Fig. 1. There was a strong negative linear trend between log10 inoculum on source and log10 percent transfer rate for almost all data. When the population of bacteria on the source surface was high, the log10 percent transfer was relatively low. Where the population on the source surface was lower, the log10 percent transfer tended to be higher. The effect of inoculum size on these data was highly significant (P < 0.0001). ANOVA analysis also showed transfer type to be significant (P < 0.0001), but it was impossible to completely separate the effect of inoculum size from the effect of transfer type because different transfer activities involved different inoculum levels on the source surface. The effects of inoculum size and transfer type on amount of E. aerogenes transferred were also significant (P < 0.0001), although it was again impossible to separate the effect of inoculum size from the effect of transfer type.
FIG. 1.
Log10 percent transfer versus log10 CFU in inoculum on source for transfer of E. aerogenes between various surfaces (352 observations). Chicken was artificially inoculated with 108 E. aerogenes cells, and contamination was monitored through subsequent food service tasks. Cross contamination activities included transfers from chicken to bare hand, bare hand to lettuce, hand to spigot, spigot to hand, cutting board to lettuce, chicken to cutting board, chicken to hands through a glove, and hands to lettuce through a glove.
The effect of inoculum size on both log10 percent transfer and log10 CFU transferred was determined for each individual rate. Inoculum size had a significant effect on log10 percent transfer for all cross contamination activities, except chicken to hand through a glove (P = 0.1643). However, inoculum size had no effect on log10 CFU transferred except for transfers from bare hand to lettuce (P < 0.0001), cutting board to lettuce (P < 0.0001), hand to lettuce through a glove (P = 0.0021), and spigot to hand (P = 0.0077).
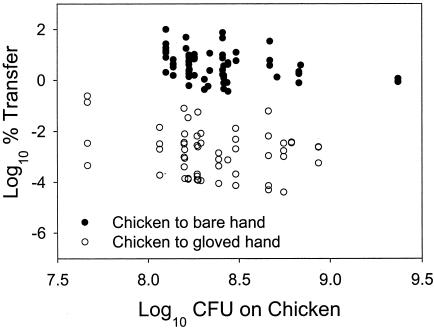
A comparison of percent transfer rates for chicken to bare hands and chicken to hands through a glove is given in Fig. 2. The effect of inoculum size on transfer from chicken to bare hand was significant (P = 0.0006), but it was not significant for transfer from chicken to hand through a glove (P = 0.1643). The range of inoculum sizes on the source was very small in both cases because the amount of inoculum deposited on chicken was controlled. A slight linear trend is visible nonetheless. The dynamics of this transfer activity are also likely to be different from others because it involved an artificially inoculated surface, one which had a high moisture content. It is clear from Fig. 2 that percent transfer of bacteria from chicken to hands was greatly reduced with the use of a glove barrier. This effect is independent of any inoculum size effect, since the chicken had similar initial concentration levels in both experiments.
FIG. 2.
Log10 CFU of E. aerogenes on source surface and corresponding log10 percent transfer rates for transfer from chicken to bare hands and from chicken to hands through a glove barrier.
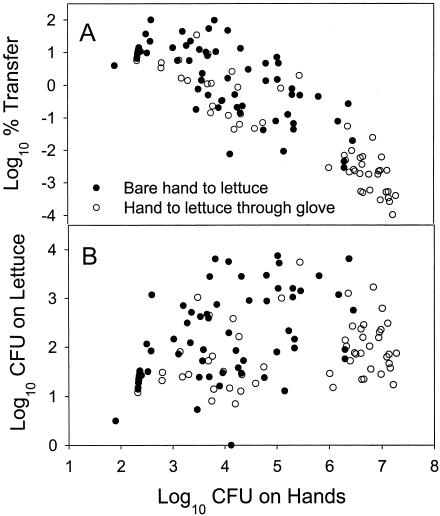
Data for transfer of E. aerogenes from bare hands to lettuce and from hands to lettuce through a glove are presented in Fig. 3. Visually, the two sets of data appear essentially to be part of the same population, despite the use of a glove barrier in one case. The effectiveness of the glove barrier observed for the chicken-to-hand transfer (Fig. 2) is not as obvious for the hand-to-lettuce bacterial transfer (Fig. 3). Figure 3A shows data presented as log10 percent transfer, and Fig. 3B shows the same data presented as log10 CFU transferred. There is a pronounced negative linear effect between log10 inoculum on source (hands, in this case) and log10 (percent) transfer rate (Fig. 3A). Even though the effect was not as obvious for the total number of cells transferred (Fig. 3B), the inoculum size still had a significant effect (P = 0.0012) on the amount of E. aerogenes transferred to lettuce. ANOVA analysis revealed that the two types of transfer were significantly different for both percent transferred (P < 0.0001) and amount transferred (P = 0.0094).
