Abstract
High frequency production of zebrafish germline chimeras was achieved by transplanting ovarian germ cells into sterile Danio hybrid recipients. Ovarian germ cells were obtained from 3-mo-old adult Tg(vasa:DsRed2-vasa);Tg(bactin:EGFP) double transgenic zebrafish by discontinuous Percoll gradient centrifugation. An average of 755 ± 108 DsRed-positive germ cells was recovered from each female. For transplantations, a total of approximately 620 ± 242 EGFP-positive cells of which 12 ± 4.7 were DsRed-positive germ cells were introduced into the abdominal cavity under the swim bladder of 2-wk-old sterile hybrid larvae. Six weeks after transplantation, a total of 10 recipients, obtained from 2 different transplantations, were examined, and 2 individuals (20%) were identified that possessed a large number of DsRed- and EGFP-positive cells in the gonadal region. The transplanted ovarian germ cells successfully colonized the gonads and differentiated into sperm in the male hybrid recipients. Of 67 adult recipients, 12 (18%) male chimeric fish reproduced and generated normal offspring when paired with wild-type zebrafish females. The fertilization efficiency ranged from 23% to 56%. Although the fertile male chimeras were generated by transplantation of ovarian germ cells, the F1 generation produced by the male chimeras contained both male and female progeny, indicating that male sex determination in zebrafish is not controlled by sex chromosome heterogamy. Our findings indicate that a population of ovarian germ cells that are present in the ovary of adult zebrafish can function as germline stem cells, able to proliferate and differentiate into testicular germ cells and functional sperm in male recipients. The high frequency of germline chimera formation achieved with the ovarian germ cells and the convenience of identifying the chimeras in the sterile host background should make this transplantation system useful for performing genetic manipulations in zebrafish.
Keywords: germline chimera, germline stem cells, ovary, transplantation, zebrafish
High frequency production of zebrafish germline chimeras was achieved by transplanting adult ovarian germ cells into sterile Danio hybrid recipients.
INTRODUCTION
Gonadal germline stem cells are derived from primordial germ cells (PGCs) and serve as the progenitors of functional gametes. In males of most species, the presence of spermatogonial stem cells in the testes makes the recurrent production of sperm possible throughout the reproductive life of the animal [1]. In contrast, the mammalian female possesses a finite number of mature oocytes that is determined before birth [2], implying the absence of a stem cell-based self-renewal mechanism in the mammalian ovary. Recently, this dogma has been reexamined as a result of in vivo [3] and in vitro [4] studies that have provided evidence for stem cell-like regenerative activity in the mammalian ovary. Using quantitative methods to analyze oocyte degeneration and clearance, Johnson et al. [3] have shown that ovaries of both juvenile and adult mice retain mitotically active germ cells. Also, cultures of germline stem cells have been established from ovaries of neonatal and adult mice, and the cell lines have been used to achieve germline transmission of a GFP transgene [4].
In nonmammalian vertebrates, the existence of ovarian germline stem cells is supported from several lines of evidence [5–7]. Histological studies have revealed that both mitotic germ cells, or oogonia, and meiotic oocytes exist in adult ovaries of many nonmammalian vertebrate species including fish [6]. The oogonia present in these species most likely serve as progenitor cells and perform a stem cell-like function ensuring the continued production of oocytes throughout the animals' reproductive lifetime. Genetic evidence for the existence of ovarian stem cells was provided by studies of a zebrafish mutant that carries an inactive nanos gene [7]. Although female fish that were homozygous for the nanos mutation initially produced mature oocytes, their ovaries lacked the presence of early-stage oogonia and as a result egg production ceased after a few months. The gradual loss of egg production and the absence of oogonia and early-stage oocytes in the mutant fish indicates that nanos is required to support a proliferating population of ovarian germline stem cells that maintain oocyte regeneration in zebrafish [7]. Further evidence for the presence of germline stem cells in fish was provided by recent studies with trout [8] and medaka [9]. Ovarian germ cells isolated from 6- to 9-mo-old transgenic trout that exhibited germ cell-specific expression of EGFP were transplanted into sexually undifferentiated embryos where the transplanted cells migrated into the developing gonads and colonized the tissue to produce functional gametes in the adult chimeras [8]. Nakamura et al. [9] used transgenic methods and clonal analysis to identify germline stem cells in the ovaries of adult medaka fish. Heat shock-induced loxP-mediated recombination was used to label oogonial stem cells with EGFP (nos2-EGFP) and demonstrated that the mitotic oogonial stem cells were able to continually give rise to germ cells that developed into fertile eggs [9].
Although the zebrafish is a popular model for studies in a wide variety of research areas [10–15], one deficiency of this system is the absence of a stem cell-mediated gene targeting approach for studies of gene function. The ability to generate germline chimeras at a high frequency would be a significant step towards addressing this problem. In this study, we describe the efficient production of zebrafish germline chimeras by the transplantation of cells isolated from adult ovaries into genetically sterile recipient larvae. The transplanted ovarian cells were able to colonize the recipient gonad and produce functional sperm in the male hosts. In addition to providing evidence for the existence of ovarian germline stem cells in zebrafish, the results of this study describe a cell transplantation method that can be used to efficiently produce germline chimeras and increase the utility of the zebrafish model for studies of gene function.