FIG. 3.
The effect of inoculum size on log10 percent transfer (A) and total amount of E. aerogenes transferred (B) from hands to lettuce through a glove barrier or without a glove barrier.
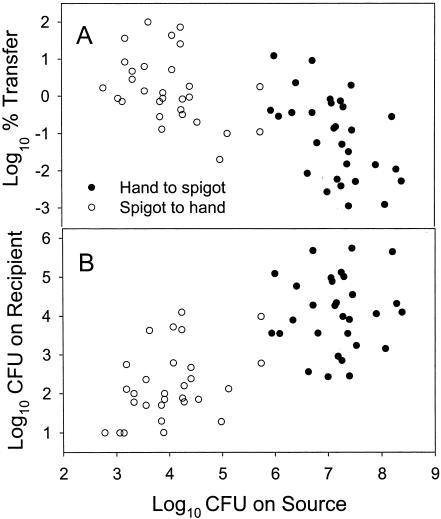
Data for transfer of E. aerogenes from a hand to a spigot and from a spigot to a hand are shown in Fig. 4. Figure 4A shows inoculum level on source surface and log10 percent transferred to the recipient surface, and Fig. 4B shows the same data presented as amount (log10 CFU) transferred to the recipient surface. A definite inoculum size effect on log10 percent transfer was observed when the transfer activities were considered separately (P = 0.0486 for spigot to hand and P = 0.0027 for hand to spigot). When the data were combined, the linear trend caused by inoculum size was much more prominent (P = 0.0005). As inoculum size decreased, the amount of bacteria transferred decreased (Fig. 4B). The effect of inoculum size on amount transferred was only statistically significant for the spigot-to-hand data set (P = 0.0077). In this case, the two experimental groups (hands to spigots and spigots to hands) had different starting populations, so while inoculum sizes on source surfaces may be responsible for some of the differences in bacterial transfer from hand to spigot and from spigot to hand, the effect is not as clear-cut as is shown in the other figures.
FIG. 4.
The effect of inoculum size on log10 percent transfer (A) and total amount of E. aerogenes transferred (B) from hand to spigot and from spigot to hand.
DISCUSSION
There was a clear, statistically significant connection between inoculum size and percent transfer for all cross contamination activities except that from chicken to hand through a glove; the trend was not as apparent when inoculum size and amount transferred were compared. However, when the range of inoculum levels on the source surface was large (e.g., from hand to lettuce with or without a glove and from spigot to hand), the effect was observed for both percent transferred and amount transferred. As inoculum size increases, the number of bacteria transferred remains approximately constant. When this is the case, then the apparent percent transfer rate will decrease.
Most research examining bacterial transfer between surfaces has utilized a small range of inoculum sizes (26) or provided only an approximation of inoculum size on source surface (10, 11, 25). Some of the earliest research on cross contamination rates between hands and food used transfer of Salmonella serotype Anatum from fingertips to corned beef as a model system. Although there was a wide range of inoculum sizes on the fingertips, final contamination levels on the corned beef and percent transfer were not presented (22). Past research has in some cases revealed a similar inoculum size phenomenon, although researchers either did not acknowledge it or did not attempt to explain it. Mackintosh and Hoffman (14) compared the transfers of Staphylococcus saprophyticus, Escherichia coli, Pseudomonas aeruginosa, Klebsiella aerogenes, Streptococcus pyogenes, and Serratia marcescens from a donor fabric to hands. The organisms with the lowest inoculum (S. saprophyticus at 4.5 × 105 CFU per cm2) had the highest transfer rate per cm2 (1.67%). The organism with the highest inoculum (S. pyogenes at 3.9 × 107 CFU per cm2) had the lowest transfer rate per square centimeter (0.007%). The same phenomenon was observed for fabric-to-fabric transfer.
Rusin et al. (24) demonstrated greater transfer efficiency for gram-positive bacteria, gram-negative bacteria, and phage from phone receivers and faucets to hands than for other surfaces. However, for all three organism types, the inoculum size on the phone receiver or the faucet was significantly less than on all other surfaces (∼3 to 5 log10 CFU less). Our results suggest that the differences observed by Rusin et al. may have been due to varying inoculum levels on the source surface and not the nature of the transfer task itself. Our findings emphasize the importance of careful data analysis; while presentation of the log percent transferred is important, some consideration of the total amount of bacteria transferred is also crucial.