MATERIALS AND METHODS
Ethics
All of the experimental protocols and procedures described in this study were approved by the Purdue University Animal Care and Use Committee and adhere to the National Research Council's Guide for Care and Use of Laboratory Animals.
Fish
Zebrafish were maintained and staged as previously described [16]. Pearl danio (Danio albolineatus) were maintained in the same conditions as zebrafish. The sterile Danio hybrids used in this study were generated by in vitro fertilization using sperm from pearl danio males to fertilize zebrafish eggs according published protocols [16].
Histology
Zebrafish and hybrids were euthanized in 0.016% tricaine (ethyl-3-aminobenzoate methanesulfonic acid) solution in water (Sigma-Aldrich), and gonads were removed and fixed with either 4% paraformaldehyde in phosphate-buffered saline (PBS; 155.17 mM NaCl, 2.97 mM Na2HPO4, 1.06 mM KH2PO4, pH 7.4) for 2 h or Bouin fixative overnight at 4°C. After two rinses in PBS, the paraformaldehyde fixed gonads were immersed in sucrose solution (30% sucrose in PBS). The following day the samples were frozen with optimal cutting temperature compound (Sakura Finetek) on dry ice, and serial cryostat sections (10 μm) were prepared using a Leica CM1850 cryostat (Leica). The Bouin-fixed gonads were processed through successive baths of ethanol (50%, 70%, 95%, and 100%) followed by two xylene baths and embedded in paraffin. The serial paraffin sections (5 μm) were prepared using an American Optical model 820 microtome (American Optical Corporation). Histological examination of the sections was performed using a Nikon Eclipse TE200 fluorescence microscope (Nikon) equipped with a RT Slider digital camera (Spot Imaging Solution), or the sections were stained with hematoxylin-eosin and examined by light microscopy.
Immunocytochemistry
A published procedure [17] was modified and used to visualize zebrafish Vasa within the gonadal tissues of Danio hybrids. For antigen retrieval, the deparaffinized and rehydrated gonadal sections were incubated with 10 mM sodium citrate (pH 6.0) for 25 min at 90°C to 95°C using a microwave. The sections were blocked for 30 min at 25°C with 3% goat serum and 2% blocking reagent (Roche) in PBS. Sections were then incubated overnight at 4°C with either a 1:5000 dilution of rabbit antiserum against zebrafish Vasa [18] or normal rabbit serum as control. Excess antibody was removed by two 15-min washes in PBS. DyLight 549 AffiniPure Dnk anti-rabbit immunoglobulin (1:1000 dilution; Jackson ImmunoResearch Lab, Inc.) and 300 nM 4′,6-diamidino-2-phenylindole dihydrochloride (Sigma-Aldrich) nuclear counterstain were used to visualize the expression of Vasa in the gonadal sections of Danio hybrids using a fluorescence microscope equipped with a digital camera.
Ovarian Germ Cell Isolation
Ovaries combined from four or five 3-mo-old adult Tg(vasa:DsRed2-vasa);Tg(bactin:EGFP) double transgenic zebrafish [19, 20] were minced with scissors and dissociated in 0.2% collagenase (Invitrogen) in PBS for 1 h at 28.5°C. The resulting ovarian cell suspension was filtered through a 60-μm mesh to remove large debris, washed with PBS, and the cell pellet was resuspended in 1 ml of PBS. The cell suspension (5.0–6.4 × 106 EGFP-positive cells) containing 4.1–5.6 × 103 DsRed-positive cells (average number obtained in three preparations using 4–5 females/preparation) was loaded onto a discontinuous Percoll (GE Healthcare) gradient (20%, 25%, 30%, 35%, 40%, 50%, and 60% in PBS) and centrifuged at 800 × g for 30 min according to published protocols [21, 22]. Individual cell fractions were removed from the gradient, transferred to a conical test tube, and washed two times with PBS. The cells were resuspended in 30 μl of PBS, and the number of DsRed-positive germ cells was counted by fluorescence microscopy using a hemocytometer. All of the cell counts are reported as mean ± standard deviation.
Ovarian Germ Cell Transplantation and Analysis
Ovarian germ cells obtained from the interfaces of the 25%–30% and 30%–35% Percoll fractions were combined, resuspended in 20 μl of Leibovitz L-15 medium (Sigma-Aldrich), and immediately transplanted into recipient hybrid larvae. Cell transplantations were performed using a glass micropipette needle (30- to 50-μm diameter borosilicate glass capillaries) under a stereomicroscope. Similar to the published methods using transplantation of trout PGCs into the peritoneal cavity [23], the ovarian germ cells were transplanted into the abdominal cavity under the swim bladder close to the gonads of 2-wk-old sterile Danio hybrid larvae. Development of the chimeric larvae was observed, and photographs were taken with a fluorescence microscope equipped with a digital camera. Estimates of the total number of cells and the germ cells transplanted into each recipient were made by counting the number of EGFP-positive and DsRed-positive cells in the cell suspension. Following transplantation, the actual number of DsRed-positive cells present in each of 10 recipients was counted, and the total number of cells transplanted into each recipient was estimated based on the ratio of DsRed-positive to EGFP-positive cells present in the initial cell suspension. Five days after transplantation, all the recipients were examined by fluorescence microscopy and the potential germline chimeras were identified based on the presence of DsRed-positive cells in the gonad region. Six weeks after transplantation, 10 recipients (from 2 transplantations) were euthanized, and the status of the transplanted ovarian germ cells and the developmental stage of the gonads were evaluated. To determine if the transplanted ovarian cells were able to generate functional gametes in the hybrid recipients, the chimeric fish were raised to sexual maturity and paired with wild-type zebrafish. Three batches of eggs from each paired fish were collected, and the efficiency of fertilization was calculated.
RESULTS
Production of Sterile Danio Hybrid Larvae
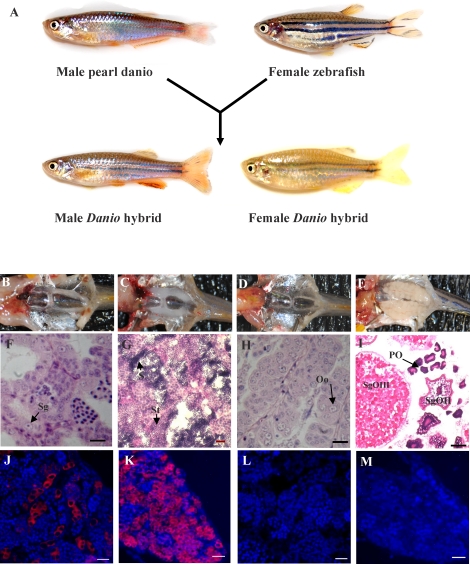
Hybrid recipient larvae used for the cell transplantation experiments were produced by in vitro fertilization using wild-type zebrafish eggs and pearl danio sperm (Fig. 1A). Development of the hybrid embryos was normal, and the adult males possessed a distinguishable phenotype (Fig. 1A). The majority of the male hybrids (90%) began to display normal mating behavior by 3 mo of age based on their ability to induce both zebrafish and pearl danio females to spawn although the eggs were not able to be fertilized. Delayed spawning behavior was observed at 5 mo in approximately 10% of the hybrid male fish. When hybrid females were paired with either zebrafish or pearl danio males, spawning behavior was induced in the males although eggs were not produced by the hybrid females. Dissection of the hybrid fish revealed that the adults possessed paired, normal shaped gonads that were smaller than the gonads present in comparably sized zebrafish (Fig. 1, B–E), and the partially developed testes of male hybrids (Fig. 1B) were approximately 60% smaller than those of adult zebrafish (Fig. 1C). Histological analysis showed that the testis of adult hybrid fish (Fig. 1F) possessed some degree of development based on the formation of seminiferous lobules, spermatogonia, and cysts of proliferated spermatogonia. Unlike the zebrafish testes (Fig. 1G), however, no spermatid or spermatozoa were found in the testes of adult hybrid fish, and sperm could not be expressed by gently pressing on the abdomen of the hybrid males. Gonad development was more severely reduced in the female hybrids because each ovary consisted of only a very thin filament-shaped tissue that was surrounded by adipose tissue (Fig. 1D). Histological examination revealed that the ovaries of hybrid fish contained oogonia (Fig. 1H) but lacked the large number of oocytes at various stages of maturation that were found in the adult zebrafish ovary (Fig. 1I). Vasa-expressing germ cells were present in the testes (Fig. 1J) and ovaries (Fig. 1K) of the adult hybrids even though gametogenesis was arrested in both tissues. Neither male nor female hybrids were able to produce functional gametes.
FIG. 1.
Production of sterile Danio hybrid fish. A) Hybrid fish were produced by in vitro fertilization of zebrafish eggs with pearl danio sperm. Gonad development in the adult hybrid male (B), zebrafish male (C), hybrid female (D), and zebrafish female (E). Transverse sections of gonads from adult hybrid male (F), Bouin-paraffin section, zebrafish male (G), hybrid female (H), and Bouin-paraffin section, zebrafish female (I). Fluorescence photomicrographs showing the expression of Vasa protein (red) in hybrid testis (J) and hybrid ovary (K); normal rabbit serum as controls in hybrid testis (L) and hybrid ovary (M). Sg = spermatogonium; St = spermatid; S = spermatozoa; Oo = oogonia; PO = primary oocytes; SgOII = secondary-growth oocyte phase II; SgOIII = secondary-growth oocyte phase III. Bars = 20 μm (F–H, J–L, M) and 100 μm (I).