The exact details of the mechanisms responsible for these phenomena are still unknown and are complicated by the usual host of factors known to be important in studying cross contamination, including surface type, bacterial species, moisture level, pressure, and friction. One possible cause for reduced transfer at high inoculum level could be improved attachment to the donor surface when microbial concentrations are high. Higher inoculum levels of E. coli O157:H7 exhibited better attachment to lettuce leaves, for example (28). Such an effect is clearly not universal, however, because attachments of Salmonella enterica serovar Typhimurium and L. monocytogenes to glass (7) and E. coli O157:H7 (9) to beef tissue were found to increase proportionally to inoculum size.
Inoculum size influences transfer between surfaces, but this effect has largely gone unnoticed in the published literature. We suspect that this effect has not been detected for two key reasons: (i) transfer rates between surfaces may be quite variable and span several orders of magnitude (5), and (ii) most studies have tended to examine only a single inoculum size. Indeed, if our original experimental design (5) had not followed initial contamination through a series of subsequent transfers with many replicates for each rate, this inoculum size effect may not have been discovered. Experiments to determine cross contamination rates must consider inoculum size to be a significant factor that can affect transfer rates and the amount of bacteria transferred. Experiments must be designed carefully to account for the potential effect of inoculum size, and published data should include analysis of both the amount of bacteria transferred and the percent of bacteria transferred.
Acknowledgments
We acknowledge Yuhuan Chen for her careful planning of the original experimental design and meticulous data collection.
REFERENCES
- 1.Bainton, N. J., B. W. Bycroft, S. R. Chhabra, P. Stead, L. Gledhill, P. J. Hill, C. E. D. Rees, M. K. Winson, G. P. C. Salmond, G. S. A. B. Stewart, and P. Williams. 1992. A general role for the lux autoinducer in bacterial cell signalling: control of antibiotic synthesis in Erwinia. Gene 116:87-91. [DOI] [PubMed] [Google Scholar]
- 2.Barwick, R. S., D. A. Levy, G. F. Craun, M. J. Beach, and R. L. Calderon. 2000. Surveillance for waterborne-disease outbreaks—United States, 1997-1998. Morb. Mortal. Wkly. Rep. 49:1-21. [PubMed] [Google Scholar]
- 3.Cain, R. M., and H. Steele. 1953. The use of calcium alginate soluble wool for the examination of cleansed eating utensils. Can. J. Pub. Health 44:464-467. [PubMed] [Google Scholar]
- 4.Caipo, M. L., S. Duffy, L. Zhao, and D. W. Schaffner. 2002. Bacillus megaterium spore germination is influenced by inoculum size. J. Appl. Microbiol. 92:879-884. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Y., K. M. Jackson, F. P. Chea, and D. W. Schaffner. 2001. Quantification and variability analysis of bacterial cross contamination rates in common foodservice tasks. J. Food Prot. 64:72-80. [DOI] [PubMed] [Google Scholar]
- 6.Crockford, A. J., G. A. Davis, and H. D. Williams. 1995. Evidence for cell-density-dependent regulation of catalase activity in Rhizobium leguminosarum bv. phaseoli. Microbiology 141:843-851. [Google Scholar]
- 7.Dickson, J. S., and E. K. Daniels. 1991. Attachment of Salmonella typhimurium and Listeria monocytogenes to glass as affected by surface film thickness, cell density, and bacterial motility. J. Ind. Microbiol. 8:281-284. [Google Scholar]
- 8.Engebrecht, J., K. Nealson, and M. Silverman. 1983. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell 32:773-781. [DOI] [PubMed] [Google Scholar]
- 9.Fratamico, P. M., F. J. Schultz, R. C. Benedict, R. L. Buchanan, and P. H. Cooke. 1996. Factors influencing attachment of Escherichia coli O157:H7 to beef tissues and removal using selected sanitizing rinses. J. Food Prot. 59:453-459. [DOI] [PubMed] [Google Scholar]
- 10.Gill, C. O., and T. Jones. 2002. Effects of wearing knitted or rubber gloves on the transfer of Escherichia coli between hands and meat. J. Food Prot. 65:1045-1048. [DOI] [PubMed] [Google Scholar]
- 11.Hilton, A. C., and E. Austin. 2000. The kitchen dishcloth as a source of and vehicle for foodborne pathogens in a domestic setting. Int. J. Environ. Health Res. 10:257-261. [Google Scholar]