Ovarian Germ Cell Isolation and Transplantation
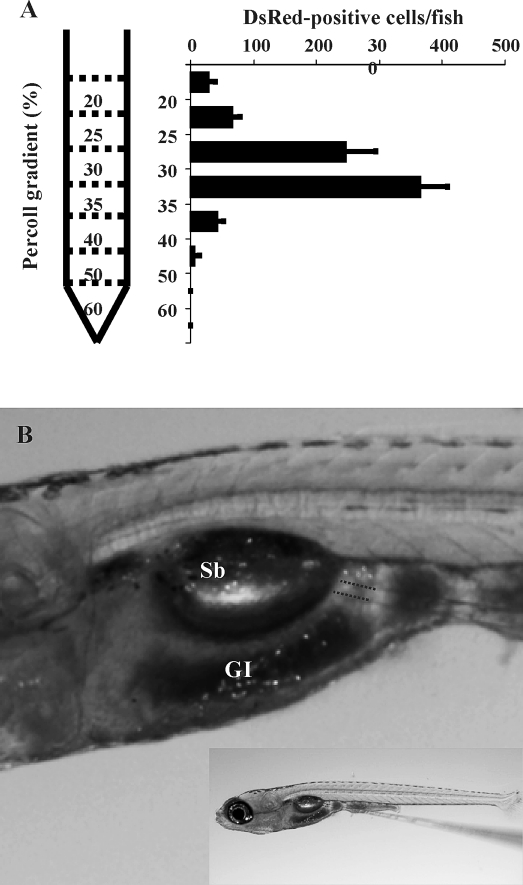
Cell transplantation experiments were performed using donor cells obtained from 3-mo-old adult Tg(vasa:DsRed2-vasa);Tg(bactin:EGFP) double transgenic zebrafish that express EGFP throughout their bodies under the control of the zebrafish bactin promoter and DsRed specifically in the germ cells driven by the zebrafish vasa promoter. Using this strategy, we were able to visualize the entire population of transplanted cells (EGFP-positive) and also identify the ovarian germ cells (DsRed-positive) among the transplanted cell population. DsRed is expressed in ovarian germ cells at different developmental stages in the transgenic fish (Fig. 2, A and B). In order to obtain a cell fraction for transplantation that is enriched with the germ cells, whole ovarian tissue obtained from the transgenic fish was dissociated and the cells fractionated on a discontinuous Percoll gradient. Using this method, an average of 755 ± 108 DsRed-positive ovarian germ cells were recovered from each female. The majority of DsRed-positive cells were obtained in two fractions taken at the 30%–35% (364 ± 44 cells) and 25%–30% (247 ± 48 cells) Percoll interface (Fig. 3A). Cells contained in these two fractions were combined and used for transplantation experiments. The cells were transplanted directly into the abdominal cavity under the swim bladder (Fig. 3B) of 2-wk-old hybrid larvae. Immediately after transplantation, the number of DsRed-positive germ cells present in the abdominal cavity of each larva was 12 ± 4.7 (n = 10) based on direct counts made by fluorescence microscopy. The total number of cells transplanted into each host was estimated to be 620 ± 242 based on the number of DsRed-positive cells that were counted in the abdominal cavity and the ratio of EGFP-positive to DsRed-positive cells in the cell suspension for transplantation. Use of the Percoll gradient centrifugation step increased the ratio of DsRed-positive and EGFP-positive cells from 8.9 ± 2.1 to 193.3 ± 40.4 per 10 000 cells in the initial cell suspension and combined Percoll fractions, respectively.
FIG. 2.
A) Photomicrograph showing the expression of DsRed (white arrows) in ovarian germ cells in the ovary of a Tg(vasa:DsRed2-vasa);Tg(bactin:EGFP) double transgenic zebrafish. B) Same ovary shown at higher magnification. Bar = 100 μm.
FIG. 3.
The isolation and transplantation of ovarian germ cells. DsRed-expressing ovarian cells from Tg(vasa:DsRed2-vasa);Tg(bactin:EGFP) double transgenic zebrafish were partially purified by discontinuous Percoll gradient centrifugation. A) The number of DsRed-positive cells obtained from each gradient fraction (mean ± SD). B) Isolated ovarian germ cells were transplanted into the abdominal cavity between the swim bladder (SB) and gastrointestinal (GI) tract of 2-wk-old sterile hybrid larvae. Location of transplanted cells (dotted lines). Length of larva = 5 mm.
Gonadal Development and Fertility of the Chimeras
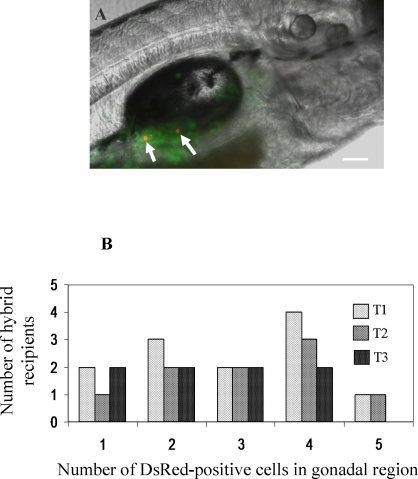
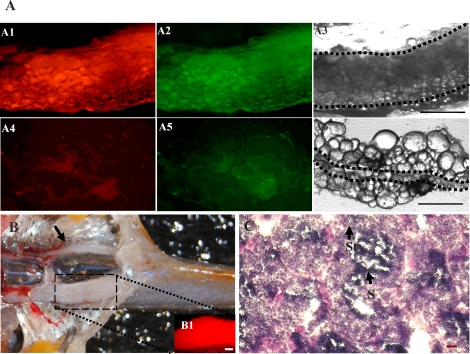
Five days after transplantation, the recipient larvae were examined by fluorescence microscopy. A total of 104 recipients were examined from 3 different transplantations, and 31 (29.9 ± 3.9%) individuals that possessed an average of 2.8 ± 1.4 DsRed-positive cells (1–5 cells per embryo) in the gonadal region were identified (Fig. 4, A and B, and Table 1). Six weeks after transplantation, a group of the 8-wk-old fish from the second and third experiments were euthanized. The gonads were dissected and examined for the presence of DsRed-positive cells. Two positive fish were identified among 10 recipients that were examined. Inspection of the two fish revealed that the transplanted ovarian germ cells had colonized the gonad of the hybrid hosts and proliferated extensively based on the presence of DsRed-positive and EGFP-positive cells throughout the tissue (Fig. 5A, A1–A3). In the remaining eight recipients that were examined, neither bright DsRed-positive nor EGFP-positive cells were detected in the gonadal region (Fig. 5A, A4–A6). All of the remaining recipient fish from the transplantation experiments were raised to sexual maturity and bred pairwise with zebrafish mates. A total of 67 adult recipients were bred, and embryos were obtained from 12 of the male hybrid chimeric fish paired with zebrafish females. The fertile male chimeras continued to produce normal embryos at 6 and 9 mo of age when they were bred with female zebrafish. The fertile male chimeras also produced numerous offspring when paired with female pearl danio mates (data not shown). Embryos were not obtained from any of the female recipient fish bred with male zebrafish or pearl danio mates even after 6 and 9 mo. The pairing of female recipient hybrids with male pearl danio mates did not result in successful egg production. Examination of the gonads from the hybrid recipient fish revealed that the infertile male and female chimeras possessed undersized gonads that closely resembled those of the nonrecipient hybrid fish in size and degree of gametogenesis (Fig. 1). In contrast, all six of the fertile male chimeric hybrid fish that were examined possessed one partially developed and one fully developed testis, the latter were completely colonized with DsRed-positive cells (Fig. 5, B1 inset). Histological analysis revealed that the fully developed testis from each of the fertile male chimeras resembled a wild-type adult zebrafish testis containing spermatid and spermatozoa (Fig. 5C). Sperm was expressed from the fertile chimeric hybrids by gently pressing on the abdomen.
FIG. 4.
A) Photomicrograph showing the incorporation of transplanted ovarian germ cells into the gonad of a recipient larva 5 days after transplantation. The arrows point to individual DsRed-positive cells. Bar = 100 μm. B) The number of recipient larvae with up to 5 DsRed-positive cells present in the gonadal region 5 days after transplantation. Data from three transplantation experiments (T1–T3) are shown.
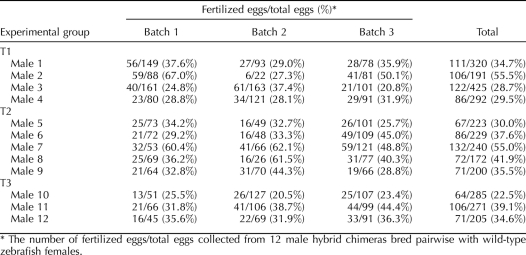
TABLE 1.
Results from three transplantation experiments using ovarian germ cells isolated from adult zebrafish females introduced into 2-wk-old sterile hybrid larvae recipients.*
FIG. 5.
Colonization of the recipient gonad by transplanted ovarian germ cells. A) Fluorescence photomicrograph of a recipient gonad 6 wk after transplantation showing that the DsRed-expressing ovarian germ cells have proliferated and completely colonized the tissue (A1–A3). A1: red fluorescence, A2: green fluorescence, A3: bright field. Fluorescence photomicrograph of a nonchimeric recipient (A4–A6). A4: red fluorescence, A5: green fluorescence, A6: bright field. Area enclosed inside the dotted lines represents the region of gonadal tissues. B) After reaching sexual maturity at 3 mo of age, the germline chimeric fish possessed one fully developed testis that contained a large number of DsRed-expressing cells derived from the transplanted germ cells (B1 inset, gonads were dissected for fluorescence detection). The other testis in the chimeric fish (black arrow) was underdeveloped. C) Transverse section of the fully developed testis from a fertile male chimera that contains spermatid (St) and spermatozoa (S). Bars = 100 μm (A3, A6, B1) and 20 μm (C).