- 12.Hoppe, J. E., U. Theurer-Mainka, and M. Stern. 1995. Comparison of three methods for culturing throat swabs from cystic fibrosis patients. J. Clin. Microbiol. 33:1896-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jochimsen, E. M., C. Frenette, M. Delorme, M. Arduino, S. Aguero, L. Carson, J. Ismail, S. Lapierre, E. Czyziw, J. I. Tokars, and W. R. Jarvis. 1998. A cluster of bloodstream infections and pyrogenic reactions among hemodialysis patients traced to dialysis machine waste-handling option units. Am. J. Nephrol. 18:485-489. [DOI] [PubMed] [Google Scholar]
- 14.Mackintosh, C. A., and P. N. Hoffman. 1984. An extended model for transfer of micro-organisms via the hands: differences between organisms and the effect of alcohol disinfection. J. Hyg. Camb. 92:345-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maki, D. G., F. S. Rhame, D. C. Mackel, and J. V. Bennett. 1976. Nationwide epidemic of septicemia caused by contaminated intravenous products. I. Epidemiologic and clinical features. Am. J. Med. 60:471-485. [DOI] [PubMed] [Google Scholar]
- 16.Montville, R., Y. Chen, and D. W. Schaffner. 2001. Glove barriers to bacterial cross-contamination between hands to food. J. Food Prot. 64:845-849. [DOI] [PubMed] [Google Scholar]
- 17.Nazarowec-White, M., and J. M. Farber. 1997. Enterobacter sakazakii: a review. Int. J. Food Microbiol. 34:103-113. [DOI] [PubMed] [Google Scholar]
- 18.Niskanen, A., and M. S. Pohja. 1977. Comparative studies on the sampling and investigation of microbial contamination of surfaces by the contact and swab methods. J. Appl. Bacteriol. 42:53-63. [DOI] [PubMed] [Google Scholar]
- 19.Pascual, C., T. P. Robinson, M. J. Ocio, O. O. Aboaba, and B. M. Mackey. 2001. The effect of inoculum size and sublethal injury on the ability of Listeria monocytogenes to initiate growth under suboptimal conditions. Lett. Appl. Microbiol. 33:357-361. [DOI] [PubMed] [Google Scholar]
- 20.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260:1127-1130. [DOI] [PubMed] [Google Scholar]
- 21.Paulson, D. S. 1993. Evaluation of three microorganism recovery procedures used to determine handwash efficacy. Dairy Food Environ. Sanit. 13:520-523. [Google Scholar]
- 22.Pether, J. V. S., and R. J. Gilbert. 1971. The survival of salmonellas on finger-tips and transfer of the organisms to food. J. Hyg. 69:673-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson, T. P., O. O. Aboaba, A. Kaloti, M. J. Ocio, J. Baranyi, and B. M. Mackey. 2001. The effect of inoculum size on the lag phase of Listeria monocytogenes. Int. J. Food Microbiol. 70:163-173. [DOI] [PubMed] [Google Scholar]
- 24.Rusin, P., S. Maxwell, and C. Gerba. 2002. Comparative surface-to-hand and fingertip-to-mouth transfer efficiency of gram-positive bacteria, gram-negative bacteria, and phage. J. Appl. Microbiol. 3:585-592. [DOI] [PubMed] [Google Scholar]
- 25.Sattar, S. A., S. Springthorpe, S. Mani, M. Gallant, R. C. Nair, E. Scott, and J. Kain. 2001. Transfer of bacteria from fabrics to hands and other fabrics: development and application of a quantitative method using Staphylococcus aureus as a model. J. Appl. Microbiol. 90:962-970. [DOI] [PubMed] [Google Scholar]
- 26.Scott, E., and S. Bloomfield. 1990. The survival and transfer of microbial contamination via cloths, hand and utensils. J. Appl. Bacteriol. 68:271-277. [DOI] [PubMed] [Google Scholar]
- 27.Scott, E., S. F. Bloomfield, and C. G. Barlow. 1984. A comparison of contact plate and calcium alginate swab techniques for quantitative assessment of bacteriological contamination of environmental surfaces. J. Appl. Bacteriol. 56:317-320. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi, K., and J. F. Frank. 2000. Penetration of Escherichia coli O157:H7 into lettuce tissues as affected by inoculum size and temperature and the effect of chlorine treatment on cell viability. J. Food Prot. 63:434-440. [DOI] [PubMed] [Google Scholar]
- 29.Withers, H. L., and K. Nordstrom. 1998. Quorum-sensing acts at initiation of chromosomal replication in Escherichia coli. Proc. Natl. Acad. Sci. USA 95:15694-15699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao, L., T. J. Montville, and D. W. Schaffner. 2000. Inoculum size of Clostridium botulinum 56A spores influence time-to-detection and percent growth-positive samples. J. Food Sci. 65:1369-1375. [Google Scholar]
- 31.Zhao, P., T. Zhao, M. P. Doyle, J. R. Rubino, and J. Meng. 1998. Development of a model for evaluation of microbial cross-contamination in the kitchen. J. Food Prot. 61:960-963. [DOI] [PubMed] [Google Scholar]