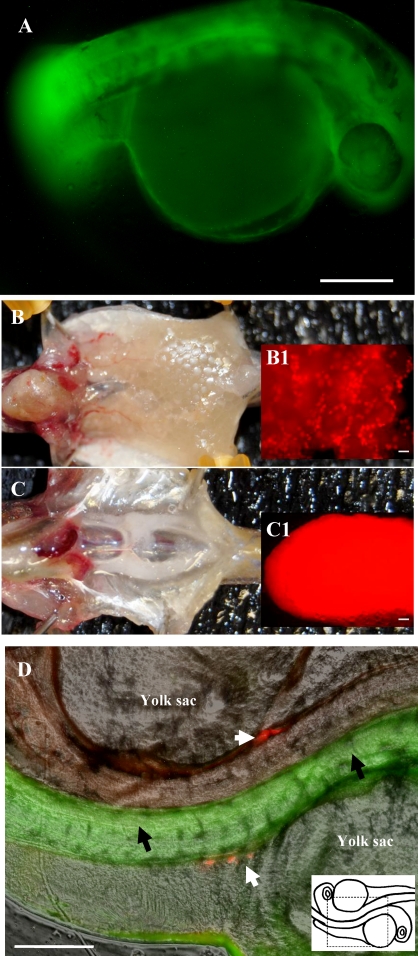
Fertility of the chimeric male fish was examined in more detail by breeding 12 of the fertile males with wild-type zebrafish females and counting the number of embryos produced (Table 2). Compared to control matings of wild-type male and female zebrafish (70% to 95% fertilization rate), the chimeric males bred with zebrafish females produced fewer fertilized embryos (23% to 56% fertilization rate, Table 2). The embryos fertilized by the chimeric males possessed normal development and expressed EGFP in all their tissues (Fig. 6A), confirming that they were fertilized by sperm derived from the transplanted ovarian germ cells. As previously reported, zygotic expression of the transgenic vasa promoter does not occur in the zebrafish embryo until 21 days postfertilization (dpf) [24, 25]. As a result, the vasa-DsRed expression was not observed in any of the F1 embryos produced by the chimeric males and wild-type female zebrafish. Vasa-DsRed expression was observed in the F1 individuals after 21 days (Fig. 6, B and C). The combined sex ratio determined for a group of F1 fish obtained from each of the two transplantation experiments was 31 males and 52 females. The F1 adult fish possessed paired gonads that were of normal size (Fig. 6, B and C,) when compared to wild-type zebrafish and expressed DsRed throughout the tissue (Fig. 6, B1 inset and C1 inset). The F1 fish were fertile and exhibited a higher fertilization rate (an average of 82%) than the founder chimeric fish (23% to 56%, Table 2). An F2 generation was produced by crossing male and female F1 fish. Fluorescence microscopy revealed that all 108 F2 embryos examined at 2 dpf expressed DsRed in the gonadal region from maternal RNA, and 76 of the embryos expressed EGFP throughout their bodies (Fig. 6D).
TABLE 2.
Fertilization efficiency of male germline chimeric fish.
FIG. 6.
A) Fluorescence photomicrograph of an F1 embryo produced by a germline chimeric father showing EGFP expression throughout its body indicating that the embryo was produced from sperm derived from the transplanted Tg(vasa:DsRed2-vasa);Tg(bactin:EGFP) double transgenic ovarian germ cells. Because vasa-DsRed expression in the embryo is from maternal RNA, DsRed expression was not observed in the F1 embryos. B, C) The F1 adult fish possessed paired gonads that were of normal size (B, ovary; C, testis) and expressed DsRed (B1 inset and C1 inset, gonads were dissected for fluorescence detection) throughout the tissue. D) The F2 embryos produced by mating heterozygous F1 siblings did express maternal vasa-DsRed in the gonadal region (white arrows) and EGFP expression (black arrows) throughout their bodies in approximately 75% of the F2 embryos. Right bottom corner: schematic drawing of the area where the photo was taken. Bars = 200 μm.
DISCUSSION
The results of this study demonstrate that germ cells obtained from ovaries of adult zebrafish can be transplanted into recipient larvae to generate germline chimeras at a high frequency. Identification of the germline chimeras was facilitated by the use of Danio hybrid recipient larvae. Since the hybrid adult fish are sterile, the germline chimeras were conveniently identified by reversal of the infertile phenotype resulting in successful embryo production. An important aspect of this strategy is the fact that the Danio hybrids are able to induce mating behavior in zebrafish mates even though zebrafish do not respond when paired with the pearl danio. Confirmation that the F1 embryos produced by the chimeric hybrids were derived from the transplanted germ cells was obtained by using donor cells isolated from Tg(vasa:DsRed2-vasa);Tg(bactin:EGFP) double transgenic zebrafish. The F1 embryos produced by the germline chimeric parent expressed EGFP driven by the zebrafish bactin promoter throughout their bodies. Because vasa-DsRed transgene expression in the early embryo is from maternal RNA [24, 25], the F1 embryos generated by germline chimeric fathers did not express the fluorescent protein until after 21 days when zygotic vasa-DsRed expression occurs. Maternal vasa-DsRed expression was observed in all of the F2 embryos produced by crossing F1 siblings. As expected, bactin-EGFP expression was observed in about 75% of the F2 embryos produced by breeding heterozygous F1 siblings.
Of the 12 fertile male germline chimeras produced in our study, 6 of the fish that were randomly sacrificed and examined possessed only one fully developed testis. The second testis was underdeveloped, being similar in size and appearance to the testes found in the infertile hybrid males. Because the cell transplantations were conducted by inserting the needle into one side of the recipient larva's body, it is likely that most of the transplanted germ cells were incorporated into only one of the developing gonads. The gonad that did not receive the transplanted cells developed into the smaller testis. The fertile male germline chimeras were found to exhibit a fertilization efficiency that was approximately 60% lower than wild-type zebrafish. The lower fertility is most likely due to the presence of only one fully developed gonad. The transplanted germ cells had to compete with the large number of nonfunctional spermatogonia that were also present in the testes of the hybrid recipients.
Of the 67 potential germline chimeric fish that survived to sexual maturity, only 9 were females and all 12 of the fertile germline chimeras were males. A similar low percentage of females was also found in the general population of hybrid fish in our laboratory. Examination of each female recipient revealed that the gonads were underdeveloped and had a filamentlike appearance similar to those found in the nonrecipient hybrid females. There were no brightly fluorescent DsRed-positive or EGFP-positive cells found in any of the recipient female gonads. The lack of female germline chimeras may be a result of the low percentage of females in the general population of recipient fish. It could also be a result of the rudimentary ovary development that was observed in the female hybrid, which may have provided a poor environment for the transplanted cells compared to the relatively well-developed testes found in the male hybrids.
Our results demonstrating complete colonization of an infertile host gonad by a small number of transplanted adult ovarian germ cells is consistent with the existence of a stem cell population in the fish ovary [7–9]. The male germline chimeric fish that were produced in this study were able to generate an average of 47 embryos each day when they were paired with individual zebrafish females. After 1 yr, the chimeric males continued to produce an average of 120 embryos each day when paired with groups of 3 pearl danio females. Also, examination of transverse tissue sections made from the testes dissected from the fertile male chimeras showed the presence of thousands of spermatids and spermatozoa, confirming that the transplanted ovarian cells were able to completely colonize the testis and recapitulate each stage of spermatogenesis.
Recently, Yoshizaki et al. [8] described a similar ovarian germ cell transplantation system in rainbow trout. The authors transplanted cells isolated from ovaries of 6- to 9-mo-old rainbow trout into recipient fry resulting in the production of one female and three male germline chimeras. All of the F1 individuals produced by the male trout chimeras were female, which was consistent with heterogametic (XY) male sex determination. In contrast, the F1 generation produced by the zebrafish male chimeras in our study consisted of both male (38%) and female (62%) individuals. Because the entire population of germ cells in the male chimera was derived from transplanted ovarian cells, the production of male F1 progeny indicates that zebrafish male sex determination is not controlled by sex chromosome heterogamy.
Germ cell transplantation has become a very useful technology in the field of reproductive biology. The technology was first developed by Brinster and Avarbock [26] in 1994 using mouse spermatogonial stem cells and has since been applied to other species including fish [8, 23, 27–32]. In fish, germ cell transplantation has been performed using PGCs [23, 27], spermatogonia [28–32], and oogonia [8]. In addition to the transplantation of fish germ cells to a recipient gonad, dissociated zebrafish testes has been inserted under the abdominal skin of a recipient fish to generate functional sperm [33]. The cell transplantation system described in this study provides an efficient strategy to generate zebrafish germline chimeras. The use of infertile Danio hybrid recipients makes it convenient to identify germline chimeras by the reversal of the infertile phenotype. The infertile recipients offer a useful system to optimize the efficiency of germline chimera production and to produce only donor-derived gametes. A similar approach has been reported by others using trout [8, 29] and zebrafish [27]. Another strategy that has been applied to fish is the depletion of endogenous germ cells by treatment with busulfan [30–32]; however, this approach resulted in a low transplantation efficiency in each species. The Percoll gradient centrifugation step used in our study provided a convenient method to obtain cell fractions that were enriched approximately 20-fold with ovarian germ cells. When we performed two initial transplantation experiments using nonfractionated cell suspensions, the DsRed-positive ovarian cells were difficult to find in the abdominal cavity of the recipient fish and no germline chimeras were produced. During our attempts to optimize the transplantation technique, we performed experiments using recipient embryos at the blastula stage, that is, 1, 2, and 7 dpf; however, the studies were not successful due to the inability to introduce a sufficient number of cells and the high mortality that resulted from manipulating the early-stage embryos. Use of the recipient larvae at an appropriate development stage, 14-dpf in zebrafish, makes it possible to introduce large number of cells into the host, and the direct isolation of germ cells from dissociated gonadal tissue without the need to use fluorescence-activated flow cytometry will make this transplantation approach applicable to other fish species.
ACKNOWLEDGMENT
We thank Dr. Holger Knaut for antiserum against zebrafish Vasa.
Footnotes
Supported by a grant (NIHR01GM069384) from the National Institutes of Health.
REFERENCES
- Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A 1994; 91: 11298 11302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S. The number of oocytes in the mature ovary. Recent Prog Horm Res 1951; 6: 63 109 [Google Scholar]
- Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004; 428: 145 150 [DOI] [PubMed] [Google Scholar]
- Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y, Hou R, Wu J. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol 2009; 11: 631 636 [DOI] [PubMed] [Google Scholar]
- Wallace RA, Selman K. Ultrastructural aspects of oogenesis and oocyte growth in fish and amphibians. J Electron Microsc Tech 1990; 16: 175 201 [DOI] [PubMed] [Google Scholar]
- McMillan DB. Ovarian follicles. McMillan DB. Fish Histology: Female Reproductive Systems. Dordrecht, The Netherlands: Springer; 2007: 67 208 [Google Scholar]
- Draper BW, McCallum CM, Moens CB. nanos1 is required to maintain oocyte production in adult zebrafish. Dev Biol 2007; 305: 589 598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki G, Ichikawa M, Hayashi M, Iwasaki Y, Miwa M, Shikina S, Okutsu T. Sexual plasticity of ovarian germ cells in rainbow trout. Development 2010; 137: 1227 1230 [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kobayashi K, Nishimura T, Higashijima S, Tanaka M. Identification of germline stem cells in the ovary of the teleost medaka. Science 2010; 328: 1561 1563 [DOI] [PubMed] [Google Scholar]
- Eisen JS. Zebrafish make a big splash. Cell 1996; 87: 969 977 [DOI] [PubMed] [Google Scholar]
- Zon LI. Zebrafish: a new model for human disease. Genome Res 1999; 9: 99 100 [PubMed] [Google Scholar]
- Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov 2005; 4: 35 44 [DOI] [PubMed] [Google Scholar]
- Briggs JP. The zebrafish: a new model organism for integrative physiology. Am J Physiol Regul Integr Comp Physiol 2002; 282: R3 R9 [DOI] [PubMed] [Google Scholar]
- Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Malicki J, Stemple DL, Stainier DY, Zwartkruis F, Abdelilah S, Rangini Z, Belak J, Boggs C. A genetic screen for mutations affecting embryogenesis in zebrafish. Development 1996; 123: 37 46 [DOI] [PubMed] [Google Scholar]
- Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, van Eeden FJ, Jiang YJ, Heisenberg CP, Kelsh RN, Furutani-Seiki M, et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 1996; 123: 1 36 [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book : A Guide for the Laboratory Use of Zebrafish (Danio rerio). Eugene, OR: University of Oregon Press; 2000. [Google Scholar]
- Wong TT, Zohar Y. Novel expression of gonadotropin subunit genes in oocytes of the gilthead seabream (Sparus aurata). Endocrinology 2004; 145: 5210 5220 [DOI] [PubMed] [Google Scholar]
- Knaut H, Pelegri F, Bohmann K, Schwarz H, Nusslein-Volhard C. Zebrafish vasa RNA but not its protein is a component of the germ plasm and segregates asymmetrically before germline specification. J Cell Biol 2000; 149: 875 888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Moon J, Wong TT, Crodian J, Collodi P. Zebrafish primordial germ cell cultures derived from vasa::RFP transgenic embryos. Stem Cells Dev 2008; 17: 585 597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima S, Okamoto H, Ueno N, Hotta Y, Eguchi G. High-frequency generation of transgenic zebrafish which reliably express GFP in whole muscles or the whole body by using promoters of zebrafish origin. Dev Biol 1997; 192: 289 299 [DOI] [PubMed] [Google Scholar]
- Morena AR, Boitani C, Pesce M, De Felici M, Stefanini M. Isolation of highly purified type A spermatogonia from prepubertal rat testis. J Androl 1996; 17: 708 717 [PubMed] [Google Scholar]
- Yoshikawa H, Morishima K, Fujimoto T, Saito T, Kobayashi T, Yamaha E, Arai K. Chromosome doubling in early spermatogonia produces diploid spermatozoa in a natural clonal fish. Biol Reprod 2009; 80: 973 979 [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Yoshizaki G, Takeuchi T. Generation of live fry from intraperitoneally transplanted primordial germ cells in rainbow trout. Biol Reprod 2003; 69: 1142 1149 [DOI] [PubMed] [Google Scholar]
- Krovel AV, Olsen LC. Expression of a vas::EGFP transgene in primordial germ cells of the zebrafish. Mech Dev 2002; 116: 141 150 [DOI] [PubMed] [Google Scholar]
- Krovel AV, Olsen LC. Sexual dimorphic expression pattern of a splice variant of zebrafish vasa during gonadal development. Dev Biol 2004; 271: 190 197 [DOI] [PubMed] [Google Scholar]
- Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A 1994; 91: 11303 11307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Goto-Kazeto R, Arai K, Yamaha E. Xenogenesis in teleost fish through generation of germ-line chimeras by single primordial germ cell transplantation. Biol Reprod 2008; 78: 159 166 [DOI] [PubMed] [Google Scholar]
- Okutsu T, Suzuki K, Takeuchi Y, Takeuchi T, Yoshizaki G. Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish. Proc Natl Acad Sci U S A 2006; 103: 2725 2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okutsu T, Shikina S, Kanno M, Takeuchi Y, Yoshizaki G. Production of trout offspring from triploid salmon parents. Science 2007; 317: 1517 [DOI] [PubMed] [Google Scholar]
- Majhi SK, Hattori RS, Yokota M, Watanabe S, Strussmann CA. Germ cell transplantation using sexually competent fish: an approach for rapid propagation of endangered and valuable germlines. PLoS One 2009; 4: e6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda SM, Batlouni SR, Costa GM, Segatelli TM, Quirino BR, Queiroz BM, Kalapothakis E, Franca LR. A new and fast technique to generate offspring after germ cells transplantation in adult fish: the Nile tilapia (Oreochromis niloticus) model. PLoS One 2010; 5: e10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega RH, Greebe CD, van de Kant H, Bogerd J, de Franca LR, Schulz RW. Spermatogonial stem cell niche and spermatogonial stem cell transplantation in zebrafish. PLoS One 2010; 5: e12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Saito K, Shinya M, Olsen LC, Sakai N. Regeneration of spermatogenesis and production of functional sperm by grafting of testicular cell aggregates in zebrafish (Danio rerio). Biol Reprod 2010; 83: 533 539 [DOI] [PubMed] [Google Scholar]